Abstract
Aim
To assess the relationship between weight change and glycated haemoglobin (HbA1c) change in dulaglutide‐treated patients by analysing data from six head‐to‐head phase III AWARD clinical trials.
Methods
At 26 weeks, the relationship between weight and HbA1c was analysed in each trial rather than by pooling data because of differences in design and background therapy. The effect of baseline characteristics was also evaluated with regard to weight and HbA1c response.
Results
Across the studies, 87–97% and 83–95% of patients treated with dulaglutide 1.5 and 0.75 mg, respectively, had reductions in HbA1c levels, while 57–88% and 43–84% of patients treated with dulaglutide 1.5 and 0.75 mg, respectively, experienced weight loss. The majority (55–83%) of patients receiving dulaglutide 1.5 mg experienced weight loss and HbA1c reductions, while 41–79% of patients in the dulaglutide 0.75 mg arm lost weight and had reductions in HbA1c level. A weak and inconsistent correlation was observed between the changes in weight and HbA1c (range from −0.223 to 0.267) in patients treated with dulaglutide. The baseline characteristics of gender, age, duration of diabetes, HbA1c, body weight and BMI were not related to different combinations of weight and HbA1c responses.
Conclusions
Dulaglutide is an effective treatment option across the type 2 diabetes treatment spectrum. Dulaglutide showed dose‐dependent effects on both weight loss and HbA1c reduction. These effects had a weak correlation and appeared to be independent.
Keywords: dulaglutide, GLP‐1 analogueincretin therapy, incretin therapy, type 2 diabetes
Introduction
The risk of developing type 2 diabetes (T2D) increases dramatically in obese people. Adults with a body mass index (BMI) >35 kg/m2 are 20 times more likely to develop T2D than those with a BMI between 18.5 and 24.9 kg/m2 1. Obesity also complicates the management of T2D by causing insulin resistance and increasing blood glucose, and many antihyperglycaemic medications (thiazolidinediones, sulphonylureas and insulin) potentiate further weight gain 2, 3, 4. By contrast, weight reduction improves glycaemic control and other cardiovascular risk factors in patients with T2D 4, 5, 6. Intentional weight loss can decrease glycated haemoglobin (HbA1c) levels in overweight or obese patients with T2D 7, therefore, weight control is an important component of the individualized treatment of patients with T2D.
The American Diabetes Association and the European Association for the Study of Diabetes recommend glucagon‐like peptide‐1 (GLP‐1) receptor agonists as one of the second‐agent options added on to metformin or as a third agent within more complex treatment regimens for T2D 4, 5. GLP‐1 receptor agonists have the advantages of being associated with weight loss as well as lowering blood glucose in patients with T2D 4. The relationship between weight change and glycaemic control of GLP‐1 receptor agonists has been investigated with other GLP‐1 receptor agonists such as exenatide and liraglutide 8, 9, 10.
Dulaglutide, a GLP‐1 receptor agonist recently approved for the treatment of T2D, exhibits both the effect of glycaemic control and the potential for weight loss. The efficacy and safety of dulaglutide 1.5 and 0.75 mg were explored across the diabetes treatment continuum in six Assessment of Weekly AdministRation of LY2189265 in Diabetes (AWARD) trials. In these studies, it was used as monotherapy, combination therapy with one or two oral antihyperglycaemic medications, or combination therapy with mealtime insulin lispro 11, 12, 13, 14, 15, 16. At the primary end point, dulaglutide 1.5 mg showed significantly greater reductions in HbA1c levels than metformin 11, sitagliptin 12, exenatide twice daily 14 or insulin glargine 15, 16, and similar reductions in comparison to liraglutide 13. Dulaglutide 0.75 mg resulted in significantly greater reductions in HbA1c levels compared with metformin 11, sitagliptin 12, exenatide twice daily 14 and insulin glargine in combination with insulin lispro, with or without metformin 16, and similar reductions compared with insulin glargine added on to metformin and sulphonylurea 15. In addition, a weight reduction was observed with dulaglutide that was consistent with the GLP‐1 receptor agonist class, and the incidence of hypoglycaemia in dulaglutide‐treated patients was similar to or lower than that in patients treated with the active comparators 11, 12, 13, 14, 15, 16.
Given the importance of HbA1c control and weight management in people with T2D and the effect of dulaglutide on these variables, we sought to further understand the relationship, if any, between these two important clinical measures. The objective of the present analysis was to assess the relationship between changes in weight and glycaemic control in patients treated with dulaglutide at 26 weeks. Specifically, we analysed the relationship between weight change and HbA1c change, the effect of baseline characteristics on different weight and HbA1c responses, and the effect of nausea and/or vomiting on weight loss.
Research Design and Methods
Design of Clinical Trial Programme
The designs of the six clinical trials included in the present analysis are shown in Table 1. The AWARD clinical trials were randomized controlled clinical studies, ranging from 26 to 104 weeks' duration, with a total of 5171 patients. They were designed to evaluate the safety and efficacy of dulaglutide in adult patients with T2D, with primary endpoints at 26 or 52 weeks, depending on the individual study. The trials used HbA1c reduction from baseline as the primary efficacy measure, and were designed to either assess the superiority of dulaglutide compared with placebo, or the non‐inferiority of dulaglutide compared with active comparators, with a subsequent test for superiority if non‐inferiority was met. These studies did not include any specific recommendation regarding diet and exercise beyond the usual practice of each study centre, and concomitant therapy with prescription or over‐the‐counter drugs that promote weight loss was not allowed. The detailed methods used in the individual trials have been previously published 11, 12, 13, 14, 15, 16.
Table 1.
Baseline characteristics and demographics.
| Umpierrez et al. | Nauck et al. | Dungan et al. | Wysham et al. | Giorgino et al. | Blonde et al. | |
|---|---|---|---|---|---|---|
| AWARD‐3 | AWARD‐5 | AWARD‐6 | AWARD‐1 | AWARD‐2 | AWARD‐4 | |
| Concomitant medication | None | Metformin | Metformin | Metformin + pioglitazone | Metformin + glimepiride | Insulin lispro w/wo metformin |
| Active comparator | Metformin* | Sitagliptin 100 mg | Liraglutide 1.8 mg | Exenatide 10 µg twice daily | Insulin glargine | Insulin glargine |
| N, intention‐to‐treat | 807 | 1098 | 599 | 976 | 807 | 884 |
| Sex, female, % | 56.3 | 52.6 | 52.1 | 41.6 | 48.7 | 46.5 |
| Age, years | 55.6 | 54.1 | 56.7 | 55.6 | 56.7 | 59.4 |
| Diabetes duration, years | 2.6 | 7.1 | 7.2 | 8.8 | 9.1 | 12.7 |
| HbA1c, % | 7.6 | 8.1 | 8.1 | 8.1 | 8.1 | 8.5 |
| Weight, kg | 92.3 | 86.4 | 94.1 | 96.0 | 86.3 | 91.1 |
| BMI, kg/m2 | 33.3 | 31.2 | 33.6 | 33.2 | 31.5 | 32.5 |
DU, dulaglutide; HbA1c, glycated haemoglobin; w/wo, with/without.
Patients received 1500–2000 mg/day, according to tolerability.
Statistical Analyses
Because of differences in design and background therapy, the AWARD‐1 to ‐6 trials were analysed separately using data at 26 weeks, a time point common to all of the studies at which, in general, a maximum effect of dulaglutide on HbA1c and weight was observed. To better understand the potential impact of concomitant medications associated with weight gain, two pooled analyses were conducted on monotherapy and metformin add‐on trials together, as well as the trials with concomitant medications associated with weight gain together. All analyses were conducted on the intention‐to‐treat population consisting of all randomized patients who received at least one dose of study treatment. The correlation coefficient of weight change and HbA1c change was calculated. A logistic regression analysis, adjusted for country, baseline HbA1c and baseline body weight, was performed on the composite endpoint of achieving weight change ≤0 kg and HbA1c change ≤0%. Means of baseline characteristics including gender, age, duration of diabetes, HbA1c, body weight and BMI were compared among patient groups with HbA1c reduction and weight loss, HbA1c reduction and weight gain, HbA1c increase and weight loss, or HbA1c increase and weight gain. We also investigated the relationship between weight and HbA1c changes through summarizing HbA1c changes according to category of weight changes at week 26: weight gain; weight loss of ≥0 to ≤3%; weight loss of >3 to ≤5%; weight loss of >5 to ≤10%; and weight loss >10%. Weight changes at 26 weeks were compared between patients with and without nausea and/or vomiting. The last post‐baseline observation was carried forward (LOCF) in the case of missing 26‐week HbA1c or body weight measurements.
Mean and standard deviation values, and correlations are presented for continuous variables, as well as scatterplots of changes from baseline in HbA1c versus changes from baseline in weight. Percentages and bar graphs are presented for categorical variables.
Results
A total of 5171 patients with T2D were enrolled in the six trials, and 1719 and 1417 patients received dulaglutide 1.5 and 0.75 mg treatment, respectively. The baseline characteristics and demographics of these patients are shown in Table 1; within each study, baseline characteristics were similar across the treatment arms.
Glycated Haemoglobin Change and Weight Change from Baseline at 26 Weeks
Dulaglutide treatment was found to have a dose‐dependent effect on HbA1c reduction. Both doses of dulaglutide had a significant HbA1c reduction from baseline across the continuum of diabetes treatments. HbA1c reductions for dulaglutide 1.5 mg ranged from 0.78% in the monotherapy study to 1.64% in the basal‐bolus trial (in combination with insulin lispro with or without metformin) and for dulaglutide 0.75 mg ranged from 0.71% in the monotherapy study to 1.59% in the basal‐bolus trial (Figure 1A). At 26 weeks, dulaglutide 1.5 mg resulted in a significantly greater reduction in HbA1c in comparison to metformin, sitagliptin, exenatide twice daily and insulin glargine, and a similar reduction in comparison to liraglutide (Figure 1A). Dulaglutide 0.75 mg resulted in a significantly greater reduction in HbA1c compared with metformin, sitagliptin, exenatide twice daily and insulin glargine at 26 weeks (Figure 1A).
Figure 1.
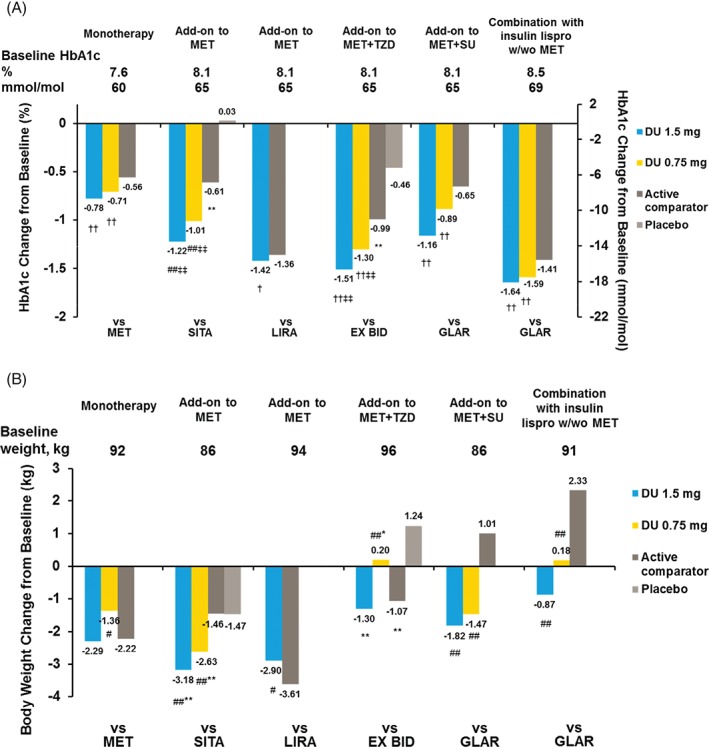
Glycated haemoglobin (HbA1c) (A) and weight (B) changes from baseline at 26 weeks. Data presented are least‐squares means, intention‐to‐treat, LOCF analysis of covariance. ††p < 0.025 superiority versus active comparator; †p < 0.001 non‐inferiority versus active comparator, margin = 0.4%; #p < 0.05, ##p < 0.001 versus active comparator; ‡‡p < 0.001 superiority versus placebo; *p < 0.05, **p < 0.001 versus placebo.
Weight changes were also dose‐dependent and varied based on the background therapy but not according to baseline BMI (data not shown). Weight change for dulaglutide 1.5 mg ranged from −0.87 kg in the basal‐bolus study to −3.18 kg in the trial comparing it with sitagliptin added on to metformin, while, for dulaglutide 0.75 mg, the weight change ranged from +0.20 kg in the study comparing it with exenatide twice daily with maximally tolerated doses of metformin and pioglitazone to −2.63 kg in the trial with sitagliptin added on to metformin as the comparator (Figure 1B). The greatest weight reductions were observed when dulaglutide was added to background metformin. With this combination, more patients (24–34%) lost >5% weight than with dulaglutide monotherapy (12–17%) or with other background therapies (9–18%; Table S1, Supporting Information).
Relationship Between Weight Change and Change in Glycated Haemoglobin at 26 Weeks
Across the studies, 87–97% and 83–95% of patients treated with dulaglutide 1.5 and 0.75 mg, respectively, had reduced HbA1c levels (Figure 2), while 57–88% and 43–84% of patients treated with dulaglutide 1.5 and 0.75 mg, respectively, experienced weight loss. The majority of patients (55–83%) receiving dulaglutide 1.5 mg experienced both weight loss and HbA1c reduction while 41–79% in the dulaglutide 0.75 mg arm experienced both weight loss and HbA1c reduction (Figure 2). Dulaglutide 1.5 mg resulted in a greater proportion of patients with both weight and HbA1c reductions compared with metformin (p = 0.04), sitagliptin (p < 0.001), exenatide twice daily (p = 0.023) and insulin glargine (p < 0.001) and a similar proportion compared with liraglutide (p = 0.115). Dulaglutide 0.75 mg resulted in a greater proportion of patients with both weight and HbA1c reductions compared with sitagliptin (p < 0.001) and insulin glargine (p < 0.001) and a similar proportion compared with metformin (p = 0.061) and exenatide twice daily (p = 0.107). Low and inconsistent correlation coefficients for each of the six studies (−0.223 to 0.267 for dulaglutide, 0.248 for liraglutide, and 0.202 for exenatide twice daily) were observed between the changes in weight and HbA1c (Figure 3).
Figure 2.
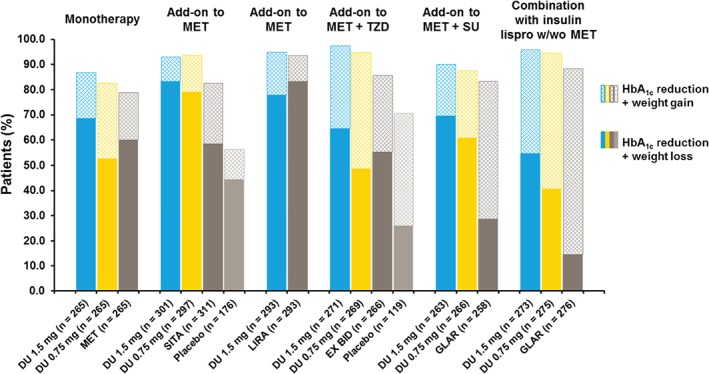
Proportions of patients with glycated haemoglobin (HbA1c) reduction and either weight reduction or weight gain at 26 weeks. DU, dulaglutide; MET, metformin; TZD, thiazolidinedione; SU, sulphonylurea; SITA, sitagliptin; LIRA, liraglutide; EX, exenatide; GLAR, insulin glargine; BID, twice daily; w/wo, with/without.
Figure 3.
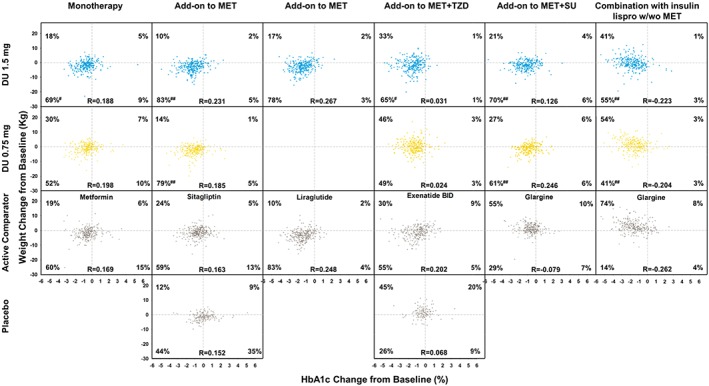
Weight change with glycated haemoglobin (HbA1c) change at 26 weeks. DU, dulaglutide; MET, metformin; TZD, thiazolidinedione; SU, sulphonylurea. #p < 0.05 and ##p < 0.001 versus active comparator.
In a categorical analysis presented in Figure 4, at 26 weeks, trends of greater HbA1c reduction were observed with more weight loss for each dulaglutide arm in the monotherapy study as well as in studies in comparison to sitagliptin and liraglutide both as add‐on to metformin. These trends were not observed in the other studies, with concomitant medications associated with weight gain. To better understand the findings in Figure 4 with respect to the potential impact of concomitant medications associated with weight gain, despite the differences in study designs, we conducted two pooled analyses. One assessed the monotherapy and the studies with therapy added on to metformin together, and the other combined the three studies with concomitant medications associated with weight gain. The correlation coefficients for the monotherapy and the add‐on‐to‐metformin trials were 0.254 and 0.237 for dulaglutide 1.5 and 0.75 mg, respectively, while the correlation coefficient of the other three studies was −0.072 for both dulaglutide doses (Figure S1, Supporting Information).
Figure 4.
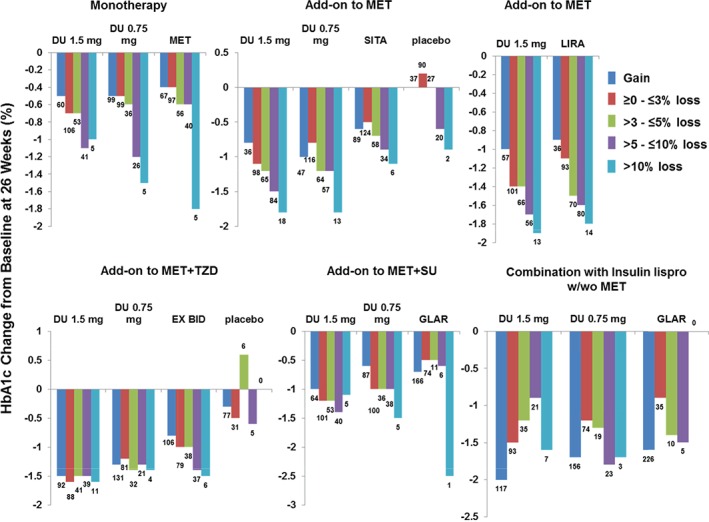
Glycated haemoglobin (HbA1c) change by weight loss at 26 weeks. DU, dulaglutide; MET, metformin; TZD, thiazolidinedione; SU, sulphonylurea; SITA, sitagliptin; LIRA, liraglutide; EX, exenatide; GLAR, insulin glargine; BID, twice daily; w/wo, with/without.
Effect of Baseline Characteristics on Weight Change and Glycated Haemoglobin Change at 26 Weeks
The baseline characteristics of gender, age, duration of diabetes, HbA1c, body weight and BMI were compiled for each quadrant of the scatter plots (HbA1c reduction and weight loss, HbA1c reduction and weight gain, HbA1c increase and weight loss, or HbA1c increase and weight gain as shown in Figure 3) by treatment arm in each of the six trials. No meaningful differences were observed in the baseline characteristics of the patients who had both HbA1c and weight reduction (lower left quadrant of the scatter plots in Figure 3) in comparison with patients in the other three quadrants (data not shown).
Effect of Nausea and/or Vomiting on Weight Change at 26 Weeks
As gastrointestinal adverse events are associated with GLP‐1 receptor agonist treatment, we assessed the impact of nausea and/or vomiting on weight change. In patients treated with dulaglutide, 8–33% had at least one occurrence of nausea and/or vomiting, whereas 43–88% of patients treated with dulaglutide experienced weight loss. Reduction in body weight was observed in patients treated with dulaglutide, irrespective of nausea and/or vomiting, although the reduction was numerically larger in the group with nausea and/or vomiting (Figure S2, Supporting Information).
Discussion
Maintaining a healthy weight is a key therapeutic goal in the management of T2D 17; however, use of some conventional antihyperglycaemic agents, such as sulphonylureas, thiazolidinediones and insulin, commonly results in weight gain; therefore, glucose‐lowering agents without weight gain effects, or those that offer the potential for weight loss, such as GLP‐1 receptor agonists, are an advantageous T2D therapy. In this post hoc analysis of six head‐to‐head trials, dulaglutide showed a weak correlation between HbA1c reduction and weight effects.
Across the studies, the majority (55–83%) of patients receiving dulaglutide 1.5 mg experienced weight loss and HbA1c reduction, while 41–79% in the dulaglutide 0.75 mg arm lost weight and had an HbA1c reduction. A significantly greater proportion of patients had both weight and HbA1c reduction in the dulaglutide 1.5 mg arm compared with metformin, sitagliptin, exenatide twice daily, and insulin glargine, and a similar proportion compared with liraglutide. A greater proportion of patients had both weight and HbA1c reduction in the dulaglutide 0.75 mg arm compared with sitagliptin and insulin glargine and a similar proportion compared with metformin and exenatide twice daily. While the majority of patients in each of the studies experienced an HbA1c reduction, body weight changes varied based on background therapy. In studies with concomitant sulphonylurea, thiazolidinedione and insulin, which are usually associated with weight gain, the percentage of patients with weight reduction was lower. Weight gain generally associated with these concomitant therapies might have been attenuated by dulaglutide treatment. In addition, more patients experienced >5% body weight loss when dulaglutide was added on to metformin (24–34%) compared with when used with prandial insulin, or maximally tolerated doses of pioglitazone or glimepiride (9–18%).
We found a weak and inconsistent correlation between weight and HbA1c reductions. When examining the correlations and the percentages of patients with both weight and HbA1c reduction, a small correlation between weight change and HbA1c change with dulaglutide treatment was observed in the studies with greater weight reduction, that is the monotherapy study and add‐on‐to‐metformin studies. Because correlations may be affected by patient‐to‐patient variability, to investigate this further we also evaluated the mean HbA1c change by weight‐change categories at 26 weeks. While there were relatively few patients with weight loss >10%, we observed greater HbA1c reductions with increasing weight loss in the monotherapy study and in the trials of treatment added on to metformin. The trends were not observed in other studies with background therapies associated with weight gain. This is consistent with the correlation coefficient values and percentages of patients with both weight and HbA1c reduction. Notably, in the AWARD‐6 study, liraglutide added on to metformin showed the same trend of greater HbA1c reduction associated with greater weight loss. In the additional pooled analyses, as expected because of the individual study results, we found weak correlation coefficient values of 0.254 and 0.237 when combining monotherapy and add‐on‐to‐metformin studies and no correlations (r = −0.072 for both dulaglutide doses) when combining studies with concomitant therapies associated with weight gain. This finding helps explain the variations in correlations between studies with different background therapies. Similar findings have been seen with previous reports on other GLP‐1 receptor agonists in a real‐world setting as well as clinical trial programme analyses 8, 9, 10, 18.
In AWARD clinical trials, consistent with the GLP‐1 receptor agonist class, dulaglutide was associated with gastrointestinal adverse events, such as nausea and vomiting, which may decrease food intake, nutrient absorption, and consequently body weight. Importantly, the incidence of nausea and/or vomiting typically peaked within 1–2 weeks of dulaglutide treatment and then rapidly declined over the next 4 weeks, while weight change with dulaglutide continued well beyond this time frame 13, 14, 15, 19. In addition, the incidence of nausea across the programme (6–28%) was much lower than the proportion of patients with weight loss (43–88%). While numerically greater weight reduction was observed in patients with nausea, reduction in body weight was observed irrespective of nausea and/or vomiting. This is consistent with observations of other GLP‐1 receptor agonists (Nixwender, Bydureon SmPC, AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA).
As a post hoc analysis, interpretation of these data does have limitations. Given the confounding effect of concomitant medications on the outcomes of interest and differences in patient populations across the AWARD studies, we were not able to combine the six trials into a meta‐analysis. Prospective studies designed to investigate this would further inform these important questions.
In conclusion, although dulaglutide was found to have dose‐dependent effects on both weight loss and HbA1c reduction, the relationship was weak in the present analysis and may have been confounded by concomitant medications associated with weight gain. Dulaglutide is an effective treatment option, with both HbA1c reductions and weight loss observed across the type 2 diabetes treatment spectrum.
Conflict of Interest
G. U. is supported in part by research grants from the American Diabetes Association (7‐03‐CR‐35), and Public Health Service Grant UL1 RR025008 from the Clinical and Translational Science Award Program (M01 RR‐00039), National Institutes of Health, National Center for Research Resources. G. U. has received research grant support (to Emory University) from Novo Nordisk, Merck, Boehringer Ingelheim, Astra Zeneca and Sanofi, and has received consulting fees or/and honoraria for membership in advisory boards from Novo Nordisk, Sanofi, Merck, Regeneron, and Boehringer Ingelheim. K. P. has received honoraria as a member of the AstraZeneca, Eli Lilly, Merck, and Novo Nordisk speaker bureaus, consulting fees from Novo Nordisk, Eli Lilly, and Merck, as well as research support from Novo Nordisk and Merck in the past 12 months. A. K., A. Z. and L. F. are full‐time employees of and own stock or stock options for Eli Lilly and Company. N. Z. is a full‐time employee of Eli Lilly and Company.
G. U. contributed to design, conduct/data collection, and analysis of the paper. K. P. contributed to analysis of the paper. A. K. contributed to design and analysis of the paper. A. Z. contributed to analysis of the paper. N. Z. contributed to design, analysis, and writing of the paper. L. F. contributed to design and analysis of the paper.
Supporting information
Table S1. Proportion of patients by weight changes at 26 weeks.
Figure S1. Pooled analysis on weight change with glycated haemoglobin change at 26 weeks.
Figure S2. Weight change at 26 weeks in patients with and without nausea and/or vomiting.
Acknowledgements
This work was sponsored by Eli Lilly and Company.
References
- 1. Klein S, Sheard NF, Pi‐Sunyer X et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care 2004; 27: 2067–2073. [DOI] [PubMed] [Google Scholar]
- 2. Maggio CA, Pi‐Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care 1997; 20: 1744–1766. [DOI] [PubMed] [Google Scholar]
- 3. Russell‐Jones D, Khan R. Insulin‐associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab 2007; 9: 799–812. [DOI] [PubMed] [Google Scholar]
- 4. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012; 35: 1364–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015; 38: 140–149. [DOI] [PubMed] [Google Scholar]
- 6. Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care 2010; 33: 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shantha GP, Kumar AA, Kahan S, Cheskin LJ. Association between glycosylated hemoglobin and intentional weight loss in overweight and obese patients with type 2 diabetes mellitus: a retrospective cohort study. Diabetes Educ 2012; 38: 417–426. [DOI] [PubMed] [Google Scholar]
- 8. Klonoff DC, Buse JB, Nielsen LL et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 2008; 24: 275–286. [DOI] [PubMed] [Google Scholar]
- 9. Niswender K, Pi‐Sunyer X, Buse J et al. Weight change with liraglutide and comparator therapies: an analysis of seven phase 3 trials from the liraglutide diabetes development programme. Diabetes Obes Metab 2013; 15: 42–54. [DOI] [PubMed] [Google Scholar]
- 10. Blonde L, Pencek R, MacConell L. Association among weight change, glycemic control, and markers of cardiovascular risk with exenatide once weekly: a pooled analysis of patients with type 2 diabetes. Cardiovasc Diabetol 2015; 14: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Umpierrez G, Tofe Povedano S, Perez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care 2014; 37: 2168–2176. [DOI] [PubMed] [Google Scholar]
- 12. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care 2014; 37: 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dungan KM, Povedano ST, Forst T et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet 2014; 384: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 14. Wysham C, Blevins T, Arakaki R et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care 2014; 37: 2159–2167. [DOI] [PubMed] [Google Scholar]
- 15. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care 2015; 38: 2241–2249. [DOI] [PubMed] [Google Scholar]
- 16. Blonde L, Jendle J, Gross J et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet 2015; 385: 2057–2066. [DOI] [PubMed] [Google Scholar]
- 17. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015; 38: 1161–1172. [DOI] [PubMed] [Google Scholar]
- 18. Fadini GP, Simioni N, Frison V et al. Independent glucose and weight‐reducing effects of Liraglutide in a real‐world population of type 2 diabetic outpatients. Acta Diabetol 2013; 50: 943–949. [DOI] [PubMed] [Google Scholar]
- 19. Kuritzky L, Umpierrez G, Ekoe JM, Mancillas‐Adame L, Lando LF. Safety and efficacy of dulaglutide, a once weekly GLP‐1 receptor agonist, for the management of type 2 diabetes. Postgrad Med 2014; 126: 60–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Proportion of patients by weight changes at 26 weeks.
Figure S1. Pooled analysis on weight change with glycated haemoglobin change at 26 weeks.
Figure S2. Weight change at 26 weeks in patients with and without nausea and/or vomiting.


