Abstract
The special anatomical position accounts for unusual clinicopathological features of uncinate process cancer. This study aimed to compare clinicopathological features of patients with uncinate process cancer to patients with non-uncinate process pancreatic head cancer. Total 160 patients with pancreatic head cancer were enrolled and classified into two groups: uncinate process cancer and non-uncinate process pancreatic head cancer. We found that the ratio of vascular invasion was significantly higher in patients with uncinate process cancer than in patients with non-uncinate process pancreatic head cancer. In addition, the rate of R1 resection was significantly higher in patients with uncinate process cancer. Furthermore, the median disease-free survival (11 months vs. 15 months, p=0.043) and overall survival (15 months vs. 19 months, p=0.036) after R0 resection were lower for uncinate process cancer. Locoregional recurrence was more frequent (p=0.017) and earlier (12 months vs. 36 months; p=0.002) in patients with uncinate process cancer than in patients with non-uncinate process pancreatic head cancer. In conclusion, uncinate process cancer is more likely to invade blood vessel and has worse prognosis due to the earlier and more frequent locoregional recurrence.
Keywords: pancreatic head cancer, uncinate process, survival, prognosis, recurrence.
Introduction
The uncinate process is a hook like extension from the lower part of the head of the pancreas, and it extends superiorly and posteriorly behind the superior mesenteric vein (SMV), and ends either to the right side or extending behind the superior mesenteric artery (SMA) 1. Compared with the other parts of the head, the uncinate process is closer to the mesentery. The special anatomical position of uncinate process accounts for the peculiar clinicopathological features of the uncinate process cancer 1-9. However, few reports have compared the clinicopathological features and prognosis of uncinate process pancreas cancer with those of non-uncinate process pancreatic cancer. Therefore, in this study we investigated the clinicopathological features and prognosis in patients with uncinate process cancer compared to patients with non-uncinate process pancreatic head cancer.
Materials and methods
Subjects
From January 2010 to December 2014, 160 patients with pancreatic head cancer referred to Peking University First Hospital were included in this study. Among them, 85 patients were pathologically confirmed as pancreatic ductal adenocarcinoma and the other 75 patients were diagnosed by CT imaging and CA 19-9. All patients diagnosed other than ductal adenocarcinoma such as ductal-endocrine carcinoma, intraductal papillary mucinous carcinoma, and signet ring cell carcinomas were excluded. The clinicopathological data were collected from electronic medical record system, and patients who underwent surgical resection were followed up every 3 months.
Based on dedicated pancreatic multi-detector computed tomography (MDCT), patients were classified into two groups: the uncinate process cancer and the non-uncinate process pancreatic head cancer. All CT scans were reviewed blindly by two experienced radiologists, and interpretations were achieved by consensus. Of these 160 patients, 55 patients had tumors located in the uncinate process of the pancreas, including 31 men and 24 women who aged from 23 to 83 years (mean age 63.8 years), and the other 105 patients had non-uncinate process pancreatic head cancers, including 65 men and 40 women who aged from 39 to 85 years (mean age 63.1 years).
Treatment
Of the 55 patients with uncinate process cancer, 24 patients received classical pancreatoduodenectomy, one patient received total pancreatectomy, 11 patients received open bypass, and 19 patients had no operation. In addition, 6 patients underwent combined partial or segmental resection of SMV/PV and reconstruction. Of the 105 patients with non-uncinate process pancreatic head cancer, 36 patients received classical pancreatoduodenectomy, 2 patients received total pancreatectomy, 2 patients received pylorus preserving pancreatoduodenectomy, 19 patients received open bypass and biopsy, 1 patient received only open biopsy without bypass, and 45 patients had no operation. In addition, 7 patients underwent combined partial or segmental resection of SMV/PV and reconstruction (Table 1).
Table 1.
The types of treatments for the patients.
| Uncinate (n=55) | Non-uncinate (n=105) | P-value | |
|---|---|---|---|
| Operation | 36 (65.5%) | 60 (57.1%) | 0.396 |
| Open bypass | 11 (20.0%) | 19 (18.1%) | 0.832 |
| Open biopsy only | 0 | 1 (1.0%) | |
| Resection | 25 (45.5%) | 40 (38.1%) | 0.400 |
| R0 resection | 16 (29.1%) | 35 (33.3%) | 0.599 |
| R1 resection | 9 (16.4%) | 5 (4.8%) | 0.019 |
| Non-resection | 30(54.5%) | 65(61.9%) | 0.400 |
| PPPD | 0 | 2 (1.9%) | |
| Whipple's operation | 24 (43.6%) | 36 (34.3%) | |
| Total pancreatectomy | 1 (1.8%) a | 2 (1.9%) b | |
| SMV and/or PV resection | 6 (10.9%) | 7 (6.6%) | 0.372 |
PPPD, pylorus-preserving Pancreatoduodenectomy; a synchronous ductal adenocarcinoma located at body; b synchronous IPMN located at body and tail.
Adjuvant chemotherapy including six cycles of gemcitabine (1000 mg/m2, 30 min intravenous infusion on day 1, 8, and 15) was performed in 17 patients of the 65 patients who underwent resection. 5 patients received only 1-2 cycle chemotherapy and the other 43 patients refused adjuvant chemotherapy. Among the other 95 patients who did not undergo curative resection, only 10 patients received chemotherapy, and the other 85 patients did not receive chemotherapy due to a poor performance or refusing chemotherapy.
Statistical analysis
SPSS version 19.0 (IBM Corp.) was used for statistical analysis and data management. The values were presented as the mean ± SD, medians (interquartile range), or numbers and percentages. Chi-Square analysis or Fisher's exact test were used to compare categorical data. Continuous variables were compared using the t test or Mann-Whitney test. Survival curves were constructed using the Kaplan-Meier method and the statistical significance of differences in survival was determined using the log-rank test. The Cox-Hazard model was used for the multivariate analysis. A multivariate analysis was conducted using the factors that were significant in the univariate analysis. A two-sided P value less than 0.05 indicated significant difference.
Results
Clinical characteristics of the patients
The main clinical characteristics of the patients were shown in Table 2. Upper abdominal pain, weight loss and jaundice were the three main symptoms, and were not significantly different between the patients with uncinate process cancer and the patients with non-uncinate process pancreatic head cancer. In addition, there were no significant differences in serum bilirubin level, liver function or CA 19-9 between the two groups.
Table 2.
Clinical characteristics of the patients.
| Uncinate (n=55) | Non-uncinate (n=105) | P-value | |
|---|---|---|---|
| Age (mean, years) | 63.8±11.1 | 63.1±10.5 | 0.701 |
| Gender (M:F) | 1.29:1 | 1.63:1 | 0.503 |
| Symptoms | |||
| abdominal pain | 27 (49.1%) | 55 (52.4%) | 0.741 |
| jaundice | 38 (69.1%) | 66 (62.9%) | 0.488 |
| weight loss | 36 (65.5%) | 58 (54.7%) | 0.239 |
| anorexia | 11 (20.0%) | 17 (16.2%) | 0.662 |
| nausea | 10 (18.2%) | 16 (15.2%) | 0.656 |
| Symptom duration (months) | 1.0 (0.7-3.0) | 1.0 (0.7-4.0) | 0.504 |
| Laboratory examination | |||
| ALT (IU/L) | 102.0 (53.0-198.0) | 91.0 (33.0-202.0) | 0.417 |
| AST (IU/L) | 84.0 (34.0-133.0) | 72.0 (28.0-130.5) | 0.461 |
| TBIL (umol/L) | 121.8 (22.7-198.3) | 115.0 (18.1-216.1) | 0.986 |
| DBIL (umol/L) | 89.9 (6.8-131.0) | 69.0(8.7-161.4) | 0.857 |
| CA 19-9 (U/ml) | 375.3 (131.2-828.8) | 295.5 (119.5-1000) | 0.889 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; TBIL, total bilirubin; DBIL, direct bilirubin; CA 19-9, carbohydrate antigen.
Clinical and pathological features of the tumors
The mean size of tumors in the uncinate process was 3.43 cm (range 1.0-7.3 cm) (Table 3). In the non-uncinate process pancreatic head, the mean size of tumors was 3.44 cm (range 1.0-7.6 cm). The tumor size was not significantly different between the two groups, as well as the dilation of pancreatic duct and dilation of common bile duct. However, the percentage of vascular (SMV/PV and/or SMA) invasion was significantly higher (P=0.019) in patients with the uncinate process cancer (32/55 patients, 58.2%) than in patients with non-uncinate process pancreatic head cancer (40/105 patients, 38.1%). Moreover, SMA encasement was diagnosed more often (P=0.011) in patients with uncinate process cancer (17/55 patients, 30.9%) than in patients with non-uncinate process pancreatic head cancer (14/105 patients, 13.3%). In addition, the chance of SMV invasion (P=0.026) and PV (P=0.001) invasion were significantly higher in the patients with the uncinate process cancer.
Table 3.
The features of the tumors.
| Uncinate (n=55) | Non-uncinate(n=105) | P-value | |
|---|---|---|---|
| Tumor size (cm) | 3.43±1.32 | 3.44±1.31 | 0.890 |
| Vascular invasion | 32 (58.2%) | 40 (38.1%) | 0.019 |
| SMV invasion | 30 (54.5%) | 38 (36.2%) | 0.026 |
| PV invasion | 25 (45.5%) | 21 (20.0%) | 0.001 |
| SMA encasement c | 17 (30.9%) | 14 (13.3%) | 0.011 |
| Duodenal invasion | 22 (40.0%) | 36 (34.3%) | 0.493 |
| Distant metastasis | 12 (21.8%) | 29 (27.6%) | 0.453 |
| Pancreatic duct dilation | 30 (54.5%) | 62 (59.0%) | 0.616 |
| Common bile duct dilation | 45 (81.8%) | 78 (74.3%) | 0.328 |
| biliary drainage | 14 (25.5%) | 29 (27.6%) | 0.852 |
| AJCC stage(7th edition) | 0.556 | ||
| I/II | 32 (58.2%) | 61 (58.1%) | |
| III | 11 (20.0%) | 15 (14.3%) | |
| IV | 12 (21.8%) | 29 (27.6%) |
c tumor contact with SMA >180°. AJCC, American Joint Committee on Cancer.
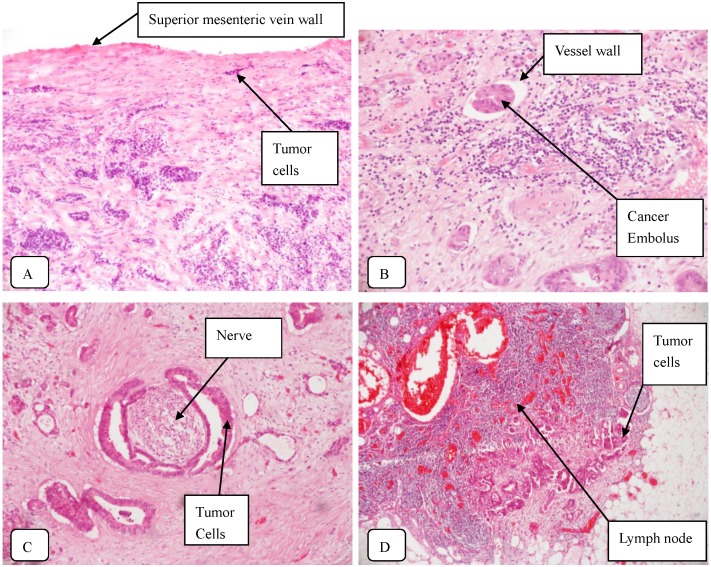
According to the AJCC TNM Staging of Pancreatic Cancer (seventh edition, 2010), 23 uncinate process cancer patients and 44 non-uncinate process pancreatic head cancer patients were diagnosed with stage III or IV disease, respectively. The rate of R0 resection was not significantly different between two groups. However, the rate of R1 resection (with microscopically residual tumor) was significantly higher (P=0.019) in patients with uncinate process cancer (9/55 patients, 16.4%) than in patients with non-uncinate process cancer (5/105 patients, 4.8%). Typical staining of the uncinate process cancer was shown in Fig. 1.
Figure 1.
Typical staining of the uncinate process cancer. Magnifications: 1:200 (A, B, C) and 1:40 (D). A: SMV invasion; B: Vascular cancer embolus; C: Perineural invasion; D: Lymph node metastasis.
Survival of the patients
The median follow-up was 15.0 months (range 5-46 months) for the 65 patients who underwent curative resection. At the last follow-up, 48 patients (73.8%) died of pancreatic head cancer recurrence, 3 patients (4.6%) died of other causes, 2 patients (3.1%) were alive with disease and 12 patients (18.5%) were alive with no clinical or radiographic evidence of disease. The median overall survival after resection (R0 and R1) was 16.0 months (95% CI: 13.5-18.5 months).
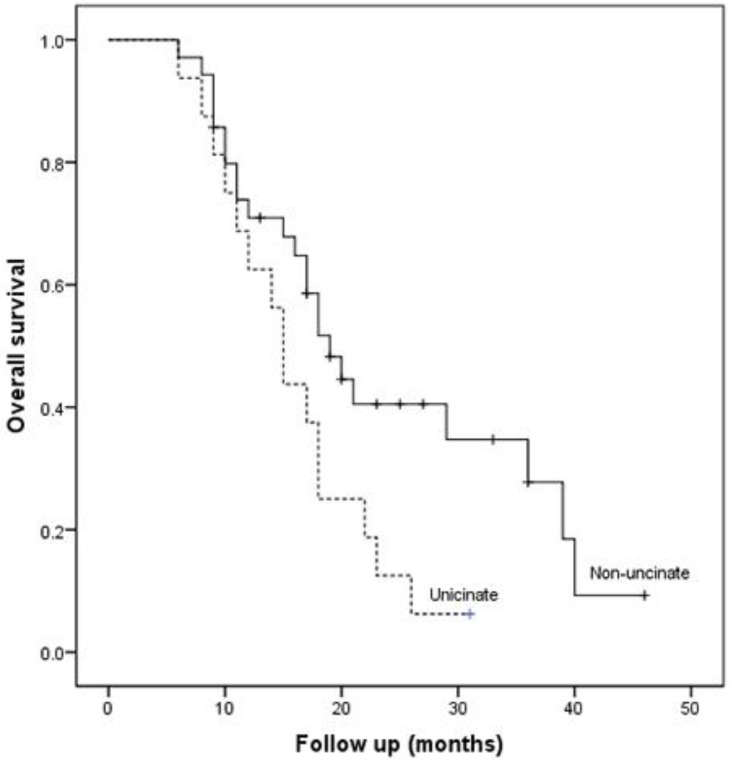
The median overall survival of patients after R0 resection was 18.0 months (95% CI: 16.2-19.8 months), significant higher than that of patients after R1 resection (11.0 months, 95% CI: 7.4-14.6 months; P=0.001). The overall 1- and 3-year survival rate of the patients after R0 resection was 68.3% and 19.2%, respectively. Although we found no significant differences in clinicopathological characteristics between the two groups after R0 resection (Table 4), the median overall survival after R0 resection was lower for uncinate process cancer than non-uncinate process pancreatic head cancer (15 months vs. 19 months, P=0.036, Fig. 2).
Table 4.
Clinicopathological characteristics of patients after R0 resection.
| Uncinate (n=16) | Non-uncinate (n=35) | P-value | |
|---|---|---|---|
| Age (mean, years) | 67.2±7.4 | 65.5±8.9 | 0.506 |
| Gender (M:F) | 1.3:1 | 2.9:1 | 0.329 |
| Operative time (min) | 397.3±98.1 | 396.6±139.0 | 0.986 |
| Peri-operative bleeding (ml) | 400 (300-875) | 400 (200-600) | 0.758 |
| SMV and/or PV resection | 5 (31.3%) | 6 (17.1%) | 0.288 |
| Complication | 5 (31.3%) | 16 (45.7%) | 0.375 |
| Pathological tumor size (cm) | 3.70±1.73 | 3.35±1.80 | 0.515 |
| Pathological tumor stage | 0.694 | ||
| T1/T2 | 13 (81.3%) | 30(85.7%) | |
| T3 | 3 (18.8%) | 5 (14.3%) | |
| LN metastasis (+) | 4 (25.0%) | 16 (45.7%) | 0.221 |
| Number of lymph nodes examined | 14.7±9.1 | 12.5±6.0 | 0.597 |
| Number of positive lymph nodes | 0.5±1.0 | 1.0±1.3 | 0.195 |
| Histology | 0.742 | ||
| well/well-moderate | 5 (31.3%) | 9 (25.7%) | |
| moderate/moderate-poor | 11(68.8%) | 26 (74.3%) | |
| Perineural invasion(+) | 13 (81.3%) | 27 (77.1%) | >0.99 |
| Vascular cancer embolus | 4 (25.0%) | 8 (22.9%) | >0.99 |
| AJCC stage | 0.322 | ||
| IB | 3 (18.8%) | 3 (8.6%) | |
| IIA | 9 (56.3%) | 16 (45.7%) | |
| IIB | 4 (25.0%) | 16 (45.7%) | |
| Chemotherapy | 6 (37.5%) | 11 (31.4%) | 0.671 |
| Follow-up time (median, months) |
13 (range, 5-31) | 15 (range, 3-46) |
AJCC, American Joint Committee on Cancer.
Figure 2.
Kaplan-Meier curve for overall survival in patients after R0 resection according to the tumor location.
Recurrence of the tumors
The median disease-free survival was higher in patients after R0 resection than in patients after R1 resection (median, 13 months vs. 6 months; P<0.001). The disease-free 1 and 3-year survival rate of the patients after R0 resection was 50.8% and 13.4%, respectively. Follow -up data showed that recurrence occurred in 37 patients (72.5%) of the 51 patients after R0 resection (Table 5). Notably, locoregional recurrence was the most common for the uncinate process pancreatic head caner, and systemic recurrence was the most common for the non-uncinate process cancer.
Table 5.
Recurrence after R0 resection.
| Uncinate (n=16) | Non-uncinate (n=35) | P-value | |
|---|---|---|---|
| Overall recurrence | 14 (87.5%) | 23 (65.7%) | 0.176 |
| Locoregional recurrence | 12 (75.0%) | 13 (37.1%) | 0.017 |
| Systemic recurrence | 10 (62.5%) | 14 (40%) | 0.226 |
| Liver | 9 (56.3%) | 13 (37.1%) | 0.235 |
| lung | 0 | 2 (5.7%) | >0.99 |
| bone | 2 (12.5%) | 2 (5.7%) | 0.581 |
| Locoregional and sychronous systemic recurrence | 8 (50.0%) | 8 (22.9%) | 0.102 |
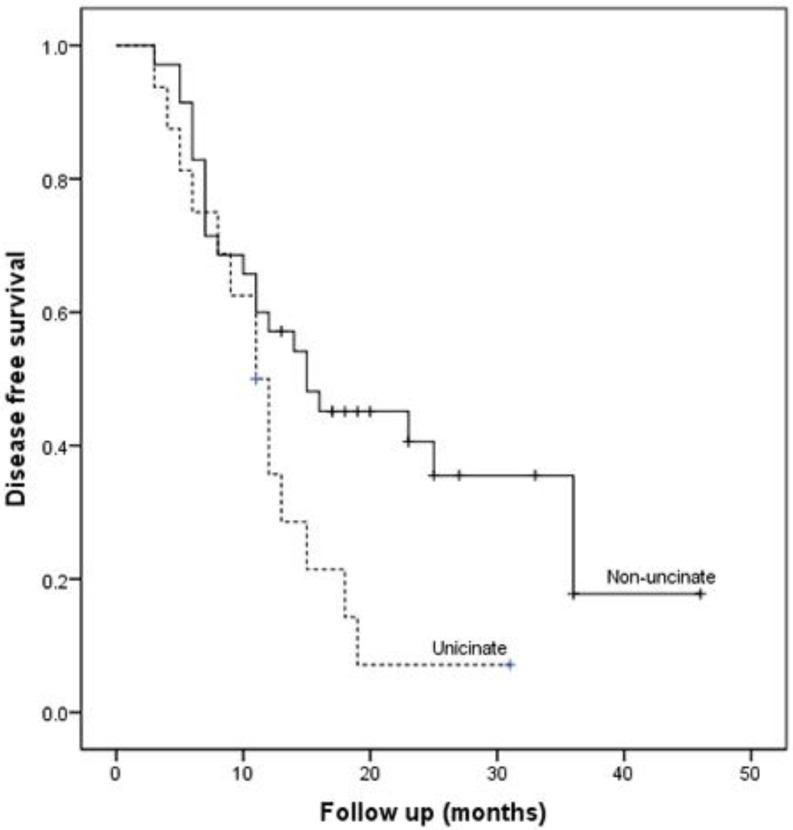
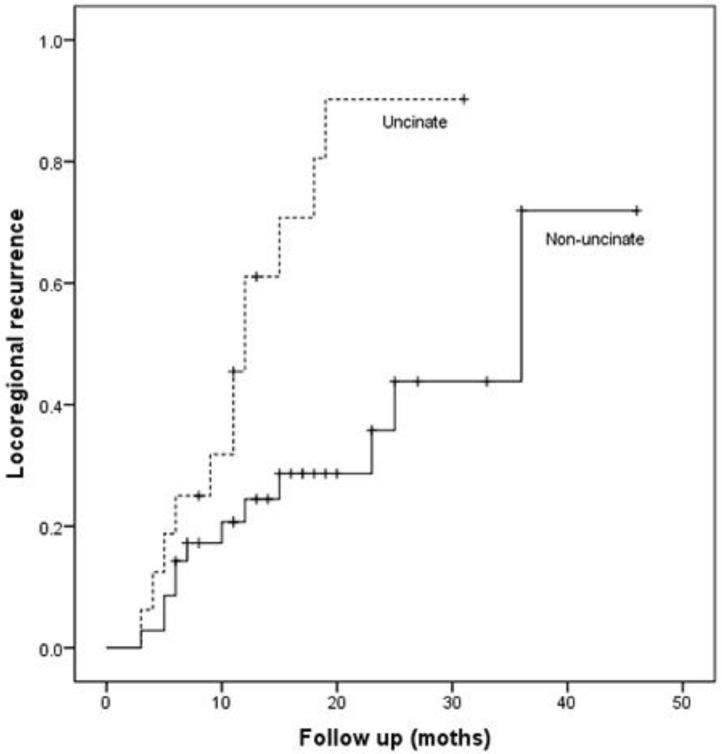
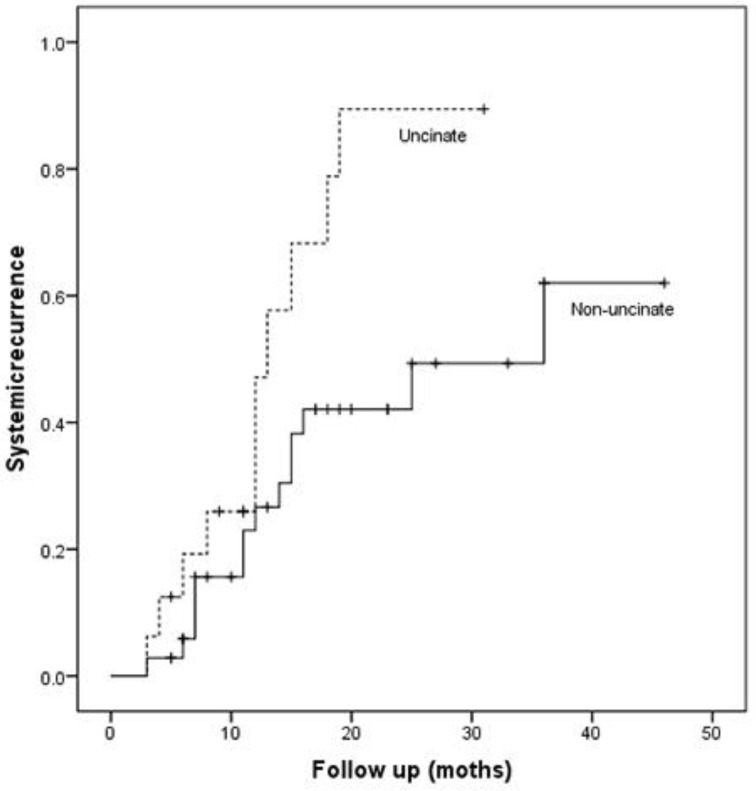
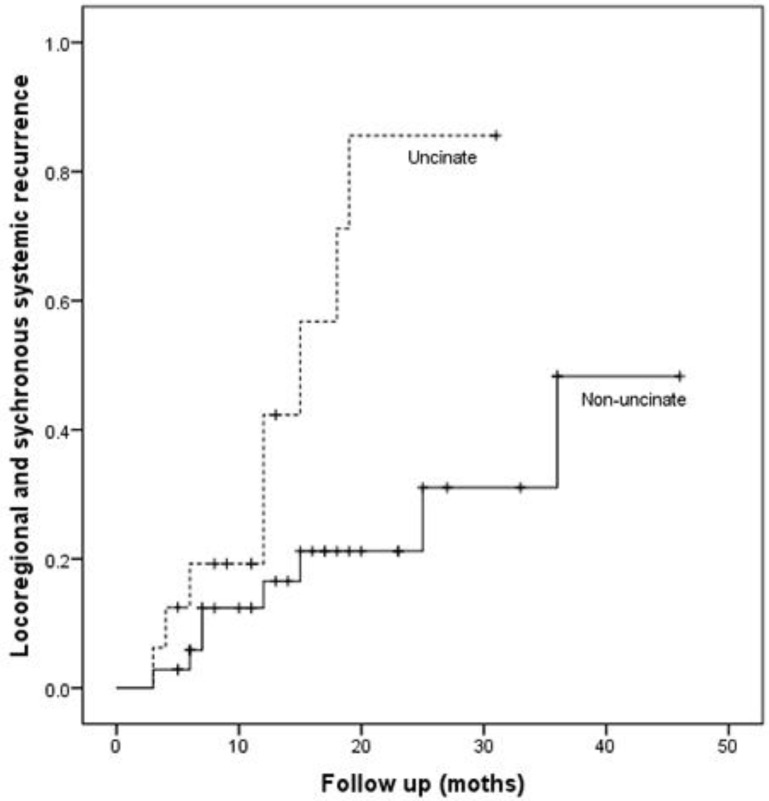
Patients with uncinate process cancer had significantly worse disease-free survival (median 11 months) than those with non-uncinate process pancreatic head cancer (median 15 months, P=0.043, Fig. 3). Locoregional recurrence had higher frequency (P=0.017) and earlier development (median, 12 months vs. 36 months; P=0.002, Fig. 4) in patients with uncinate process cancer than in those with non-uncinate process pancreatic head caner. In addition, systemic recurrence had earlier development (median, 13 months vs. 36 months; P=0.027, Fig. 5) in patients with uncinate process cancer, as well as locoregional and sychronous systemic recurrence (P=0.007; Fig. 6).
Figure 3.
Kaplan-Meier curve for disease-free survival after R0 resection according to the tumor location.
Figure 4.
Locoregional recurrence in patients after R0 resection according to the tumor location.
Figure 5.
Systemic recurrence in patients after R0 resection according to the tumor location.
Figure 6.
Locoregional and sychronous systemic recurrence in patients after R0 resection according to the tumor location.
Prognostic factors
Univariate survival analysis shows that the location, margin status, LN metastasis, and the number of positive lymph node were significant predictors for both disease-free survival and overall survival (Table 6). Tumor differentiation and vascular cancer embolus correlated with disease-free survival, but not with overall survival. CA 19-9 correlated with overall survival, but not with disease-free survival. Multivariate analysis showed that the location, margin status and LN metastasis were independent prognostic factors for disease-free survival, and the location and LN metastasis were independent prognostic factors for overall survival (Table 7).
Table 6.
Univariate analyses of disease-free and overall survival of patients after resection.
| disease-free survival | overall survival | |||||
|---|---|---|---|---|---|---|
| No | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age | 1.004 (0.972-1.036) | 0.829 | 1.012 (0.980-1.045) | 0.465 | ||
| Location | uncinate | 25 | 1 | 1 | ||
| non-uncinate | 50 | 0.405 (0.227-0.721) | 0.002 | 0.409 (0.231-0.724) | 0.002 | |
| CA 19-9 | 1.001 (1.000-1.002) | 0.093 | 1.001 (1.000-1.002) | 0.046 | ||
| Venous resection | no | 52 | 1 | 1 | ||
| yes | 13 | 0.995 (0.496-1.997) | 0.989 | 1.353 (0.701-2.611) | 0.367 | |
| T stage | T1/T2 | 55 | 1 | 1 | ||
| T3 | 10 | 0.976 (0.456-2.092) | 0.951 | 0.910 (0.425-1.951) | 0.809 | |
| Tumor size (mm) | 1.007 (0.992-1.023) | 0.372 | 1.011 (0.996-1.027) | 0.144 | ||
| Tumor differentiation | Well-Moderate | 16 | 1 | 1 | ||
| Moderate-Poor | 49 | 2.278 (1.102-4.4707) | 0.026 | 1.721 (0.860-3.447) | 0.125 | |
| Margins | R0 | 51 | 1 | 1 | ||
| R1 | 14 | 3.401 (1.716-6.741) | 0.001 | 2.879 (1.478-5.612) | 0.002 | |
| LN metastasis | negative | 35 | 1 | 1 | ||
| positive | 30 | 2.378 (1.330-4.253) | 0.003 | 2.633 (1.451-4.779) | 0.001 | |
| N0. of positive LN | 1.365 (1.176-1.584) | <0.001 | 1.253 (1.093-1.437) | 0.001 | ||
| AJCC stage | IB | 7 | 1 | 1 | ||
| IIA | 28 | 0.860 (0.316-2.341) | 0.768 | 0.891 (0.325-2.442) | 0.822 | |
| IIB | 30 | 2.115 (0.809-5.531) | 0.127 | 2.411 (0.919-6.323) | 0.074 | |
| Perineural invasion | no | 14 | 1 | 1 | ||
| yes | 51 | 1.360 (0.660-2.805) | 0.405 | 1.200 (0.598-2.409) | 0.607 | |
| Vascular cancer embolus | no | 50 | 1 | 1 | ||
| yes | 15 | 2.072 (1.103-3.894) | 0.024 | 1.663 (0.880-3.144) | 0.118 | |
| Chemotherapy | no | 43 | 1 | 1 | ||
| yes | 22 | 0.767 (0.413-1.424) | 0.401 | 0.826 (0.452-1.512) | 0.536 | |
HR, Hazard ratio; 95% CI, 95% confidence interval; AJCC, American Joint Committee on Cancer.
Table 7.
Multivariate analysis of disease-free and overall survival of patients after resection.
| disease-free survival | overall survival | |||||
|---|---|---|---|---|---|---|
| No. | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Location | <0.001 | <0.001 | ||||
| uncinate | 25 | 1 | 1 | |||
| non-uncinate | 50 | 0.299 (0.155-0.577) | 0.309 (0.160-0.596) | |||
| CA 19-9 | 1.000 (0.999-1.001) | 0.488 | 1.000 (1.000-1.001) | 0.331 | ||
| Tumor differentiation | 0.076 | 0.394 | ||||
| well | 16 | 1 | 1 | |||
| moderate/ poor |
49 | 1.959 (0.932-4.116) | 1.3378 (0.660-2.880) | |||
| Margins | 0.028 | 0.263 | ||||
| R0 | 51 | 1 | 1 | |||
| R1 | 14 | 2.278 (1.092-4.750) | 1.523 (0.729-3.181) | |||
| LN metastasis | 0.002 | 0.002 | ||||
| negative | 35 | 1 | 1 | |||
| positive | 30 | 2.847 (1.456-5.566) | 3.084 (1.524-6.242) | |||
| Vascular cancer embolus | 0.067 | 0.352 | ||||
| no | 50 | 1 | 1 | |||
| yes | 15 | 1.852 (0.958-3.580) | 1.366 (0.708-2.638) | |||
HR, Hazard ratio; 95% CI, 95% confidence interval; AJCC, American Joint Committee on Cancer.
Discussion
The pancreatic primordium consists of two buds: dorsal and ventral. The uncinate process is derived from the ventral bud. Anatomically, the uncinate process represents a hook like extension of the lower part of the pancreatic head and is located closely to the superior mesenteric artery and vein 1. This particular anatomical feature may result in specific clinicopathological features of the uncinate process. Previous studies showed that vascular invasion was frequent in patients with the uncinate process cancer, and the rate of R1 resection was significantly higher in these patients 7-9. In this study, we confirmed that the percentage of vascular invasion, especially SMA encasement was significantly higher in patients with uncinate process cancer. Furthermore, more patients with uncinate process cancer underwent R1 resection. However, no significant differences were found between the two groups in the incidence of jaundice and the dilatation of common bile duct, inconsistent with previous studies 7-9.
It was suggested that patients with uncinate process cancer had worse prognosis accompanied by earlier and more recurrence than patients with non-uncinate process pancreatic head cancer 7, 8. In this study, we found no significant difference in clinicopathological characteristics between the two groups after R0 resection, but the overall survival was lower for the patients with uncinate process cancer, and more and earlier locoregional recurrence was observed in the patients with uncinate process cancer. So far, little is known about the mechanism and cause of recurrence of uncinate process cancer.
Currently, the impact of margin status on overall and disease-free survival remains controversial 10. In this study, we found that patients who underwent R0 resection had better overall survival and disease-free survival than those who underwent R1 resection, in agreement with previous studies 11, 12. Furthermore, resection margin was an independent significant prognostic factor in pancreatic cancer 13, 14. The goal of operation treatment is to achieve R0 resection. For patients with venous involvement, en bloc resection of PV/SMV was not associated with increased morbidity or mortality compared with patients who did not require vein resection, and the rate of R0 resection and overall survival were not different between the two groups 15-18.
SMA (retroperitoneal/uncinate) margin clearance has become a major concern in the operation treatment for uncinate process cancer. The lymph node metastases and extrapancreatic nerve plexus invasion alongside the SMA have been recognized in numerous studies 19-23. Furthermore, a meta-analysis of radical resection rates and margin assessment in pancreatic cancer indicated that the most commonly involved margins are the SMA, PV/SMV, and posterior margins 24. However, the division of the retroperitoneal tissues between the uncinate process and SMA with a stapler may leave some areolar and lymphatic tissue and increase the risk of R1 resection 25. Jing et al. demonstrated that extended pancreaticoduodenectomy combined with full-length SMA isolation could achieve the goal of R0 resection, and improve the quality of life and survival rate of patients 26.
However, the reported R1 rate showed high variation (17-85%) due to the lack of consensus on the definition of microscopic margin involvement and standardized protocols for pathological examination 10, 27. R1 rate was highly underreported in some studies, which may lead to the discrepancy between margin status and clinical outcome 28-31. A series of studies have supported that patients with wider margins (> 1 mm) have better overall survival and disease-free survival than patients with direct invasion of tumor cells within 1 mm of the resection margin, and many centers have adopted a minimum clearance of more than 1 mm to define R0 resection 32-34. However, a recent study reported that a margin clearance of 2 mm or more was significantly linked to improved overall survival and the R1 definition might have to be adapted 35. In this study, resection margins including SMA, SMV/PV, pancreatic neck, bile duct, enteric and anterior surface margins were evaluated independently, and R0 resection was defined as a minimum clearance of more than 1 mm.
The limitation of this study is that many patients did not receive adjuvant therapies. Recurrence rate of pancreatic cancer was still high. Adjuvant therapies that impact on recurrence were necessary. It has been proved that adjuvant therapies can improve outcomes, although there is no consensus of standard adjuvant treatment after resection.
In conclusion, compared with non-uncinate process pancreatic head cancer, uncinate process cancer had a significantly higher incidence of vascular invasion. Specifically, the chance of SMA encasement, SMV invasion and PV invasion was significantly higher in patients with uncinate process cancer. In addition, the rate of R1 resection (with microscopically residual tumor) was significantly higher in patients with uncinate process cancer. Furthermore, after R0 resection, patients with uncinate process cancer had a worse prognosis than patients with non-uncinate process pancreatic cancer, which was related to the earlier and more frequent locoregional recurrence.
Acknowledgments
This work was supported by the grants from the Beijing Municipal Science & Technology Commission (Z131107002213043 and Z161100000516038).
References
- 1.O'Sullivan AW, Heaton N, Rela M. Cancer of the uncinate process of the pancreas: surgical anatomy and clinicopathological features. Hepatobiliary Pancreat Dis Int. 2009;8:569–574. [PubMed] [Google Scholar]
- 2.Suzuki T, Kuratsuka H, Uchida K. et al. Carcinoma of the pancreas arising in the region of the uncinate process. Cancer. 1972;30:796–800. doi: 10.1002/1097-0142(197209)30:3<796::aid-cncr2820300330>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi K. Carcinoma of the uncinate process of the pancreas with a peculiar clinical manifestation. Am J Gastroenterol. 1992;87:1046–1050. [PubMed] [Google Scholar]
- 4.Birk D, Schoenberg MH, Gansauge F. et al. Carcinoma of the head of the pancreas arising from the uncinate process. Br J Surg. 1998;85:498–501. doi: 10.1046/j.1365-2168.1998.00629.x. [DOI] [PubMed] [Google Scholar]
- 5.Li S, Pei YQ, Du FT. et al. Surgical treatment for uncinate process carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2002;1:592–594. [PubMed] [Google Scholar]
- 6.Ye C, Xi PC, Hu XG. Clinical analysis of uncinate process carcinoma of the pancreas. Hepatobiliary Pancreat Dis Int. 2003;2:605–608. [PubMed] [Google Scholar]
- 7.Kang MJ, Jang JY, Lee SE. et al. Comparison of the long-term outcomes of uncinate process cancer and non-uncinate process pancreas head cancer: poor prognosis accompanied by early locoregional recurrence. Langenbecks Arch Surg. 2010;395:697–706. doi: 10.1007/s00423-010-0593-6. [DOI] [PubMed] [Google Scholar]
- 8.El Nakeeb A, Roshdy S, Ask W. et al. Comparative study between uncinate process carcinoma and pancreatic head carcinoma after pancreaticodudenectomy (clincopathological features and surgical outcomes) Hepatogastroenterology. 2014;61:1748–1755. [PubMed] [Google Scholar]
- 9.Padilla-Thornton AE, Willmann JK, Jeffrey RB. Adenocarcinoma of the uncinate process of the pancreas: MDCT patterns of local invasion and clinical features at presentation. Eur Radiol. 2012;22:1067–1074. doi: 10.1007/s00330-011-2339-4. [DOI] [PubMed] [Google Scholar]
- 10.Verbeke CS. Resection margins in pancreatic cancer. Surg Clin North Am. 2013;93:647–662. doi: 10.1016/j.suc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Rau BM, Moritz K, Schuschan S. et al. R1 resection in pancreatic cancer has significant impact on long-term outcome in standardized pathology modified for routine use. Surgery. 2012;152(3 Suppl 1):S103–S111. doi: 10.1016/j.surg.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 12.Kimbrough CW, St HC, Martin RC. et al. Tumor-positive resection margins reflect an aggressive tumor biology in pancreatic cancer. J Surg Oncol. 2013;107:602–607. doi: 10.1002/jso.23299. [DOI] [PubMed] [Google Scholar]
- 13.Perini MV, Montagnini AL, Jukemura J. et al. Clinical and pathologic prognostic factors for curative resection for pancreatic cancer. HPB (Oxford) 2008;10:356–362. doi: 10.1080/13651820802140752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westgaard A, Tafjord S, Farstad IN. et al. Resectable adenocarcinomas in the pancreatic head: the retroperitoneal resection margin is an independent prognostic factor. BMC Cancer. 2008;8:5. doi: 10.1186/1471-2407-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly KJ, Winslow E, Kooby D. et al. Vein involvement during pancreaticoduodenectomy: is there a need for redefinition of "borderline resectable disease"? J Gastrointest Surg. 2013;17:1209–1217. doi: 10.1007/s11605-013-2178-5. [DOI] [PubMed] [Google Scholar]
- 16.Hartel M, Niedergethmann M, Farag-Soliman M. et al. Benefit of venous resection for ductal adenocarcinoma of the pancreatic head. Eur J Surg. 2002;168:707–712. doi: 10.1080/00000000000000007. [DOI] [PubMed] [Google Scholar]
- 17.Tseng JF, Raut CP, Lee JE. et al. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935–950. doi: 10.1016/j.gassur.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 18.Riediger H, Makowiec F, Fischer E. et al. Postoperative morbidity and long-term survival after pancreaticoduodenectomy with superior mesenterico-portal vein resection. J Gastrointest Surg. 2006;10:1106–1115. doi: 10.1016/j.gassur.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Kitagawa H, Ohta T, Makino I. et al. Carcinomas of the ventral and dorsal pancreas exhibit different patterns of lymphatic spread. Front Biosci. 2008;13:2728–2735. doi: 10.2741/2880. [DOI] [PubMed] [Google Scholar]
- 20.Makino I, Kitagawa H, Ohta T. et al. Nerve plexus invasion in pancreatic cancer: spread patterns on histopathologic and embryological analyses. Pancreas. 2008;37:358–365. doi: 10.1097/mpa.0b013e31818166e6. [DOI] [PubMed] [Google Scholar]
- 21.Noto M, Miwa K, Kitagawa H. et al. Pancreas head carcinoma: frequency of invasion to soft tissue adherent to the superior mesenteric artery. Am J Surg Pathol. 2005;29:1056–1061. [PubMed] [Google Scholar]
- 22.Jin G, Sugiyama M, Tuo H. et al. Distribution of lymphatic vessels in the neural plexuses surrounding the superior mesenteric artery. Pancreas. 2006;32:62–66. doi: 10.1097/01.mpa.0000194607.16982.d7. [DOI] [PubMed] [Google Scholar]
- 23.Mitsunaga S, Hasebe T, Kinoshita T. et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31:1636–1644. doi: 10.1097/PAS.0b013e318065bfe6. [DOI] [PubMed] [Google Scholar]
- 24.Chandrasegaram MD, Goldstein D, Simes J. et al. Meta-analysis of radical resection rates and margin assessment in pancreatic cancer. Br J Surg. 2015;102:1459–1472. doi: 10.1002/bjs.9892. [DOI] [PubMed] [Google Scholar]
- 25.Baque P, Iannelli A, Delotte J. et al. Division of the right posterior attachments of the head of the pancreas with a linear stapler during pancreaticoduodenectomy: vascular and oncological considerations based on an anatomical cadaver-based study. Surg Radiol Anat. 2009;31:13–17. doi: 10.1007/s00276-008-0353-2. [DOI] [PubMed] [Google Scholar]
- 26.Jing W, He T, Hu X. et al. The clinical efficacy and safety of modified Miwa's augmented regional pancreatoduodenectomy in the treatment of ductal adenocarcinoma of the pancreas in the uncinate process. Hepatogastroenterology. 2013;60:268–272. doi: 10.5754/hge12713. [DOI] [PubMed] [Google Scholar]
- 27.Schlitter AM, Esposito I. Definition of microscopic tumor clearance (r0) in pancreatic cancer resections. Cancers (Basel) 2010;2:2001–2010. doi: 10.3390/cancers2042001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbeke CS, Menon KV. Redefining resection margin status in pancreatic cancer. HPB (Oxford) 2009;11:282–289. doi: 10.1111/j.1477-2574.2009.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito I, Kleeff J, Bergmann F. et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. doi: 10.1245/s10434-008-9839-8. [DOI] [PubMed] [Google Scholar]
- 30.Verbeke CS, Leitch D, Menon KV. et al. Redefining the R1 resection in pancreatic cancer. Br J Surg. 2006;93:1232–1237. doi: 10.1002/bjs.5397. [DOI] [PubMed] [Google Scholar]
- 31.Liszka L, Pajak J, Zielinska-Pajak E. et al. Different approaches to assessment of lymph nodes and surgical margin status in patients with ductal adenocarcinoma of the pancreas treated with pancreaticoduodenectomy. Pathology. 2010;42:138–146. doi: 10.3109/00313020903494060. [DOI] [PubMed] [Google Scholar]
- 32.Hartwig W, Hackert T, Hinz U. et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 33.Frampton AE, Gall TM, Krell J. et al. Is there a 'margin' for error in pancreatic cancer surgery? Future Oncol. 2013;9:31–34. doi: 10.2217/fon.12.175. [DOI] [PubMed] [Google Scholar]
- 34.Gnerlich JL, Luka SR, Deshpande AD. et al. Microscopic margins and patterns of treatment failure in resected pancreatic adenocarcinoma. Arch Surg. 2012;147:753–760. doi: 10.1001/archsurg.2012.1126. [DOI] [PubMed] [Google Scholar]
- 35.Gebauer F, Tachezy M, Vashist YK. et al. Resection margin clearance in pancreatic cancer after implementation of the Leeds Pathology Protocol (LEEPP): clinically relevant or just academic? World J Surg. 2015;39:493–499. doi: 10.1007/s00268-014-2808-4. [DOI] [PubMed] [Google Scholar]