abstract
There is increasing evidence that cancers are heterogeneous and contain a hierarchical organization consisting of cancer stem cells and their differentiated cell progeny. These cancer stem cells are at the core of the tumor as they represent the clonogenic cells within a tumor. Moreover, these cells are considered to contain selective therapy resistance, which suggests a pivotal role in therapy resistance and tumor relapse. Here we show that differentiated cells can re-acquire stemness through factors secreted from fibroblasts. This induced CSC state also coincides with re-acquisition of resistance to chemotherapy. Resistance induced in newly formed CSCs is mediated by the anti-apoptotic molecule BCLXL and inhibition of BCLXL with the BH3 mimetic ABT-737 sensitizes these cancer cells toward chemotherapy. These data point to an important interplay between tumor cells and their microenvironment in the regulation of stemness and therapy resistance.
Keywords: apoptosis, ABT-737, BCLXL, colorectal cancer, cancer stem cell, chemotherapy, fibroblast, microenvironment, resistance
Introduction
Over the last decade it has become increasingly clear that a variety of cancers is hierarchically organized and cancer stem cells (CSCs) can be found at the top of this hierarchy. CSCs are, in contrast to their differentiated daughter cells, highly tumorigenic and besides their self-renewal capacity CSCs can differentiate in more differentiated cell types.1 In colorectal cancer CSCs were identified by 2 independent groups that made use of an antibody that recognize the cell surface molecule CD133.2,3 More recent data provided for evidence for other CSC markers in colon, such as LGR5 expression and Wnt pathway activity.4,5 Especially the latter was surprising as this occurred even in tumors where all cells contained a mutated APC, indicating that not all cells have the same level of Wnt pathway activation, despite this mutation.5 When primary colon cancer spheroid lines were dissociated and transduced with a Wnt activity reporter that directs the expression of enhanced green fluorescent protein (TOP-GFP), cells expressing high TOP-GFP levels (TOP-GFPhi) are shown to be the CSCs. In contrast, cells with low Wnt pathway activity (TOP-GFPlo) are not tumorigenic upon xenotransplantation and express markers of differentiated colon cells.5 Using these TOP-GFP spheroid cultures we have designed flow cytometer based assays in which cell death in CSCs and their differentiated progeny can be measured simultaneously and importantly under the same conditions. This assay showed that differentiated cells are killed by chemotherapy, while CSCs were resistant to all therapies that we have tested.6 Resistance was due to a disturbed apoptotic balance in CSCs in favor of anti-apoptotic molecules.6 This suggests that targeting this balance could be an effective therapy. However, we have recently also shown that signals emanating from the microenvironment are crucial in the regulation of stemness and can direct the acquisition of stemness features in more differentiated tumor cells.5,7,8 This suggests that the microenvironment is a pivotal player in the hierarchy within a tumor and could therefore also regulate chemotherapy sensitivity. Here we show that factors derived from myofibroblasts can not only dedifferentiate non-CSCs into CSCs, but also direct a resistance toward chemotherapy. Furthermore, this fibroblast induced resistance can be reverted by inhibition of BCLXL with the BH3 mimetic ABT-737.
Materials and methods
Cell culture and reagents
Colon spheroid cultures were derived from colorectal cancer patients and maintained as previously described6 in stem cell medium (advanced DMEM/F12 (Gibco) supplemented with N2 Supplement (Gibco), 6 mg/ml glucose, 5 mM HEPES, 2 mM l-glutamine, 4 μg/ml heparin, epidermal growth factor (50 ng/ml) and basic fibroblast growth factor (10 ng/ml). After lentiviral transduction with TCF/LEF reporter driving expression of GFP (TOP-GFP) cells were single-cell plated in 96-well ultra-low adhesion plates (Corning) with FACSaria (BD Biosciences). This TOP-GFP vector was a gift from Dr. Laurie Ailles and was described previously.9
Myofibroblast 18Co cells were purchased from the American Type Culture Collection and maintained in DMEM medium supplemented with 10% FCS and 1% glutamine. To obtain MFCM, 1.5 × 106 18Co cells were seeded in 75-cm2 flasks. The next day, cells were washed 5 times with PBS and incubated for 24 h with 10 ml of CSC medium without EGF and bFGF. MFCM was then collected, cleared by centrifugation and supplemented with EGF (50 ng/ml) and bFGF (10 ng/ml) prior to addition to the cells.
RNA extraction, cDNA synthesis and RT-PCR
Extraction of RNA from cells was performed with Trizol reagent (Invitrogen) in accordance with the manufacturer's protocol. After quantification of RNA using NanoDrop ND-2000 (Thermo Scientific) 2 μg of RNA was used to synthesis cDNA using SuperScript III in accordance with the manufacturer's protocol (Invitrogen). RT-PCR was performed with LC480 SYBR green (Roche) in accordance with the manufacturer's instructions on a LC480. The following primers were used: 18 S sense: 5′-AGACAACAAGCTCCGTGAAGA-3′, 18 S antisense: 5′-CAGAAGTGACGCAGC-CCTCTA-3′, Mucin 2 sense: 5′-CGA-AACCACGGCCACAACGT-3′, Mucin 2 anti-sense: 5′-GACCACGGCCCCGTTAAGCA -3′, Ck20 sense: 5′- TGTCC-TGCAAATTGATAATGCT -3′, and Ck20 anti-sense: 5′- AGACGTATTCC-TCTCTCACTCTCATA -3′.
Immunoblotting and antibodies
Spheroid cultures were grown in MFCM or control medium and 24 h later cultures were lysed in 1 × RIPA lysis and extraction buffer (Thermo Fisher Scientific) supplemented with complete protease inhibitor (Roche). Subsequently, lysates were cleared by centrifugation (14 000 rpm, 10 min, 4°C). After quantification of protein concentration using BCA protein assay (Thermo Scientific), 20 μg extracted protein was separated on 12% precast gels (Bio-Rad) and transferred to Hybond-P membranes (Amersham). Membranes were blocked with 5% milk in phosphate-buffered saline solution containing 0.2% TWEEN (PBS-T) for 1 h. The membranes were incubated with anti-BCLXL (clone S18, 1:500, Santa-Cruz) in 2.5% milk/PBS-T overnight at 4°C and subsequently washed 3 times with PBS-T. Membranes were then incubated with anti-mouse IgG horseradish peroxidase conjugates for 1 h, washed 3 times and detection of bound antibody was performed with ECLplus reagents (Amersham). Western blots were analyzed by LAS4000. For loading control, blots were incubated with anti-ERK1/2 (1 : 10 000, Cell Signaling).
Limiting-dilution assay
Spheroid cultures were dissociated and CSCs (10% TOP-GFPhi) or differentiated (10%TOP-GFPlo) cells were FACS deposited using the FACSaria (BD Biosciences) in a limiting dilution fashion at 1, 2, 4, 8, 16, 24, 32, 40, and 64 cells per well in ultra-low 96-well plates (Corning). CSCs were sorted in control medium, while differentiated cells were sorted in either control medium or MFCM. Clonal frequency was evaluated after 2 weeks and calculated using the Extreme Limiting Dilution Analysis ‘limdil’ function as described.10
FACS staining
Colon spheroid cultures were grown in control medium or MFCM. After 24 h cells were dissociated and stained with AC133/CD133-APC antibody (1 : 25, Miltenyi Biotec) or anti-LGR5-biotin antibody (4D11F8, 1:100, BD Biosciences) in PBS containing 1% bovine serum albumin (PBS-B) for 30 min at 4°C. Subsequently, cells were washed with PBS-B and resuspended in PBS-B. For LGR5 staining, cells were incubated with APC conjugated streptavidin (1:500, E biosciences) and washed twice with PBS-B. Dead cells were excluded with 7-AAD (BD Biosciences). Stainings were analyzed on a FASCanto (BD Biosciences).
Cell death assay
TOP-GFP transduced spheroid culture Co100 was seeded as single cells on an adherent cell culture 12-well plate (Greiner) overnight. The next day, adherent cells were refreshed with control medium or MFCM and 24 h later cells were left untreated or treated with 100 nM ABT-737 (Selleck Chemicals) and/or 50 μM oxaliplatin. After 24 h treatment cells were resuspended to single cells using trypsin-EDTA. 50 000 cells were washed with PBS and stained with Annexin V-APC (BD biosciences) and 7-AAD (BD biosciences) for 15 min at RT, followed by flow cytometry performed with FACSCanto (BD biosciences). Cell death (AnnexinV+) was measured in CSCs by gating on TOP-GFPhi cells and in differentiated tumor cells by gating on TOP-GFPlo cells.
Results
Factors secreted from fibroblasts can induce dedifferentiation of non-CSC
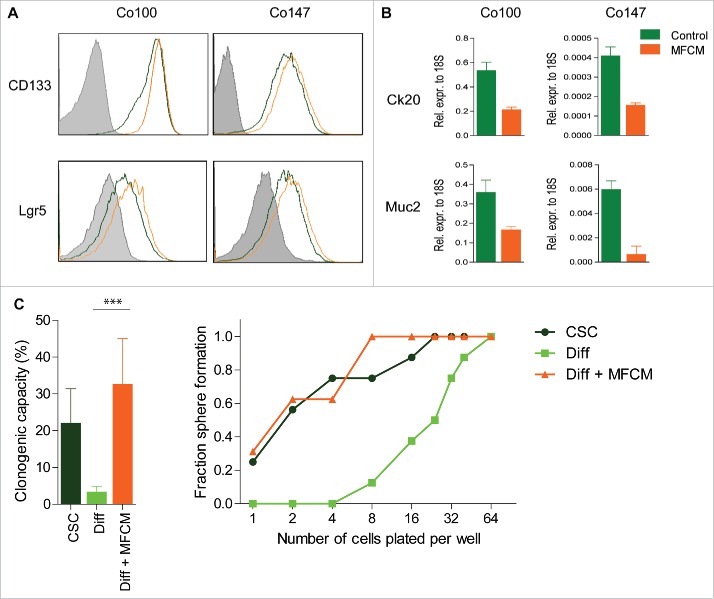
Myofibroblasts make up an important part of the microenvironment in colon cancers and direct CSC features.5,11,12 To study the effect of myofibroblast-secreted factors on CSCs, myofibroblast-conditioned medium (MFCM) was derived from human colon 18Co fibroblasts. These fibroblasts model cancer-associated fibroblasts (CAFs) and similar observations can be obtained using CAFs (not shown). Spheroid cultures, containing CSCs and more differentiated tumor cells, were grown in the absence or presence of this MFCM and CSC markers CD133 and LGR5 were measured using flow cytometry. This revealed a clear increase in cells expressing these CSC markers (Fig. 1A). In line with the idea that CSC numbers increased in these cultures, a concomitant decrease in the expression of differentiation markers Cytokeratin 20 (Ck20) and Mucin2 was observed after MFCM exposure (Fig. 1B). Increased stem cell marker expression in combination with decreased differentiation marker expression suggest a MFCM-induced increase in the stem cell fraction in our spheroid cultures. However, marker expression by itself is not sufficient to determine stem cell capacity, which in vitro can only be quantified using a limiting dilution assay. Spheroid cultures were therefore sorted in a limiting dilution fashion of either the CSCs (GFPhi) or the differentiated cells (GFPlo) cells. Confirming previous data, only CSCs were clonogenic while differentiated cells failed to grow. However, when differentiated cells were sorted in plates containing MFCM, clonogenicity was restored to levels of CSCs (Fig. 1C), confirming the idea that fibroblast secrete factors that promote dedifferentiation of differentiated cells into CSCs. Previously, we observed that this reversal even resulted in the re-acquisition of tumor-initiating potential confirming the idea that MFCM can re-install cancer stemness in more differentiated cells.5
Figure 1.
MFCM dedifferentiate differentiated cells to CSCs Spheroid cultures grown in control medium (red) or MFCM (brown) for 24 h and were (A) stained with stem cell marker Lgr5, CD133 or (B) qrt-pcr was performed on differentiation markers Ck20 and Mucin2 (Muc2). (C) Limiting dilutions experiments were performed and clonogenic fraction was calculated in CSC (TOP-GFPhi), Differentiated cell (TOP-GFPlo), and differentiated cells deposited in MFCM (Diff + MFCM). Significance is shown as ***P < 0.001.
Conditioned medium increases BCLXL protein expression and induces resistance to chemotherapy
Recently, we have described an assay that allows for the study of chemotherapy sensitivity in differentiated cells and CSCs simultaneously.6 Using this assay we have shown that a differential sensitivity exists between CSCs, which are resistant and differentiated cells, which are chemosensitive. To study if factors secreted by myofibroblasts were also capable of inducing therapy resistance in differentiated cells, spheroid cultures were grown in control or in MFCM and subsequently treated with chemotherapy. In control medium, differentiated cells were sensitive to oxaliplatin when compared with CSCs. However, when pretreated for only 24 hours with MFCM oxaliplatin-induced cell death was significantly blocked, suggesting that, similar to the induction of clonogenicity, also therapy resistance can be restored by the microenvironment (Fig. 2A). To further investigate the mechanism of chemotherapy resistance the anti-apoptotic molecule BCLXL was analyzed, as we have previously shown that colorectal CSCs are dependent on BCLXL for survival and therapy resistance.6 Upon MFCM exposure of spheroid cultures BCLXL protein expression was increased (Fig. 2B and C), which is sufficient to block oxaliplatin-induced cell death in spheroid cultures.6
Figure 2.
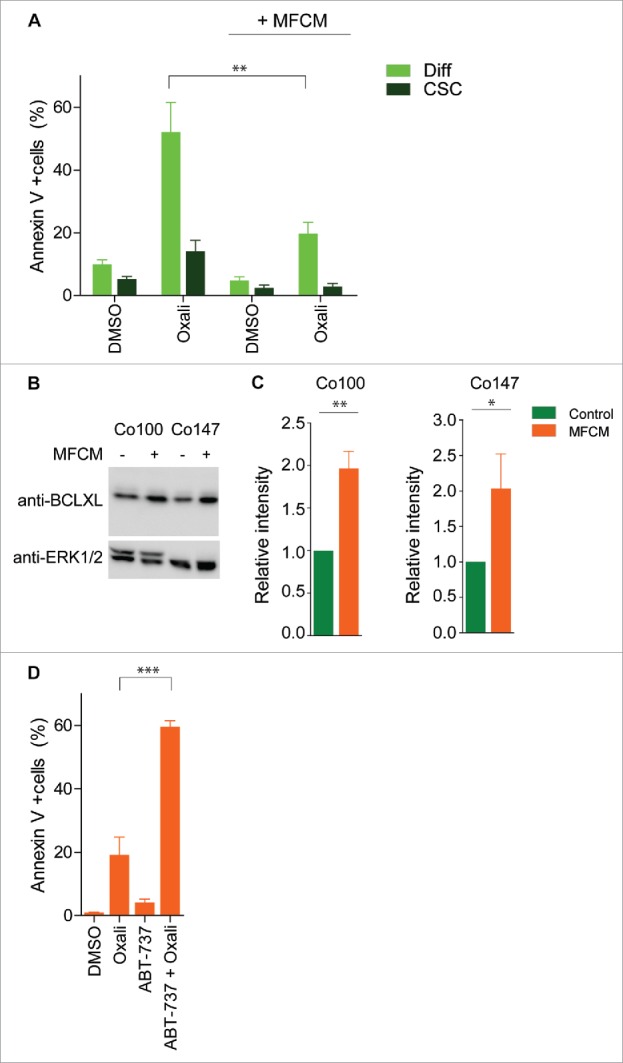
BCLXL is required for MFCM induced resistance toward oxaliplatin (A) Primary spheroid culture transduced with TOP-GFP construct (Co100) was exposed to MFCM or control medium for 24 h. Subsequently, cells were treated for 24 h with oxaliplatin and Annexin V/ 7AAD staining was performed. CSCs and differentiated cells were defined by gating on TOP-GFPhi and TOP-GFPlo expressing cells, respectively. MFCM treatment decreases oxaliplatin induced cell death. (B) Western blot analysis of BCLXL protein in spheroid cultures grown in control or MFCM for 24 h. Increased BCLXL expression in spheroid culture treated with MFCM compared to control medium. Control western blots for ERK1/2 is shown in lower panel. (C) Quantification of BCLXL protein intensity relative to ERK1/2 protein expression. Average of 3 independent experiment is shown. (D) Co100 spheroid culture was exposed to MFCM for 24 h. Cells were treated with 100 nM ABT-737 in combination with or without oxaliplatin. Cell death was measured with AnnexinV/7-AAD staining in TOP-GFPlo cells. Significance is indicated (*P < 0.05, **P < 0.01, ***P < 0.001).
ABT-737 reverts MFCM induced chemotherapy resistance
Inhibition of apoptosis is one of the hallmarks of cancer. Therefore there are small molecule inhibitors in clinical trials that inhibit anti-apoptotic BCL2 molecules. ABT-737 inhibits BCLXL, BCL2 and BCLW and displays anti-tumorigenic activity.13,14 To investigate if inhibition of these anti-apoptotic molecules is sufficient to sensitize cells that were exposed to MFCM cells were treated with ABT-737. Interestingly, differentiated cells that were exposed to MFCM were resistant to oxaliplatin. However, this road block in chemotherapy-induced apoptosis was lifted by ABT-737 (Fig. 2D). This suggested that anti-apoptotic BCL2 proteins are crucial in MFCM induced-resistance and inhibition of these proteins using small molecule inhibitors is sufficient to sensitize to chemotherapy. Moreover, our data provide compelling evidence that factors secreted by fibroblast are able to induce CSC features in differentiated tumor cells, which include therapy resistance. These factors therefore are interesting candidates for therapeutic targeting in colorectal cancers.
Discussion
Despite the fact that only CSCs and not the more differentiated cancer cells are highly tumorigenic in xenotransplantation assays, differentiated cells can indirectly become tumorigenic by dedifferentiation.5,7,8,15,16 Here we show that human colonic fibroblast secrete factors that increases the stem cell fraction in spheroid cultures. In addition, increased anti-apoptotic BCLXL protein expression and resistance toward chemotherapy were observed. This MFCM induces resistance could be overcome by the BH3 mimetic ABT-737. Inhibition of BCLXL by ABT-737 is therefore likely to be sufficient to revert the effects of myofibroblasts .
There is evidence that depletion of colorectal CSCs does not block tumor growth.17 This is analogous to normal intestinal stem cells. In the healthy intestine stem cells have been identified using LGR5 as marker.18 Depletion of LGR5-positive stem cells in the intestinal epithelium using a diphtheria toxin receptor driven by the LGR5-promoter, does not disturb homeostasis as would be expected after killing a stem cell population. Distinct stem cells, namely Bmi1-expressing stem cells were able to compensate for the loss of LGR5-expressing cells.19 Besides highly proliferative LGR5-positive stem cells, there are also quiescent LGR5 expressing cells, also called label-retaining cells, that express Paneth and enteroendocrine cell markers. These cells are able to repopulate intestinal epithelial cells when there is intestinal damage, suggesting that these label-retaining cells are reserve stem cell population in the intestine.20 In addition, high Notch ligand expressing cells are described in the mouse intestine, which represent progenitor cells derived from the LGR5-stem cells. These cells are already specified to a secretory lineage, but can dedifferentiate and replenish the pool of LGR5-positive stem cells.21 The same phenomenon of a bidirectional relationship between stem cells and differentiated progeny is observed during intestinal tumorigenesis. APC mutations in stem cells was suggested to effectively lead to intestinal tumors, while more differentiated cells would not fully transform.22 However, differentiated cells can be the tumor initiating cells when the NFkB pathway is co-activated, leading to enhanced nuclear β-catenin activity. In other words dedifferentiation can occur under the right conditions and result in tumor-initiating capacities.7
Tumors are surrounded by stromal cells, including myofibroblasts, which we have previously shown to install a CSC-phenotype in differentiated tumor cells, restoring their clonogenic potential.5 The dominant factor in MFCM was shown to be hepatocyte growth factor (HGF). Next to HGF, osteopontin and stromal-derived factor 1a can revert differentiated colorectal cancer cells into metastatic CSCs.23 In addition, it was shown that the microenvironment can even enhance the metastatic potential of non-metastatic progenitors and as such also form metastatic CSCs.23 Similar observations of plasticity have been made in other tissues. For instance, in mammary epithelium, stochastic conversion of differentiated cells occurs at low levels during homeostatic conditions.8 Moreover, oncogenic mutations increase the conversion of differentiated cells into stem cells, which then can act as tumor-initiating cells.8 Similarly, activation of the RAS signaling pathway, by NF1 deletion, and inhibition of p53 tumor suppressor in terminally differentiated neurons and astrocytes can also induce stem cell markers and tumorigenicity.16 Finally, in breast cancer induction of epithelial-mesenchymal transition (EMT) by overexpression of TWIST or SNAIL or exposing cells to TGFB strongly enhances the CSCs phenotype24 while, mesenchymal stem cells and cancer stem cells regulate stemness by a network of CXCL6 and IL6.25
Importantly, transitions between more differentiated cancer cells and CSCs may also represent a more stochastic process as suggested by the group of Lander. When differentiated breast cancer cells were sorted, these cells were able to form stem cells. Interestingly, these differentiated are not tumorigenic in vivo, but become tumorigenic when injected with irradiated carrier cells, which gives them the time to revert to CSCs in vivo.15 In line with these observations, we have shown that differentiated glioblastoma cells can re-acquire stem cell traits under the influence of endothelial like cells (unpublished observations).
Therefore, these lines of evidence suggest that plasticity in the form of a bidirectional relationship between stem cells and their differentiated progeny is present and is increased in the presence of oncogenic mutations, as is the case within a tumor.
Besides the fact that CSCs are tumorigenic it is now well accepted that CSCs are also resistant to tumor therapy.6,26-29 By measuring mitochondrial priming in CSCs and differentiated cells we have shown that mitochondria of CSCs are less primed to death, which is due to an elevated anti-apoptotic machinery. Targeting the anti-apoptotic molecule BCLXL directly, resulted in cell death of CSCs, while sublethal doses of the BH3 mimetics allowed for mitochondrial priming of CSCs, which then sensitizes CSCs toward various chemotherapies.6 Likewise to our previous study, here we used sublethal doses of ABT-737 that did not induce cell death but sensitized chemotherapy resistant cells toward oxaliplatin. This combination treatment resulted in more than just an additional effect of ABT-737 on oxaliplatin. It is worth to mention that there is increasing evidence that also CSCs from other cancers may depend on BCLXL. In a recent study it is shown that chemotherapy resistant lung CSCs require BCLXL for resistance and treatment with ABT-737 eliminated these CSCs in vitro and in vivo.30 This indicates that our findings are not only restricted to colorectal cancer, but can also be important for other cancers.
Therapy resistance of CSCs and plasticity has important implications when treating cancer. Therapy aimed at CSCs might not be effective, since CSC-pools will readily be restored by reversion of the differentiated progeny. Therefore, to kill all tumor cells and overcome relapses caused by remaining or reverted CSCs, more study needs to be done on regulation of plasticity of CSCs and their tumor therapy resistance mechanisms. Nevertheless, targeting microenvironmental factors or the anti-apoptotic features induced by the microenvironment could prove useful new avenues for therapy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Abbreviations
- CSC
cancer stem cell
- MFCM
myofibroblast conditioned medium.
Acknowledgments
We thank the members of the laboratory for useful discussion. In addition, we thank Kate Cameron, Berend Hooibrink and Toni van Capel for assistance with fluorescence-activated cell sorting experiments. We also thank Laurie Ailles (University of Toronto) for providing Tcf/Lef reporter construct.
Funding
JPM is sponsored by grants from the Netherlands Organization for Scientific Research (NWO; Gravitation-Cancer Genomics Center The Netherlands Zwaartekracht), from the Dutch Cancer Society (UVA2009-4416 and UVA2012-5735), from MLDS (FP13-07) and Alpe dHuzes/KWF (CONNECTION).
References
- [1]. Medema JP. Cancer stem cells: the challenges ahead. Nature Cell Biol 2013; 15:338-44; PMID:23548926; http://dx.doi.org/ 10.1038/ncb2717 [DOI] [PubMed] [Google Scholar]
- [2]. Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445:111-5; PMID:17122771; http://dx.doi.org/ 10.1038/nature05384 [DOI] [PubMed] [Google Scholar]
- [3]. O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445:106-10; PMID:17122772; http://dx.doi.org/ 10.1038/nature05372 [DOI] [PubMed] [Google Scholar]
- [4]. Kemper K, Prasetyanti PR, De Lau W, Rodermond H, Clevers H, Medema JP. Monoclonal antibodies against Lgr5 identify human colorectal cancer stem cells. Stem Cells 2012; 30:2378-86; PMID:22969042; http://dx.doi.org/ 10.1002/stem.1233 [DOI] [PubMed] [Google Scholar]
- [5]. Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol 2010; 12:468-76; PMID:20418870; http://dx.doi.org/ 10.1038/ncb2048 [DOI] [PubMed] [Google Scholar]
- [6]. Colak S, Zimberlin CD, Fessler E, Hogdal L, Prasetyanti PR, Grandela CM, Letai A, Medema JP. Decreased mitochondrial priming determines chemoresistance of colon cancer stem cells. Cell Death Differ 2014; 21:1170-7; PMID:24682005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Goktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, et al. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell 2013; 152:25-38; PMID:23273993; http://dx.doi.org/ 10.1016/j.cell.2012.12.012 [DOI] [PubMed] [Google Scholar]
- [8]. Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, Brooks M, Reinhardt F, Su Y, Polyak K, et al. Normal and neoplastic nonstem clls can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A 2011; 108:7950-5; PMID:21498687; http://dx.doi.org/ 10.1073/pnas.1102454108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003; 423:409-14; PMID:12717450; http://dx.doi.org/ 10.1038/nature01593 [DOI] [PubMed] [Google Scholar]
- [10]. Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods 2009; 347:70-8; PMID:19567251; http://dx.doi.org/ 10.1016/j.jim.2009.06.008 [DOI] [PubMed] [Google Scholar]
- [11]. Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 2002; 126:829-36; PMID:12088453 [DOI] [PubMed] [Google Scholar]
- [12]. Yeung TM, Buskens C, Wang LM, Mortensen NJ, Bodmer WF. Myofibroblast activation in colorectal cancer lymph node metastases. British J Cancer 2013; 108:2106-15; PMID:23652304; http://dx.doi.org/ 10.1038/bjc.2013.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Zhai D, Jin C, Satterthwait AC, Reed JC. Comparison of chemical inhibitors of antiapoptotic Bcl-2-family proteins. Cell Death Differ 2006; 13:1419-21; PMID:16645636; http://dx.doi.org/ 10.1038/sj.cdd.4401937 [DOI] [PubMed] [Google Scholar]
- [14]. Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435:677-81; PMID:15902208; http://dx.doi.org/ 10.1038/nature03579 [DOI] [PubMed] [Google Scholar]
- [15]. Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell 2011; 146:633-44; PMID:21854987; http://dx.doi.org/ 10.1016/j.cell.2011.07.026 [DOI] [PubMed] [Google Scholar]
- [16]. Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science 2012; 338:1080-4; PMID:23087000; http://dx.doi.org/ 10.1126/science.1226929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014; 14:149-59; PMID:24332836; http://dx.doi.org/ 10.1016/j.stem.2013.11.008 [DOI] [PubMed] [Google Scholar]
- [18]. Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007; 449:1003-7; PMID:17934449; http://dx.doi.org/ 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- [19]. Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 2011; 478:255-9; PMID:21927002; http://dx.doi.org/ 10.1038/nature10408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Buczacki SJ, Zecchini HI, Nicholson AM, Russell R, Vermeulen L, Kemp R, Winton DJ. Intestinal label-retaining cells are secretory precursors expressing Lgr5. Nature 2013; 495:65-9; PMID:23446353; http://dx.doi.org/ 10.1038/nature11965 [DOI] [PubMed] [Google Scholar]
- [21]. van Es JH, Sato T, van de Wetering M, Lyubimova A, Nee AN, Gregorieff A, Sasaki N, Zeinstra L, van den Born M, Korving J, et al. Dll1 +secretory progenitor cells revert to stem cells upon crypt damage. Nat Cell Biol 2012; 14:1099-104; PMID:23000963; http://dx.doi.org/ 10.1038/ncb2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature 2009; 457:608-11; PMID:19092804; http://dx.doi.org/ 10.1038/nature07602 [DOI] [PubMed] [Google Scholar]
- [23]. Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell 2014; 14:342-56; PMID:24607406; http://dx.doi.org/ 10.1016/j.stem.2014.01.009 [DOI] [PubMed] [Google Scholar]
- [24]. Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133:704-15; PMID:18485877; http://dx.doi.org/ 10.1016/j.cell.2008.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res 2011; 71:614-24; PMID:21224357; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-0538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133 +HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene 2008; 27:1749-58; PMID:17891174; http://dx.doi.org/ 10.1038/sj.onc.1210811 [DOI] [PubMed] [Google Scholar]
- [27]. Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, et al. Highly tumorigenic lung cancer CD133 +cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A 2009; 106:16281-6; PMID:19805294; http://dx.doi.org/ 10.1073/pnas.0905653106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Eramo A, Ricci-Vitiani L, Zeuner A, Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C, De Maria R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ 2006; 13:1238-41; PMID:16456578; http://dx.doi.org/ 10.1038/sj.cdd.4401872 [DOI] [PubMed] [Google Scholar]
- [29]. Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 2007; 1:389-402; PMID:18371377; http://dx.doi.org/ 10.1016/j.stem.2007.08.001 [DOI] [PubMed] [Google Scholar]
- [30]. Zeuner A, Francescangeli F, Contavalli P, Zapparelli G, Apuzzo T, Eramo A, Baiocchi M, De Angelis ML, Biffoni M, Sette G, et al. Elimination of quiescent/slow-proliferating cancer stem cells by Bcl-X inhibition in non-small cell lung cancer. Cell Death Differ 2014; PMID:25034785; http://dx.doi.org/10.1038/cdd.2014.105 [DOI] [PMC free article] [PubMed] [Google Scholar]