The centriole is a conserved organelle in most animal cells. It is built with numerous proteins including a 9-fold symmetrical microtubule wall. Centrioles usually exist in pairs and constitute the core of the centrosome, the major microtubule-organizing center of the cell. In line with DNA replication, the centriole duplicates once every cell cycle. Beginning in G1 phase, a newly born daughter centriole assembles perpendicularly to the mother centriole, and subsequently elongates throughout S and G2. Interestingly, the daughter centriole can neither duplicate itself nor nucleate pericentriolar material (PCM), a cloud of proteins that enhances the microtubule nucleating ability of the centrosome, until the centriole has passed through mitosis.1-3 This process enabling the daughter centriole to acquire motherhood and be able to duplicate and recruit PCM has been named “centriole-to-centrosome conversion”.1 In a recent study, we demonstrated that centriole-to-centrosome conversion relies upon the building of a protein complex comprising Cep135, Ana1/Cep295 and Asl/Cep152 onto the nascent centriole in both Drosophila melanogaster and human cells.4
Understanding centriole to centrosome conversion requires knowledge of the centriolar components that are loaded during mitosis, how they are organized in space and the dependencies of their recruitment. We first utilized 3D structured illumination super-resolution microscopy (3D-SIM) on cultured Drosophila cells to establish a timeline of the recruitment of proteins that are critical for centriole duplication, which had been identified from previous genome-wide screens.5,6 This revealed the sequential recruitment of Cep135, Ana1 (Anastral spindle 1) and Asl (Asterless) onto newly assembled daughter centrioles as they matured from late interphase through mitosis. Notably, these proteins each displayed elongated configurations within single centriole, as indicated by attaching GFP fluorophore at the different ends of each protein. Together the 3 proteins formed partially overlapping, concentric circles within the daughter centriole cylinder, with C terminus of Cep135 the most centrally located, and moving sequentially outwards to be followed by Cep135's N-terminus, Ana1's N-terminus, Ana1's C-terminus, Asl's C-terminus, and finally with Asl's N-terminus being the most peripherally located.
Biochemical investigations and cell experiments revealed the existence of a protein network, in which Cep135 and Asl were linked together through direct interaction with Ana1 via their overlapping termini. Stripping off Asl abolished the very ability of the mature mother centriole to duplicate, as indicated by a failure to recruit Sas-6, one of the earliest factors participating in building the daughter. Asl was recruited to the centriole apparently via Ana1, which in turn required interaction with Cep135 to localize to the centriole. Cep135 depended on Ana1 for its proper concentration on the centriole thus indicating a mutual dependence reflected by the similar timing of their loading. In addition, depletion of Ana1 also affected the accumulation of gamma-tubulin on the daughter centriole in anaphase, indicating another aspect of the failure in the daughter's conversion. Intriguingly, we were able to engineer a form of Ana1 in which we replaced its localizing domain by GBP (GFP binding protein). This was able to bind Cep135 tagged with GFP and completely rescue centriole duplication. However, to do this, the Ana1 fragment had to be positioned at a correct radial diameter so that the centriole could duplicate efficiently. This is a direct pointer to the importance of the spatial organization that is provided by this protein network.
This system has been highly conserved and notably, we uncovered a similar linear network of molecules required for the conversion process in mammalian cells. Cep135, Cep295 (the human ortholog of Ana1) and Cep152 (the human ortholog of Asl) presented similar localization patterns within centriole, spanning from the center of the centriole to its outside region. Moreover, these 3 proteins showed linear co-dependency for loading to the newly assembled daughters similar to their Drosophila counterparts and the loss of any one of them led to failure of daughter centriole conversion. As a result, when the daughter was separated from the mother in the next cell cycle, it could not duplicate and remained a singlet. Thus, the Cep135-Cep295-Cep152 protein network is conserved both in Drosophila and human cells.
In summary, our findings account for the final stages in the assembly of daughter centriole that convert it into a mother able to duplicate (Fig. 1). Cep135, Ana1/Cep295 and Asl/Cep152 sequentially decorate the daughter centriole during its conversion, forming a molecular network. Since Asl serves as a scaffold for the recruitment of Plk4 kinase (Polo-like kinase 4) - the master regulator of centriole duplication pathway,7 this network enables the conversion of a daughter to a duplication-competent mother centriole. Future work will be needed to investigate how this protein cascade fits into the bigger picture of centriole assembly and how it is connected to the very early steps of procentriole formation. Centriole-to-centrosome conversion follows a strict timetable, and it has been suggested that Plk1 (Polo-like kinase 1) plays an important role in regulating the process in human cells.1 Indeed, it seems highly likely that the protein-protein interactions we described can be further regulated by phosphorylation both in time and space to control the sequence of events. Thus, unraveling the potential roles of Polo/Plk1 in regulating these interactions will be an intriguing topic for future study.
Figure 1.
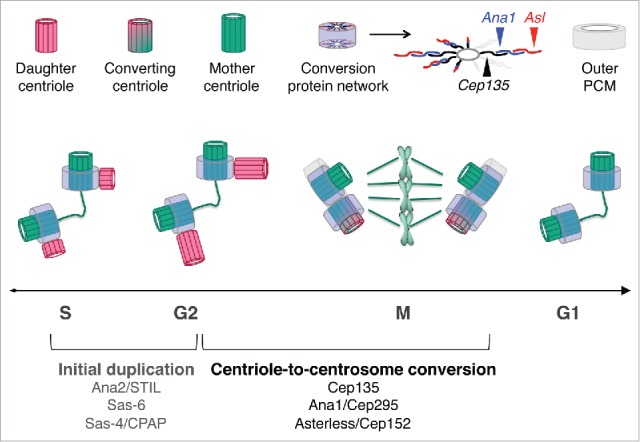
The newly assembled centriole is converted into duplication-competent mother through a cascade of protein interactions between Cep135, Ana1/Cep295 and Asl/Cep152, controlled both in time and space.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Wang WJ, et al.. J Cell Biol 2011; 193:727-39; PMID:21576395; http://dx.doi.org/ 10.1083/jcb.201101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fu J, et al.. Open Biol 2012; 2:120104; PMID:22977736; http://dx.doi.org/ 10.1098/rsob.120104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Izquierdo D, et al.. Cell Rep 2014; 8:957-65; PMID:25131205; http://dx.doi.org/ 10.1016/j.celrep.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fu J, et al.. Nat Cell Biol 2016; 18:87-99; PMID:26595382; http://dx.doi.org/ 10.1038/ncb3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dobbelaere J, et al.. PLoS Biol 2008; 6:e224; PMID:18798690; http://dx.doi.org/ 10.1371/journal.pbio.0060224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goshima G, et al.. Science 2007; 316:417-21; PMID:17412918; http://dx.doi.org/ 10.1126/science.1141314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fu J, et al.. Cold Spring Harb Perspect Biol 2015; 7:a015800; PMID:25646378; http://dx.doi.org/ 10.1101/cshperspect.a015800 [DOI] [PMC free article] [PubMed] [Google Scholar]


