ABSTRACT
Cytokinesis is the final step in cell division that results in the separation of a parent cell into daughter cells. Unlike somatic cells that undergo symmetric division, meiotic division is highly asymmetric, allowing the preservation of maternal resources for embryo development. Beclin-1/BECN1, the mammalian homolog of yeast Atg6, is a key molecule of autophagy. As part of a class III phosphatidylinositol 3-kinase (PI3K-III) complex, BECN1 initiates autophagosome formation by coordinating membrane trafficking. However, emerging evidence suggests that BECN1 regulates chromosome segregation and cytokinesis during mitosis. Thus, we investigated the function of BECN1 during oocyte meiotic maturation. BECN1 was widely distributed during meiotic maturation forming small vesicles. Interestingly, BECN1 is also detected at the midbody ring during cytokinesis. Depletion of BECN1 impaired the cytokinetic abscission, perturbing the recruitment of ZFYVE26 at the midbody. Similar phenotypes were observed when PI3K-III activity was inhibited. However, inhibition of autophagy by depleting Atg14L did not disturb meiotic maturation. Therefore, our results not only demonstrate that BECN1 as a PI3K-III component is essential for cytokinesis, but also suggest that BECN1 is not associated with autophagy pathway in mouse oocytes.
KEYWORDS: autophagy, Beclin-1, cytokinetic abscission, meiosis, oocyte
Introduction
Cytokinesis is the final step of cell division, in which the cytoplasm of a single parental cell is divided into 2 daughter cells.1-3 At the end of cell division, the constriction of the contractile ring drives the formation of a cleavage furrow, leaving 2 daughter cells connected by a narrow intercellular cytoplasmic bridge known as midbody. The midbody consists of a thin tube of plasma membrane filled with bundled anti-parallel microtubules and associated proteins. Completion of cytokinesis to 2 separate daughter cells requires the midbody to be severed in a final step termed abscission.4 Defects in this process have been shown to promote cell death and genetic instability associated with tumorigenesis.5,6 Therefore, the process of cytokinesis must be spatially and temporally controlled in a highly structured manner. However, the molecular mechanisms involved in this process are not yet clearly understood.
Autophagy is an evolutionarily conserved cellular process that degrades long-lived proteins and damaged organelles.7,8 This process involves sequestration of cellular constituents in double-membraned cytoplasmic vesicles called autophagosomes, with subsequent fusion to the lysosome where they are degraded and recycled. Becn1, the mammalian homolog of Atg6, is the first identified mammalian gene with a role in mediating autophagy.9,10 BECN1 is a coiled-coil protein that contains a Bcl-2 homology-3 (BH3) domain. BECN1 was found to restore starvation-induced autophagy in Atg6-disrupting yeast and human breast cancer cells lacking Becn1, whereas Becn1 overexpression activates autophagy, implicating central roles of Becn1 in autophagy.11 Recent studies reveal that BECN1 interacts with the class III phosphatidylinositol 3-kinase (PI3K-III) VPS34/PIK3C3 and governs autophagy induction by regulating the generation of phosphatidylinositol 3-phosphate (PI(3)P) and subsequent recruitment of additional autophagic proteins.12-15 In addition to its core functions in autophagy control, a growing body of evidence suggests that BECN1 appears to have several non-autophagy functions.16-18 For instance, BECN1 knockdown has been shown to cause cytokinesis arrest and increased multinuclear cells.16 BECN1 has also been shown to regulate chromosome congression and kinetochore assembly during mitosis.18
Despite the apparent importance of BECN1 in the regulation of several biological processes, little is known about its function during oocyte meiosis. In this study, we investigated the function of BECN1 during oocyte meiotic maturation. Our data not only demonstrates that BECN1 plays a critical role in cytokinesis during meiosis as a component of PI3K-III, but also suggests that the BECN1 is not associated with autophagy pathway during meiotic maturation.
Results
Expression and subcellular localization of BECN1 during meiotic maturation
We first determined the expression of BECN1 during meiotic maturation. Oocytes were cultured for 0, 4, 8 and 13 hours, corresponding to germinal vesicle (GV), GV breakdown (GVBD), metaphase I (MI), and metaphase II (MII) stages, respectively and collected for immunoblotting analysis. Immunoblotting results showed that BECN1 was expressed at all stages of oocyte maturation and gradually increased from GV to MII stages (Fig. 1A).
Figure 1.

Expression and subcellular localization of BECN1 during meiotic maturation (A) Oocytes at GV, GVBD, MI, and MII stages were collected and subjected to immunoblot analysis with anti-BECN1 antibody. β-actin was used as a loading control. Each lane contains 50 oocytes. Normalized expression of BECN1 was quantified and expressed as the mean ± SEM from 3 independent experiments. (B) Immunostaining of BECN1 during oocyte meiotic maturation. Each stage of oocytes were fixed and stained with anti-BECN1 antibody. Oocytes at MI stage were fixed and stained with normal rabbit IgG as a negative control. DNA and spindle were stained with DAPI and anti-tubulin antibody, respectively. Representative images are shown from 3 independent experiments with at least 30 oocytes. Bar, 10 μm.
We next determined the subcellular localization of BECN1 by immunofluorescence staining during meiotic maturation. BECN1 showed a vesicular punctate pattern of localization distributed throughout the cytoplasm and nucleus at GV stages. After GVBD, BECN1 was widely distributed at the cytoplasm. At the MI and MII stages, BECN1 showed uniform localization throughout the cytoplasm (Fig. 1B).
BECN1 knockdown impairs cytokinesis
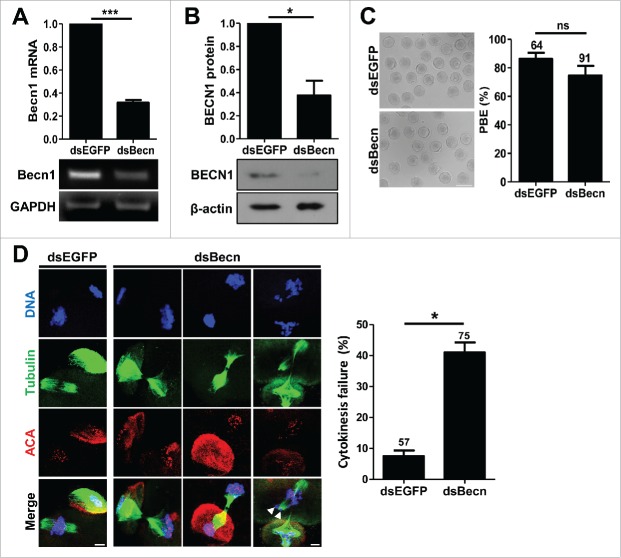
To assess the physiological function of BECN1 during meiotic maturation, we injected long double-stranded RNA targeting Becn1 (dsBecn). EGFP double-stranded RNA (dsEGFP) was used as a control. RT-PCR and immunoblotting analyses showed that BECN1 expression was significantly reduced following dsBecn injection (Fig. 2A, B). Interestingly, BECN1 knockdown oocytes showed no discernible morphological defects (Fig. 2C). However, confocal microscopy analysis of chromosomes and spindle configuration revealed that a significant portion of oocytes arrested at the telophase I with abscission failure, leaving thin microtubule bundles at the midbody bridge with some decondensed or misaligned chromosomes (Fig. 2D).
Figure 2.
Knockdown of BECN1 impairs cytokinesis (A, B) GV oocytes injected with indicated dsRNAs were cultured for 24 hours in the presence of IBMX. Knockdown of BECN1 was confirmed by either RT-PCR (A) or immunoblot analysis (B). GAPDH and β-actin were used as a loading control for RT-PCR and immunoblot, respectively. Quantification of the BECN1 level is shown above the representative images. The data are expressed as the mean ± SEM from 3 independent experiments. *p < 0.05; ***p < 0.0001. (C) BECN1 knockdown oocytes were cultured in IBMX-free medium for 13 hours and polar body extrusion (PBE) was scored to assess meiotic maturation. Representative images from at least 3 independent experiments are shown. Bar, 100 μm. The data are expressed as the mean ± SEM. The number of oocytes analyzed was shown above the bar. ns, not significant. (D) BECN1 knockdown oocytes were fixed and stained with anti-tubulin and anti-centromere antibodies (ACA) with DAPI for DNA staining. Representative images from 3 independent experiments are shown. Bar, 10 μm. Arrows indicate the misaligned and lagging chromosomes. The chromosome misalignment and cytokinesis failure were quantified and shown in right panel of images. The data are expressed as the mean ± SEM from 3 independent experiments. The number of oocytes analyzed was shown above the bar. *p < 0.05.
Cytokinesis failure at abscission is associated with inhibition of PI3K-III but not autophagy
Because BECN1 is a well-known key autophagy regulator, we investigated whether the observed phenotype following BECN1 knockdown is associated with defects in autophagy during meiotic maturation. To this end, we first examined the localization of BECN1 with autophagosomes. Surprisingly, we observed that BECN1 is not colocalized with the autophagosome marker microtubule-associated protein 1 light chain 3B (MAP1LC3B)/LC3, implying a non-autophagy function for BECN1 during oocyte meiosis (Fig. 3A). Next, we treated oocytes with autophagy inhibitors, either 3-methyladenine (3-MA) or bafilomycin A1 (Baf A1), and observed meiotic maturation. As with BECN1 knockdown oocytes, the polar body was normally extruded without any discernible morphological defect following treatment (Fig. 3B). However, immunostaining analysis showed that oocytes treated with 3-MA failed to complete cytokinesis, as in BECN1 knockdown oocytes (Fig. 3C). In contrast, oocytes treated with Baf A1 showed normal spindle and chromosome configurations (Fig. 3C).
Figure 3.
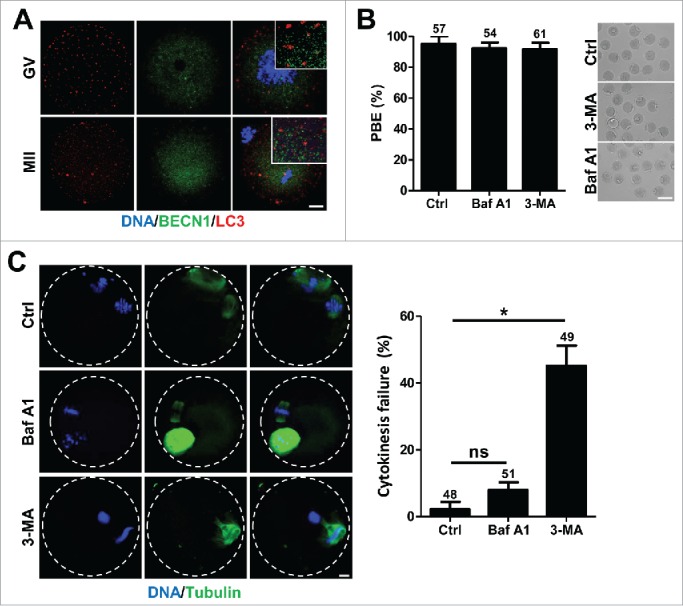
BECN1 is not associated with autophagy during meiotic maturation (A) Oocytes injected with mRNAs encoding BECN1 tagged with V5 were co-stained with anti- MAP1LC3B/LC3 antibody. Note that BECN1 and MAP1LC3B/LC3 are not colocalized. Bar, 10 μm. (B) Oocytes at the MI stage (8 hours after IBMX release) were cultured with either 100 nM Baf A1 or 2 mM 3-MA for 6 hours and polar body extrusion (PBE) was scored. Data are the mean ± SEM from 3 independent experiments with the indicated number of oocytes. The representative images are shown in the right panel of the bar graph. Bar, 100 μm. (C) Oocytes treated with either Baf A1 or 3-MA were stained with DAPI and anti-tubulin antibody to visualize DNA and spindle, respectively. Bar, 10 μm. Quantification of cytokinesis failure is shown in the right panel of the images. Data are the mean ± SEM from 3 independent experiments with the indicated number of oocytes. *p < 0.05; ns, not significant.
Given that 3-MA inhibits autophagy by blocking autophagosome formation via the inhibition of PI3K-III, while Baf A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes,19,20 it is likely that cytokinetic abscission failure might be associated with defects in PI3K-III activation rather than autophagy defects. To test this possibility, we selectively disrupted autophagy-associated function of PI3K-III by depleting Atg14L, a sub-component of PI3K-III exclusively associated with autophagy (Fig. 4A).21 Following Atg14L knockdown, oocytes normally extruded the polar body without any discernible defects in spindle and chromosome configuration (Figs. 4B, C). In contrast, significant portions of oocytes showed abscission failure when catalytic subunit of PI3K-III (Pik3c3)/Vps34 was depleted (Figs. 4A-C). This result, along with the observation that 3-MA but not Baf A1 disturbs cytokinesis abscission during oocyte meiosis, suggests that oocyte cytokinesis is regulated by PI3K-III and that also autophagy seems not to be essential for meiotic maturation. Collectively, our results suggest that BECN1 as part of a PI3K-III complex plays a critical role in cytokinetic abscission but not in autophagy during meiotic maturation.
Figure 4.

Cytokinesis failure at abscission is mediated by Pik3c3/Vps34, but not by Atg14L (A) GV oocytes injected with indicated dsRNAs were cultured for 24 hours in the presence of IBMX. Knockdown of Atg14L and Pik3c3/Vps34 was confirmed by RT-PCR analysis. GAPDH was used as a loading control. The normalized expression level of Atg14L and Pik3c3/Vps34 is shown in the right panel of the representative images. The data are expressed as the mean ± SEM from 2 independent experiments. (B) Oocytes depleting Atg14L or Pik3c3/Vps34 were cultured in IBMX-free medium for 13 hours and polar body extrusion (PBE) was scored. The data are expressed as the mean ± SEM from 3 independent experiments. The number of oocytes analyzed is shown above the bar. (C) Oocytes depleting Atg14L or Pik3c3/Vps34 were fixed and stained with anti-tubulin antibody and DAPI for spindle and DNA staining, respectively. Representative images from 3 independent experiments are shown. Bar, 10 μm. Cytokinesis failure was quantified and is shown in the right panel of images. Data are the mean ± SEM from 3 independent experiments with the indicated number of oocytes. **p < 0.05; ns, not significant.
BECN1 knockdown impairs recruitment of ZFYVE26 to the midbody
It has been known that ZFYVE26 is a PtdIns(3)P-binding protein recruited to the midbody during cytokinesis and is required for cytokinetic abcission.16,22-24 Given that BECN1 as a subunit of the PI3K-III complex is responsible for generating PI(3)P, we assumed that depletion of BECN1 impaired the generation of PtdIns(3)P and thereby prevented ZFYVE26 recruitment to the midbody during cytokinesis, leading to abscission failure. To investigate this possibility, we first determined BECN1 localization during cytokinesis. Consistent with the observation that BECN1 depletion impairs cytokinetic abscission during oocyte meiosis, BECN1 was detected at the midbody bridge during cytokinesis (Fig. 5A). In contrast, BECN1 was disappeared at the midbody following BECN1 knockdown, further confirming the efficient knockdown (Fig. 5A). We next investigated whether BECN1 regulates ZFYVE26 recruitment to the midbody during cytokinesis. Whereas ZFYVE26 was clearly detectable at the midbody during cytokinesis in control oocytes, the signal of ZFYVE26 was dramatically reduced in BECN1 knockdown oocytes, suggesting that BECN1 is required to localize ZFYVE26 at the midbody (Fig. 5B). Consistent with this, we observed abscission failure when ZFYVE26 was depleted using specific dsRNA (dsZFYVE) (Figs. 5C-G). Moreover, BECN1 was significantly reduced at the midbody in ZFYVE26 knockdown oocytes (Fig. 5G). Taken together, our results suggest that BECN1 is involved in ZFYVE26 localization to the midbody, which plays a critical role in cytokinetic abscission.
Figure 5.

BECN1 is required to localize ZFYVE26 to the midbody (A) BECN1 knockdown oocytes were fixed and stained with anti-BECN1 antibody. Non-injected oocytes at cytokinesis were used as a control. DNA and spindle were stained with DAPI and anti-tubulin antibody, respectively. Bar, 10 μm. (B) BECN1 knockdown oocytes were fixed and stained with anti-ZFYVE26 antibody. DNA and spindle were stained with DAPI and anti-tubulin antibody, respectively. Bar, 10 μm. (C, D) Oocytes injected with dsRNA targeting Zfyve26 (dsZFYVE) were cultured for 24 hours in the presence of IBMX. Knockdown of ZFYVE26 was confirmed by either RT-PCR (C) or immunoblot analysis (D). GAPDH and β-actin were used as a loading control for RT-PCR and immunoblot, respectively. Normalized expression level of ZFYVE26 is shown above the representative images. The data are expressed as the mean ± SEM from 2 independent experiments. *p < 0.05; **p < 0.001. (E, F) Oocytes depleting ZFYVE26 were fixed and stained with anti-tubulin antibody with DAPI for DNA staining. The representative images from at least 3 independent experiments are shown. Bar, 10 μm. (F) Cytokinesis failure was quantified. Data are the mean ± SEM from 3 independent experiments with the indicated number of oocytes. *p < 0.05. (G) Oocytes depleting ZFYVE26 were fixed at cytokinesis and stained with anti-BECN1 antibody. DNA and spindle were stained with DAPI and anti-tubulin antibody, respectively. Bar, 10 μm.
Discussion
During meiosis, cytokinesis is an essential step for asymmetric division that allows the retention of maternal components required for early development.25 However, the mechanisms controlling cytokinesis during meiosis are not well understood. In this study, we show that BECN1, a well-known regulator of autophagy, is required to complete cytokinesis as a key component of the PI3K-III complex during meiotic maturation in mouse oocytes. BECN1 knockdown impaired cytokinetic abscission, disturbing ZFYVE26 recruitment at midbody during oocyte meiotic maturation. Similar defects were observed when PI3K-III activity was disturbed. In contrast, inhibition of the autophagic pathway did not affect meiotic maturation. Therefore, our data not only demonstrate that oocyte cytokinesis is regulated by PI3K-III complex, but also suggest that autophagy pathway seems not to be essential for meiotic maturation.
Autophagy is an essential catabolic process involved in maintaining cellular homeostasis and function. BECN1 is a coiled-coil protein that is well known as a regulator of autophagy in mammalian cells. However, our results show that BECN1 is not colocalized with MAP1LC3B/LC-3, suggesting that BECN1 is not associated with autophagosome formation. Autophagy pathway inhibition by either Atg14L depletion or Baf A1 treatment did not disturb meiotic maturation, suggesting that autophagy is dispensable for meiotic maturation. Consistent with our results, oocytes lacking Atg5 have been known to develop and be fertilized normally,26 supporting the dispensable role of autophagy during oocyte maturation. However, we could not entirely exclude the possibility that alternative autophagy pathways independent of known regulators, such as BECN1, Atg5 or MAP1LC3B/LC3, may participate in meiotic maturation. Indeed, at least 2 alternative autophagy pathways have been recently uncovered: an Atg5/Atg7-independent pathway and the BECN1-independent noncanonical autophagy pathway.27-30 Therefore, it is of interest to determine whether alternative autophagic pathways are present in oocytes during meiotic maturation. Given that autophagy is a primary response to cellular stress in an attempt to survive unfavorable conditions such as starvation, heat, or hypoxia, it is possible that autophagy is normally repressed but activated in response to stress conditions during oocyte meiosis. Consistent with this, autophagy activation has been reported in vitrified-warmed oocytes.31 Also, rapamycin treatment has been shown to induce autophagy, improving in vitro maturation of porcine oocytes.32
In addition to cytokinetic failure, we found a few misaligned chromosomes in BECN1 knockdown oocytes. Consistent with our results, BECN1 has been recently shown to regulate chromosome congression and kinetochore assembly during mitosis.18 Interestingly, this mitosis-specific function of BECN1 is independent of the PI3K-III complex and is instead mediated by interaction with kinetochore protein Zwint-1. In conjunction with INCENP, Borealin and Survivin, Aurora B/C kinases form the chromosome passenger complex and regulate various chromosome dynamics, including chromosome congression, alignment, and segregation.33 Given that Zwint-1 is required for proper function of Aurora B/C kinases during chromosome segregation in both mitosis and meiosis,34,35 it could be possible that chromosome defects following BECN1 knockdown might be due to the improper function of Aurora B/C kinases. The notion that BECN1 interacts with Survivin further suggests a possible association between BECN1 and Aurora B/C kinases.36 Indeed, BECN1 and Aurora B kinase are colocalized at the midbody during cytokinesis.16,37
It was recently shown that perturbation of zinc homeostasis during oocyte maturation led to a block in meiosis following telophase I, showing cytokinetic abscission failure.38 We found that BECN1 is required to recruit ZFYVE26 to the midbody. Given that ZFYVE26 contains several zinc binding domains and is essential for cytokinetic abscission,16,22-24 it is likely that cytokinetic defects following zinc depletion might be associated with ZFYVE26. Further studies are required to elucidate how ZFYVE26 and zinc facilitate cytokinesis.
In summary, we found that BECN1 is required for cytokinetic abscission during oocyte meiosis. Depletion of BECN1 impaired oocyte maturation, perturbing cytokinetic abscission. Although BECN1 is a well-known regulator of autophagy, cytokinetic defects following BECN1 knockdown might be not associated with the autophagy pathway. Instead, it is most likely due to defects in PI3K-III activity. Taken together, our findings not only demonstrate that BECN1 is required to complete cytokinesis as a component of the PI3K-III complex, but reveal that BECN1 is not associated with autophagy pathway in mouse oocytes.
Methods and materials
Antibodies
The primary antibodies used for immunoblotting were anti-BECN1 (Cell Signaling, 1:500) and anti-β actin (Cell Signaling, 1:500). HRP-labeled mouse and rabbit antibodies (Jackson ImmunoResearch) were used as the secondary antibodies for immunoblotting. The primary antibodies for immunostaining were anti-BECN1 (Cell Signaling, 1:100), anti- MAP1LC3B/LC3B (Cell Signaling, 1:500), anti-ZFYVE26 (Cell Signaling, 1:500), anti-centromere (Antibodies Inc., 1:200), anti-acetylated anti-α-tubulin (Sigma, 1:500) and anti-V5 (Invitrogen, 1:500). The secondary antibodies used for immunostaining were Alexa Fluor-conjugated 488 and 594 secondary antibodies (Jackson ImmunoResearch).
Oocyte collection and culture
All procedures for mouse care and use were conducted in accordance with guidelines from and approved by the Institutional Animal Care and Use Committees of Sungkyunkwan University. GV oocytes were recovered from the ovaries of 3-4 week old CD-1 female mice (Koatech) that had been administrated 5 IU of pregnant mare's serum of gonadotrophin (PMSG). Oocytes were released from the ovaries by puncturing with a fine needle and were placed in M2 medium (Sigma or Zenith Bio.) supplemented with 200 μM 3-isobutyl-1-methylxanthine (IBMX) to prevent meiotic resumption. Only oocytes with an intact layer of cumulus cells were subsequently recovered and cumulus cells were removed by repeated pipetting with a mouth-operated micropipette. For in vitro maturation, oocytes were washed and cultured in IBMX-free M16 medium in a 5% CO2 atmosphere at 37°C. All reagents and media were from Sigma unless otherwise stated.
For treatment, 2 mM 3-MA or 100 nM Baf A1 dissolved in dimethyl sulfoxide (DMSO) was added to the culture medium. Control oocytes were treated with an equivalent volume of DMSO.
Microinjection
Approximately 5–10 pl of constructed dsRNA or mRNA were microinjected into the cytoplasm of oocytes using a FemtoJet microinjector (Eppendorf) with a Leica inverted microscope (DMIRB) equipped with a micromanipulator (Narishige). Following microinjection, oocytes were incubated for 22 to 24 hours in M16 medium containing IBMX at 37°C in 5% CO2 atmosphere. Oocytes were then transferred to IBMX-free medium and cultured under mineral oil at 37°C in an atmosphere of 5% CO2 in air.
Cloning and in vitro mRNA or dsRNA synthesis
Full-length cDNA encoding Becn1 was synthesized from mouse total cDNA by PCR. PCR products were cloned into pcDNA3.1/V5-His using the BamHI and NotI sites and sequenced. The cDNA clones encoding either mouse Zfyve26 or Pik3c3/Vps34 were purchased from the Origene or the Korea Human Gene Bank, respectively. mRNA for microinjection was prepared in vitro using an mMessage mMachine kit (Ambion), polyadenylated, and then purified with a Nucleospin RNA clean-up kit (Macherey-Nagel). For double stranded RNA, PCR products were used as a template for in vitro transcription using a MEGAscript Kit (Ambion). The primers used were: EGFP, ATTAATACGACTAACTATAGGGAGAATGGTGAGCAAGGGCGAG and ATTAATACGACTCACTATAGGGAGAGCTCGTCCATGCCGAGAG; Becn1, ATTAATACGACTCACTATAGGGAGAGAGGATGACAGTGAGCAG and ATTAATACGACTCACTATAGGGAGAACAAGTCGGTACCTCTG; Atg14L, ATTAATACGACTCACTATAGGGAGACCCTGCAGACGTG and ATTAATACGACTCACTATAGGGAGACCAGTCTGTGCCCAGG; Pik3c3/Vps34, ATTAATACGACTCACTATAGGGAGAAGCCTTGCTCAAGGGTG and ATTAATACGACTCACTATAGGGAGAGGTTATCCAGGTGCCG; Zfyve26, ATTAATACGACTCACTATAGGGAGAAGCCGCCAGTGGAGTTCG and ATTAATACGACTCACTATAGGGAGAGCTGGCTGCAGGATCTGATC.
Reverse transcription-PCR
Total RNAs were extracted from oocytes using the RNeasy Plus Mini Kit (Qiagen) followed by reverse transcription (RT) using a Sensiscript RT kit (Qiagen). PCR was performed using the following primers: for Becn1, GAAGGTCCAGGCTGAGGC and AGGAACACTGGGCAAGCG; for Zfyve26, GTCCCTCGACCAGCACC and GTGGACAGGCTCCTGGAG; for Pik3c34/Vps34, GTGGAGTGTGAAGATCAGGAC and CACCTGTGGCTCCAGCG; for Atg14L, CTGACACGCGCAGTGAG and CGGCTGGGAAGGGATGTC; for GAPDH, ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA. PCR conditions were as follows: denaturation at 95°C for 5 min, followed by 30 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, extension at 72°C for 30 sec, and final extension at 72°C for 10 min.
Immunoblotting analysis
Oocytes were lysed in SDS sample buffer and subjected to SDS-PAGE. After transfer, the membranes were blocked in TBST (0.1% Tween-20, 3% BSA) at room temperature for 1 hour, and then incubated with primary antibodies overnight at 4°C. After washing 3 times in TBST, membranes were incubated with secondary antibodies for 1 hour. The blots were developed with the ECL Plus Western Blotting Detection kit (GE Healthcare).
Immunostaining and confocal microscopy
For immunostaining, oocytes at specific stages were fixed in 4% paraformaldehyde in PBS for 20 min and permeabilized in PBS with 0.1% Triton X-100 for 15 min. After blocking with PBS containing 3% BSA for 1 hour, oocytes were incubated with primary antibodies followed by Alexa Fluor-conjugated 488 and 594 secondary antibodies. DAPI was used for DNA counterstaining. These oocytes were mounted on glass slides and examined with a confocal laser-scanning microscopy (LSM 700; Zeiss) equipped with a C-Apochromat 63× /1.2 water immersion objective. Optical sections were obtained at 1-μm intervals and converted into maximum intensity projections. Data analysis was performed using the ZEN 2010 LSM software (Zeiss) and ImageJ software (National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software). Data represent at least 3 independent experiments unless otherwise specified. The significance of differences between groups was analyzed by the Student's t-test and p-values less than 0.05 were considered statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Authors' contributions
JSO and JSK conceived and designed the experiments. SYY, YSP and HJJ performed the experiments. SYY and JSO analyzed the data. DHC, HBJ, SHK and JWC contributed reagents/materials/analysis tools. JSO wrote the paper. All authors reviewed the manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number : HI12C0737) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1004766).
References
- [1].Barr FA, Gruneberg U. Cytokinesis: placing and making the final cut. Cell 2007; 131:847-60; PMID:18045532; http://dx.doi.org/ 10.1016/j.cell.2007.11.011 [DOI] [PubMed] [Google Scholar]
- [2].Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem 2006; 75:543-66; PMID:16756502; http://dx.doi.org/ 10.1146/annurev.biochem.74.082803.133425 [DOI] [PubMed] [Google Scholar]
- [3].Glotzer M. The molecular requirements for cytokinesis. Science 2005; 307:1735-9; PMID:15774750; http://dx.doi.org/ 10.1126/science.1096896 [DOI] [PubMed] [Google Scholar]
- [4].Steigemann P, Gerlich DW. Cytokinetic abscission: cellular dynamics at the midbody. Trends Cell Biol 2009; 19:606-16; PMID:19733077; http://dx.doi.org/ 10.1016/j.tcb.2009.07.008 [DOI] [PubMed] [Google Scholar]
- [5].Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005; 437:1043-7; PMID:16222300; http://dx.doi.org/ 10.1038/nature04217 [DOI] [PubMed] [Google Scholar]
- [6].Steigemann P, Wurzenberger C, Schmitz MH, Held M, Guizetti J, Maar S, Gerlich DW. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 2009; 136:473-84; PMID:19203582; http://dx.doi.org/ 10.1016/j.cell.2008.12.020 [DOI] [PubMed] [Google Scholar]
- [7].Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell 2004; 6:463-77; PMID:15068787; http://dx.doi.org/ 10.1016/S1534-5807(04)00099-1 [DOI] [PubMed] [Google Scholar]
- [8].Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol 2010; 12:814-22; PMID:20811353; http://dx.doi.org/ 10.1038/ncb0910-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol 1998; 72:8586-96; PMID:9765397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Klionsky DJ, Cregg JM, Dunn WA Jr., Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, et al.. A unified nomenclature for yeast autophagy-related genes. Dev Cell 2003; 5:539-45; PMID:14536056; http://dx.doi.org/ 10.1016/S1534-5807(03)00296-X [DOI] [PubMed] [Google Scholar]
- [11].Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999; 402:672-6; PMID:10604474; http://dx.doi.org/ 10.1038/45257 [DOI] [PubMed] [Google Scholar]
- [12].Funderburk SF, Wang QJ, Yue Z. The Beclin 1-VPS34 complex–at the crossroads of autophagy and beyond. Trends Cell Biol 2010; 20:355-62; PMID:20356743; http://dx.doi.org/ 10.1016/j.tcb.2010.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep 2001; 2:330-5; PMID:11306555; http://dx.doi.org/ 10.1093/embo-reports/kve061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy 2005; 1:46-52; PMID:16874027; http://dx.doi.org/ 10.4161/auto.1.1.1542 [DOI] [PubMed] [Google Scholar]
- [15].Jaber N, Dou Z, Chen JS, Catanzaro J, Jiang YP, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al.. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proc Natl Acad Sci U S A 2012; 109:2003-8; PMID:22308354; http://dx.doi.org/ 10.1073/pnas.1112848109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sagona AP, Nezis IP, Pedersen NM, Liestol K, Poulton J, Rusten TE, Skotheim RI, Raiborg C, Stenmark H. PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat Cell Biol 2010; 12:362-71; PMID:20208530; http://dx.doi.org/ 10.1038/ncb2036 [DOI] [PubMed] [Google Scholar]
- [17].Thoresen SB, Pedersen NM, Liestol K, Stenmark H. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp Cell Res 2010; 316:3368-78; PMID:20643123; http://dx.doi.org/ 10.1016/j.yexcr.2010.07.008 [DOI] [PubMed] [Google Scholar]
- [18].Fremont S, Gerard A, Galloux M, Janvier K, Karess RE, Berlioz-Torrent C. Beclin-1 is required for chromosome congression and proper outer kinetochore assembly. EMBO Rep 2013; 14:364-72; PMID:23478334; http://dx.doi.org/ 10.1038/embor.2013.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem 2010; 285:10850-61; PMID:20123989; http://dx.doi.org/ 10.1074/jbc.M109.080796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 1998; 23:33-42; PMID:9639028; http://dx.doi.org/ 10.1247/csf.23.33 [DOI] [PubMed] [Google Scholar]
- [21].Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 2008; 19:5360-72; PMID:18843052; http://dx.doi.org/ 10.1091/mbc.E08-01-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gaullier JM, Simonsen A, D'Arrigo A, Bremnes B, Stenmark H. FYVE finger proteins as effectors of phosphatidylinositol 3-phosphate. Chem Phys Lipids 1999; 98:87-94; PMID:10358931; http://dx.doi.org/ 10.1016/S0009-3084(99)00021-3 [DOI] [PubMed] [Google Scholar]
- [23].Stenmark H, Aasland R. FYVE-finger proteins–effectors of an inositol lipid. J Cell Sci 1999; 112(Pt 23):4175-83; PMID:10564636 [DOI] [PubMed] [Google Scholar]
- [24].Stenmark H, Aasland R, Driscoll PC. The phosphatidylinositol 3-phosphate-binding FYVE finger. FEBS Lett 2002; 513:77-84; PMID:11911884; http://dx.doi.org/ 10.1016/S0014-5793(01)03308-7 [DOI] [PubMed] [Google Scholar]
- [25].Maro B, Verlhac MH. Polar body formation: new rules for asymmetric divisions. Nat Cell Biol 2002; 4:E281-3; PMID:12461532; http://dx.doi.org/ 10.1038/ncb1202-e281 [DOI] [PubMed] [Google Scholar]
- [26].Tsukamoto S, Kuma A, Mizushima N. The role of autophagy during the oocyte-to-embryo transition. Autophagy 2008; 4:1076-8; PMID:18849666; http://dx.doi.org/ 10.4161/auto.7065 [DOI] [PubMed] [Google Scholar]
- [27].Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 2009; 461:654-8; PMID:19794493; http://dx.doi.org/ 10.1038/nature08455 [DOI] [PubMed] [Google Scholar]
- [28].Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of non-canonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ 2008; 15:1318-29; PMID:18421301; http://dx.doi.org/ 10.1038/cdd.2008.51 [DOI] [PubMed] [Google Scholar]
- [29].Grishchuk Y, Ginet V, Truttmann AC, Clarke PG, Puyal J. Beclin 1-independent autophagy contributes to apoptosis in cortical neurons. Autophagy 2011; 7:1115-31; PMID:21646862; http://dx.doi.org/ 10.4161/auto.7.10.16608 [DOI] [PubMed] [Google Scholar]
- [30].Smith DM, Patel S, Raffoul F, Haller E, Mills GB, Nanjundan M. Arsenic trioxide induces a beclin-1-independent autophagic pathway via modulation of SnoN/SkiL expression in ovarian carcinoma cells. Cell Death Differ 2010; 17:1867-81; PMID:20508647; http://dx.doi.org/ 10.1038/cdd.2010.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bang S, Shin H, Song H, Suh CS, Lim HJ. Autophagic activation in vitrified-warmed mouse oocytes. Reproduction 2014; 148:11-9; PMID:24760879; http://dx.doi.org/ 10.1530/REP-14-0036 [DOI] [PubMed] [Google Scholar]
- [32].Song BS, Kim JS, Kim YH, Sim BW, Yoon SB, Cha JJ, Choi SA, Yang HJ, Mun SE, Park YH, et al.. Induction of autophagy during in vitro maturation improves the nuclear and cytoplasmic maturation of porcine oocytes. Reprod Fertil Dev 2014; 26:974-81; PMID:23902659; http://dx.doi.org/ 10.1071/RD13106 [DOI] [PubMed] [Google Scholar]
- [33].Carmena M, Wheelock M, Funabiki H, Earnshaw WC. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nat Rev Mol Cell Biol 2012; 13:789-803; PMID:23175282; http://dx.doi.org/ 10.1038/nrm3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Woo Seo D, Yeop You S, Chung WJ, Cho DH, Kim JS, Su Oh J. Zwint-1 is required for spindle assembly checkpoint function and kinetochore-microtubule attachment during oocyte meiosis. Sci Rep 2015; 5:15431; PMID:26486467; http://dx.doi.org/ 10.1038/srep15431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kasuboski JM, Bader JR, Vaughan PS, Tauhata SB, Winding M, Morrissey MA, Joyce MV, Boggess W, Vos L, Chan GK, et al.. Zwint-1 is a novel Aurora B substrate required for the assembly of a dynein-binding platform on kinetochores. Mol Biol Cell 2011; 22:3318-30; PMID:21775627; http://dx.doi.org/ 10.1091/mbc.E11-03-0213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Niu TK, Cheng Y, Ren X, Yang JM. Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL-induced apoptosis. FEBS Lett 2010; 584:3519-24; PMID:20638385; http://dx.doi.org/ 10.1016/j.febslet.2010.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sagona AP, Nezis IP, Bache KG, Haglund K, Bakken AC, Skotheim RI, Stenmark H. A tumor-associated mutation of FYVE-CENT prevents its interaction with Beclin 1 and interferes with cytokinesis. PLoS One 2011; 6:e17086; PMID:21455500; http://dx.doi.org/ 10.1371/journal.pone.0017086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim AM, Vogt S, O'Halloran TV, Woodruff TK. Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 2010; 6:674-81; PMID:20693991; http://dx.doi.org/ 10.1038/nchembio.419 [DOI] [PMC free article] [PubMed] [Google Scholar]