The outer segment (OS) is the specialized light sensing organelle of vertebrate rod photoreceptor cells. It is cylindrical in shape and full of stacked disc membranes that, in rods, are enclosed by the plasma membrane and are abundant in the light sensitive pigment rhodopsin. Due to the metabolically demanding process of phototransduction, the discs undergo daily renewal. The distal end is shed and new discs are replenished at the OS base. The mechanism of transport of disc constituents from their site of synthesis in the inner segment (IS) through the connecting cilium (CC) to the OS and how they are assembled into new discs has been a subject of intense debate. This has recently led us,1 as well as the groups of David Williams2 and Vadim Arshavsky3 to examine the structure of the newly developing discs in detail. The results of these studies provide strong evidence that newly developing discs form from the OS plasma membrane rather than by vesicle fusion at the base of the OS.
Previous studies have resulted in 2 main models of disc renewal. The ‘evagination model’ proposes that the discrete enclosed discs form from outgrowths of the plasma membrane at the base of the OS that are initially open to the extracellular space.4 The ‘fusion model’ proposes that discs are formed by progressive fusion of cytoplasmic vesicles that are either transported directly from the IS or pinched off from the plasma membrane at the OS base.5,6 To evaluate these models, electron tomography was used to generate 3D reconstructions of the IS:OS interface,1,2 providing a higher resolution and better vizualization of membrane continuity than has been previously achieved by conventional electron microscopy. Reconstructions clearly showed that the newly forming discs are continuous with the OS plasma membrane and, thus, exposed to the extracellular space.1,2 This is consistent with the study from the Arshavsky group who showed that the newly formed ‘open’ discs, but not the mature ‘closed’ discs were accessible to tannic acid stain.3 In addition, the latter study investigated the orientation of rhodopsin in the new discs using antibodies against the C- and N- terminus of the protein. They showed that the N-terminus (extracellular domain) is outside and the C-terminus (cytoplasmic domain) inside newly forming discs, in contrast to the mature discs when the N-terminus is inside, facing the disc lumen3 (Fig. 1). This topological inversion occurs as a result of fusion of adjacent newly formed disc membranes to form discrete discs, a fusion event visualised in tomographic series in Burgoyne et al.1
Figure 1.
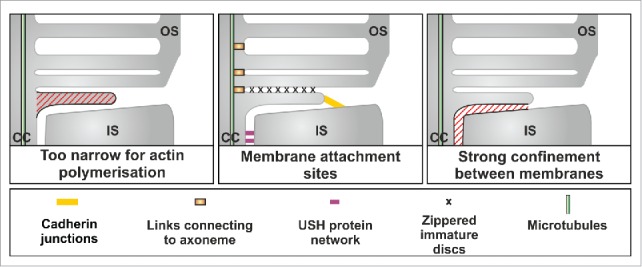
Features of the IS:OS interface that support the hypothesis that disc extension is driven by blebbing
The continuity of the forming discs with the plasma membrane, together with their topology, is not consistent with the vesiculation model, which would predict that the nascent discs would be cytoplasmic vesicles, rather than exposed to the extracellular space. Consistently we did not find rhodopsin-bearing cytoplasmic vesicles in either the base of the OS or in the connecting cilium. Within the CC, rhodopsin was confined to the ciliary plasma membrane, in agreement with previous studies,7 and so in the right place to be incorporated into new discs.
All three recent studies addressed the issue of how previous studies had given rise to such different models and all concluded that sample preservation is key. Although the different researchers found different preservation methods to be optimal, they all concluded that a failure to preserve the structural integrity of the delicate IS:OS interface may explain some of the observations that have led to the proposal of the vesicular fusion model.
Distinction between the evagination and vesiculation models was important as the 2 models predict the involvement of very different molecular machineries. Although one study proposed that the initial plasma membrane deformation is invagination of the OS plasma membrane,2 all 3 studies involve evagination of the plasma membrane to form flattened discs. What might drive this evagination? We noted that the dimensions of the flattened nascent discs preclude actin polymerisation as a driving force but conditions at the IS:OS interface might favor blebbing, whereby the plasma membrane detaches from the actin cortex and protrudes in a manner driven by the intracellular hydrostatic pressure. We identified features that might facilitate this process and help to generate and maintain the correct disc shape (Fig. 1). Adjacent plasma membrane evaginations appear to be closely associated, giving a zippered appearance but their leading edges are free and interact with the plasma membrane of the IS via cadherin-based junctions. The strong confinement to which the evaginating disc is subject between the existing OS and the periciliary ridge of the IS could both promote blebbing and maintain the flattened shape.
Much remains to be established about the molecular mechanisms underlying plasma membrane evagination and the fusion between adjacent evaginations that leads to the generation of mature discrete rod OS discs but the identification of the genes mutated in retinal degenerations where OS disc structure is impaired will continue to provide candidate components of this machinery.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Burgoyne T, et al.. Proc Natl Acad Sci U S A 2015; 112:15922-7; PMID:26668363; http://dx.doi.org/ 10.1073/pnas.1509285113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Volland S, et al.. Proc Natl Acad Sci U S A 2015; 112:14870-5; PMID:26578801; http://dx.doi.org/ 10.1073/pnas.1516309112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ding JD, et al.. J Cell Biol 2015; 211:495-502; PMID:26527746; http://dx.doi.org/ 10.1083/jcb.201508093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Steinberg RH, et al.. J Comp Neurol 1980; 190:501-8; PMID:6771304; http://dx.doi.org/ 10.1002/cne.901900307 [DOI] [PubMed] [Google Scholar]
- [5].Obata S, Usukura J. Cell Tissue Res 1992; 269:39-48; PMID:1423483; http://dx.doi.org/ 10.1007/BF00384724 [DOI] [PubMed] [Google Scholar]
- [6].Chuang JZ, et al.. Cell 2007; 130:535-47; PMID:17693260; http://dx.doi.org/ 10.1016/j.cell.2007.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wolfrum U, Schmitt A. Cell Motil Cytoskeleton 2000; 46:95-107; PMID:10891855; http://dx.doi.org/ 10.1002/1097-0169(200006)46:2%3c95::AID-CM2%3e3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]


