Abstract
Objective:
To assess the availability of fluoride concentration in saliva following the use of fluoride mouthrinse and dentifrice.
Materials and Methods:
The experiment was carried out in 7–15 year-old school children of Chandigarh (n = 90). The children were nonfluoride users. Baseline saliva samples were collected. The subjects were exposed to two test agents, i.e., fluoride mouthrinse (0.05%, 225 ppm F) and dentifrice (1000 ppm F) for 7 days and on the day 8, saliva samples were collected over a 20 hrs period. Wash out period of 31/2 months was there before the subjects were exposed to the second test agent. Fluoride in saliva was estimated using fluoride ion-specific electrode. Written informed consent was taken.
Statistical Analysis:
Kolmogorov–Smirnov test was applied to test the normality of the variables. Mann–Whitney U-test was used to compare the fluoride concentration available in saliva at respective time intervals subsequent to use of the two test agents.
Results:
Fluoride concentration was elevated in saliva compared to baseline for both the test agents. Fluoride mouthrinse (0.05% sodium fluoride [NaF]) and dentifrice (1000 ppm monofluorophosphate [MFP]) showed a biphasic clearance. Peak in saliva occurred at 15 mins postuse. Night-time use resulted in higher concentration of fluoride in saliva compared to baseline. There was statistically significantly higher fluoride concentration available in saliva for the dentifrice at 5 hrs, 10 hrs, and 20 hrs postuse (P < 0.05).
Conclusion:
Subsequent to the use of NaF (0.05%) daily mouthrinse and MFP dentifrice (1000 ppm) the fluoride concentration in saliva remained elevated to a level of 0.12 ppm for mouthrinse and 0.14 ppm for dentifrice compared to baseline (0.03 ppm) up to 20 hrs postuse. The therapeutic window though not yet established but suggested is 0.1–1 ppm for prevention of demineralization, indicating that daily use of fluoride mouthrinse and dentifrice provides fluoride concentration in saliva for the prevention of demineralization.
Keywords: Fluoride dentifrice, fluoride mouthrinse, prevention of demineralization, saliva
INTRODUCTION
Cariostatic efficacy of topical fluorides is attributed to their ability to decrease the rate of demineralization[1,2,3,4,5,6,7] of enamel and enhance the rate of remineralization.[1,2,4,6,7] The most effective topical fluorides are dentifrices and mouthrinses used daily.[8,9] There is a current consensus that bacteria-mediated tooth destruction can be arrested or even reversed by adopting fluorides.[10] The fluoride concentration in unstimulated whole saliva is a cumulative reflection of the sum of fluoride present in ductal saliva and various hard and soft tissue retention sites in the mouth.[9] Availability of fluoride in the oral environment is a dynamic process determined on the one hand by the concentration, quantity, frequency duration of the topical fluoride used[11,12] and factors affecting retention and clearance. The clearance and availability of fluoride in saliva subsequent to the use of topical fluoride agents have been studied by various investigators for different time intervals after the use, namely, 30 mins,[13] 120 mins,[14] 3 hrs,[15] 6 hrs, 7 days.[16] The majority of these studies have been conducted in adults. Studies in this direction in children would help depict availability of fluoride concentration in saliva to rationalize the frequency of use. Studies have been conducted in children, but the subjects have been followed up for a short time such as 30 mins and 120 mins.[17,18] Mostly, these populations have been using fluoride in one form or the other that may have a masking effect. Keeping the above in mind, this study was undertaken in children aged 7–15 years with no previous history of exposure to topical fluorides or water fluoridation.
Objectives of the study were:
To study the fluoride concentration available in saliva following the use of daily prescribed fluoride agents, namely, 0.05% sodium fluoride (NaF) daily mouthrinse (225 ppm fluoride) and fluoridated dentifrice (1000 ppm fluoride)
To compare the concentration of fluoride available in saliva subsequent to use of NaF daily mouthrinse and fluoridated dentifrice and the duration of its availability in saliva.
MATERIALS AND METHODS
The experiment was carried out in 90, 7–15 year old school children of Chandigarh residing in hostels. The children were healthy and had no history of exposure to either topical (fluoride dentifrice, tea) or systemic fluoride. Medically compromised children and those undergoing orthodontic treatment were excluded from the study. Water fluoride of the area was 0.34–0.38 ppm. Written informed consent along with the permission to conduct the study was taken from the school authorities. The study was crossover in design. Two test agents were used in the same subjects. Routinely prescribed topical preventive fluoride agents were used in this study. The first test agent was daily fluoride mouthrinse (0.05% NaF) and the second test agent was 1000 ppm monofluorophosphate (MFP) dentifrice. Before the start of the study, baseline saliva samples were collected. As the subjects were nonfluoride users, they were exposed to the NaF (once) daily mouthrinse for 7 days before sampling. The subjects were exposed to the first test agent initially, and saliva sampling was done. After a washout period of (3½ months), the same subjects were exposed to fluoride dentifrice twice daily for 1 week as the use of fluoride dentifrice is routinely advised twice daily after meals. Of the ninety subjects who were exposed to the mouthrinse (0.05%), 81 subjects were exposed to the fluoride dentifrice (1000 ppm) whereas nine subjects were lost to follow-up. Baseline early morning unstimulated saliva samples were collected in screw-capped plastic vials of 15 ml capacity. The subjects unscrewed the caps, bent their head forward which facilitated pooling of saliva in the oral cavity. After the collection of saliva, the caps were replaced. Subjects were instructed not to touch the inside of the bottles. The vials were transported within 30 mins of the collection to the Department of Dentistry at PGI, Chandigarh and stored in a refrigerator at 4°C till analysis (Zero et al. 1988).
Estimation of fluoride in saliva
The saliva samples were brought to room temperature and treated with TISAB III (ORION). To 10 parts of saliva, 1 part TISAB III buffer (5 ml for 50 ml of sample) was added in a plastic vial and shaken before fluoride analysis. The addition of TISAB to saliva samples was done to liberate free fluoride ions (i.e., to break the aluminum and fluoride-ion complexes and to adjust the pH between 5 and 5.5). At a pH below 5.0, H+ form complexes (HF, HF2−) that interfere in fluoride estimation. Likewise, if the pH of the solution is above 7.0, there is interference by OH− ions during fluoride estimation. The AMS P507 ion-analyzer with fluoride combination electrode (Orion model 94-09, 96-09) was used for the estimation of fluoride in saliva samples. The instrument was calibrated in incremental order, i.e., 0.01, 0.03, 0.1, 0.3–1 ppm with temperature remaining constant.
Test agent 1 (0.05% NaF daily mouthrinse)
The subjects were instructed to carry out rinsing once daily with 0.05% NaF mouthrinse for 2 mins from the day 1 to 7 before retiring to bed and abstain from eating and drinking anything postrinse. A demonstration was given to the children and the week long use was supervised by the hostel warden. On the 8th day, unstimulated whole saliva samples were collected between 6 and 6.30 a.m. referred to as the cumulative baseline that reflects the fluoride concentration available in saliva after 7 days postuse. On the 8th day itself, the subjects were asked to do their routine brushing with a nonfluoridated toothpaste, have their breakfast and at 9 a.m., they were asked to do fluoride mouthrinse with 0.05% NaF solution under professional supervision for 2 mins and expectorate. Postrinse unstimulated saliva samples were collected at 15, 30, 45, 60 mins, before lunch (5 hrs), before dinner (10 hrs), and early next morning (20 hrs). Oral intake in between sample collection was allowed except in the 1st hr of sampling.
Test agent 2 (1000 ppm monofluorophosphate dentifrice)
The same subjects were exposed to the second test agent, namely, 1000 ppm MFP dentifrice. There was a time internal of 3½ months before exposure to the second test agent. A 200 g tube of Cibaca fluoride dentifrice (1000 ppm F), and a Cibaca junior toothbrush (standard size) was given to the subjects. They were instructed to brush their teeth with half-length ribbon twice in a day, once in the morning, and once at bedtime from the day 1 to day 7. On the day 8, early morning unstimulated whole saliva samples were collected between 6 and 6.30 a.m. Subsequently, the subjects brushed their teeth with a nonfluoride paste, had breakfast, and were asked to brush their teeth with a half-length ribbon of Cibaca fluoride dentifrice for 3 mins under supervision. The subjects expectorated the saliva-dentifrice slurry and rinsed with tap water for 5 secs from plastic vials (50 ml capacity) in a standardized manner. Unstimulated whole saliva samples were collected at 15, 30, 45, 60 mins, before lunch (5 hrs), before dinner (10 hrs), and early next morning (20 hrs). Oral intake in between sample collection was allowed except in the 1st hr of sampling.
Statistical analysis
Fluoride concentration in saliva at various intervals, namely, baseline, cumulative baseline, postuse, i.e., 15, 30, 45, 60 mins, at 5 hrs (before lunch), at 10 hrs (before dinner), and at 20 hrs (early next morning) termed variables was tested for normality using Kolmogorov–Smirnov test. It was observed that none of the variables followed normal distribution (P < 0.05). Comparison of fluoride concentration in saliva for the two test agents (mouthrinse and dentifrice) with respect to the various time intervals, i.e., cumulative baseline, 15, 30, 60 mins, before lunch (5 hrs), before dinner (10 hrs), and early next morning (20 hrs) postuse was carried out using Mann–Whitney U-test [Table 2].
Table 2.
Comparison of two test agents using Mann-Whitney U-test

RESULTS
Descriptive statistics which include mean, standard deviation, standard error of mean, and 95% confidence interval for the fluoride concentration available in saliva at different time intervals is presented in [Table 1]. The number of subjects varies due to loss during collection; some samples were lost or could not be collected in a few subjects due to nonavailability. Comparison of two test agents was carried out using the Mann–Whitney U-test because of the nonnormal distribution [Table 2]. It was observed that there is no significant difference between the two test agents at baseline (P = 0.879), 45 mins (P = 0.08) before lunch (P = 0.055). At all other time intervals, there was a statistically significantly higher fluoride concentration available in saliva. A line graph was plotted for the two test agents of the time intervals and fluoride concentration available in saliva [Figure 1]. The results indicate that mean salivary fluoride concentration subsequent to the use of mouthrinse from the day 1 to day 7 referred to as cumulative baseline and at time intervals of 15, 30, 45 mins, before lunch (5 hrs), before dinner (10 hrs), and 20 hrs (early next morning) postrinse were elevated above baseline. Similar, findings were observed for the fluoride dentifrice. The number of subjects initially recruited in the study was ninety. The number of subjects had reduced from 90 to 81 due to attrition in the sample. While the collection of saliva samples, a few subjects were not available and at times the saliva sample collected was insufficient for analysis; hence, the variation in number of saliva samples collected.
Table 1.
Descriptive statistics of variables
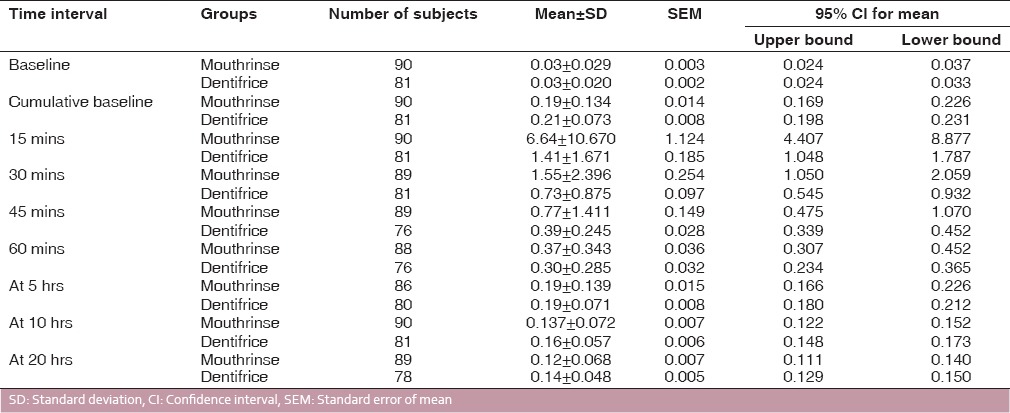
Figure 1.
Salivary fluoride clearance curves
DISCUSSION
Fluoride mouthrinse (0.05% NaF) and dentifrice (1000 ppm MFP) showed a biphasic clearance pattern. There is an initial rapid increase with peak at 15 mins followed by a decline at 45 mins. After 60 mins, there was a slow and steady decrease. In a study by Nuca et al.,[10] it was observed that after peak increase of fluoride in saliva the fluoride concentration decreases slowly. While Naumova et al.[18] found peak increase of salivary fluoride concentration immediately after brushing and lasting at least 30 mins. Studies have been conducted by several authors in which almost similar time intervals have been used. Design of this experiment was different in comparison with previous studies in that it was carried out in subjects who had not used fluoride in any form before the experimental period and were asked to use the fluoride agents, namely, 0.05% NaF daily mouthrinse (225 ppm) and 1000 ppm MFP dentifrice for 1 week before the experiment.
Salivary fluoride concentration remained elevated above baseline even after 20 hrs of use of the mouthrinse and dentifrice. Cumulative baseline values for the mouthrinse and dentifrice group were comparable.[19] The salivary fluoride concentration postrinse at 15, 30, 45, and 60 mins were higher for the mouthrinse group compared to the dentifrice group [Figure 1]. At 10 hrs and 20 hrs, the fluoride concentration in saliva is statistically significantly (P < 0.05) higher for the dentifrice group [Table 2]. This could be due to the fact that dentifrice is used twice and mouthrinse only once, in spite of the fact that mouthrinse is used undiluted while dentifrice slurry is expectorated and controlled rinsing carried out with water postuse. Another factor could be that fluoride concentration in the dentifrice is 1000 ppm while in the mouthrinse, it is 225 ppm.
The presence of fluoride ions in the oral cavity at the time when the pH is decreasing and the carious lesion is starting, inhibits demineralization of enamel by promoting remineralization.[20,21,22] The presence of low but constant concentration of fluoride in saliva and plaque fluid is the most effective method to control initial dental caries.[18,23] Remineralization requires a higher concentration. Remineralization is enhanced in the presence of fluoride ions.[24] This therapeutic window has as yet not been established. Salivary fluoride concentration of 0.12 and 0.14 ppm, 20 hrs after exposure to the test agents suggests that routinely prescribed daily topical fluoride agents offer the benefit of prevention of demineralization. It is observed, in this study, that clearance of fluoride occurs relatively slowly at night, suggesting that bedtime use is more beneficial because of higher salivary fluoride concentration available for a longer period. Similar findings have been reported in a study by Zero et al.[17] Fluoride concentration in saliva can be maintained to an optimal therapeutic level with the regular use of fluoridated products.[19]
CONCLUSION
The scientific rationale for the frequency of use in this population is NaF mouthrinse (0.05%) once daily at bedtime for 1-2 mins and 1000 ppm fluoridated dentifrice half ribbon twice daily after meals for 3 mins.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Koulourides T, Phantumvanit P, Munksgaard EC, Housch T. An intraoral model used for studies of fluoride incorporation in enamel. J Oral Pathol. 1974;3:185–96. doi: 10.1111/j.1600-0714.1974.tb01710.x. [DOI] [PubMed] [Google Scholar]
- 2.Ten Cate JM, Arends J. Remineralization of artificial enamel lesions in vitro. Caries Res. 1977;11:277–86. doi: 10.1159/000260279. [DOI] [PubMed] [Google Scholar]
- 3.Van Dijk JW, Borggreven JM, Driessens FC. Chemical and mathematical simulation of caries. Caries Res. 1979;13:169–80. doi: 10.1159/000260398. [DOI] [PubMed] [Google Scholar]
- 4.Silverstone LM, Wefel JS, Zimmerman BF, Clarkson BH, Featherstone MJ. Remineralization of natural and artificial lesions in human dental enamel in vitro. Effect of calcium concentration of the calcifying fluid. Caries Res. 1981;15:138–57. doi: 10.1159/000260512. [DOI] [PubMed] [Google Scholar]
- 5.Ten Cate JM, Duijsters PP. Influence of fluoride in solution on tooth demineralization. I. Chemical data. Caries Res. 1983;17:193–9. doi: 10.1159/000260667. [DOI] [PubMed] [Google Scholar]
- 6.Gelhard TB, Arends J. In vivo remineralization of artificial subsurface lesions in human enamel. I. J Biol Buccal. 1984;12:49–57. [PubMed] [Google Scholar]
- 7.Featherstone JD, O'Reilly MM, Sheriati M, Brugler S. Leach SA. Factors Relating to Demineralization and Remineralization of the Teeth. Oxford: IRL Press; 1986. Enhancement of remineralization in vitro and in vivo; pp. 23–34. [Google Scholar]
- 8.Aoba T. Solubility properties of human tooth mineral and pathogenesis of dental caries. Oral Dis. 2004;10:249–57. doi: 10.1111/j.1601-0825.2004.01030.x. [DOI] [PubMed] [Google Scholar]
- 9.Zero DT, Fu J, Espeland MA, Featherstone JD. Comparison of fluoride concentrations in unstimulated whole saliva following the use of a fluoride dentifrice and a fluoride rinse. J Dent Res. 1988;67:1257–62. doi: 10.1177/00220345880670100201. [DOI] [PubMed] [Google Scholar]
- 10.Nuca C, Aniarizi C, Gaita A, Diaconu C. Salivary fluoride concentration after professional topical fluoride applications. OHDMBSC 2003-2 (4) [Google Scholar]
- 11.Gupta B, Anegundi R, Sudha P. Study of salivary retention after the application of various topical reagents and their effect on Streptococcus mutans. Internet J Dent Sci. 2006;5 DOI:10.5580/27ca. [Google Scholar]
- 12.Zero DT, Creeth JE, Bosma ML, Butler A, Guibert RG, Karwal R, et al. The effect of brushing time and dentifrice quantity on fluoride delivery in vivo and enamel surface microhardness in situ. Caries Res. 2010;44:90–100. doi: 10.1159/000284399. [DOI] [PubMed] [Google Scholar]
- 13.Paul S, Tandon S, Murthy K. Effect of fluoride dentifrices on salivary fluoride levels in children. Indian J Dent Res. 1993;4:95–101. [PubMed] [Google Scholar]
- 14.Petersson LG, Ludvigsson N, Ullbro C, Gleerup A, Koch G. Fluoride clearance of whole saliva in young school children after topical application. Swed Dent J. 1987;11:95–101. [PubMed] [Google Scholar]
- 15.Duckworth RM, Knoop DT, Stephen KW. Effect of mouthrinsing after toothbrushing with a fluoride dentifrice on human salivary fluoride level. Caries Res. 1991;25:289–91. doi: 10.1159/000261378. [DOI] [PubMed] [Google Scholar]
- 16.Aasenden R, Brudevold F, Richardson B. Clearance of fluoride from the mouth after topical treatment or the use of a fluoride mouthrinse. Arch Oral Biol. 1968;13:625–36. doi: 10.1016/0003-9969(68)90141-6. [DOI] [PubMed] [Google Scholar]
- 17.Zero DT, Raubertas RF, Fu J, Pederson AM, Hayes AL, Featherstone JD. Fluoride concentration in plaque, whole saliva and ductal saliva after application of home use topical fluorides. J Dent Res. 1992;71:1768–75. doi: 10.1177/00220345920710110201. [DOI] [PubMed] [Google Scholar]
- 18.Naumova EA, Arnold WH, Gaengler P. Fluoride bioavailability in saliva using Dentttabs® compared to dentifrice. Cent Eur J Med. 2010;5:375–80. [Google Scholar]
- 19.Campus G, Lallai MR, Carboni R. Fluoride concentration in saliva after use of oral hygiene products. Caries Res. 2003;37:66–70. doi: 10.1159/000068220. [DOI] [PubMed] [Google Scholar]
- 20.Murray JJ, Rugg-Gunn AJ, Jenkins GN. Fluorides in caries prevention. 3rd ed. ©Butterworth - Heinemann, Ltd; 1991. pp. 295–318. [Google Scholar]
- 21.Duckworth RM, Jones Y, Nicholson J, Jacobson AP, Chestnutt IG. Studies on plaque fluoride after use of F-containing dentifrices. Adv Dent Res. 1994;8:202–7. doi: 10.1177/08959374940080021101. [DOI] [PubMed] [Google Scholar]
- 22.Twetman S, Sköld-Larsson K, Modéer T. Fluoride concentration in whole saliva and separate gland secretions after topical treatment with three different fluoride varnishes. Acta Odontol Scand. 1999;57:263–6. doi: 10.1080/000163599428670. [DOI] [PubMed] [Google Scholar]
- 23.Ogaard B, Seppä L, Rølla G. Professional topical fluoride applications – Clinical efficacy and mechanism of action. Adv Dent Res. 1994;8:190–201. doi: 10.1177/08959374940080021001. [DOI] [PubMed] [Google Scholar]
- 24.Ten Cate JM. Fluorides in caries prevention and control: Empiricism or science. Caries Res. 2004;38:254–7. doi: 10.1159/000077763. [DOI] [PubMed] [Google Scholar]


