Abstract
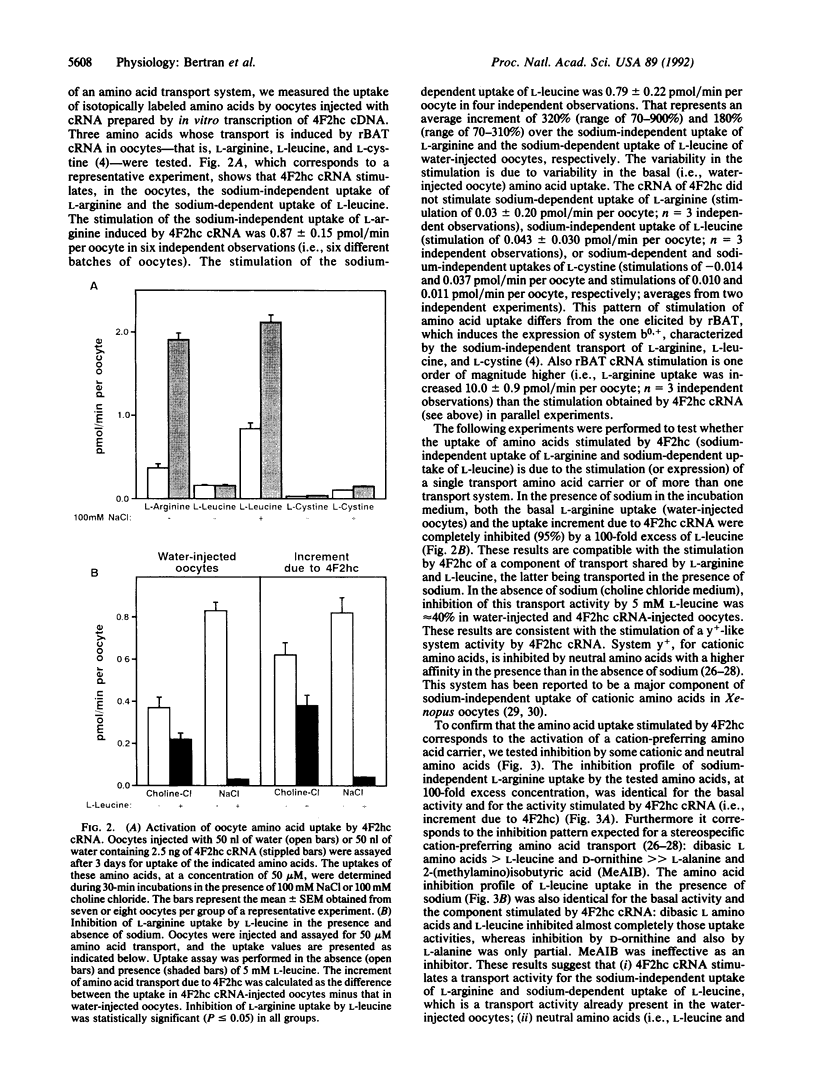
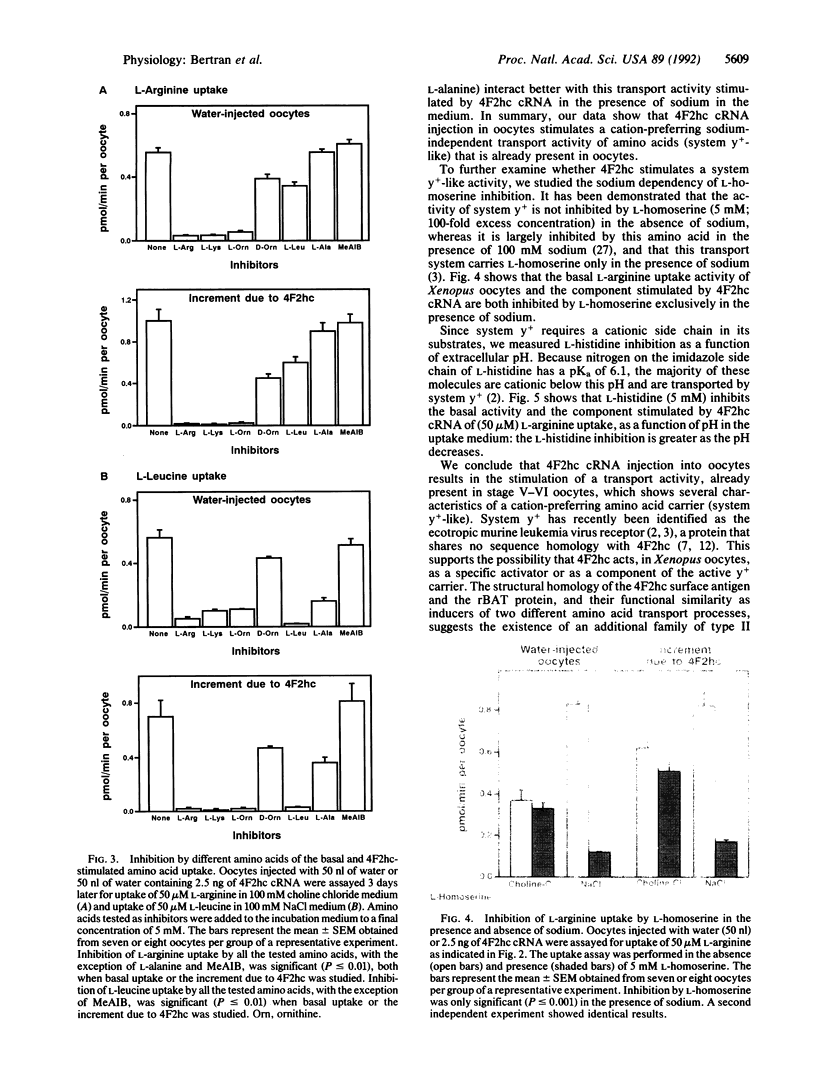
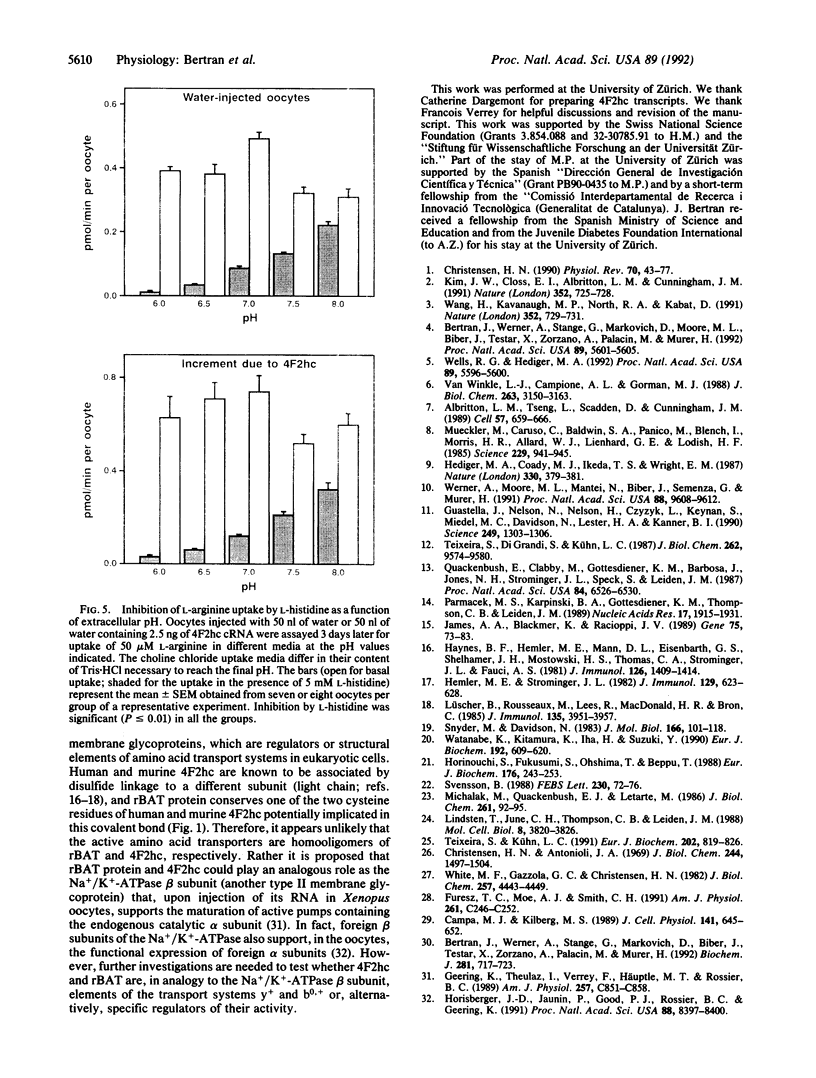
A kidney cortex cDNA clone (rBAT) has recently been isolated, which upon in vitro transcription and capping complementary RNA (cRNA) and injection into Xenopus laevis oocytes induces a system b0,(+)-like amino acid transport activity. This cDNA encodes a type II membrane glycoprotein that shows significant homology to another type II membrane glycoprotein, the heavy chain of the human and mouse 4F2 surface antigen (4F2hc). Here we demonstrate that injection of human 4F2hc cRNA into oocytes results in the activation of a cation-preferring amino acid transport system that appears to be identical to the y(+)-like transport already present in the oocyte. This is based on the following results: (i) Injection of in vitro transcripts from 4F2hc cDNA (4F2hc cRNA) into oocytes stimulates up to 10-fold the sodium-independent uptake of L-arginine and up to 4.1-fold the sodium-dependent uptake of L-leucine. In contrast, 4F2hc cRNA does not increase the basal sodium-independent uptake of L-leucine. (ii) Basal and 4F2hc cRNA-stimulated sodium-independent uptake of L-arginine is completely inhibited by L-leucine in the presence of sodium. Similarly, the basal and 4F2hc cRNA-stimulated sodium-dependent uptake of L-leucine is entirely inhibited by L-arginine. (iii) The stimulation of sodium-independent uptake of L-arginine and the stimulation of sodium-dependent uptake of L-leucine induced by injection of 4F2hc cRNA are both completely inhibited by dibasic L amino acids and to a lesser extent by D-ornithine. (iv) Both basal and 4F2hc cRNA-stimulated sodium-independent uptake of L-arginine show two additional characteristics of the system y+ transport activity: inhibition of L-arginine uptake by L-homoserine only in the presence of sodium and an increase in the inhibition exerted by L-histidine as the extracellular pH decreased. Our results allow us to propose that an additional family of type II membrane glycoproteins (composed by rBAT and 4F2hc) is involved in amino acid transport, either as specific activators or as components of amino acid transport systems.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton L. M., Tseng L., Scadden D., Cunningham J. M. A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell. 1989 May 19;57(4):659–666. doi: 10.1016/0092-8674(89)90134-7. [DOI] [PubMed] [Google Scholar]
- Bertran J., Werner A., Moore M. L., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression cloning of a cDNA from rabbit kidney cortex that induces a single transport system for cystine and dibasic and neutral amino acids. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5601–5605. doi: 10.1073/pnas.89.12.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran J., Werner A., Stange G., Markovich D., Biber J., Testar X., Zorzano A., Palacin M., Murer H. Expression of Na(+)-independent amino acid transport in Xenopus laevis oocytes by injection of rabbit kidney cortex mRNA. Biochem J. 1992 Feb 1;281(Pt 3):717–723. doi: 10.1042/bj2810717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa M. J., Kilberg M. S. Characterization of neutral and cationic amino acid transport in Xenopus oocytes. J Cell Physiol. 1989 Dec;141(3):645–652. doi: 10.1002/jcp.1041410324. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Antonioli J. A. Cationic amino acid transport in the rabbit reticulocyte. Na+-dependent inhibition of Na+-independent transport. J Biol Chem. 1969 Mar 25;244(6):1497–1504. [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Furesz T. C., Moe A. J., Smith C. H. Two cationic amino acid transport systems in human placental basal plasma membranes. Am J Physiol. 1991 Aug;261(2 Pt 1):C246–C252. doi: 10.1152/ajpcell.1991.261.2.C246. [DOI] [PubMed] [Google Scholar]
- Geering K., Theulaz I., Verrey F., Häuptle M. T., Rossier B. C. A role for the beta-subunit in the expression of functional Na+-K+-ATPase in Xenopus oocytes. Am J Physiol. 1989 Nov;257(5 Pt 1):C851–C858. doi: 10.1152/ajpcell.1989.257.5.C851. [DOI] [PubMed] [Google Scholar]
- Guastella J., Nelson N., Nelson H., Czyzyk L., Keynan S., Miedel M. C., Davidson N., Lester H. A., Kanner B. I. Cloning and expression of a rat brain GABA transporter. Science. 1990 Sep 14;249(4974):1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Characterization of antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J Immunol. 1982 Aug;129(2):623–628. [PubMed] [Google Scholar]
- Horinouchi S., Fukusumi S., Ohshima T., Beppu T. Cloning and expression in Escherichia coli of two additional amylase genes of a strictly anaerobic thermophile, Dictyoglomus thermophilum, and their nucleotide sequences with extremely low guanine-plus-cytosine contents. Eur J Biochem. 1988 Sep 15;176(2):243–253. doi: 10.1111/j.1432-1033.1988.tb14275.x. [DOI] [PubMed] [Google Scholar]
- Horisberger J. D., Jaunin P., Good P. J., Rossier B. C., Geering K. Coexpression of alpha 1 with putative beta 3 subunits results in functional Na+/K+ pumps in Xenopus oocytes. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8397–8400. doi: 10.1073/pnas.88.19.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. A., Blackmer K., Racioppi J. V. A salivary gland-specific, maltase-like gene of the vector mosquito, Aedes aegypti. Gene. 1989 Jan 30;75(1):73–83. doi: 10.1016/0378-1119(89)90384-3. [DOI] [PubMed] [Google Scholar]
- Kim J. W., Closs E. I., Albritton L. M., Cunningham J. M. Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature. 1991 Aug 22;352(6337):725–728. doi: 10.1038/352725a0. [DOI] [PubMed] [Google Scholar]
- Lindsten T., June C. H., Thompson C. B., Leiden J. M. Regulation of 4F2 heavy-chain gene expression during normal human T-cell activation can be mediated by multiple distinct molecular mechanisms. Mol Cell Biol. 1988 Sep;8(9):3820–3826. doi: 10.1128/mcb.8.9.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Rousseaux M., Lees R., MacDonald H. R., Bron C. Cell surface glycoproteins involved in the stimulation of interleukin 1-dependent interleukin 2 production by a subline of EL4 thymoma cells. II. Structure, biosynthesis, and maturation. J Immunol. 1985 Dec;135(6):3951–3957. [PubMed] [Google Scholar]
- Michalak M., Quackenbush E. J., Letarte M. Inhibition of Na+/Ca2+ exchanger activity in cardiac and skeletal muscle sarcolemmal vesicles by monoclonal antibody 44D7. J Biol Chem. 1986 Jan 5;261(1):92–95. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Parmacek M. S., Karpinski B. A., Gottesdiener K. M., Thompson C. B., Leiden J. M. Structure, expression and regulation of the murine 4F2 heavy chain. Nucleic Acids Res. 1989 Mar 11;17(5):1915–1931. doi: 10.1093/nar/17.5.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quackenbush E., Clabby M., Gottesdiener K. M., Barbosa J., Jones N. H., Strominger J. L., Speck S., Leiden J. M. Molecular cloning of complementary DNAs encoding the heavy chain of the human 4F2 cell-surface antigen: a type II membrane glycoprotein involved in normal and neoplastic cell growth. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6526–6530. doi: 10.1073/pnas.84.18.6526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Davidson N. Two gene families clustered in a small region of the Drosophila genome. J Mol Biol. 1983 May 15;166(2):101–118. doi: 10.1016/s0022-2836(83)80001-1. [DOI] [PubMed] [Google Scholar]
- Svensson B. Regional distant sequence homology between amylases, alpha-glucosidases and transglucanosylases. FEBS Lett. 1988 Mar 28;230(1-2):72–76. doi: 10.1016/0014-5793(88)80644-6. [DOI] [PubMed] [Google Scholar]
- Teixeira S., Di Grandi S., Kühn L. C. Primary structure of the human 4F2 antigen heavy chain predicts a transmembrane protein with a cytoplasmic NH2 terminus. J Biol Chem. 1987 Jul 15;262(20):9574–9580. [PubMed] [Google Scholar]
- Teixeira S., Kühn L. C. Post-transcriptional regulation of the transferrin receptor and 4F2 antigen heavy chain mRNA during growth activation of spleen cells. Eur J Biochem. 1991 Dec 18;202(3):819–826. doi: 10.1111/j.1432-1033.1991.tb16438.x. [DOI] [PubMed] [Google Scholar]
- Van Winkle L. J., Campione A. L., Gorman J. M. Na+-independent transport of basic and zwitterionic amino acids in mouse blastocysts by a shared system and by processes which distinguish between these substrates. J Biol Chem. 1988 Mar 5;263(7):3150–3163. [PubMed] [Google Scholar]
- Wang H., Kavanaugh M. P., North R. A., Kabat D. Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature. 1991 Aug 22;352(6337):729–731. doi: 10.1038/352729a0. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kitamura K., Iha H., Suzuki Y. Primary structure of the oligo-1,6-glucosidase of Bacillus cereus ATCC7064 deduced from the nucleotide sequence of the cloned gene. Eur J Biochem. 1990 Sep 24;192(3):609–620. doi: 10.1111/j.1432-1033.1990.tb19267.x. [DOI] [PubMed] [Google Scholar]
- Wells R. G., Hediger M. A. Cloning of a rat kidney cDNA that stimulates dibasic and neutral amino acid transport and has sequence similarity to glucosidases. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5596–5600. doi: 10.1073/pnas.89.12.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Moore M. L., Mantei N., Biber J., Semenza G., Murer H. Cloning and expression of cDNA for a Na/Pi cotransport system of kidney cortex. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9608–9612. doi: 10.1073/pnas.88.21.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F., Gazzola G. C., Christensen H. N. Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J Biol Chem. 1982 Apr 25;257(8):4443–4449. [PubMed] [Google Scholar]