Abstract
Aim:
We aimed to identify the predictors of coronary artery disease (CAD) in patients with type 2 diabetes mellitus (type 2 DM).
Methods:
About fifty Asian Indian patients with type 2 DM patients aged >40 years and fifty sex- and age-matched nondiabetic controls were enrolled for this study. Following complete medical history and baseline clinical data, laboratory investigations were performed to assess fasting and postprandial plasma glucose levels, lipid profile, blood urea, serum creatinine, and serum uric acid levels.
Results:
Body mass index (BMI), waist-to-hip ratio, serum uric acid, serum total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, very LDL cholesterol were significantly higher among diabetic patients compared to controls. On univariate analysis, serum LDL cholesterol (odds ratio [OR]: 29.67, P < 0.001), serum uric acid (OR: 25.65, P < 0.001), low high-density lipoprotein (HDL) cholesterol (OR: 21.12, P < 0.001), hypertension (OR: 17.06, P < 0.001), family history of cardiovascular disease (CVD) (OR: 9.43, P = 0.002), and duration of diabetes (OR: 4.65, P = 0.03) were identified as predictors of CVD among diabetic patients. On multivariate regression, only LDL cholesterol (OR: 1.51, P = 0.002) and serum uric acid (OR: 1.21, P = 0.01) were the independent predictors of CAD among diabetic patients. Significant positive correlation of serum uric acid with duration of diabetes (r = 0.38, P = 0.006), BMI (r = 0.35, P = 0.01), triglycerides (r = 0.356, P = 0.01), LDL cholesterol (r = 0.38, P = 0.007), HDL cholesterol (r = −0.514, P < 0.001), and hypertension (r = 0.524, P < 0.001) was observed.
Conclusion:
Serum LDL cholesterol and hyperuricemia may serve as independent predictors of CAD among Asian Indian subjects with type 2 DM.
Key words: Coronary artery disease, cardiovascular disease, hyperuricemia, low-density lipoprotein cholesterol, type 2 diabetes mellitus
INTRODUCTION
Diabetes mellitus (DM) refers to a group of common metabolic disorders that share a phenotype of hyperglycemia. Currently, the prevalence of diabetes worldwide is estimated to be around 415 million (8.8% of the whole population) and is predicted to reach 642 million by 2040. India has the second highest number of diabetics with estimated prevalence of 69.3 million, which is expected to reach 123.5 million by 2040.[1] DM increases the risk of developing cardiovascular diseases (CVD). Incidence of sudden cardiac death is on the rise, especially in the urban regions of India, which may largely be attributed to the increase in prevalence of coronary artery disease (CAD), diabetes, and hypertension.[2] Indeed, type 2 DM (type 2 DM) is associated with a 2-4 fold increase in the risk of developing CAD. However, such an increased risk cannot be explained by hyperglycemia alone, as other cardiovascular risk factors such as hypertension and dyslipidemia also play a major role.[3,4] Moreover, it is observed that the prevalence of other cardiovascular risk factors such as smoking, sedentary lifestyle, and obesity are higher among diabetic compared to nondiabetic patients.[5]
The role of some risk factor such as serum uric acid levels in developing CVD is still controversial. Framingham study has suggested that hyperuricemia is only a bystander in CVD and its association with the disease is indirectly influenced by confounding factors such as obesity, dyslipidemia, hypertension, use of diuretics, and insulin resistance.[6] On the contrary, several other studies have identified serum uric acid levels as an independent predictor of CVD in various population groups.[7] Such contrasting reports make it difficult to delineate the specific role of hyperuricemia in CVD, thus limiting its clinical application. Nevertheless, understanding the association between hyperuricemia and CVD, especially in patients with type 2 DM in the absence of other confounding factors may be a worthy consideration. Hence, we evaluated the risk factors of CVD including serum uric acid levels among subjects with type 2 DM.
METHODS
A prospective case-control study was designed at the Vydehi Institute of Medical Sciences and Research Centre, Bengaluru, India, after obtaining approval from the Institutional Ethics Review Board. About fifty type 2 DM patient's aged >40 years and fifty age- and sex-matched nondiabetic controls were included in the study after obtaining written informed consent. DM was diagnosed according to the criteria given by the American diabetes association.[8] Pregnant and lactating mothers, patients with renal failure, renal transplant, hepatic and metabolic disorders, and gout were excluded from the study. Patients on long-term thiazide diuretics, antitubercular drugs, glucocorticoids, chemotherapy, and regularly consuming alcohol were also excluded.
The clinical data obtained included the following: Body weight, height, waist-to-hip ratio (WHR), systolic and diastolic blood pressure, duration of diabetes, history of smoking, family history of CVD, and the presence of ischemic heart disease. Laboratory investigations included the following: Fasting and postprandial plasma glucose levels, blood urea, serum creatinine, and serum uric acid levels. Fasting serum lipid parameters were noted from the tests done during statin-naïve period.
Statistical analysis
Data were analyzed using SPSS, Version 21. Continuous variables are represented as mean ± standard deviation and categorical data as percentages. Continuous variables between two groups were analyzed using unpaired t-test and categorical variables by Chi-square test or by Fisher's exact test (when sample size was small). Binary logistic regression was used to analyze the predictors of CVD among diabetics. Pearson correlation coefficient (r) was used to study the correlation between serum uric acid and other variables. A P < 0.05 was considered statistically significant.
RESULTS
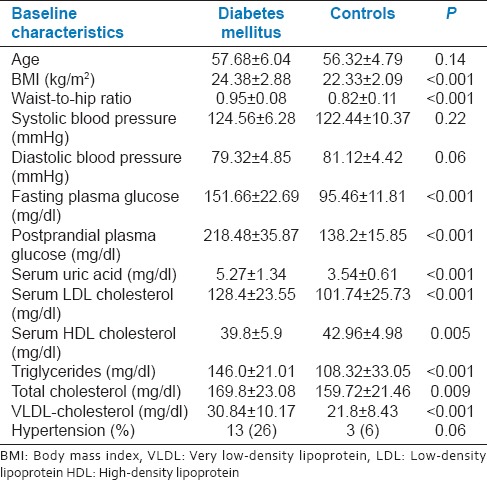
The baseline characteristics of the diabetic and nondiabetic subjects are summarized in Table 1. The age and gender distribution did not differ between both groups. The clinical characteristics of the two groups were however significantly different. Body mass index (BMI), WHR, serum uric acid, serum total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, very LDL cholesterol levels were significantly higher, whereas serum high-density lipoprotein (HDL) cholesterol levels were significantly lower among diabetic patients. The glycemic parameters such as fasting plasma glucose and postprandial glucose were significantly higher among diabetic subjects.
Table 1.
Comparison of the baseline characteristics of the diabetics with controls
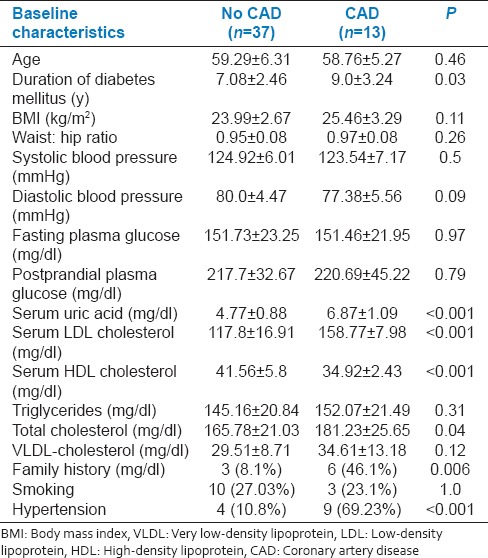
A 26% prevalence rate of CAD was observed among diabetic patients. Patients with CAD had significantly higher incidence of hypertension, family history of CAD and higher duration of diabetes and higher serum uric acid, total cholesterol, LDL cholesterol levels, and lower serum HDL cholesterol levels [Table 2].
Table 2.
Comparison of baseline characteristics of diabetic patients with and without coronary artery disease
On univariate analysis, serum LDL cholesterol (odds ratio [OR]: 29.67, P < 0.001), serum uric acid (OR: 25.65, P < 0.001), low HDL cholesterol (OR: 21.12, P < 0.001), hypertension (OR: 17.06, P < 0.001), family history of CVD (OR: 9.43, P = 0.002), and duration of diabetes (OR: 4.65, P = 0.03) were observed as possible predictors of CVD among diabetic patients. On multivariate regression, only LDL cholesterol (OR: 1.51, P = 0.002) and serum uric acid (OR: 1.21, P = 0.01) were the independent predictors of CAD among diabetic patients.
Serum uric acid levels significantly and positively correlated with duration of diabetes (r = 0.38, P = 0.006), BMI (r = 0.35, P = 0.01), triglycerides (r = 0.356, P = 0.01), LDL cholesterol (r = 0.38, P = 0.007), HDL cholesterol (r = −0.514, P < 0.001), and hypertension (r = 0.524, P < 0.001).
DISCUSSION
We observed a higher BMI and WHR among type 2 diabetics than nondiabetics. High BMI is the strongest modifiable risk factor for diabetes. The BMI cut-off for diabetes risk in Asian-Indians is less when compared to that of Caucasians. In a multicenter randomized clinical trial, an increased likelihood for the development of type 2 DM was observed among South Asians with a BMI value of >22 kg/m2, whereas in urban Indian population, a BMI of >23 kg/m2 significantly increased the risk of diabetes.[9,10] Despite low rates of general obesity among Indians, central obesity, measured as WHR, is common among Asian Indians. This android pattern of body fat, typified by more upper body adiposity (increased WHR), was a greater risk factor for diabetes as compared to general obesity. High BMI (BMI >40 kg/m2 ) in diabetic women is also associated with increased risk of complications including CVD.[11] Surprisingly, in our study, BMI was not significantly associated with CVD, this may be due to lower BMI of our diabetic cohort than that of Caucasian diabetic patients. In our study, diabetic patients had higher total cholesterol, triglycerides, LDL cholesterol, prevalence of hypertension and lower HDL cholesterol than nondiabetic patients, which is consistent with published reports.[12] We also observed higher serum uric acid levels among diabetics than nondiabetics with significant positive correlation between serum uric acid levels and duration of diabetes. Hyperglycemia is a well-known risk factor for hyperuricemia[13] and also hyperuricemia is a risk factor for the development of diabetes. Such counter influence leads to a vicious cycle, which may drive the development of co-morbidities such as CVD in general and CAD in specific. Indeed, in a study of 3,681 Japanese adults, hyperuricemia significantly increased the risk of type 2 diabetes.[14]
Serum LDL cholesterol, hypertension, and family history of CVD are the proven risk factors for CAD among diabetics and reduction of LDL cholesterol and control of hypertension have proven to protect against CAD.[8] Although low HDL cholesterol has been shown to be a risk factor for CVD, attempts to decrease CVD by increasing HDL cholesterol have not shown clinical benefits. However, in our study, hypertension, low HDL cholesterol, family history of CVD and smoking were not identified as independent predictors although they are proven CV risk factors. This could probably be due to small sample size of our study and warrants further evaluation.
Serum uric acid levels were observed as an independent predictor of CAD among diabetics, thereby indicating the role of uric acid in promoting vascular inflammation. However, the role of serum uric acid as a risk factor for CVD is debatable. Higher serum uric acid levels in patients with established CAD is previously reported.[15] However, it is difficult to delineate the role of uric acid in predicting CAD due to its association with other CAD risk factors including obesity, insulin resistance, hypertriglyceridemia, and hypercholesterolemia. Each of these components of metabolic syndrome is demonstrated to be an independent risk factor for CVD and synergistically accelerate both nondiabetic atherosclerosis and the atheroscleropathy associated with type 2 DM. In the MONICA Augsburg cohort involving 1044 males, a significant association between hyperuricemia and cardiovascular mortality was observed, which was independent of BMI, serum cholesterol concentration, hypertension, diuretic use, alcohol intake, and smoking habits.[7] In the Gothenburg prospective study evaluating 1462 women aged 38-60 years, a significant correlation was observed between serum uric acid concentration and total mortality during 12 years follow-up, which was independent of BMI, serum lipid concentrations, smoking habit, blood pressure, and age.[16] In contrast, association of hyperuricemia with fatal and nonfatal CAD was not observed in the British Regional Heart Study (7688 men, aged 40-59 years) after correcting for other risk factors, particularly serum cholesterol concentration.[17] The Coronary Drug Project Research Group evaluating 2789 men, aged 30-64 years, did not find any association between increased cardiovascular risk and hyperuricemia.[18] Such contrasting observations may be influenced by genetic and ethnic differences.
Association of hyperuricemia with BMI is reported in a recent study.[19] In concurrence with previous reports, we observed significant positive correlation of serum uric acid with serum triglycerides, serum total cholesterol, LDL cholesterol, and negative correlation with HDL cholesterol levels.[20,21] The association of uric acid and hypertension is well described in the literature.[22] Preclinical studies have demonstrated the development of hypertension by inducing hyperuricemia, which is associated with hyperreninemia and decreased nitric oxide synthesis in the juxtaglomerular apparatus.[22,23] Nevertheless, our study does have some limitations such as small sample size, exclusion of other risk factors such as hyperhomocysteinemia, increased reactive oxygen species, and high sensitivity C-reactive protein. Hence, our observations must be interpreted considering these limitations.
CONCLUSION
Well-established cardiovascular risk factors such as total cholesterol, triglycerides, LDL cholesterol, and prevalence of hypertension were higher and HDL cholesterol was lower among diabetic patients. Serum LDL cholesterol and hyperuricemia were identified as independent predictors of CAD among Asian Indian subjects with type 2 DM in this small cohort of prospective study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Diabetes Atlas. International Diabetes Federation. 7th ed. 2015. [Last cited on 2016 Jan 06]. Available from: https://www.idf.org/diabetesatlas .
- 2.Honnekeri BS, Lokhandwala D, Panicker GK, Lokhandwala Y. Sudden cardiac death in India: A growing concern. J Assoc Physicians India. 2014;62:36–40. [PubMed] [Google Scholar]
- 3.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 4.Stern MP. Glycemia and cardiovascular risk. Diabetes Care. 1997;20:1501–2. doi: 10.2337/diacare.20.10.1501. [DOI] [PubMed] [Google Scholar]
- 5.Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23) BMJ. 1998;316:823–8. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: The Framingham Heart Study. Ann Intern Med. 1999;131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Liese AD, Hense HW, Löwel H, Döring A, Tietze M, Keil U. Association of serum uric acid with all-cause and cardiovascular disease mortality and incident myocardial infarction in the MONICA Augsburg cohort. World Health Organization Monitoring Trends and Determinants in Cardiovascular Diseases. Epidemiology. 1999;10:391–7. doi: 10.1097/00001648-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Executive summary: Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S4–10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The Diabetes Prevention Program: Baseline characteristics of the randomized cohort. The Diabetes Prevention Program Research Group. Diabetes Care. 2000;23:1619–29. doi: 10.2337/diacare.23.11.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–6. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 11.Gray N, Picone G, Sloan F, Yashkin A. Relation between BMI and diabetes mellitus and its complications among US older adults. South Med J. 2015;108:29–36. doi: 10.14423/SMJ.0000000000000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Gupta R, Sharma KK, Lodha S, Achari V, Asirvatham AJ, et al. Prevalence of diabetes and cardiovascular risk factors in middle-class urban participants in India. BMJ Open Diabetes Res Care. 2014;2:e000048. doi: 10.1136/bmjdrc-2014-000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo HG, Lee SI, Chae HJ, Park SJ, Lee YC, Yoo WH. Prevalence of insulin resistance and metabolic syndrome in patients with gouty arthritis. Rheumatol Int. 2011;31:485–91. doi: 10.1007/s00296-009-1304-x. [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol. 2003;18:523–30. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 15.Rakic MT, Valkenburg HA, Davidson RT, Engels JP, Mikkelsen WM, Neel JV, et al. Observations on the natural history of hyperuricemia and gout. I. An eighteen year follow-up of nineteen gouty families. Am J Med. 1964;37:862–71. doi: 10.1016/0002-9343(64)90129-9. [DOI] [PubMed] [Google Scholar]
- 16.Bengtsson C, Lapidus L, Stendahl C, Waldenström J. Hyperuricaemia and risk of cardiovascular disease and overall death. A 12-year follow-up of participants in the population study of women in Gothenburg, Sweden. Acta Med Scand. 1988;224:549–55. [PubMed] [Google Scholar]
- 17.Wannamethee SG, Shaper AG, Whincup PH. Serum urate and the risk of major coronary heart disease events. Heart. 1997;78:147–53. doi: 10.1136/hrt.78.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Coronary Drug Project Research Group. Serum uric acid: Its association with other risk factors and with mortality in coronary heart disease. J Chronic Dis. 1976;29:557–69. doi: 10.1016/0021-9681(76)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Bonora E, Targher G, Zenere MB, Saggiani F, Cacciatori V, Tosi F, et al. Relationship of uric acid concentration to cardiovascular risk factors in young men. Role of obesity and central fat distribution. The Verona Young Men Atherosclerosis Risk Factors Study. Int J Obes Relat Metab Disord. 1996;20:975–80. [PubMed] [Google Scholar]
- 20.Conen D, Wietlisbach V, Bovet P, Shamlaye C, Riesen W, Paccaud F, et al. Prevalence of hyperuricemia and relation of serum uric acid with cardiovascular risk factors in a developing country. BMC Public Health. 2004;4:9. doi: 10.1186/1471-2458-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogbera AO, Azenabor AO. Hyperuricaemia and the metabolic syndrome in type 2 DM. Diabetol Metab Syndr. 2010;2:24. doi: 10.1186/1758-5996-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin KC, Tsao HM, Chen CH, Chou P. Hypertension was the major risk factor leading to development of cardiovascular diseases among men with hyperuricemia. J Rheumatol. 2004;31:1152–8. [PubMed] [Google Scholar]
- 23.Wu X, Wakamiya M, Vaishnav S, Geske R, Montgomery C, Jr, Jones P, et al. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994;91:742–6. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]


