Abstract
We report a very rare case of urinary bladder stone in a laboratory rat, which was associated with severe prostatitis and seminal vesiculitis. Importantly, the histopathological analysis revealed the rare variety of keratinizing desquamative squamous metaplasia of bladder, prostate, and seminal vesicle epithelium. Immunohistochemistry for alpha smooth muscle actin protein and aniline blue staining for collagen clearly showed interstitial prostate fibrosis. The detail information about these findings and subsequent discussion are provided here.
Key words: Bladder stone, fibrosis, infection, metaplasia, prostate, rat
INTRODUCTION
Like human beings, the incidence of bladder stone or urolithiasis is a common finding in domestic animals such as dogs and cats. In laboratory animals such as mice and rats, the incidence of bladder stone is not very common. Sporadic and outbreak of kidney and bladder stone in laboratory rodents have been reported earlier, and the most of them have been linked to dietary ingredients.[1] Intravesical obstructions, such as prostatic hyperplasia and neurogenic bladder are two known risk factors for bladder stone in elderly men. Several other predisposing factors are also responsible for formation of bladder stones in humans and animals, that is, chronic urinary tract infection (UTI), presence of intravesical foreign substances, bladder diverticula, and upper urinary tract stones. In a rat experimental model, UTI induced by Proteus mirabilis produced kidney fibrosis and stones.[2] Moreover, rats have been used to develop a spinal cord injury animal model for the study of bladder stones.[3] Animals with bladder stone may display dysuria, irritating urine voiding symptoms, and macroscopic hematuria.
CASE REPORT
To understand the role of bacterial infection in prostate cancer pathogenesis; currently, we are conducting multiple animal studies. Mostly, we are using Copenhagen rats for our experimental studies. With the permission of Institutional Animal Ethical Committee; till date, we have already received and used more than thirty adult male Copenhagen rats. Coincidentally, among the animals that we had received for our study, one animal had lethargic posture and rough body coat. While holding the animal, we notice a nodular solid mass inside the abdomen of the animal. From the exterior palpation, it was difficult to know the exact origin or location of the solid mass. Therefore, with the consultation of veterinarian in-charge of the animal house we decided to sacrifice the animal and conduct a thorough necropsy.
Upon excision of the ventral abdomen, immediately we noticed two highly engorged seminal vesicles and an unusually enlarged bladder. There was a very few fecal material inside the stomach and intestine; which, might be due to less food intake. The gross appearance of kidney and liver were looking normal. Palpation of the bladder was giving gritty feeling, and was clearly suggesting the presence of bladder stones. We excised prostate, seminal vesicle, and bladder together, and captured the photograph [Figure 1]. Investigation of the prostate showed some enlargement and had a solid feeling. Exploratory puncture of the seminal vesicle showed the presence of yellow caseous exudates with the purulent smell. The urinary bladder was highly stretched and had lost its tonicity. Upon opening of the bladder, we noticed around fifteen round shaped, white colored stones. The gross investigation clearly suggested this case as a complicated case of urinary bladder stone accompanied by urinary tract and accessory sex glands infection. To understand the histological changes that might have occurred due to these complications, bladder, prostate, and seminal vesicle tissues were separated from each other and fixed in buffered formalin.
Figure 1.
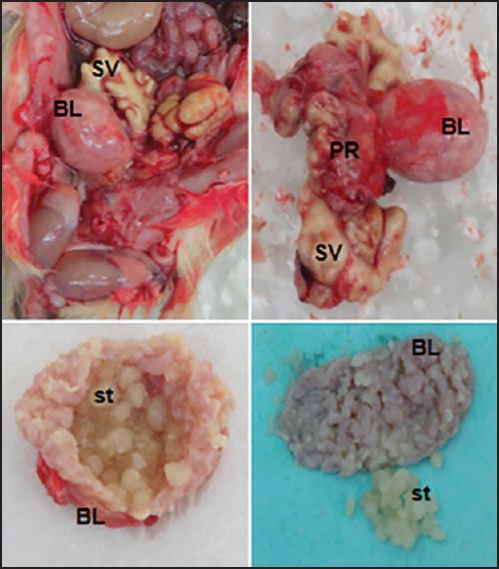
Gross images of the rat bladder (BL) filled with multiple bladder stones (st), seminal vesicles filled with caseous mass (SV), and enlarged prostate (PR)
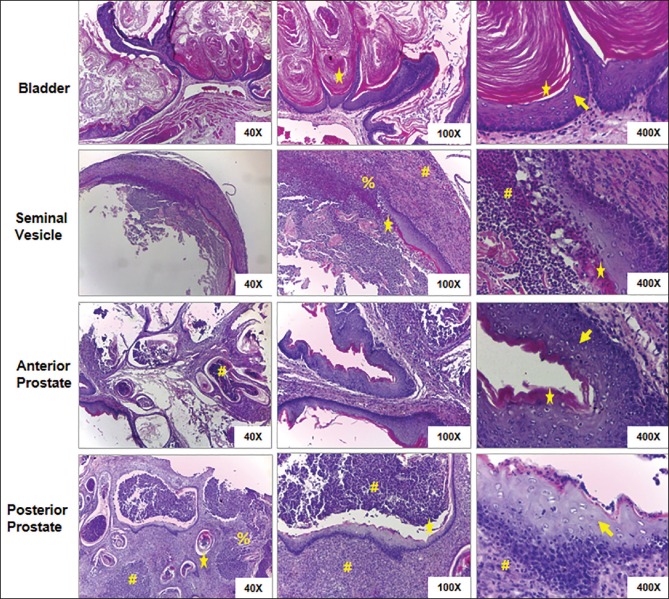
Microscopy of bladder tissue samples revealed transitional epithelium replaced by squamous epithelium (squamous metaplasia) and covered by laminated keratin [Figure 2]. There was an excessive accumulation of denudated keratin lamella inside the lumen, which is a typical feature of keratinizing desquamative squamous metaplasia (KDSM). The stromal region had moderate inflammation compare to other organs (prostate and seminal vesicle). There was no evidence of ulceration. The anterior and posterior prostate were processed separately, and histological analysis showed the presence of an excessive number of inflammatory cells in both anterior and posteriors glands [Figure 2]. The lumen of the prostatic glands and the surrounding stroma was filled with the inflammatory cells and debris. In posterior prostate, most of the glands have KDSM of epithelial cells. The denudated keratin lamella covered the inflammatory cells inside the lumen of the glands. At many places, there was a loss of epithelial and/or basement membrane. At certain places, immune cells have invaded basement membranes. A similar type of alteration was also noticed in seminal vesicle [Figure 2]. The presence of more number of alpha smooth muscle actin expressing myofibroblast like cells, and deposition of excessive collage in the stromal region indicates its reactive nature and confirms prostatic fibrosis [Figure 3].
Figure 2.
Histology of bladder, seminal vesicle, anterior, and posterior prostate tissues stained with H and E. There is a distinct keratinizing desquamative squamous metaplasia of normal epithelium in all the organs (arrow heads). Excessive accumulation of denudated keratin lamella is clearly visible all-over the bladder lumen, and other organs also have keratin lamellae at many places of their lumens (stars). Lumens and surrounding stroma of prostate glands and seminal vesicles are filled with immune cells (hashes). In the lumens of some prostate glands, the immune cells are covered with keratin lamellae (star). In most of the prostate glands and some places of seminal vesicles, the epithelial cells, and basement membrane were totally destroyed by invading immune cells (percentage)
Figure 3.
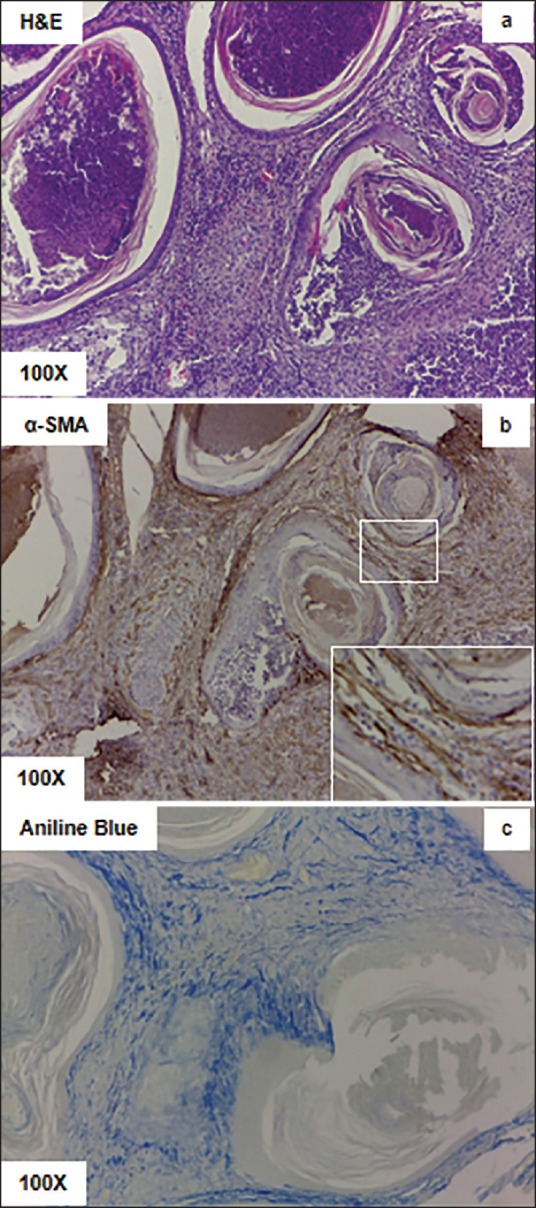
Histology of the posterior prostate tissue shows interstitial fibrosis. (a) H and E staining shows the presence of desquamated keratin lamella covering immune cells inside the lumens, and the presence of excessive immune cells in inside the stroma. (b) Alpha smooth muscle actin immunohistochemistry shows the presence of multiple myofibroblast like cells in the stromal regions. (c) Aniline blue staining shows accumulation of collagen in the stromal region
DISCUSSION
Urinary bladder infection is a predisposing factor for stone formation. At the same time, the urinary stone can promote further infection of other adjacent organs like prostate and seminal vesicles. In this particular case, it is difficult to figure out the origin of infection; however, co-existence of bladder stone, prostitis and seminal vesiculitis might have mutually promoted each other. Though dietary factors and genetic predisposition are some of the risk factors for bladder stone, but absence of this kind of complicacies in other animals of the same age group (even older) partially rules out this possibilities. However, further biochemical analysis of the uterine stones might have provided some important clues about the possible cause of stone formation. KDSM otherwise known as upper urinary tract leukoplakia or cholesteatoma is a very rare disease.[4,5] The hyperkeratosis and squamous metaplasia noticed in almost all the epithelia is a natural protective event to withstand the harsh condition produced by urinary stone and/or infection-induced chronic inflammation. The etiology of KDSM includes the presence of chronic irritation/inflammation[5] or secondary to Vitamin A deficiency.[5] Though squamous metaplasia is nonmalignant in nature, but keratinizing squamous metaplasia of the bladder is a significant risk factor for bladder cancer.[6] Likewise, it has also been believed that under adverse conditions the normal columnar prostatic epithelial cells might undergo squamous metaplasia, which later on could form squamous cell carcinoma.[7] However, more experimental evidences are required to justify this poorly established fact. There are reports that Copenhagen rats could develop spontaneous prostate cancer. Including our lab many other labs have used Copenhagen prostate cancer cell lines that were originated from a spontaneous tumor.[8] The spontaneous origin of a prostate tumor in rats and similar genetic makeup of the derived cancer cell lines with human prostate cancer cells suggests a similarity between the pathophysiology of this disease both in human and rats.
Metaplasia is known as the reversible replacement of one differentiated cell type with another mature differentiated cell type. Metaplasia mostly occurs in response to chronic inflammation or irritation and leads to a change of original cells with cells that are capable enough to withstand the new adverse environment. Metaplasia is a benign or noncancerous change and reversible in nature. However, upon sustained irritation metaplastic cells might develop dysplasia and then neoplasia. Metaplasia occurs due to reprograming of progenitor or stem cells that are present in the normal tissues. The normal prostate consists of luminal, basal, neuroendocrine, and stromal cells. Recently, a series of experiments have demonstrated that murine prostate basal and luminal cell lineage are independently sustained (lineage restricted unipotent stem cells), and in rare cases some basal cells are capable of differentiate into luminal cells.[9,10] Moreover, the study conducted by Kwon et al., 2014 provides the experimental evidence that bacterial infection stimulates differentiation of basal cells to luminal cells. Therefore, the metaplasia observed in the aforementioned rat prostate might have originated from basal cells or from the self-sustained luminal cells. Kwon et al., have used a mouse model of prostitis in which infection of mouse prostate (C57BL/6) was induced with an uropathogenic Escherichia coli strain, CP9. In this model the authors have observed almost all kind of histopathological changes that we observed in the infected rat prostate tissue (immune cell infiltration, expansion of myofibrobalst stromal cells, fibrosis, etc.); however, no report has been made about the possible metaplastic changes in prostate epithelium. Rather the authors have observed an epithelial reactive hyperplasia. The infection could induce both hyperplasia and metaplasia of human and rodent cells. At the same time, both hyperplasia and metaplasia are two completely different cellular events. Therefore, in future information about the factors that decide the fate of luminal cells upon chronic prostate infection might be interesting. Taken together, this particular case has provided a hope that these strain of rats might be a nice model to induce infection-mediated prostatic metaplasia and observe its effect on the incidence of prostate cancer.
CONCLUSION
Copenhagen rats could develop spontaneous urinary bladder stones.
Urinary bladder obstruction in rats might get associated with prostate and seminal vesicle infection.
Chronic infection of the prostate might induce KDSM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Klurfeld DM. Kidney and bladder stones in rodents fed purified diets. J Nutr. 2002;132:3784. doi: 10.1093/jn/132.12.3784. [DOI] [PubMed] [Google Scholar]
- 2.Nickel JC, Olson M, McLean RJ, Grant SK, Costerton JW. An ecological study of infected urinary stone genesis in an animal model. Br J Urol. 1987;59:21–30. doi: 10.1111/j.1464-410x.1987.tb04573.x. [DOI] [PubMed] [Google Scholar]
- 3.Linsenmeyer TA, Ottenweller J. Bladder stones following SCI in the Sprague-Dawley rat. J Spinal Cord Med. 2003;26:65–8. doi: 10.1080/10790268.2003.11753663. [DOI] [PubMed] [Google Scholar]
- 4.Jain P, Mishra A, Misra DS. Keratinizing squamous metaplasia of the upper urinary tract in a child with a solitary kidney. Indian J Urol. 2014;30:230–2. doi: 10.4103/0970-1591.126916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hertle L, Androulakakis P. Keratinizing desquamative squamous metaplasia of the upper urinary tract: Leukoplakia - cholesteatoma. J Urol. 1982;127:631–5. doi: 10.1016/s0022-5347(17)53967-1. [DOI] [PubMed] [Google Scholar]
- 6.Khan MS, Thornhill JA, Gaffney E, Loftus B, Butler MR. Keratinising squamous metaplasia of the bladder: Natural history and rationalization of management based on review of 54 years experience. Eur Urol. 2002;42:469–74. doi: 10.1016/s0302-2838(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 7.Lager DJ, Goeken JA, Kemp JD, Robinson RA. Squamous metaplasia of the prostate. An immunohistochemical study. Am J Clin Pathol. 1988;90:597–601. doi: 10.1093/ajcp/90.5.597. [DOI] [PubMed] [Google Scholar]
- 8.Jain S, Suklabaidya S, Das B, Raghav SK, Batra SK, Senapati S. TLR4 activation by lipopolysaccharide confers survival advantage to growth factor deprived prostate cancer cells. Prostate. 2015;75:1020–33. doi: 10.1002/pros.22983. [DOI] [PubMed] [Google Scholar]
- 9.Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21:253–65. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon OJ, Zhang L, Ittmann MM, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. Proc Natl Acad Sci U S A. 2014;111:E592–600. doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]