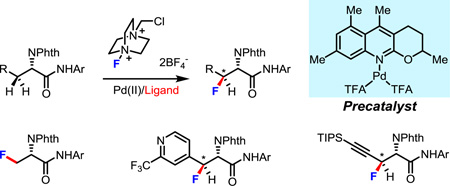
Abstract
A quinoline-based ligand was shown to promote palladium-catalyzed β-C(sp3)–H fluorination for the first time. A range of unnatural enantiopure fluorinated α-amino acids were obtained through sequential β-C(sp3)–H arylation and subsequent stereoselective fluorination from readily available L-alanine.
Graphical abstract
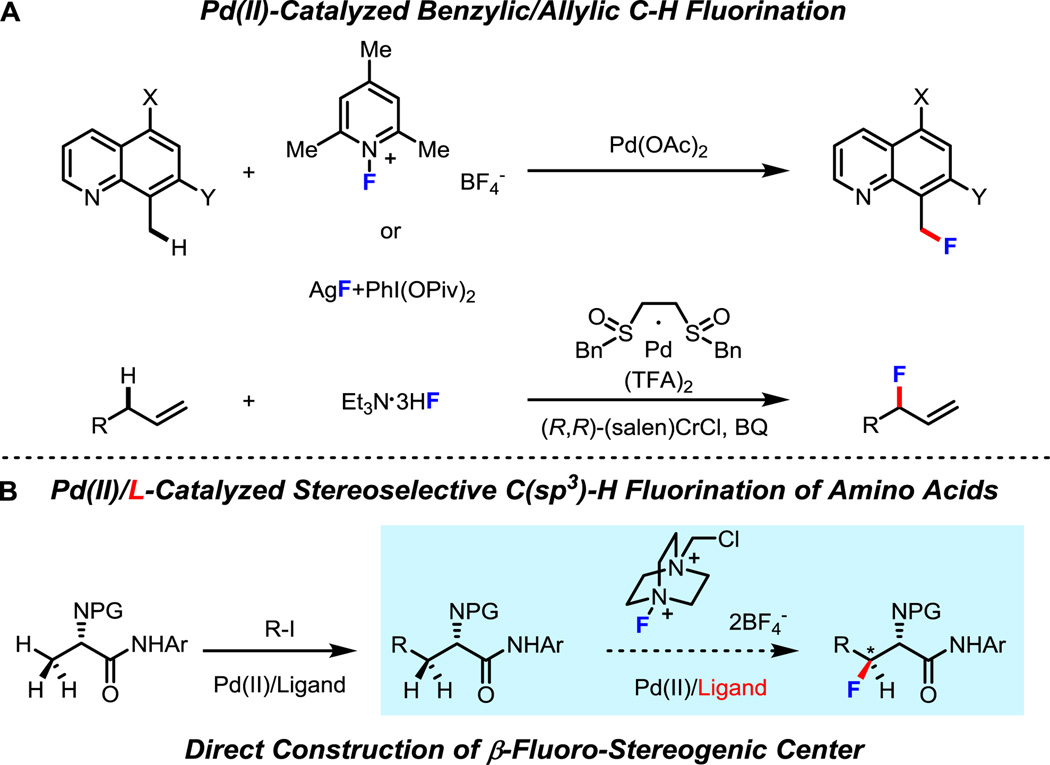
Incorporating a fluorine atom into an amino acid molecule can dramatically influence the lipophilicity, conformational flexibility and, metabolic stability of a compound, which can be very useful when attempting to modulate biological activity of a preclinical drug candidate.1 Therefore, fluorine-containing amino acids could serve as a versatile class of building blocks for peptide and protein modification.2 However, despite the potential utility of these valuable compounds, to the best of our knowledge, there is no method available for the synthesis of anti-β-fluoro-α-amino acids through C–H activation. In the past decade, the development of new methods for both aromatic and aliphatic C–H fluorination has been an area of great interest. Although many catalytic C(sp2)–H fluorination reactions have been reported,3 the success of C(sp3)–H fluorination is still rare (Scheme 1, A).3a,4 The first transition-metal-catalyzed benzylic C–H fluorination of 8-methylquinolines was reported by Sanford and co-workers.3a Recently, allylic C–H fluorination of terminal olefins was achieved by Doyle and co-workers.4b However, for inert primary or methylene C(sp3)–H fluorination, there are only a few stoichiometric reactions5 and no catalytic reaction has been demonstrated with the exception of radical C–H abstraction/fluorination.6 Though radical C–H abstraction/fluorination represents a useful strategy for methylene C–H fluorination, it is important to develop complementary methods that can address some of the shortcomings of this methodology such as lack of regio- and stereo-selectivity.
Scheme 1.
Catalytic C(sp3)–H Fluorination
In our continued efforts to develop C–H activation reactions using weakly coordinating substrates, C(sp3)–H fluorination has presented us with tremendous difficulties. First, achieving C(sp3)–H activation in the presence of coordinative fluorinating reagents can be problematic; second, the C(sp3)–F reductive elimination from a high-valent transition-metal center is also challenging due to a a formidable kinetic barrier.7 Recent success in the ligand promoted C(sp3)–H arylation and alkylation of amino acids8 inspired us to revisit the fluorination of inert C(sp3)–H bond by employing pyridine- and quinoline-based ligands. Herein we report fluorination of both primary and methylene C(sp3) C–H bonds enabled by a quinoline ligand. Fluorinated α-amino acid derivatives are rapidly prepared through sequential β-arylation and -fluorination of L-alanine using this method (Scheme 1, B).
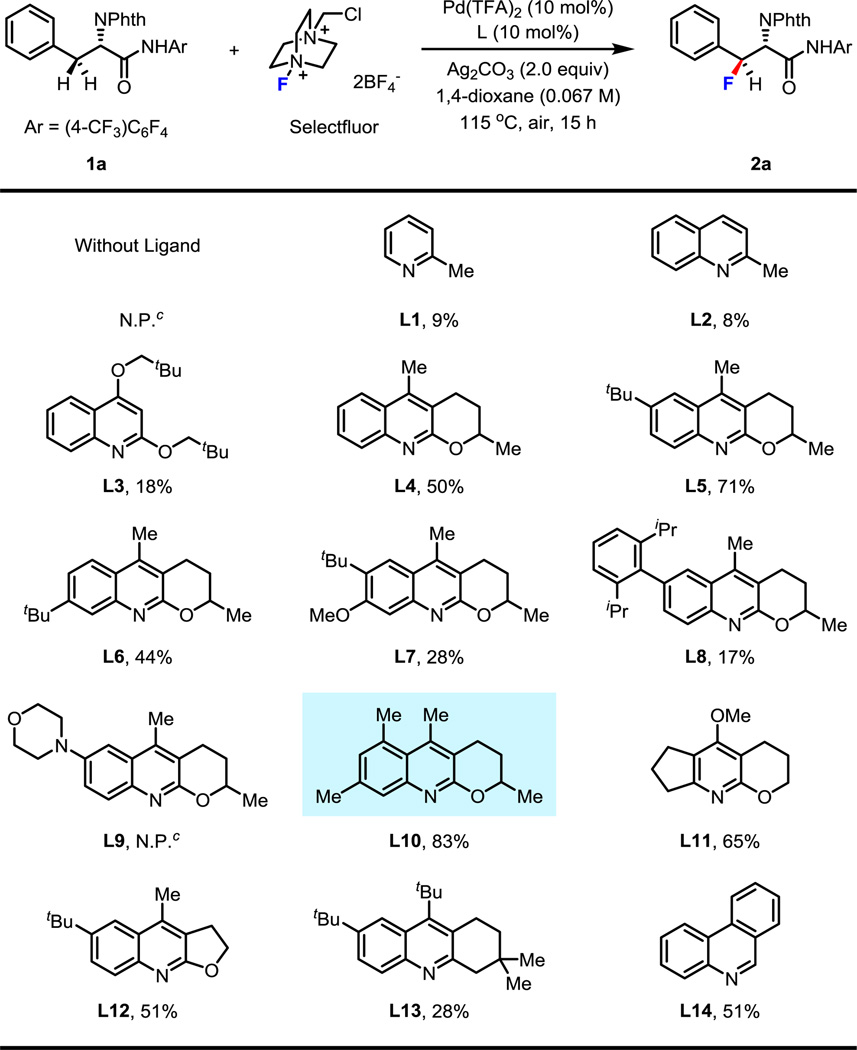
As we envision fluorinated α-amino acids are becoming rapidly adopted by medicinal chemistry and biochemistry efforts, we sought to design a sequential C–H activation approach that would provide a diverse library of fluorinated α-amino acid derivatives quickly and efficiently. In particular, we sought to install the first aryl group at the β-position of L-alanine via the first C–H activation, followed by incorporation of the fluorine atom at the β-position of the β-arylated amino acid through the second C–H activation building the β-fluorinated stereogenic carbon center in this step. With this consideration in mind, our study began by utilizing L-phenylalanie derived amide (1a) as a model substrate to develop this fluorination reaction. Among several commonly used electrophilic fluorine reagents, Selectfluor is a prefered choice as it is relatively cheap and is unlikely to cause the deactivation of the palladium catalyst in comparison to other pyridine-containing fluorinating reagents. We also anticipated that a sterically hindered pyridine-type ligand could potentially promote the C(sp3)–F bond-forming reductive elimination from the Pd(IV) center. Encouragingly, we found that treatment of 1a with 1.5 equiv. of Selectfluor, 10 mol% of Pd(TFA)2, 10 mol% of 2-picoline (L1, a common starting point for pyridine-ligand promoted reactions within our group), and 2.0 equiv. of Ag2CO3 in 1,4-dioxane at 115 °C for 15 h gave the desired product in 9% yield (Table 1). Solvent screening disclosed that 1,4-dioxane was the most efficient solvent for this reaction. The presence of Ag2CO3 was also crucial for this reaction to proceed. As we expected, no desired product was formed without the ligand. Notably, this fluorination reaction was quite sensitive to the amounts of Selectfluor, Ag2CO3 and 1,4-dioxane. However, after extensive screening, we failed to improve the reactivity by further manipulating the reaction conditions. We thus turned our attention to ligand screening in hopes of finding a ligand that would sufficiently promote this reaction. The observable impact of the ligand on this fluorination reaction encouraged us to further tune the structures of several pyridine- and quinoline-based ligands (Table 1). To begin screening, 2-methylquinoline (L2) was tested and gave a similar result with 2-picoline (L1). Next, we tried to utilize a more electron-rich ligand (L3) to enhance the coordinating ability of the ligand to palladium. Delightfully, the yield was improved to 18%. Interestingly L4, which had previously been successfully employed for methylene C(sp3)–H arylation,8a proved to be effective in this fluorination reaction. With this promising result in hand, we began to tune the substituents on the quinoline ring. When a tert-butyl group was installed at the6-position, the yield further increased to 71%while moving the tert-butyl group to the 7-position resulted in lower yield (L6). Modifying the structure of L5 by installation of a methoxy group at the 7 position failed to improve the efficiency of the reaction (L7). Substituting the 6-position with aryl or amino groups was detrimental to the reaction (L8–9). Eventually, we discovered that when 5,7-dimethylquinoline was used as the ligand (L10) the product was delivered in 83% yield. Further investigation of ligands showed that both the aryl ring and the aliphatic ring of quinoline-based ligands were important to the reactivity (L11–14).
Table 1.
Conditions: 0.1 mmol of 1a, 1.5 equiv of Selectfluor, 10 mol% of Pd(TFA)2, 10 mol% of ligand, 2.0 equiv of Ag2CO3, 1.5 mL of 1,4-dioxane, 115 °C, under air, 15 h.
The yield was determined by 1H NMR analysis of the crude product using CH2Br2 as the internal standard.
N.P. means no desired product.
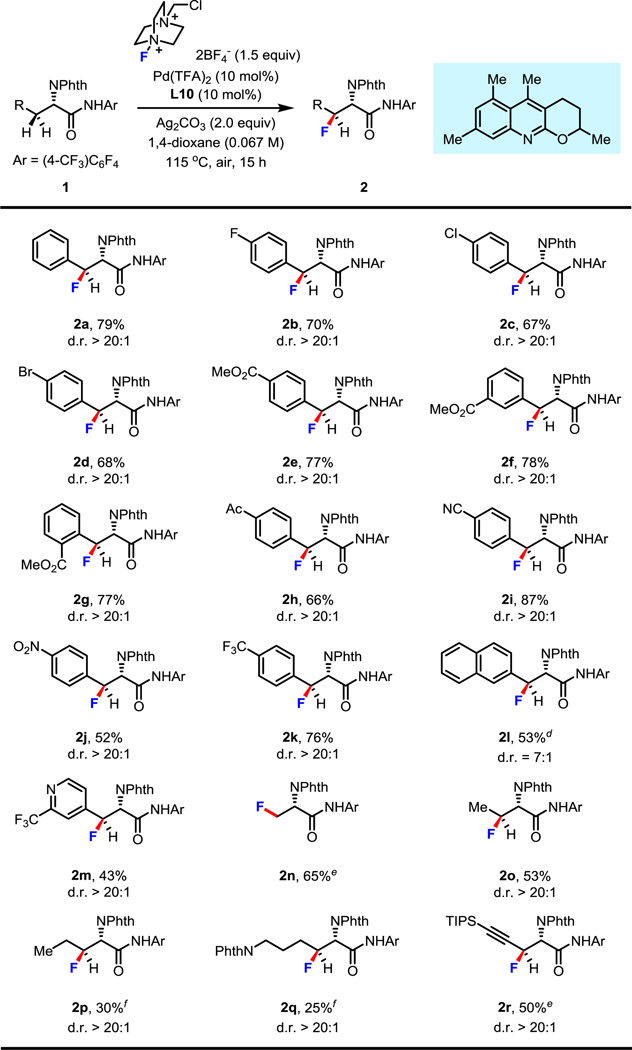
With the optimal ligand and reaction conditions in hand, a series of L-phenylalanine derivatives synthesized through C–H arylation of L-analine were fluorinated at the β-position regioselectively and stereoselectively (Table 2). L-phenylalanine was smoothly fluorinated in 79% isolated yield to provide product 2a. The reaction was found to be compatible with fluorine, bromine and chlorine groups on the aryl ring and substrates containing these functional groups gave good yields (2b–d). A methyl ester group substituted at different positions did not have much impact on the reactivity and the corresponding products were formed in good yields (2e–g). Diverse functionality such as acetyl, cyano, nitro, trifluoromethyl, and naphthyl groups were well tolerated and afforded the products in moderate to excellent yields (2h–l). Importantly, the pyridine moiety was demonstrated to be compatible with this reaction when the 2-position of the pyridine was appropriately substituted (2j). The fluorination was also suitable with the primary C–H bonds of L-alanine. Using simplified conditions, β-fluorinated L-alanine was obtained in 65% yield (2n). It is important to note that β-fluoroalanine and its analogues can deactivate alanine racemase, which provides D-alanine for bacterial cell wall formation.9 This potential chemotherapeutic property could be potentially attractive. Not only benzylic and the primary C–H bonds could be fluorinated, but more inert methylene C–H bonds could also be cleaved and subsequently transformed into a fluorine atom at the β-position stereoselectively (2o–q). Notably, L-lysine derivative was fluorinated selectively, albeit in low yield (2q). Importantly, a propargylic C–H bond was successfully fluorinated as well, (2r) providing a synthetically useful fluorinated synthon.
Table 2.
Condition A: 0.1 mmol of 1, 1.5 equiv of Selectfluor, 10 mol% of Pd(TFA)2, 10 mol% of L10, 2.0 equiv of Ag2CO3, 1.5 mL of 1,4-dioxane, 115 °C, under air, 15 h.
Isolated yields.
The d.r. value was estimated by 1H NMR analysis of the crude product.
Isolated yield of major diastereomer.
Condition B: 0.1 mmol of 1, 1.25 equiv of Selectfluor, 10 mol% of Pd(OAc)2, 20 mol% of L5, 1.25 mL of 1,4-dioxane, 115 °C, under air, 15 h.
Condition A, but using 20 mol% of L10 and adding 1.0 equiv of KHCO3.
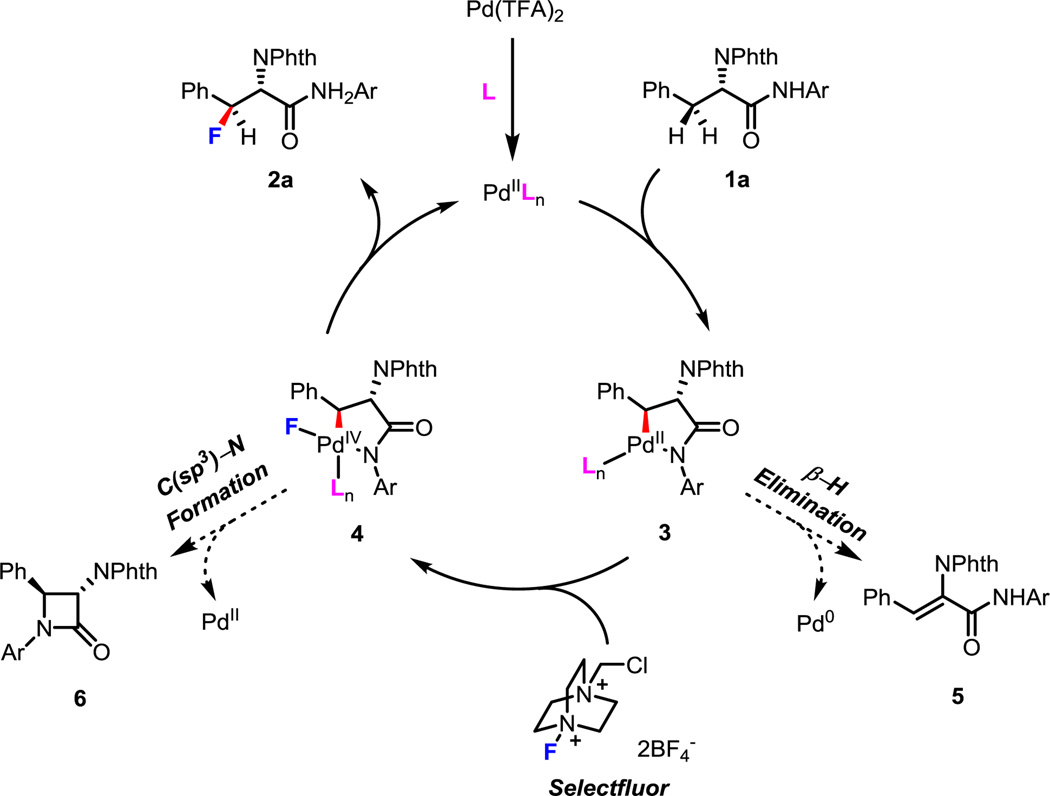
Based on previous literature,3–5 we reasoned that the reaction may proceeded through a Pd(II/IV) catalytic cycle. First, Pd(TFA)2 was coordinated by a quinoline ligand to form the active catalyst PdIILn. In the presence of ligand, intermediate 3 was obtained through C(sp3)–H activation. The stereogenic center was built during the C(sp3)–H activation step and was controlled by the favorability of a trans substituted 5-membered palladacycle. Next, the oxidative addition of intermediate 3 with Selectfluor led to intermediate 4 with a fluorine atom attached to the Pd(IV) center. Notably, we did not detect any β-hydride elimination byproduct 5. This step in the catalytic cycle is particularly prone to side reactions as the intermediate 4 could undergo direct C(sp3)–N bond-forming reductive elimination.5e,10 However, the lactam byproduct 6 was not observed in the reaction. Instead, the reductive elimination of C(sp3)–F bond formation outcompeted the C(sp3)–N bond-forming reductive elimination and resulted in corresponding fluorinated product exclusively. While the role of the ligand in each step of the catalytic cycle remains to be ascertained, the ligand effect might be crucial in favoring the C(sp3)–F bond-forming reductive elimination over the intramolecular C(sp3)–N bond-forming reductive elimination.
In summary, we describe the first example of a ligand-enabled primary and methylene C(sp3)–H stereoselective fluorination of valuable α-amino acids and synthesized various unnatural enantipure anti-β-fuoro-α-amino acids. The ligand effect is crucial for this C(sp3)–H fluorination to occur. Future work will focus on revealing the role of ligand in this reaction and develop chiral ligand-controlled asymmetric methylene C(sp3)–H fluorination.
Supplementary Material
Scheme 2.
Proposed Mechanism
Acknowledgments
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 2R01GM084019) for financial support.
Footnotes
Supporting Information Available: Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews: Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. Purser S, Moore PR, Swallow S, Gouverneur V. Chem. Soc. Rev. 2008;37:320. doi: 10.1039/b610213c.
- 2.(a) Soloshonok VA. Chapter 35 Fluorine-Containing Synthons. Washington, DC: American Chemical Society; 2005. [Google Scholar]; (b) Salwiczek M, Nyakatura EK, Gerling UIM, Ye S, Koksch B. Chem. Soc. Rev. 2012;41:2135. doi: 10.1039/c1cs15241f. [DOI] [PubMed] [Google Scholar]; (c) Marsh ENG. Acc. Chem. Res. 2014;47:2878. doi: 10.1021/ar500125m. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hull KL, Anani WQ, Sanford MS. J. Am. Chem. Soc. 2006;128:7134. doi: 10.1021/ja061943k. [DOI] [PubMed] [Google Scholar]; (b) Wang X, Mei T-S, Yu J-Q. J. Am. Chem. Soc. 2009;131:7520. doi: 10.1021/ja901352k. [DOI] [PubMed] [Google Scholar]; (c) Chan KSL, Wasa M, Wang X, Yu J-Q. Angew. Chem., Int. Ed. 2011;50:9081. doi: 10.1002/anie.201102985. [DOI] [PubMed] [Google Scholar]; (d) Lou S-J, Xu D-Q, Xia A-B, Wang Y-F, Liu Y-K, Du X-H, Xu Z-Y. Chem. Commun. 2013;49:6218. doi: 10.1039/c3cc42220h. [DOI] [PubMed] [Google Scholar]; (e) Truong T, Klimovica K, Daugulis O. J. Am. Chem. Soc. 2013;135:9342. doi: 10.1021/ja4047125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Lou S-J, Xu D-Q, Xu Z-Y. Angew. Chem., Int. Ed. 2014;53:10330. doi: 10.1002/anie.201404423. [DOI] [PubMed] [Google Scholar]; (g) Lou S-J, Chen Q, Wang Y-F, Xu D-Q, Du X-H, He J-Q, Mao Y-J, Xu Z-Y. ACS Catal. 2015;5:2846. [Google Scholar]
- 4.(a) McMurtrey KB, Racowski JM, Sanford SM. Org. Lett. 2012;14:4094. doi: 10.1021/ol301739f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Braun M-G, Doyle AG. J. Am. Chem. Soc. 2013;135:12990. doi: 10.1021/ja407223g. [DOI] [PubMed] [Google Scholar]
- 5.(a) Kaspi AW, Goldberg I, Vigalok A. J. Am. Chem. Soc. 2010;132:10626. doi: 10.1021/ja101436w. [DOI] [PubMed] [Google Scholar]; (b) Zhao S-B, Becker JJ, Gagné MR. Organometallics. 2011;30:3926. doi: 10.1021/om200515f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Racowski JM, Kampf JW, Sanford MS. Angew. Chem., Int. Ed. 2012;51:3414. doi: 10.1002/anie.201107816. [DOI] [PubMed] [Google Scholar]; (d) Mankad NP, Toste FD. Chem. Sci. 2012;3:72. doi: 10.1039/C1SC00515D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Pérez-Temprano MH, Racowski JM, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2014;136:4097. doi: 10.1021/ja411433f. [DOI] [PubMed] [Google Scholar]
- 6.For selected examples: Liu W, Huang X, Cheng M-J, Nielsen RJ, Goddard WA, III, Groves J-T. Science. 2012;337:1322. doi: 10.1126/science.1222327. Liu W, Groves JT. Angew. Chem., Int. Ed. 2013;52:6024. doi: 10.1002/anie.201301097. Bloom S, Pitts CR, Miller DC, Haselton N, Holl MG, Urheim E, Lectka T. Angew. Chem., Int. Ed. 2012;51:10580. doi: 10.1002/anie.201203642. Bloom S, Pitts CR, Woltornist R, Griswold A, Holl MG, Lectka T. Org. Lett. 2013;15:1722. doi: 10.1021/ol400424s. Xia J-B, Zhu C, Chen C. J. Am. Chem. Soc. 2013;135:9342. doi: 10.1021/ja410815u.
- 7.For selected reviews: Engle KM, Mei T-S, Wang X, Yu J-Q. Angew. Chem., Int. Ed. 2011;50:1478. doi: 10.1002/anie.201005142. Furuya T, Kamket AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108. Grushin VV. Acc. Chem. Res. 2010;43:160. doi: 10.1021/ar9001763. Vigalok A, Kaspi AW. Top. Organomet. Chem. 2010;31:19.
- 8.(a) He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu J-Q. Science. 2014;343:1216. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhu R-Y, He J, Wang X-C, Yu J-Q. J. Am. Chem. Soc. 2014;136:13194. doi: 10.1021/ja508165a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen G, Shigenari T, Jain P, Zhang Z, Jin Z, He J, Li S, Mapelli C, Miller MM, Poss MA, Scola PM, Yeung K-S, Yu J-Q. J. Am. Chem. Soc. 2015;137:3338. doi: 10.1021/ja512690x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Esaki N, Walsh CT. Biochemistry. 1986;25:3261. doi: 10.1021/bi00359a027. [DOI] [PubMed] [Google Scholar]; (b) Flynn GA, Beight DW, Bohme EHW, Metcalf BW. Tetrahedron Lett. 1985;26:285. [Google Scholar]
- 10.For selected C(sp3)–N bond forming examples: Nadres ET, Daugulis O. J. Am. Chem. Soc. 2012;134:7. doi: 10.1021/ja210959p. He G, Zhao Y, Zhang S, Lu C, Chen G. J. Am. Chem. Soc. 2012;134:6. doi: 10.1021/ja210660g. Zhang Q, Chen K, Rao W-H, Zhang Y, Chen F-J, Shi B-F. Angew. Chem., Int. Ed. 2013;52:13588. doi: 10.1002/anie.201306625.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.