Abstract
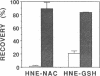
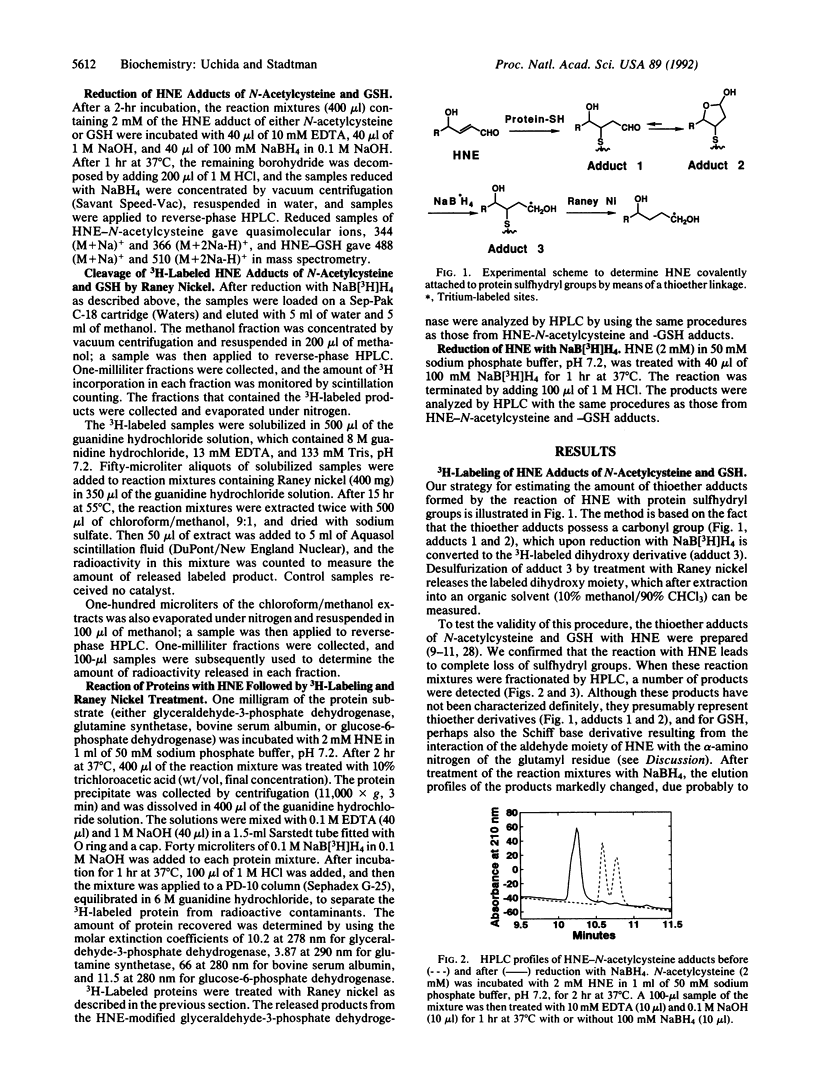
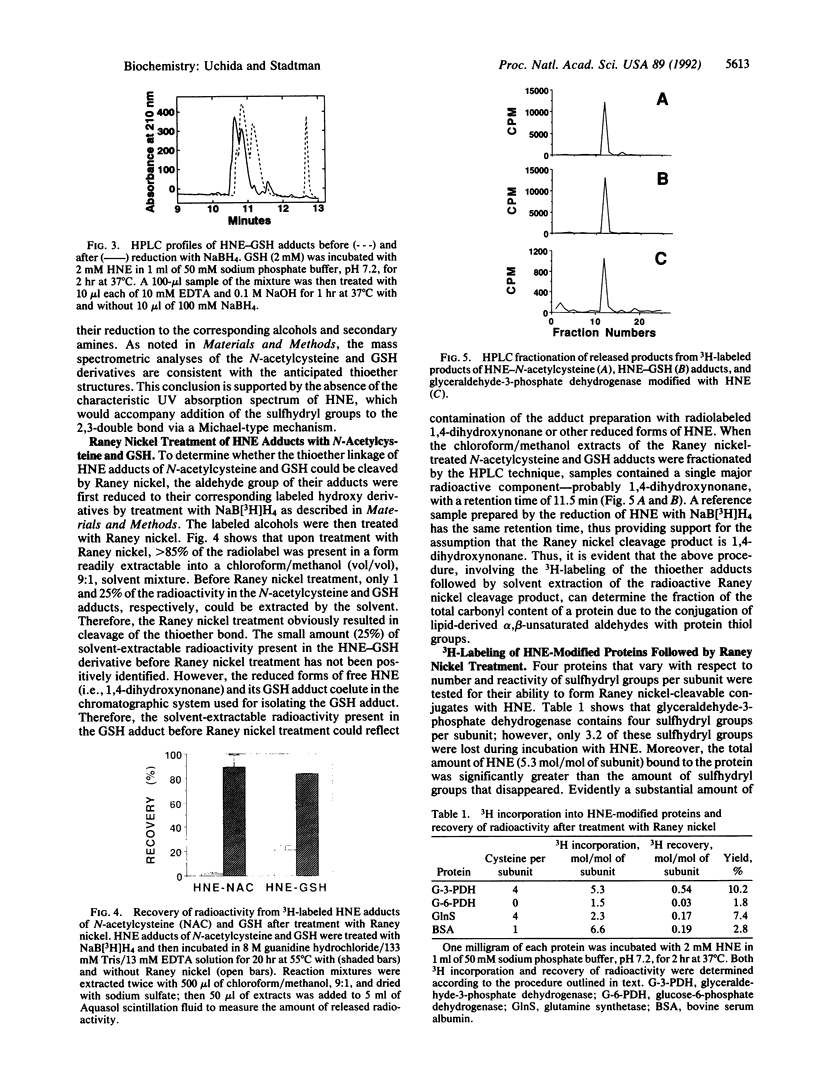
The peroxidation of polyunsaturated fatty acids leads to numerous products, including 4-hydroxynonenal (HNE). That 4-hydroxy-2-alkenal compounds react with sulfhydryl groups of proteins to form thioether adducts possessing a carbonyl function has been established [Schauenstein, E. & Esterbauer, H. (1979) Ciba Found. Symp. 67, 225-244]. Taking advantage of the fact that Raney nickel catalyzes cleavage of thioether bonds, we have developed a procedure to quantitate the amount of HNE moiety bound to protein by means of a thioether linkage. Adducts of HNE with N-acetylcysteine and glutathione were prepared, labeled with NaB[3H]H4, and then treated with Raney nickel. The 3H-labeled product was recovered in 85-90% yield from both HNE-N-acetylcysteine and HNE-glutathione adducts in a solvent [10% (vol/vol) methanol/chloroform]-estractable form. Treatment of proteins with HNE led to the disappearance of protein sulfhydryl groups. However, less than 10% of the labeled adducts obtained after subsequent reduction with NaB[3H]H4 could be released in a solvent-extractable form upon treatment with Raney nickel. This and the observation that HNE reacts with proteins lacking a sulfhydryl group attests to the fact that HNE can react with amino acid residues other than cysteinyl residues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amici A., Levine R. L., Tsai L., Stadtman E. R. Conversion of amino acid residues in proteins and amino acid homopolymers to carbonyl derivatives by metal-catalyzed oxidation reactions. J Biol Chem. 1989 Feb 25;264(6):3341–3346. [PubMed] [Google Scholar]
- Bateman R. C., Jr, Youngblood W. W., Busby W. H., Jr, Kizer J. S. Nonenzymatic peptide alpha-amidation. Implications for a novel enzyme mechanism. J Biol Chem. 1985 Aug 5;260(16):9088–9091. [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980 Nov 7;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Esterbauer H., Ferrali M., Fulceri R., Comporti M. Evidence for aldehydes bound to liver microsomal protein following CCl4 or BrCCl3 poisoning. Biochim Biophys Acta. 1982 May 13;711(2):345–356. doi: 10.1016/0005-2760(82)90044-3. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Pompella A., Fulceri R., Romani A., Comporti M. Detection of 4-hydroxynonenal and other lipid peroxidation products in the liver of bromobenzene-poisoned mice. Biochim Biophys Acta. 1986 May 21;876(3):658–666. doi: 10.1016/0005-2760(86)90055-x. [DOI] [PubMed] [Google Scholar]
- Carney J. M., Starke-Reed P. E., Oliver C. N., Landum R. W., Cheng M. S., Wu J. F., Floyd R. A. Reversal of age-related increase in brain protein oxidation, decrease in enzyme activity, and loss in temporal and spatial memory by chronic administration of the spin-trapping compound N-tert-butyl-alpha-phenylnitrone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzio M., Esterbauer H., Di Mauro C., Cecchini G., Dianzani M. U. Chemotactic activity of the lipid peroxidation product 4-hydroxynonenal and homologous hydroxyalkenals. Biol Chem Hoppe Seyler. 1986 Apr;367(4):321–329. doi: 10.1515/bchm3.1986.367.1.321. [DOI] [PubMed] [Google Scholar]
- Dunn J. A., Ahmed M. U., Murtiashaw M. H., Richardson J. M., Walla M. D., Thorpe S. R., Baynes J. W. Reaction of ascorbate with lysine and protein under autoxidizing conditions: formation of N epsilon-(carboxymethyl)lysine by reaction between lysine and products of autoxidation of ascorbate. Biochemistry. 1990 Dec 11;29(49):10964–10970. doi: 10.1021/bi00501a014. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K. H., Dianzani M. U., Poli G., Slater T. F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem J. 1982 Oct 15;208(1):129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Lang J. Metabolism of the lipid peroxidation product 4-hydroxynonenal by isolated hepatocytes and by liver cytosolic fractions. Biochem J. 1985 Jun 1;228(2):363–373. doi: 10.1042/bj2280363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Scholz N. Reaction of glutathione with conjugated carbonyls. Z Naturforsch C. 1975 Jul-Aug;30(4):466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- Farnsworth C. C., Gelb M. H., Glomset J. A. Identification of geranylgeranyl-modified proteins in HeLa cells. Science. 1990 Jan 19;247(4940):320–322. doi: 10.1126/science.2296721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnsworth C. C., Wolda S. L., Gelb M. H., Glomset J. A. Human lamin B contains a farnesylated cysteine residue. J Biol Chem. 1989 Dec 5;264(34):20422–20429. [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Esterbauer H., Sies H. Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem. 1986 Feb 5;261(4):1576–1581. [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- Oliver C. N., Starke-Reed P. E., Stadtman E. R., Liu G. J., Carney J. M., Floyd R. A. Oxidative damage to brain proteins, loss of glutamine synthetase activity, and production of free radicals during ischemia/reperfusion-induced injury to gerbil brain. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5144–5147. doi: 10.1073/pnas.87.13.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling H. C., Breunger E., Epstein W. W., Crain P. F. Prenylated proteins: the structure of the isoprenoid group. Science. 1990 Jan 19;247(4940):318–320. doi: 10.1126/science.2296720. [DOI] [PubMed] [Google Scholar]
- Roseman J. E., Levine R. L. Purification of a protease from Escherichia coli with specificity for oxidized glutamine synthetase. J Biol Chem. 1987 Feb 15;262(5):2101–2110. [PubMed] [Google Scholar]
- Schaffer M. H., Stark G. R. Ring cleavage of 2-iminothiazolidine-4-carboxylates by catalytic reduction. A potential method for unblocking peptides formed by specific chemical cleavage at half-cystine residues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1040–1047. doi: 10.1016/0006-291x(76)90759-2. [DOI] [PubMed] [Google Scholar]
- Schauenstein E., Esterbauer H. Formation and properties of reactive aldehydes. Ciba Found Symp. 1978;(67):225–244. doi: 10.1002/9780470720493.ch15. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. Regulation of glutamine synthetase. IX. Reactivity of the sulfhydryl groups of the enzyme from Escherichia coli. J Biol Chem. 1967 Nov 10;242(21):5069–5079. [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Starke-Reed P. E., Oliver C. N., Stadtman E. R., Floyd R. A., Markesbery W. R. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R. Covalent modification reactions are marking steps in protein turnover. Biochemistry. 1990 Jul 10;29(27):6323–6331. doi: 10.1021/bi00479a001. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med. 1990;9(4):315–325. doi: 10.1016/0891-5849(90)90006-5. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Starke-Reed P. E., Oliver C. N. Protein oxidation and proteolysis during aging and oxidative stress. Arch Biochem Biophys. 1989 Dec;275(2):559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Uchida K., Stadtman E. R. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4544–4548. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]