INTRODUCTION
Cryosurgery (also known as cryoablation or cryotherapy) is a surgical technique that utilizes extreme cold to destroy diseased tissues in the body. This is achieved by the removal of heat from the tissue when placed in contact with a cold surface. Arnott reported the first use of cryosurgery in 1840's, using iced saline solutions at −20°C to treat breast and uterine cancers [1]. Until the early 1900's the use of cryosurgery was limited to the treatment of superficial surface malignancies using liquefied gases such as nitrogen, carbon dioxide and cholorofluorocarbons. A partial list of the current diseases that have cryosurgery as a treatment option are given in Table 1. While cryosurgery has for decades been a standard mode of treatment for non-melanoma dermatologic conditions with a 90-95% cure rate, the inability to deliver cryogens to subdermal regions prevented its application to treat other conditions [2]. The development of the first cryosurgical probe system capable of delivering liquid nitrogen to an insulated Linde cryosurgical probe in the 1960's allowed for the first time, the capacity to treat cancers present deep within the body [3-5]. The use of cryosurgery then spread to treatment of malignancies present in prostate, liver, uterus, bone and eye [6-10]. However poor monitoring of the procedure resulted in major complications and morbidity, thereby limiting the acceptance of cryosurgery as an alternative therapeutic modality.
Table 1.
Summary of the current patient base for various diseases that have cryosurgery as one treatment option.
| Targets | New Cases/Deaths* | Cryosurgical Outcomes | Alternatives | References |
|---|---|---|---|---|
| Cancer Targets | ||||
| Skin Cancer# | 65,000 / 10,900 | 95-98% cure rates | Surgery, Rad, Chemo | [101, 114-119] |
| Prostate Cancer | 218,900 / 27,000 | 1-2 year no PSA | Surgery, Rad, Chemo, Heat | [120-124] |
| Breast Cancer** | 180,510 / 40,910 | Size reduction | Surgery, Rad, Chemo, Heat | [125-128] |
| Liver (Colon***) Cancer | 19,200 (112, 300) / 16,800 (52,200) | 5 years survivals | Surgery, Heat | [124, 129-134] |
| Kidney Cancer | 51,200 / 12,900 | 5 year survivals | Surgery, Heat | [135-140] |
| Lung and Bronchus | 213,400 / 160,400 | Palliation | Surgery, Heat | [141-144] |
| Non-Cancer Targets | ||||
| Skin: Keloids/Scars | Unknown | Cosmetic | Excision / Grafting | [145-147] |
| Uterus: abnormal bleeding | Unknown | Improved lifestyle | Surgery, Heat | [148-150] |
| Uterus: uterine fibroids | >60% in women above 45 | Maintain uterus | Surgery | [151-154] |
| Cardiac Arrythmia | >2.2 million | Improved lifestyle | Surgery, Heat | [155-158] |
| Vascular Disease | 12-20% above 65 | Reduce arteriosclerosis | Angioplasty, Stenting | [159-161] |
Sources of incidence:
ACS statistics 2007, AHA statistics 2007.
cure rates apply to non-melanoma skin cancers.
fibroadenomas – a non-malignant tumor.
liver cancers with colon metastases.
Technological advancements in early 1980's and 1990's in two areas lead to a renewed interest in cryosurgery: (1) intraoperative monitoring by medical imaging and, (2) advances in cryosurgical equipment [11-14]. Intra-operative monitoring for cryosurgery is now well established using Ultrasonography (US), Computed Tomography (CT) and Magnetic Resonance (MR). These imaging modalities provide the surgeon with the ability to visualize the 3D geometry of the frozen region (−0.5°C contour) and get simultaneous feedback of the procedure [12, 13]. Cryosurgical instrumentation has also improved dramatically in the last several decades.
Cooling of the tissue in cryosurgery is achieved by delivering various cryogens (Table 2A) inside a metal, ceramic or balloon probe tip usually several mm in diameter, where they undergo expansion or vaporization, thereby extracting heat from the surrounding tissue to form an iceball, as shown in Figure 1. Table 2 lists some of the cryosurgical devices and cryogens that are available for several indicated treatments. Historically much of the innovation in cryosurgical technology has come in the form of novel cryogen compositions to achieve lower subzero temperatures (Table 2A). Additionally, much effort went into engineering new cryoprobes for efficient heat extraction from tissue such as vacuum insulated Linde cryoprobes in 1961. Several important design innovations that improve the original Linde probe include: 1. Subcooled nitrogen delivery to insulated probes, 2. Argon Joule-Thomson (JT) probes with faster tip cooling and adjustable cryogen delivery hoses and probe sizes (EndoCare, Galil, CryoCath, ATS), 3. Closed loop refrigeration of gaseous cryogen mixtures (AMS), 4. Ballon-shaped probes for cardiac and intra-arterial use [15-17]. Cryotherapy can now be delivered to complex anatomic locations such as the prostate or the heart. For example Atrial Fibrillation can now be treated in a minimally invasive manner using steerable transcatheter cryoprobes or by open surgery with fixed cryoprobes [18-21]. Lesions can be created in the shape of arcs, semicircles or long straight lines [18, 19]. The placement of multiple probes can also be used to create a desired shape of the frozen region that conforms to the complex anatomy of a diseased tissue such as the prostate [22].
Table 2.
A) List of cryogens that have been used in cryosurgical instruments; B) Summary of cryosurgical devices available in clinic and the various indications for which they are used.
| Cryogen | Lowest Achievable Temperature (°C) |
|---|---|
| Ice-saline | −21.2 |
| Dichlorotetrafluoromethane (Freon 114) | 3.8 |
| Dichlorodifluoromethane (Freon 12) | −29.8 |
| Chlorodifluoromethane (Freon 22) | −40.8 |
| Solid Carbon Dioxide (CO2) | −78.5 |
| Liquid Nitrous Oxide (N2O) | −89.5 |
| Argon (compressed) | −185.7 |
| Liquid Nitrogen (N2) | −195.8 |
| Probe | Company | Cryogen | Shape | Indications* | Web |
|---|---|---|---|---|---|
| Cancer Targets | |||||
| SeedNet™ | Galil Medical, Yokneam, Israel | Argon JT | Probe | Prostate, Kidney Cancers, Uterine Fibroids | www.galil-medical.com |
| CryoCare CS™ | Endocare, Irvine, CA, USA. | Argon JT | Probe | Prostate, Kidney, Lung and Liver Cancers | www.endocare.com |
| Visica 2™ | Sanarus, Pleasanton, CA, USA. | Liquid N2 | Probe | Breast fibroadenomas | www.sanarus.com |
| Various Systems | BryMill, Ellington, CT, USA. | Liquid N2 | Probe, Spray | Dermatologic Lesions | www.brymill.com |
| Erbokryo CA™ | Erbe Medical, Leeds, UK. | N2O or CO2 JT | Probe | General Surgical Use | www.erbe-med.com |
| Non-Cancer Targets | |||||
| CryoMaze™ | ATS Medical, Minneapolis, MN, USA. | Argon JT | Steerable Catheter | Cardiac Arrythmia | www.atsmedical.com |
| FrostByte™ | ATS Medical, Minneapolis, MN, USA. | Argon JT | Clamp Probe | Atrial Fibrillation | www.atsmedical.com |
| Arctic Front™ | Cryocath*, Montreal, QC, Canada. | Liquid N2O | Balloon Catheter | Atrial Fibrillation | www.cryocath.com |
| Freezor™ | Cryocath, Montreal, QC, Canada. | Liquid N2O | Steerable Catheter | Cardiac Arrythmia | www.cryocath.com |
| CryoCor™** | Boston Scientific, Natick, MA, USA. | Liquid N2O | Steerable Catheter | Atrial Flutter | www.cryocor.com |
| PolarCath™ | Boston Scientific, Natick, MA, USA. | Liquid N2O | Balloon Catheter | Peripheral Arterial Disease | www.bsci.com |
| HerOption™ | American Medical Systems, Minnetonka, MN, USA. | Mixture of cryogens | Probe | Endometrial Bleeding | www.americanmedicalsystems.com |
| CryoPac™ Systems | Cryomed LLC, Layton, UT, USA. | N2O or CO2 JT | Probe | Chronic Pain | www.cryomed.us |
Purchased by Medtronic, Inc. in 2008
Purchased by Boston Scientific in 2008.
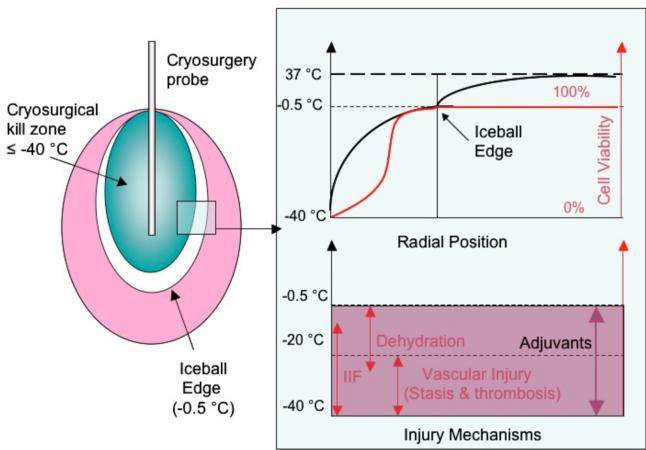
Figure 1.
Outcome of a cryosurgical procedure. Complete tissue destruction is usually obtained at temperatures less than −40°C. Direct cell injury, vascular injury and immune injury mechanisms cause incomplete cellular destruction in the region from −40° to the imaged edge of the iceball (−0.5°C).
Due to both the imaging and technological improvements cryosurgery is now less invasive and easier to perform and used on a number of deep tissue diseases as described in Table 1 [22]. Cryosurgery has been recognized by the American Urological Association (AUA) as a therapeutic option for carcinoma of the prostate and is also considered an alternative treatment for a number of other cancers including liver, kidney and breast [23-26]. Recently, there has been a surge in the use of cryosurgery to treat abnormal cardiac tissue that cause arrythmia [27-29]. Table 1 is a partial list of diseases that have cryosurgery as a treatment option. While surgery is often still the standard of care, cryosurgery or other heat therapies such as radiofrequencey, microwave, laser etc are increasingly being used as minimally invasive surgical treatments to improve patient outcomes. An important advantage of cryosurgery over other heat treatments includes both the imaging aspect as noted above and the preservation of the underlying tissue structure that induces less scarring and better healing of the wound than with heat treatments [30].
Despite these advantages cryosurgery is still not the preferred mode of treatment for most diseases and is usually employed only after other therapies fail to show an improvement. This lack of complete acceptance of cryosurgery within the medical community is due in part to the variability in outcome achieved clinically. Specifically, it is now evident that cryosurgical treatment alone causes complete tissue destruction at temperatures well within the imaged edge of the iceball (−0.5°C). In fact, the clinical guideline to assure complete cell death by this technique is −40°C [1, 31]. Thus, whereas the edge of the iceball (−0.5°C) can be visualized using US, CT or MR, there is no means to predict the treated region or the “kill zone” within the iceball (Figure 1). The effectiveness of cryosurgery is largely dependent on the ability of the surgeon to predict the −40°C critical isotherm based on the imaging feedback of the edge of the iceball (−0.5°C) and the temperature measurements from thermocouples placed at strategic anatomic locations. The need to avoid damage to critical normal adjacent tissues adds further challenges particularly in prostate and cardiac tissue.
Recent research in cryosurgery has focused on two approaches to predict the outcome and achieve greater control of the procedure. One approach is patient-specific computational modeling to predict temperature isotherms within an iceball and to estimate the tissue volume reaching temperatures lower than −40°C. Improved imaging input to models and computational methods has led to significant progress in the speed and accuracy of such predictions [32-34]. However, there is still a need for intraoperative thermal feedback (comparison and validation of models) that is difficult to achieve with the state of current imaging apparatus in the clinical setting. The second approach, and the topic of this review, has been the use of molecular adjuvants to increase cryosensitivity of the tissue at the periphery of an iceball (0 to −40°C) (Fig.1), which would otherwise remain viable [35].
Cellular and tissue level studies have provided deeper insight into the biophysical and biological changes involved during and after removal of heat from the tissue. Cryosurgery can now be directly related to cellular and vascular mechanisms of injury, with the addition of probable immune mechanisms of injury within a tissue after freezing as shown in Fig. 2 [31, 36]. By focusing on specific cellular and vascular mechanisms of injury, it is now possible to accentuate them using chemical or biological adjuvants. Specifically, adjuvants have been identified which can increase the destructive effect of freezing in the −40°C to −0.5°C region such that the kill zone can be raised to −20°C and in some cases extends up to the imaged edge of the iceball (−0.5°C). Identified adjuvants are shown in Table 3 and include anti-freeze proteins, salt solutions, chemotherapeutics such as bleomycin, 5-fluoracil and vascular targeting agents like TNF-α, which enhance the destructive effect of freezing in tissues at temperatures greater than −40°C [35, 37-44].
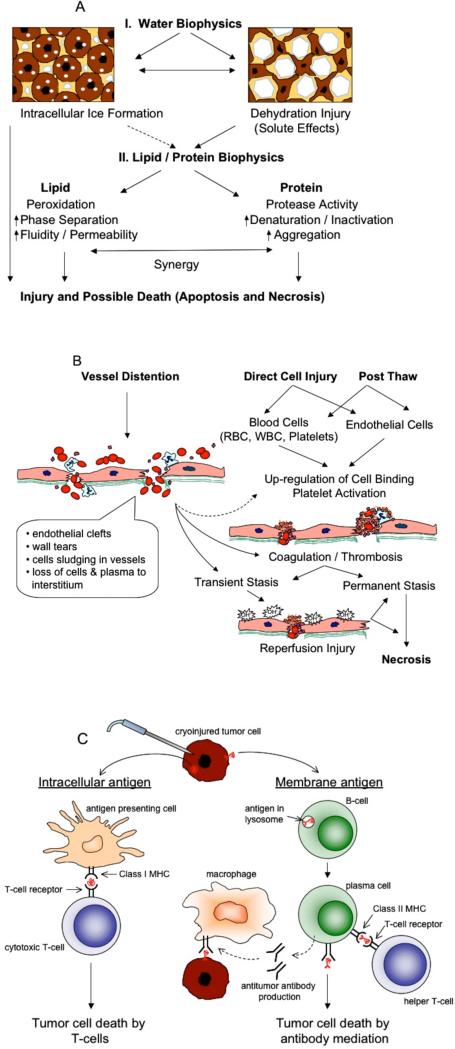
Figure 2.
Mechanisms of cryosurgical injury. Tissue destruction is achieved by A) direct cell injury, B) vascular injury and C) immunological injury.
Table 3.
A) Summary of adjuvants that have been used to enhance cryosurgical destruction in vitro; B) Summary of adjuvants that have been used to enhance cryoinjury in vivo.
| Mechanism | Model | Adjuvant | Dose | Time | Reference |
|---|---|---|---|---|---|
| Direct Cell Injury | |||||
| 1) Thermophysical Adjuvants: | |||||
| Ice crystals | Normal Rat Liver | AFP-1 | 10 mg/ml | 0-5 mins before FT | [43] |
| Rat Prostate | Eutectic salts | Eutectic conc. | 0-5 mins before FT | [84] | |
| Human Breast | Glycine | 10 g/ml | [85] | ||
| 2) Chemotherapeutics: | |||||
| Apoptosis, Antiproliferative | Human Prostate | 5-FU | 25 μg/ml | 2-4 days before FT | [38] |
| Human Prostate | 5-FU* | 25 μg/ml | 2 days before FT | [39] | |
| Human Prostate | TRAIL | 500 ng/ml | 0-5 mins before FT | [89] | |
| Human Hepatoma | Doxorubicin** | 55 μg/ml | 24 hrs before FT | [88] | |
| 3) Inflammatory cytokines: | |||||
| Apoptosis, Inflammation | Human Prostate | TNF-α | 1-1000 ng/ml | 4 hrs before FT | [99] |
| Human Endothelial | TNF-α | 1-1000 ng/ml | 4 hrs before FT | [99] | |
| Human Breast | TNF-α | 100 ng/ml | 24 hrs before FT | [113] | |
| Human Prostate | TNF-α | 1 μg/ml | 4 hrs before FT | [95] |
| Mechanism | Model | Adjuvant | Dose, Delivery | Time | Reference |
|---|---|---|---|---|---|
| Direct Cell Injury | |||||
| 1) Thermophysical adjuvants: | Human Prostate | AFP-1 | 10 mg, local | 0-5 mins before FT | [44] |
| Ice crystals. | Rat Prostate | Flounder AFP | 0.5 mg, local | 0-5 mins before FT | [83] |
| 2) Chemotherapeutics: | Human Lung | Navalbine | 120 μg, i.v | Multiple time points | [40] |
| Apoptosis, Antiproliferative | Human Lung | Vinblastine | 120 μg, i.v | 20 hours after FT, 15 days after FT | [42] |
| Human Prostate | 5-FU | 15-20 ng, local | 0-5 mins after FT | [90] | |
| Vascular Injury | |||||
| 1) Inflammatory cytokines: | Human Prostate | TNF-α | 0.2-1 μg, local | 4 hrs before FT | [98-99] |
| Apoptosis, Inflammation | 5 μg, i.v | 4 hrs before FT | |||
| Immune Injury | |||||
| Mouse Melanoma | Imiquimod | 3.12 mg, local | up to 10 days after FT | [101] | |
| Mouse Melanoma | CpG-ODN | 100 μg, local | 1 hour after FT | [102] | |
| Mouse Colon | BCG-CWS | [100] |
Enhancement was also reported with other drugs: cisplatin, etoposide and taxotere.
Enhancement was also reported with other drugs: mitomycin, 5-fluorouracil, cisplatin.
MECHANISMS OF CRYOINJURY
The clinical guideline of −40°C to ensure complete cell death largely oversimplifies the complex heat and mass transfer processes that occur upon removal of heat from the tissue. When using a cryoprobe at a sub-zero temperature to ablate a volume of tissue, the thermal conditions experienced by each cell vary depending on its location relative to the probe. For example, the region of the tissue that is closer to the probe experiences faster freezing rates and lower end temperatures as compared to the region that is further away. During this event, a cell can sustain injury by multiple mechanisms, broadly categorized into: 1) cellular, 2) vascular and 3) immunological mechanisms [1]. These have been reviewed in detail elsewhere and are discussed here briefly [1, 36, 45, 46].
Direct cell injury during freezing can occur due to the physical presence of ice (both inside the cell as well as outside) or because of a shift in the osmotic balance of the cell (Figure 2A). Once nucleated at lower temperatures near the probe, ice forms in the extracellular space of the tissue up to a temperature of −0.5°C. As a consequence of this ice growth, different biophysical phenomenon may occur within a cell depending on its location with respect to the probe. At low cooling rates, the solute concentration outside the cell rises due to the presence of extracellular ice, causing an osmotic imbalance (ΔΠ) of the cell membrane [1, 47]. Depending on the geometry (volume, V and surface area, A) of a specific tissue cell / extracellular (vascular) unit and the membrane permeability (Lp) between the cellular / extracellular compartment, cellular dehydration will occur (equation 1) as shown in Fig. 2A. This dehydration and the presence of high salt concentrations have been suggested to damage the enzymatic machinery and destabilize the cell membrane [47-50].
| (1) |
At higher cooling rates near the probe, water gets trapped within the cell, supercools, nucleates, thereby yielding intracellular ice formation (IIF), which is mechanically damaging to the cell organelles and membranes [51, 52]. The probability of IIF (PIF) is generally computed based on knowledge of the cellular supercooling (ΔT) and cell specific parameters as shown below:
| (2) |
where V and A are the same as above, Ω and κ are the kinetic and thermodynamic parameters of heterogenous nucleation respectively as originally defined by Toner [52]. The specific geometry, permeability and IIF parameters of different cell and tissue systems have been reviewed elsewhere [53, 54]. Post-thaw analysis of injury in cell suspensions suggests that both apoptosis and necrosis can occur after freeze-thaw. It is still unclear as to the extent to which biophysical events during freezing (IIF or dehydration) determine a specific cell death pathway (apoptosis or necrosis) post-thaw [1, 31]. It is also likely that any added adjuvant will affect biophysical processes at the cellular level, although these specific changes have not been documented to our knowledge.
A second mechanism of damage is termed vascular injury, caused by the shutdown of microvasculature after freezing and resultant ischemia (Figure 2B). Microvascular endothelial cells have previously been shown to be more sensitive to direct cell injury by freeze-thaw (FT) when compared to several other cancer cell lines [55]. During freezing, ice-crystals form and propagate along the vascular system and mechanically induce damage to the blood vessel wall. At low cooling rates, cells surrounding the blood vessels dehydrate resulting in expansion of the blood vessels [1, 24, 31]. This is believed to cause damage to the endothelium and exposure of underlying basement membrane, subsequently leading to thrombosis and ischemia of the surrounding tissue (Figure 2B, [56, 57]). Apart from direct cell injury to the endothelium, FT also induces free radical formation when re-reperfusion of the blood occurs at the end of the FT cycle, and also induces inflammation of the tissue causing additional damage to the remaining endothelium [1, 58-61]. The loss of microcirculatory support by a combination of direct endothelial injury, thrombosis, free-radical formation and inflammation is considered critical in defining the extent of injury after FT [1, 62].
Immunological injury is another mechanism that in a few studies has been shown to induce secondary tumor damage after FT. Cryodestruction causes cell lysis, thereby releasing the proteins (including tumor antigens) present within them into the blood stream. These antigens may induce an immune reaction against the remaining tumor by stimulating immune cells to produce antibodies against tumor cells [63-66]. Some studies have shown that cryosurgery increases the level of serum cytokines and induce maturation of dendritic cells. The dendritic cells then stimulate T-cells against the antigen. Other studies have shown that tumor antigens are processed by B cells and stimulated with additional signal from a T-helper cell to produce antibodies against those antigens (Figure 2C) [36]. This mode of cell death is still not well documented and experimental evidence in support of it is sparse [36, 67]. Several animal studies failed to demonstrate a cryo-immunologic response [36, 46]. Infact few studies have documented immunosuppressive effects after cryosurgery [46]. Some of the unresolved questions are the nature and time course of the released antigen and the resultant humoral and cellmediated immune response that contributes to immune-enhancement or immunesuppression.
The desired outcome of cryosurgery is that the targeted cells will die from the FT injury as shown in Figure 2 schematics. Histological studies of the cryosurgical lesion days or weeks after a cryosurgical treatment demonstrate cell death by necrosis [1, 36, 62, 68]. However, in recent years, another important mode of cell death by FT that appears to be active in some cases is programmed cell death or apoptosis. Cell death by apoptosis has been shown to occur after FT in several in vitro studies particularly at temperature conditions present near the periphery of an iceball (i.e > −40°C) [38, 39]. The in vivo evidence of apoptosis is not well documented although some data suggests it can occur in some neural tissues and is also expected in the cells of inflammatory infiltrate (i.e. neutrophils) [69, 70]. It still needs to be determined to what extent apoptosis (and by extension necrosis) is a direct consequence of the FT process or a secondary result of biophysical events (IIF or dehydration) that affect molecular targets (lipid and proteins) that ultimately trigger programmed cell death post thaw.
Thus, there are multiple mechanisms by which a cell can be injured by FT, but all are linked by the amount of temperature excursion in the tissue during cryosurgery. Experimentally, enhanced injury is achieved with faster cooling rates, lower end temperature, longer hold time and slower thawing rates [71, 72]. Indeed, these are the thermal conditions that are recommended clinically to induce maximal damage within the tissue [72]. However due to the complexities in relating the mechanisms of cryoinjury to the temperature profile experienced locally in the tissue, the overall effectiveness of a cryosurgical procedure is usually judged with respect to the lowest end temperature achieved in a tissue [31]. The current clinical guideline is to achieve a temperature lower than −40°C everywhere within the tissue to be destroyed [1, 31]. Vascular and direct cell injury (esp. solution effects) and possibly immunological mechanisms are important predominantly at the periphery of the iceball but result in only partial damage to the tissue between −0.5°C and −40°C. Cell death has been shown to occur by both apoptosis and necrosis but more investigations are needed to relate the injury mechanisms to the mode of cell death. In principal, the goal of adjuvant-assisted cryosurgery is to augment one or more of these mechanisms to increase tissue destruction between −0.5°C to −40°C (Fig. 2).
ADJUVANTS WITH CRYOSURGERY
The desire to use adjuvants to control and improve cryosurgical outcomes is shown schematically in Fig. 3. In the early 1970s, there were attempts to use other cancer therapies such as radiation and hyperthermia in combination with cryosurgery, to increase the size of the lesion, though there is no reference of a mechanistic interplay with any of these modalities [73-81]. Later with an increased understanding of the mechanisms of cryoinjury several efforts were undertaken to use chemical adjuvants that accentuate tissue destruction by modulating the response to known mechanisms of cryoinjury. These adjuvants can be broadly categorized into four groups: (1) thermophysical adjuvants which enhance the injury caused by ice, (2) chemotherapeutic approaches that act at a molecular level to induce apoptosis, (3) cytokines or vascular based agents that modulate the inflammatory response, and (4) immunomodulators that stimulate the immune cells to enhance tissue destruction. Several of these adjuvants have shown potential to enhance cellular destruction between −40°C and −0.5°C but still many challenges remain to develop them for clinical use. Some of the common variables that are involved in the adjuvant selection and use are: a.) the sensitivity of the tissue to the adjuvant, b.) the selectivity of the adjuvant towards the tissue, c.) the dose of the adjuvant needed to enhance injury in the temperature range (up to −0.5°C), d.) any side effects associated with the adjuvant, e.) the mode of delivery of the adjuvant, and f.) the time of application of the adjuvant relative to cryotherapy.
Figure 3.
Improvements in a cryosurgical procedure by the use of adjuvants.
Below, and summarized in Table 3, we briefly discuss the different families of adjuvants and describe their potential benefits and shortcomings for clinical use
1) Thermophysical agents
Thermophysical adjuvants such as antifreeze proteins (AFPs), salts and some amino acids modify the crystalline ice (or solid) phase during freezing thereby causing additional direct cell injury due to the presence of ice crystals. AFPs at a very high concentration (>5 mg/ml) have been shown to modify ice crystals to a spicular shape (reducing dendrite tip diameter) such that mechanical disruption of the membranes and tissue connective structures is achieved [43, 44, 82, 83]. Pham et al., showed in an in vivo prostate cancer model that the presence of AFPs can cause injury at temperatures up to −5°C [44]. The main hindrance in the clinical use of AFPs is the high concentration (> 5 mg/ml) required to achieve the desired effect. Moreover these proteins are derived from species of fish and plants and have a somewhat limited availability.
Salt solutions such as NaCl, KCl and amino acids such as glycine can induce secondary ice formation (also called eutectic solidification), which can enhance cell injury between −21°C to −5°C [35, 84, 85]. This has been shown in an in vitro prostate cancer model but in vivo feasibility is yet to be determined. Thermophysical adjuvants have the advantage that their physical presence in the proper amount (for example, the eutectic concentration of a specific salt locally [84]) produces the desired effects with cryosurgery. Therefore they can be injected locally only a few minutes before cryotreatment. The major challenge in this mode of combination treatment is to develop means of targeted delivery in the required dosages without causing toxicity to the surrounding normal tissue.
2) Chemotherapeutics
The earlier efforts of using chemotherapeutics with cryosurgery originated in 1970's when adriamycin and peplomycin were used in combination with cryosurgery of cancer [86]. This treatment, also termed as cryochemotherapy, was based on the premise that cytotoxic drugs can be more efficiently trapped in the tumor after cryosurgery because of an increase in vascular volume and vascular permeability as a result of the treatment. The identification of apoptosis as a mode of cell death in the 1990s at thermal conditions present near the periphery of the iceball (−0.5 to −40°C), led to the revival of cryochemotherapy with the use of cytotoxic drugs to modulate and enhance cell death by apoptosis. Many in vitro studies have since shown the ability of chemotherapeutics such as 5-fluorouracil, cisplatin, bleomycin to augment cell death at milder freezing conditions [38, 40, 42, 87-89]. For example, Clarke et al showed enhanced cell destruction at temperatures between −15°C and −5°C in an in vitro prostate cancer model using 5-fluorouracil, cisplatin and etoposide [38]. They also observed that this was due in part to a shift in the ratio of pro-apoptotic to anti-apoptotic protein after a 2-day exposure of the drug before FT. Mir and Rubinsky demonstrated significant improvement in cell death by using bleomycin during FT [87]. Their hypothesis was that during FT, there is a transient increase in cell permeability that permits better uptake of the drug in the cells. Forest et al. investigated the application of navelbine at different time points both before and after cryosurgery in an in vivo lung cancer model [40, 42]. In their studies they observed a benefit when the drug was delivered 20 hours after FT due to enhanced apoptosis or 15 days after FT due to an increase in necrosis. Forest et al. suggest that the mechanism of enhanced cell death could be apoptotic or antiproliferative depending on the choice of the drug and the time interval between the two treatments.
None of the studies in cryo-chemo combination therapy have shown an augmentation up to the iceball edge. Some of the limitations in this mode of combination therapy are toxicity of the drug to normal cells limiting the amount of dose that can be injected intravenously. Secondly, the choice of the drug will vary depending on the type, stage and demonstrated drug resistance of the cancer. Le Pivert et al recently used microencapsulated 5-FU injections near the periphery of prostate tumors grown in nude mice after a single FT cycle and showed a longer inhibitory effect on tumor growth [90]. This strategy offers combined advantages of increased microvessel and tumor cell permeability after FT and a longer residence time of the drug. While promising, much of the work is in vitro and more in vivo evidence is needed to address the key issues of dose, timing and selection of the drug with cryosurgery. Also more careful studies are needed to understand the mechanisms of enhanced cell death such that a synergistic benefit can be achieved.
3) Vascular Agents
Vascular agents are proteins or drugs that act as an additional stressor to the microvasculature of the tissue, making it more susceptible to the vascular mode of cryoinjury. These molecules can potentiate a number of vascular effects including blood coagulation, vascoconstriction, inflammation or free radical formation [91-94]. Previous in vivo studies have shown that injury to the endothelium and inflammation are critical in determining the extent of the lesion. Studies by our laboratory sought to identify vascular-based agents to induce inflammation prior to cryosurgery and achieve tissue destruction up to the edge of the iceball (−0.5°C). Only one such molecule, TNF-α, has been used in combination with cryosurgery and has shown augmentation of cell death up to the edge of the iceball in an in vivo model. TNF-α is closely associated with multiple vascular events such as endothelial cell apoptosis, increase in procoagulant activity, decrease in anticoagulant activity, recruitment and adherence of inflammatory cells such as neutrophils, and production of other cytokines [95-97]. In a mouse model of human prostate cancer, it was shown that TNF-α increases the thermal threshold of tissue damage in a dose-dependent manner [37, 97-99]. This augmentation was observed when TNF-α was delivered both locally or systemically, about 3-4 hours before cryosurgery. Later, it was also shown that the this enhancement was a result of direct TNF-α action on the endothelium and more importantly on the induction of inflammatory pathways leading to an improved vascular response [99]. But like many other stand–alone cytotoxic drugs used in cancer treatment, the systemic delivery of required amounts of TNF-α is a major obstacle to achieve rapid clinical translation of this finding. In a recent study, targeted delivery of TNF-α was performed using gold nanoparticles coated with the drug that achieved a similar augmentation in tissue injury and greatly reduced systemic side effects [98].More studies are needed in translational models to optimize dosage and advance this cryoadjuvant approach into clinic.
4) Immunomodulators
Immunomodulators are agents that stimulate the cells of the immune system through the production of cytokines such as TNF-α and IFN-γ. These molecules have been used to aid in the maturation of immune cells at the time of local tumor destruction during cryosurgery [100]. Redondo and co-authors demonstrated in a mouse melanoma model that cryosurgery of the tumor along with topical application of imiquimod induced potent antitumor immune responses and also protected 60% of the animals against tumor re-challenges [101]. Similarly den Brock et al observed a synergy between cryosurgery and application of CpG-oligodeoxynucleotides, a dendritic cell stimulator, in another melanaoma mouse model [102].
However, the phenomenon or factors controlling the immune response after cryotherapy are complex and not well understood. Several important basic questions need to be addressed such as the availability and source of antigens in the cancerous tissue (Figure 2C), amount of antigens needed to generate an immune response, cytokine profile triggered by cryosurgery, and the subset of phagocytic cells involved in immune response. Hence, although this mode of cryosurgical augmentation is promising, there are few if any proven or controlled results [46, 65, 67, 103]. Thus, while currently more speculative, there is considerable potential in this mechanism, which warrants further basic research.
Finally, an effort has been made to label each adjuvant in a single family above. However, it is likely that some adjuvants will have multiple effects (i.e. TNF-α is both vascular and immunologically active). Thus, these grouping should be taken as organizational not absolute.
CHALLENGES
Overall, the concept of adjuvant-assisted cryosurgery has found considerable support and interest among researchers but several challenges remain and need to be addressed as described below
-
1)
Selection: choice of the adjuvant, which may depend on the mechanism and disease type.
-
2)
Timing: time interval between the adjuvant addition and cryotherapy to achieve maximum tissue destruction.
-
3)
Dose and delivery: dose of the adjuvant to achieve maximal tissue destruction between −40°C and −0.5°C and delivery of the adjuvant to the diseased site.
As described above and in Table 3, there already exist a number of molecules that have been tested or are under investigation as an adjuvant to cryosurgery. Thermophysical solutions are the most flexible of all and would be effective to augment any kind of cryosurgical treatment. Chemotherapeutics are usually cell specific and the resistance that develops against them in advanced stage cancers may limit their effectiveness. However, there already exists a large volume of literature on the use of these drugs as a stand-alone treatment for various cancers in clinic with known benefits and well-documented safety profiles. Information can be borrowed from these resources to design a clinical investigation and optimize the use of one such cancer specific drug in combination with cryosurgery for a particular treatment. Vascular agents and immunomodulators act on the endothelium and inflammatory cells and possibly can be used to modulate the cryosurgical response in multiple tumor types and even normal or benign tissue. Apart from these, there are other classes of related molecules such as vasoconstricters, clotting factors and oxidative stressors based on the observed vascular injury events that may enhance this mode of tissue destruction but have not been explored yet.
The time interval between adjuvant delivery and cryosurgery is another constraint that may affect the clinical use of this mode of combination therapy. Here too thermophysical adjuvants have a clear advantage, as only their physical presence in the tissue before cryosurgery is necessary to demonstrate their benefit. Vascular agents, immunomodulators and chemotherapeutics require time to interact with the cell and activate specific molecular pathways to provide an advantage over cryosurgery alone. Molecules that can be injected right before, during or after cryosurgery will be easier to integrate with the already established cryotreatment planning protocols. In all cases, application of adjuvant molecules will require considerable laboratory research and an understanding of the biological phenomenon controlling their response before clinical usage.
The ultimate goal of these adjuvants is to obtain complete tissue destruction to the edge of the iceball. This will make it possible for the surgeon to control the treatment of diseased tissue i.e., imaged iceball = kill zone (Figure 3). Thus, the dose of the adjuvant needs to be determined and delivered such that complete tissue destruction can be obtained as close to the iceball edge as possible. The most critical aspect faced by all adjuvants before reaching clinical applicability is the selective delivery of the molecule to enhance sensitivity of only the target tissue. The portion of the diseased tissue that will particularly benefit from the selective enhancement of cryosurgery is the tissue near the edge that experiences temperatures > −40°C (Figure 3). The extent of this region will be a function of the cryoprobe geometry, tip temperature, freeze duration and distance from the tip. Targeted adjuvant delivery to this region can be performed either locally by direct injections and/or polymeric implants, or systemically using nanosized carriers such as liposomes, metallic or polymeric nanoparticles [104-107]. If performing a localized delivery, the number of injections or implants and their precise locations will likely require incorporation within a the 3D computerized planning of the overall treatment. For thermophysical adjuvants, delivery is a big constraint as the adjuvant dosages that have been shown to be effective in vitro are relatively higher (mg per g of tissue) than other groups (μg per g of tissue) [42, 44, 98]. Both local and systemic delivery approaches are possible options. The successful application of nanoparticles was demonstrated for TNF-α assisted cryosurgery when gold nanoparticles coated with the drug were shown to produce tumor destruction up to the iceball edge without any observed toxicity [98]. There is a considerable literature available and ongoing research efforts on targeted delivery of drugs (including adjuvants) to the tumors [108-111]. As only the periphery of the tumor and not the entire volume needs to be targeted, the use of systemic approaches such as nanocarriers should be further explored in this mode of combination therapy.
SUMMARY
Cryosurgery has evolved significantly since the use of liquid nitrogen based systems in the 1960's. Initial research emphasis was on improving device design and cryogen use to maximize heat transfer (extraction) within a given volume of tissue and to form lesions of different sizes and shapes. The current state of the art cryosurgical devices have benefited greatly from these efforts and are used broadly for treatment of cancer, cardiovascular and other diseases [22, 112]. In recent years a new research focus has emerged in cryosurgery that emphasizes on the manipulation of the biologic response of tissues to cryodestruction [39, 83, 98, 100, 113]. The goal of this latest work involves the use of adjuvants to achieve cell destruction (kill zone) as close to the edge of the iceball as possible. The proven benefits of adjuvants include: enhanced destruction and imaging (iceball = kill zone) and the potential to reduce local recurrence and enhance wound healing. Work in this area has identified adjuvants that typically work through the following mechanisms: (1) thermophysical adjuvants which enhance the injury caused by ice, (2) chemotherapeutic approaches that act at a molecular level or to induce apoptosis, (3) cytokines or vascular based agents that modulate the inflammatory response, and (4) immunomodulators that stimulate immune cells to enhance tissue destruction. This biology-based approach has shown potential but significant work remains before clinical translation. Challenges include appropriate selection of the adjuvant, the time interval between FT and adjuvant delivery, and identification of the dose and means of delivery of the adjuvant. One recent vascular based adjuvant, TNF-α, has shown the ability to achieve a kill-zone up to the imaged iceball edge in preclinical models. Exploitation of this and/or other appropriate molecular adjuvants to cryosurgery can lead to tremendous clinical benefit by improvements in the application and outcome of cryosurgery for treatment of disease.
Acknowledgement
The authors are thankful to Mithun Shenoi, Biomedical Engineering at the University of Minnesota for help with the figures and Sue Clemmings, Mechanical Engineering at the University of Minnesota for her inputs on the manuscript.
REFERENCES
- 1.Hoffmann NE, Bischof JC. The cryobiology of cryosurgical injury. Urology. 2002;60(2 Suppl 1):40–9. doi: 10.1016/s0090-4295(02)01683-7. [DOI] [PubMed] [Google Scholar]
- 2.Kuflik EG. Re: Evidence-based review of the use of cryosurgery in treatment of basal cell carcinoma. Dermatol Surg. 2004;30(3):478. doi: 10.1111/j.1524-4725.2004.30126_1.x. [DOI] [PubMed] [Google Scholar]
- 3.Cooper IS. Cryobiology as Viewed by the Surgeon. Cryobiology. 1964;51:44–51. doi: 10.1016/0011-2240(64)90019-7. [DOI] [PubMed] [Google Scholar]
- 4.Cooper IS. Cryogenic Surgery for Cancer. Fed Proc. 1965;24:S237–40. [PubMed] [Google Scholar]
- 5.Cooper IS, Stellar S. Cryogenic Freezing of Brain Tumors for Excision or Destruction in Situ. J Neurosurg. 1963;20:921–30. doi: 10.3171/jns.1963.20.11.0921. [DOI] [PubMed] [Google Scholar]
- 6.Gonder MJ, Soanes WA, Smith V. Chemical and morphologic changes in the prostate following extreme cooling. Ann N Y Acad Sci. 1965;125(2):716–29. doi: 10.1111/j.1749-6632.1965.tb45423.x. [DOI] [PubMed] [Google Scholar]
- 7.Cahan WG. Cryosurgery for cancer. CA Cancer J Clin. 1972;22(6):338–43. doi: 10.3322/canjclin.22.6.338. [DOI] [PubMed] [Google Scholar]
- 8.Marcove RC, Miller TR. The treatment of primary and metastatic localized bone tumors by cryosurgery. Surg Clin North Am. 1969;49(2):421–30. doi: 10.1016/s0039-6109(16)38799-0. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves JC. Fractional cryosurgery. A new technique for basal cell carcinoma of the eyelids and periorbital area. Dermatol Surg. 1997;23(6):475–81. [PubMed] [Google Scholar]
- 10.Korpan NN. A history of cryosurgery: its development and future. J Am Coll Surg. 2007;204(2):314–24. doi: 10.1016/j.jamcollsurg.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Daniel BL, Butts K, Block WF. Magnetic resonance imaging of frozen tissues: temperature-dependent MR signal characteristics and relevance for MR monitoring of cryosurgery. Magn Reson Med. 1999;41(3):627–30. doi: 10.1002/(sici)1522-2594(199903)41:3<627::aid-mrm28>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.Pease GR, Wong ST, Roos MS, Rubinsky B. MR image-guided control of cryosurgery. J Magn Reson Imaging. 1995;5(6):753–60. doi: 10.1002/jmri.1880050623. [DOI] [PubMed] [Google Scholar]
- 13.Sandison GA, Loye MP, Rewcastle JC, et al. X-ray CT monitoring of iceball growth and thermal distribution during cryosurgery. Phys Med Biol. 1998;43(11):3309–24. doi: 10.1088/0031-9155/43/11/010. [DOI] [PubMed] [Google Scholar]
- 14.Saliken JC, Donnelly BJ, Rewcastle JC. The evolution and state of modern technology for prostate cryosurgery. Urology. 2002;60(2 Suppl 1):26–33. doi: 10.1016/s0090-4295(02)01681-3. [DOI] [PubMed] [Google Scholar]
- 15.Chang Z, Finkelstein JJ, Ma H, Baust J. Development of a highperformance multiprobe cryosurgical device. Biomed Instrum Technol. 1994;28(5):383–90. [PubMed] [Google Scholar]
- 16.Sarabanda AV, Bunch TJ, Johnson SB, et al. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J Am Coll Cardiol. 2005;46(10):1902–12. doi: 10.1016/j.jacc.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan SA, Greenberg R, Baust JG. A comparative assessment of cryosurgical devices: application to prostatic disease. Urology. 1995;45(4):692–9. doi: 10.1016/S0090-4295(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 18.Khairy P, Dubuc M. Transcatheter cryoablation part I: preclinical experience. Pacing Clin Electrophysiol. 2008;31(1):112–20. doi: 10.1111/j.1540-8159.2007.00934.x. [DOI] [PubMed] [Google Scholar]
- 19.Lemola K, Dubuc M, Khairy P. Transcatheter cryoablation part II: clinical utility. Pacing Clin Electrophysiol. 2008;31(2):235–44. doi: 10.1111/j.1540-8159.2007.00975.x. [DOI] [PubMed] [Google Scholar]
- 20.Skanes AC, Yee R, Krahn AD, Klein GJ. Cryoablation of atrial arrhythmias. Card Electrophysiol Rev. 2002;6(4):383–8. doi: 10.1023/a:1021128207078. [DOI] [PubMed] [Google Scholar]
- 21.Friedman PL. Catheter cryoablation of cardiac arrhythmias. Curr Opin Cardiol. 2005;20(1):48–54. [PubMed] [Google Scholar]
- 22.Gage AA, Baust JG. Cryosurgery for tumors. J Am Coll Surg. 2007;205(2):342–56. doi: 10.1016/j.jamcollsurg.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Onik G. Image-guided prostate cryosurgery: state of the art. Cancer Control. 2001;8(6):522–31. doi: 10.1177/107327480100800607. [DOI] [PubMed] [Google Scholar]
- 24.Mala T. Cryoablation of liver tumours -- a review of mechanisms, techniques and clinical outcome. Minim Invasive Ther Allied Technol. 2006;15(1):9–17. doi: 10.1080/13645700500468268. [DOI] [PubMed] [Google Scholar]
- 25.Mouraviev V, Joniau S, Van Poppel H, Polascik TJ. Current status of minimally invasive ablative techniques in the treatment of small renal tumours. Eur Urol. 2007;51(2):328–36. doi: 10.1016/j.eururo.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Onik G, Gilbert J, Hoddick W, et al. Sonographic monitoring of hepatic cryosurgery in an experimental animal model. AJR Am J Roentgenol. 1985;144(5):1043–7. doi: 10.2214/ajr.144.5.1043. [DOI] [PubMed] [Google Scholar]
- 27.Aktas MK, Daubert JP, Hall B. Surgical atrial fibrillation ablation: a review of contemporary techniques and energy sources. Cardiol J. 2008;15(1):87–94. [PubMed] [Google Scholar]
- 28.Comas GM, Imren Y, Williams MR. An overview of energy sources in clinical use for the ablation of atrial fibrillation. Semin Thorac Cardiovasc Surg. 2007;19(1):16–24. doi: 10.1053/j.semtcvs.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 29.Neumann T, Vogt J, Schumacher B, et al. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J Am Coll Cardiol. 2008;52(4):273–8. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd JP, Dawber RP. Wound healing and scarring after cryosurgery. Cryobiology. 1984;21(2):157–69. doi: 10.1016/0011-2240(84)90207-4. [DOI] [PubMed] [Google Scholar]
- 31.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37(3):171–86. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 32.Baissalov R, Sandison GA, Donnelly BJ, et al. A semi-empirical treatment planning model for optimization of multiprobe cryosurgery. Phys Med Biol. 2000;45(5):1085–98. doi: 10.1088/0031-9155/45/5/301. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Sandison GA, Murthy JY, Xu LX. Numerical simulation for heat transfer in prostate cancer cryosurgery. J Biomech Eng. 2005;127(2):279–94. doi: 10.1115/1.1865193. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka D, Shimada K, Rabin Y. Two-phase computerized planning of cryosurgery using bubble-packing and force-field analogy. J Biomech Eng. 2006;128(1):49–58. doi: 10.1115/1.2136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han B, Iftekhar A, Bischof JC. Improved cryosurgery by use of thermophysical and inflammatory adjuvants. Technol Cancer Res Treat. 2004;3(2):103–11. doi: 10.1177/153303460400300203. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann NE, Bischof JC. Mechanisms of Injury Caused by In Vivo Freezing. In: Benson E, Fuller B, Lane N, editors. Life in the Frozen State. Taylor & Francis; London: 2004. pp. 455–482. [Google Scholar]
- 37.Chao BH, He X, Bischof JC. Pre-treatment inflammation induced by TNF-alpha augments cryosurgical injury on human prostate cancer. Cryobiology. 2004;49(1):10–27. doi: 10.1016/j.cryobiol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology. 2001;42(4):274–85. doi: 10.1006/cryo.2001.2333. [DOI] [PubMed] [Google Scholar]
- 39.Clarke DM, Baust JM, Van Buskirk RG, Baust JG. Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology. 2004;49(1):45–61. doi: 10.1016/j.cryobiol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Forest V, Peoc'h M, Ardiet C, Campos L, Guyotat D, Vergnon JM. In vivo cryochemotherapy of a human lung cancer model. Cryobiology. 2005;51(1):92–101. doi: 10.1016/j.cryobiol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Forest V, Peoc'h M, Campos L, Guyotat D, Vergnon JM. Effects of cryotherapy or chemotherapy on apoptosis in a non-small-cell lung cancer xenografted into SCID mice. Cryobiology. 2005;50(1):29–37. doi: 10.1016/j.cryobiol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 42.Forest V, Peoc'h M, Campos L, Guyotat D, Vergnon JM. Benefit of a combined treatment of cryotherapy and chemotherapy on tumour growth and late cryo-induced angiogenesis in a non-small-cell lung cancer model. Lung Cancer. 2006;54(1):79–86. doi: 10.1016/j.lungcan.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 43.Koushafar H, Pham L, Lee C, Rubinsky B. Chemical adjuvant cryosurgery with antifreeze proteins. J Surg Oncol. 1997;66(2):114–21. doi: 10.1002/(sici)1096-9098(199710)66:2<114::aid-jso8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Pham L, Dahiya R, Rubinsky B. An in vivo study of antifreeze protein adjuvant cryosurgery. Cryobiology. 1999;38(2):169–75. doi: 10.1006/cryo.1999.2158. [DOI] [PubMed] [Google Scholar]
- 45.Baust JG, Gage AA. The molecular basis of cryosurgery. BJU Int. 2005;95(9):1187–91. doi: 10.1111/j.1464-410X.2005.05502.x. [DOI] [PubMed] [Google Scholar]
- 46.Sabel MS. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2008 doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 47.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168(934):939–49. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 48.Mazur P. Kinetics of Water Loss from Cells at Subzero Temperatures and the Likelihood of Intracellular Freezing. J Gen Physiol. 1963;47:347–69. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazur P. The role of cell membranes in the freezing of yeast and other single cells. Ann N Y Acad Sci. 1965;125(2):658–76. doi: 10.1111/j.1749-6632.1965.tb45420.x. [DOI] [PubMed] [Google Scholar]
- 50.Mazur P. Freezing of living cells: mechanisms and implications. Am J Physiol. 1984;247(3 Pt 1):C125–42. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 51.Mazur P. The role of intracellular freezing in the death of cells cooled at supraoptimal rates. Cryobiology. 1977;14(3):251–72. doi: 10.1016/0011-2240(77)90175-4. [DOI] [PubMed] [Google Scholar]
- 52.Toner M, Cravalho EG, Karel M. Thermodynamics and kinetics of intracellular ice formation during freezing of biological cells. Journal of Applied Physics. 1990;67:1582–1593. [Google Scholar]
- 53.Bischof JC. Quantitative measurement and prediction of biophysical response during freezing in tissues. Annu Rev Biomed Eng. 2000;2:257–88. doi: 10.1146/annurev.bioeng.2.1.257. [DOI] [PubMed] [Google Scholar]
- 54.He X, Bischof JC. Quantification of temperature and injury response in thermal therapy and cryosurgery. Crit Rev Biomed Eng. 2003;31(5-6):355–422. doi: 10.1615/critrevbiomedeng.v31.i56.10. [DOI] [PubMed] [Google Scholar]
- 55.Berrada MS, Bischof JC. Evaluation of freezing effects on human microvascular-endothelial cells (HMEC). Cryo Letters. 2001;22(6):353–66. [PubMed] [Google Scholar]
- 56.Marzella L, Jesudass RR, Manson PN, Myers RA, Bulkley GB. Morphologic characterization of acute injury to vascular endothelium of skin after frostbite. Plast Reconstr Surg. 1989;83(1):67–76. doi: 10.1097/00006534-198901000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Rabb JM, Renaud ML, Brandt PA, Witt CW. Effect of freezing and thawing on the microcirculation and capillary endothelium of the hamster cheek pouch. Cryobiology. 1974;11(6):508–18. doi: 10.1016/0011-2240(74)90120-5. [DOI] [PubMed] [Google Scholar]
- 58.Barker JH, Bartlett R, Funk W, Hammersen F, Messmer K. The effect of superoxide dismutase on the skin microcirculation after ischemia and reperfusion. Prog. Appl. Microcirculation. 1987;12:276–281. [Google Scholar]
- 59.Bowers WD, Jr., Hubbard RW, Daum RC, Ashbaugh P, Nilson E. Ultrastructural studies of muscle cells and vascular endothelium immediately after freeze-thaw injury. Cryobiology. 1973;10(1):9–21. doi: 10.1016/0011-2240(73)90003-5. [DOI] [PubMed] [Google Scholar]
- 60.Bulkley GB. Free radical-mediated reperfusion injury: a selective review. Br J Cancer Suppl. 1987;8:66–73. [PMC free article] [PubMed] [Google Scholar]
- 61.Manson PN, Jesudass R, Marzella L, Bulkley GB, Im MJ, Narayan KK. Evidence for an early free radical-mediated reperfusion injury in frostbite. Free Radic Biol Med. 1991;10(1):7–11. doi: 10.1016/0891-5849(91)90015-u. [DOI] [PubMed] [Google Scholar]
- 62.Hoffmann NE, Bischof JC. Cryosurgery of normal and tumor tissue in the dorsal skin flap chamber: Part II--injury response. J Biomech Eng. 2001;123(4):310–6. doi: 10.1115/1.1385839. [DOI] [PubMed] [Google Scholar]
- 63.Ablin RJ. Cryoimmunotherapy. Br Med J. 1972;3(5824):476. doi: 10.1136/bmj.3.5824.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ablin RJ. Cryosensitivity. Factors influencing development of immunologic response following cyrosurgery of prostate. Urology. 1975;05(3):317–21. doi: 10.1016/0090-4295(75)90145-4. [DOI] [PubMed] [Google Scholar]
- 65.Ablin RJ, Fontana G. Cryoimmunotherapy: continuing studies toward determining a rational approach for assessing the candidacy of the prostatic cancer patient for cryoimmunotherapy and postoperative responsiveness. An interim report. Cryobiology. 1980;17(2):170–7. doi: 10.1016/0011-2240(80)90022-x. [DOI] [PubMed] [Google Scholar]
- 66.Soanes WA, Gonder MJ, Ablin RJ. A possible immuno-cryothermic response in prostatic cancer. Clin Radiol. 1970;21(3):253–5. doi: 10.1016/s0009-9260(70)80036-8. [DOI] [PubMed] [Google Scholar]
- 67.Hoffmann NE, Coad JE, Huot CS, Swanlund DJ, Bischof JC. Investigation of the mechanism and the effect of cryoimmunology in the Copenhagen rat. Cryobiology. 2001;42(1):59–68. doi: 10.1006/cryo.2001.2305. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann NE, Bischof JC. Cryosurgery of normal and tumor tissue in the dorsal skin flap chamber: Part I--thermal response. J Biomech Eng. 2001;123(4):301–9. doi: 10.1115/1.1385838. [DOI] [PubMed] [Google Scholar]
- 69.Bischof JC, Coad JE, Hoffmann NE, Roberts KR. Is apoptosis an important mechanism of cryoinjury in vivo? Cryo Letters. 2002;23(4):277–8. author reply 279-80. [PubMed] [Google Scholar]
- 70.Steinbach JP, Weissenberger J, Aguzzi A. Distinct phases of cryogenic tissue damage in the cerebral cortex of wild-type and c-fos deficient mice. Neuropathol Appl Neurobiol. 1999;25(6):468–80. doi: 10.1046/j.1365-2990.1999.00206.x. [DOI] [PubMed] [Google Scholar]
- 71.Bischof J, Fahssi W, Smith D, Nagel T, Swanlund D. A parametric study of freezing injury in ELT-3 uterine leiomyoma tumour cells. Hum Reprod. 2001;16(2):340–8. doi: 10.1093/humrep/16.2.340. [DOI] [PubMed] [Google Scholar]
- 72.Smith DJ, Fahssi WM, Swanlund DJ, Bischof JC. A parametric study of freezing injury in AT-1 rat prostate tumor cells. Cryobiology. 1999;39(1):13–28. doi: 10.1006/cryo.1999.2189. [DOI] [PubMed] [Google Scholar]
- 73.Herzeel R, Demols E, Brihaye-Van Geertruyden M. [Combination argon laser and cryotherapy]. Bull Soc Belge Ophtalmol. 1978;182:82–9. [PubMed] [Google Scholar]
- 74.Miehlke A, Chilla R, Vollrath M. Cryosurgery and laser surgery in the treatment of malignant and benign laryngeal processes. ORL J Otorhinolaryngol Relat Spec. 1979;41(5):273–87. doi: 10.1159/000275446. [DOI] [PubMed] [Google Scholar]
- 75.Vercellino V, Goia F, Gandolfo S, Camoletto D. [Cryotherapy, combined drug therapy and radiotherapy in the treatment of oral cavity cancer: 3 years' experience]. Minerva Stomatol. 1980;29(5):335–40. [PubMed] [Google Scholar]
- 76.Matsuura S, Satake B, Makino S, et al. [Evaluation of multi-disciplinary treatment combined with intra-arterial chemotherapy for carcinoma of the mesopharynx]. Gan To Kagaku Ryoho. 1984;11(6):1189–96. [PubMed] [Google Scholar]
- 77.Mobley WC, Loening SA, Narayana AS. Combination perineal cryosurgery and external radiation therapy for adenocarcinoma of prostate. Urology. 1984;24(1):11–4. doi: 10.1016/0090-4295(84)90377-7. [DOI] [PubMed] [Google Scholar]
- 78.Zografos L, Uffer S, Bercher L, Gailloud C. [Combined surgery, cryocoagulation and radiotherapy for treatment of melanoma of the conjunctiva]. Klin Monatsbl Augenheilkd. 1994;204(5):385–90. doi: 10.1055/s-2008-1035564. [DOI] [PubMed] [Google Scholar]
- 79.Goncalves JC, Martins C. Debulking of skin cancers with radio frequency before cryosurgery. Dermatol Surg. 1997;23(4):253–6. doi: 10.1111/j.1524-4725.1997.tb00038.x. discussion 256-7. [DOI] [PubMed] [Google Scholar]
- 80.Kuz'menko AP, Todor IN, Mosienko VS. [The effect of the combined use of cryosurgery and hyperthermia on an experimental tumor process]. Eksp Onkol. 1990;12(2):60–1. [PubMed] [Google Scholar]
- 81.Liu, Zhou, Yu, Gui, Deng, Lv Minimally invasive probe system capable of performing both cryosurgery and hyperthermia treatment on target tumor in deep tissues. Minim Invasive Ther Allied Technol. 2004;13(1):47–57. doi: 10.1080/13645700310022691. [DOI] [PubMed] [Google Scholar]
- 82.Koushafar H, Rubinsky B. Effect of antifreeze proteins on frozen primary prostatic adenocarcinoma cells. Urology. 1997;49(3):421–5. doi: 10.1016/S0090-4295(96)00572-9. [DOI] [PubMed] [Google Scholar]
- 83.Muldrew K, Rewcastle J, Donnelly BJ, et al. Flounder antifreeze peptides increase the efficacy of cryosurgery. Cryobiology. 2001;42(3):182–9. doi: 10.1006/cryo.2001.2321. [DOI] [PubMed] [Google Scholar]
- 84.Han B, Bischof JC. Direct cell injury associated with eutectic crystallization during freezing. Cryobiology. 2004;48(1):8–21. doi: 10.1016/j.cryobiol.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 85.Wang CL, Teo KY, Han B. An amino acidic adjuvant to augment cryoinjury of MCF-7 breast cancer cells. Cryobiology. 2008;57(1):52–9. doi: 10.1016/j.cryobiol.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 86.Ikekawa S, Ishihara K, Tanaka S, Ikeda S. Basic studies of cryochemotherapy in a murine tumor system. Cryobiology. 1985;22(5):477–83. doi: 10.1016/0011-2240(85)90159-2. [DOI] [PubMed] [Google Scholar]
- 87.Mir LM, Rubinsky B. Treatment of cancer with cryochemotherapy. Br J Cancer. 2002;86(10):1658–60. doi: 10.1038/sj.bjc.6600306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan F, Zhou W, Zhang J, et al. Anticancer drugs are synergistic with freezing in induction of apoptosis in HCC cells. Cryobiology. 2008;57(1):60–5. doi: 10.1016/j.cryobiol.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 89.Clarke DM, Robilotto AT, VanBuskirk RG, Baust JG, Gage AA, Baust JM. Targeted induction of apoptosis via TRAIL and cryoablation: a novel strategy for the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(2):175–84. doi: 10.1038/sj.pcan.4500920. [DOI] [PubMed] [Google Scholar]
- 90.Le Pivert P, Haddad RS, Aller A, et al. Ultrasound guided combined cryoablation and microencapsulated 5-Fluorouracil inhibits growth of human prostate tumors in xenogenic mouse model assessed by luminescence imaging. Technol Cancer Res Treat. 2004;3(2):135–42. doi: 10.1177/153303460400300206. [DOI] [PubMed] [Google Scholar]
- 91.Bettinger DA, Pellicane JV, Tarry WC, et al. The role of inflammatory cytokines in wound healing: accelerated healing in endotoxin-resistant mice. J Trauma. 1994;36(6):810–3. doi: 10.1097/00005373-199406000-00010. discussion 813-4. [DOI] [PubMed] [Google Scholar]
- 92.Feiken E, Romer J, Eriksen J, Lund LR. Neutrophils express tumor necrosis factor-alpha during mouse skin wound healing. J Invest Dermatol. 1995;105(1):120–3. doi: 10.1111/1523-1747.ep12313429. [DOI] [PubMed] [Google Scholar]
- 93.Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13(4-5):413–21. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 94.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 95.Geeslin MG, Swanlund DJ, Bischof JC. A parametric study of freezing injury in BPH1CAFTD-2 human prostate tumor cells. Cryo Letters. 2007;28(3):173–86. [PubMed] [Google Scholar]
- 96.Eggermont AM, Schraffordt Koops H, Klausner JM, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for limb salvage in 186 patients with locally advanced soft tissue extremity sarcomas. The cumulative multicenter European experience. Ann Surg. 1996;224(6):756–64. doi: 10.1097/00000658-199612000-00011. discussion 764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4(9):565–73. doi: 10.1016/s1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 98.Goel R, Swanlund D, Coad J, Paciotti GF, Bischof JC. TNF-alpha-based accentuation in cryoinjury--dose, delivery, and response. Mol Cancer Ther. 2007;6(7):2039–47. doi: 10.1158/1535-7163.MCT-06-0676. [DOI] [PubMed] [Google Scholar]
- 99.Jiang J, Goel R, Iftekhar MA, et al. Tumor necrosis factor-alpha-induced accentuation in cryoinjury: mechanisms in vitro and in vivo. Mol Cancer Ther. 2008;7(8):2547–55. doi: 10.1158/1535-7163.MCT-07-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Udagawa M, Kudo-Saito C, Hasegawa G, et al. Enhancement of immunologic tumor regression by intratumoral administration of dendritic cells in combination with cryoablative tumor pretreatment and Bacillus Calmette-Guerin cell wall skeleton stimulation. Clin Cancer Res. 2006;12(24):7465–75. doi: 10.1158/1078-0432.CCR-06-1840. [DOI] [PubMed] [Google Scholar]
- 101.Redondo P, del Olmo J, Lopez-Diaz de Cerio A, et al. Imiquimod enhances the systemic immunity attained by local cryosurgery destruction of melanoma lesions. J Invest Dermatol. 2007;127(7):1673–80. doi: 10.1038/sj.jid.5700777. [DOI] [PubMed] [Google Scholar]
- 102.den Brok MH, Sutmuller RP, Nierkens S, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66(14):7285–92. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 103.Ablin RJ, Sadoughi N, Guinan P, Bruns GR, Bush IM. Biphasic effect of cryosurgery of the prostate on lymphocyte proliferation. Cryobiology. 1977;14(1):60–7. doi: 10.1016/0011-2240(77)90123-7. [DOI] [PubMed] [Google Scholar]
- 104.Paciotti GF, Myer L, Weinreich D, et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004;11(3):169–83. doi: 10.1080/10717540490433895. [DOI] [PubMed] [Google Scholar]
- 105.Kircheis R, Ostermann E, Wolschek MF, et al. Tumor-targeted gene delivery of tumor necrosis factor-alpha induces tumor necrosis and tumor regression without systemic toxicity. Cancer Gene Ther. 2002;9(8):673–80. doi: 10.1038/sj.cgt.7700487. [DOI] [PubMed] [Google Scholar]
- 106.Kedar E, Palgi O, Golod G, Babai I, Barenholz Y. Delivery of cytokines by liposomes. III. Liposome-encapsulated GM-CSF and TNF-alpha show improved pharmacokinetics and biological activity and reduced toxicity in mice. J Immunother. 1997;20(3):180–93. doi: 10.1097/00002371-199705000-00003. 1997. [DOI] [PubMed] [Google Scholar]
- 107.Kim DW, Andres ML, Li J, et al. Liposome-encapsulated tumor necrosis factor-alpha enhances the effects of radiation against human colon tumor xenografts. J Interferon Cytokine Res. 2001;21(11):885–97. doi: 10.1089/107999001753289497. [DOI] [PubMed] [Google Scholar]
- 108.Alexis F, Rhee JW, Richie JP, Radovic-Moreno AF, Langer R, Farokhzad OC. New frontiers in nanotechnology for cancer treatment. Urol Oncol. 2008;26(1):74–85. doi: 10.1016/j.urolonc.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 109.Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10. [PubMed] [Google Scholar]
- 110.Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer Cell. 2003;4(5):337–41. doi: 10.1016/s1535-6108(03)00276-9. [DOI] [PubMed] [Google Scholar]
- 111.Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428(6982):487–92. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- 112.Gage AA. Cryosurgery in the treatment of cancer. Surg Gynecol Obstet. 1992;174(1):73–92. [PubMed] [Google Scholar]
- 113.Bassukas ID, Gamvroulia C, Zioga A, Nomikos K, Fotika C. Cryosurgery during topical imiquimod: a successful combination modality for lentigo maligna. Int J Dermatol. 2008;47(5):519–21. doi: 10.1111/j.1365-4632.2008.03562.x. [DOI] [PubMed] [Google Scholar]
- 114.Ceilley RI, Del Rosso JQ. Current modalities and new advances in the treatment of basal cell carcinoma. Int J Dermatol. 2006;45(5):489–98. doi: 10.1111/j.1365-4632.2006.02673.x. [DOI] [PubMed] [Google Scholar]
- 115.Gazzaniga S, Bravo A, Goldszmid SR, et al. Inflammatory changes after cryosurgery-induced necrosis in human melanoma xenografted in nude mice. J Invest Dermatol. 2001;116(5):664–71. doi: 10.1046/j.0022-202x.2001.01313.x. [DOI] [PubMed] [Google Scholar]
- 116.Graham GF. Cryosurgery in the management of cutaneous malignancies. Clin Dermatol. 2001;19(3):321–7. doi: 10.1016/s0738-081x(01)00171-7. [DOI] [PubMed] [Google Scholar]
- 117.Jorizzo J, Weiss J, Furst K, VandePol C, Levy SF. Effect of a 1-week treatment with 0.5% topical fluorouracil on occurrence of actinic keratosis after cryosurgery: a randomized, vehicle-controlled clinical trial. Arch Dermatol. 2004;140(7):813–6. doi: 10.1001/archderm.140.7.813. [DOI] [PubMed] [Google Scholar]
- 118.Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. Br J Dermatol. 2008;159(1):35–48. doi: 10.1111/j.1365-2133.2008.08666.x. [DOI] [PubMed] [Google Scholar]
- 119.Aus G. Current status of HIFU and cryotherapy in prostate cancer--a review. Eur Urol. 2006;50(5):927–34. doi: 10.1016/j.eururo.2006.07.011. discussion 934. [DOI] [PubMed] [Google Scholar]
- 120.Aus G. Cryosurgery for prostate cancer. J Urol. 2008;180(5):1882–3. doi: 10.1016/j.juro.2008.08.085. [DOI] [PubMed] [Google Scholar]
- 121.Han KR, Cohen JK, Miller RJ, et al. Treatment of organ confined prostate cancer with third generation cryosurgery: preliminary multicenter experience. J Urol. 2003;170(4 Pt 1):1126–30. doi: 10.1097/01.ju.0000087860.52991.a8. [DOI] [PubMed] [Google Scholar]
- 122.Long JP, Bahn D, Lee F, Shinohara K, Chinn DO, Macaluso JN., Jr Five-year retrospective, multi-institutional pooled analysis of cancer-related outcomes after cryosurgical ablation of the prostate. Urology. 2001;57(3):518–523. doi: 10.1016/s0090-4295(00)01060-8. [DOI] [PubMed] [Google Scholar]
- 123.Seifert JK, Morris DL. World survey on the complications of hepatic and prostate cryotherapy. World J Surg. 1999;23(2):109–13. doi: 10.1007/pl00013173. discussion 113-4. [DOI] [PubMed] [Google Scholar]
- 124.Kaufman CS, Bachman B, Littrup PJ, et al. Cryoablation treatment of benign breast lesions with 12-month follow-up. Am J Surg. 2004;188(4):340–8. doi: 10.1016/j.amjsurg.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 125.Kaufman CS, Littrup PJ, Freeman-Gibb LA, et al. Office-based cryoablation of breast fibroadenomas with long-term follow-up. Breast J. 2005;11(5):344–50. doi: 10.1111/j.1075-122X.2005.21700.x. [DOI] [PubMed] [Google Scholar]
- 126.Littrup PJ, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology. 2005;234(1):63–72. doi: 10.1148/radiol.2341030931. [DOI] [PubMed] [Google Scholar]
- 127.Nurko J, Mabry CD, Whitworth P, et al. Interim results from the FibroAdenoma Cryoablation Treatment Registry. Am J Surg. 2005;190(4):647–51. doi: 10.1016/j.amjsurg.2005.06.033. discussion 651-2. [DOI] [PubMed] [Google Scholar]
- 128.Biasco G, Derenzini E, Grazi G, et al. Treatment of hepatic metastases from colorectal cancer: many doubts, some certainties. Cancer Treat Rev. 2006;32(3):214–28. doi: 10.1016/j.ctrv.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 129.Derenzini E, Di Battista M, Di Marco MC, et al. [Treatment of colorectal cancer liver metastases]. Minerva Med. 2006;97(1):107–19. [PubMed] [Google Scholar]
- 130.Joosten J, Jager G, Oyen W, Wobbes T, Ruers T. Cryosurgery and radiofrequency ablation for unresectable colorectal liver metastases. Eur J Surg Oncol. 2005;31(10):1152–9. doi: 10.1016/j.ejso.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 131.Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237(2):171–9. doi: 10.1097/01.SLA.0000048443.71734.BF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Onik G, Rubinsky B, Zemel R, et al. Ultrasound-guided hepatic cryosurgery in the treatment of metastatic colon carcinoma. Preliminary results. Cancer. 1991;67(4):901–7. doi: 10.1002/1097-0142(19910215)67:4<901::aid-cncr2820670408>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 133.Seifert JK, Springer A, Baier P, Junginger T. Liver resection or cryotherapy for colorectal liver metastases: a prospective case control study. Int J Colorectal Dis. 2005;20(6):507–20. doi: 10.1007/s00384-004-0723-0. [DOI] [PubMed] [Google Scholar]
- 134.Deane LA, Clayman RV. Review of minimally invasive renal therapies: Needle-based and extracorporeal. Urology. 2006;68(1 Suppl):26–37. doi: 10.1016/j.urology.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 135.Gill IS, Novick AC. Renal cryosurgery. Urology. 1999;54(2):215–9. doi: 10.1016/s0090-4295(99)00160-0. [DOI] [PubMed] [Google Scholar]
- 136.Gill IS, Novick AC, Soble JJ, et al. Laparoscopic renal cryoablation: initial clinical series. Urology. 1998;52(4):543–51. doi: 10.1016/s0090-4295(98)00309-4. [DOI] [PubMed] [Google Scholar]
- 137.Gill IS, Remer EM, Hasan WA, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005;173(6):1903–7. doi: 10.1097/01.ju.0000158154.28845.c9. [DOI] [PubMed] [Google Scholar]
- 138.Rehman J, Landman J, Lee D, et al. Needle-based ablation of renal parenchyma using microwave, cryoablation, impedance-and temperature-based monopolar and bipolar radiofrequency, and liquid and gel chemoablation: laboratory studies and review of the literature. J Endourol. 2004;18(1):83–104. doi: 10.1089/089277904322836749. [DOI] [PubMed] [Google Scholar]
- 139.Weight CJ, Kaouk JH, Hegarty NJ, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol. 2008;179(4):1277–81. doi: 10.1016/j.juro.2007.11.075. discussion 1281-3. [DOI] [PubMed] [Google Scholar]
- 140.Homasson JP. [Cryotherapy in pneumology. Review of basic and clinical data]. Rev Pneumol Clin. 1990;46(5):189–93. [PubMed] [Google Scholar]
- 141.Maiwand MO, Asimakopoulos G. Cryosurgery for lung cancer: clinical results and technical aspects. Technol Cancer Res Treat. 2004;3(2):143–50. doi: 10.1177/153303460400300207. [DOI] [PubMed] [Google Scholar]
- 142.Maiwand O, Glynne-Jones R, Chambers J, Asimakopoulos G. Direct cryosurgery for inoperable metastatic disease of the lung. Ann Thorac Surg. 2006;81(2):718–21. doi: 10.1016/j.athoracsur.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 143.Maiwand O, Makey AR. Cryoanalgesia for relief of pain after thoracotomy. Br Med J (Clin Res Ed) 1981;282(6278):1749–50. doi: 10.1136/bmj.282.6278.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Berman B, Viera MH, Amini S, Huo R, Jones IS. Prevention and management of hypertrophic scars and keloids after burns in children. J Craniofac Surg. 2008;19(4):989–1006. doi: 10.1097/SCS.0b013e318175f3a7. [DOI] [PubMed] [Google Scholar]
- 145.Har-Shai Y, Amar M, Sabo E. Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Reconstr Surg. 2003;111(6):1841–52. doi: 10.1097/01.PRS.0000056868.42679.05. [DOI] [PubMed] [Google Scholar]
- 146.Har-Shai Y, Brown W, Labbe D, et al. Intralesional cryosurgery for the treatment of hypertrophic scars and keloids following aesthetic surgery: the results of a prospective observational study. Int J Low Extrem Wounds. 2008;7(3):169–75. doi: 10.1177/1534734608322813. [DOI] [PubMed] [Google Scholar]
- 147.Rutherford TJ. Cryosurgery Is a Simple Modality for Endometrial Ablation. J Am Assoc Gynecol Laparosc. 1996;3(4, Supplement):S44–5. doi: 10.1016/s1074-3804(96)80287-8. [DOI] [PubMed] [Google Scholar]
- 148.Rutherford TJ, Zreik TG, Troiano RN, Palter SF, Olive DL. Endometrial cryoablation, a minimally invasive procedure for abnormal uterine bleeding. J Am Assoc Gynecol Laparosc. 1998;5(1):23–8. doi: 10.1016/s1074-3804(98)80006-6. [DOI] [PubMed] [Google Scholar]
- 149.Townsend DE, Duleba AJ, Wilkes MM. Durability of treatment effects after endometrial cryoablation versus rollerball electroablation for abnormal uterine bleeding: two-year results of a multicenter randomized trial. Am J Obstet Gynecol. 2003;188(3):699–701. doi: 10.1067/mob.2003.178. [DOI] [PubMed] [Google Scholar]
- 150.Ciavattini A, Tsiroglou D, Litta P, Vichi M, Tranquilli AL. Pregnancy outcome after laparoscopic cryomyolysis of uterine myomas: report of nine cases. J Minim Invasive Gynecol. 2006;13(2):141–4. doi: 10.1016/j.jmig.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 151.Sakuhara Y, Shimizu T, Kodama Y, et al. Magnetic resonance-guided percutaneous cryoablation of uterine fibroids: early clinical experiences. Cardiovasc Intervent Radiol. 2006;29(4):552–8. doi: 10.1007/s00270-004-6163-y. [DOI] [PubMed] [Google Scholar]
- 152.Sharp HT. Assessment of new technology in the treatment of idiopathic menorrhagia and uterine leiomyomata. Obstet Gynecol. 2006;108(4):990–1003. doi: 10.1097/01.AOG.0000232618.26261.75. [DOI] [PubMed] [Google Scholar]
- 153.Zreik TG, Rutherford TJ, Palter SF, et al. Cryomyolysis, a new procedure for the conservative treatment of uterine fibroids. J Am Assoc Gynecol Laparosc. 1998;5(1):33–8. doi: 10.1016/s1074-3804(98)80008-x. [DOI] [PubMed] [Google Scholar]
- 154.Avitall B, Lafontaine D, Rozmus G, et al. Ablation of atrial-ventricular junction tissues via the coronary sinus using cryo balloon technology. J Interv Card Electrophysiol. 2005;12(3):203–11. doi: 10.1007/s10840-005-0339-5. [DOI] [PubMed] [Google Scholar]
- 155.Khargi K, Hutten BA, Lemke B, Deneke T. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27(2):258–65. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 156.Moten SC, Rodriguez E, Cook RC, Nifong LW, Chitwood WR., Jr. New ablation techniques for atrial fibrillation and the minimally invasive cryomaze procedure in patients with lone atrial fibrillation. Heart Lung Circ. 2007;16(Suppl 3):S88–93. doi: 10.1016/j.hlc.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 157.Reddy VY, Neuzil P, d'Avila A, et al. Balloon catheter ablation to treat paroxysmal atrial fibrillation: what is the level of pulmonary venous isolation? Heart Rhythm. 2008;5(3):353–60. doi: 10.1016/j.hrthm.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 158.Karthik S, Kessel DO. Cryoplasty--where is the evidence? Acta Chir Belg. 2007;107(5):500–3. doi: 10.1080/00015458.2007.11680110. [DOI] [PubMed] [Google Scholar]
- 159.Kessel DO, Samson RH. What is the evidence for the efficacy of cryoplasty? J Cardiovasc Surg (Torino) 2008;49(2):179–85. [PubMed] [Google Scholar]
- 160.Rigatelli G, Cardaioli P, Giordan M. Endovascular treatment of femoropopliteal obstructive disease. Minerva Cardioangiol. 2007;55(1):125–32. [PubMed] [Google Scholar]