Abstract
Background
Diabetes mellitus and impaired glucose metabolism are associated with increased risk for cardiovascular disease (CVD). However, it is still not clear whether glucose levels can predict CVD risk among patients without diabetes. The primary aim of this study is to assess whether normoglycemic fasting plasma glucose (FPG) is associated with increased risk of CVD outcomes in healthy patients.
Methods
We obtained blood measurements, data from physical examination, and medical and lifestyle information from 10,913 men and women who were evaluated in the Institute for Preventive Medicine of Sheba Medical Center. Enrolled were participants with FPG <100 mg/dL as well as 100 to 125 mg/dL, who were free of diagnosis of CVD. The participants were actively screened for coronary disease using a stress test. Primary end points were coronary heart disease or self-reported cerebral vascular disease.
Results
A total of 1,119 incident cases of CVD occurred during a mean follow-up of 4.3 years. Subjects with fasting glucose levels in the high normal range (95–99 mg/dL) had an increased CVD risk when compared with levels <80 mg/dL, (HR 1.53;CI 95% [1.22–1.91], P < .001). A multivariate model, adjusted for age, sex, family history of CVD, blood pressure, body mass index, smoking status, pharmacologic treatment, serum triglycerides, and high-density lipoprotein and low-density lipoprotein cholesterol levels, revealed an independent increased risk of CVD with rising FPG levels in the normal range.
Conclusion
Elevated CVD risk is strongly and independently associated with glucose levels within the normoglycemic range. Fasting plasma glucose may help in identifying apparently healthy persons with early metabolic abnormalities who are at increased risk for CVD before progression to prediabetes and overt diabetes mellitus.
Since the introduction of the concept of impaired fasting glucose (IFG), there has been considerable debate regarding where the lower limit should be set to achieve a reasonable balance between sensitivity and specificity for diabetes mellitus (DM) prediction. In 2003, the American Diabetes Association lowered its threshold for diagnosis of IFG from 110 to 100 mg/dL on the basis of evidence that DM prediction may be optimized at a lower threshold.1 However, we have previously shown that different populations may require distinct glycemic threshold when DM risk is assessed. In a young adult population, glucose levels already within the normal range independently predicted incident DM during a 6-year follow-up study.2 Although there is a wealth of epidemiological data on the correlation between fasting plasma glucose (FPG) and DM risk, its association with cardiovascular disease (CVD) risk is less clear.
Diabetes mellitus and impaired glucose metabolism are associated with increased CVD mortality.3–6 However, most studies have failed to detect evidence of FPG threshold that identifies subgroups at increased CVD risk.7,8 Cardiovascular disease mortality among individuals with IFG was found to be similar to that of patients with overt DM.9 Similar to observations with other risk factors such as elevated blood pressure and cholesterol, CVD morbidity and mortality were suggested to progress as a continuum across blood glucose concentration, from levels well below the conventional cutoff values used for the diagnosis of DM.10–12
Taken together, it is still not clear whether increased FPG within the normoglycemic range posses a significantly elevated cardiovascular risk.2,13 Therefore, the aim of this study is to characterize the role of FPG within the entire nondiabetic range (normoglycemia and IFG) in predicting CVD outcomes in nondiabetic patients. Fasting plasma glucose levels from 10,913 healthy adults (mean age 50.4 years, range 34–91 years) from the CArdiovascular Risk and Metabolic Assessment (CARMA) study were obtained at baseline, and CVD outcomes were followed up during a mean follow-up of 4.3 years.
Methods
The CARMA study
The CARMA study was designed to investigate cardiometabolic risk factors in healthy male and female adults of white ethnicity. The study has been conducted at the Executive Screening Survey (ESS) at the Sheba Medical Center in Israel. The ESS performs approximately 9,000 screening examinations of adult males and females annually. This population is composed mainly of senior executives referred for periodic health screening by their organizations and so represents a higher-than-average socioeconomic class. When compared with data available for the general Israeli population, our study population had lower rates of obesity (body mass index [BMI] ≥30 kg/m2) and active smoking. A computerized database established in 2000 is the source of data for the study. At each annual visit, participants completed a detailed questionnaire assessing demographic, nutritional, lifestyle, and medical factors. Thereafter, blood samples were drawn after a 12-hour fast and were analyzed immediately. A physician at the center performed a complete physical examination. Blood pressure measurements were performed by medical professionals with the use of mercury sphygmomanometers. All medical information was recorded in a central database, thus allowing an ongoing follow-up. The Institutional Review Board of Sheba Medical Center approved this study on the basis of strict maintenance of participants' anonymity during database analyses. Data from subjects were recorded anonymously. No individual consent was obtained.
Inclusion and exclusion criteria
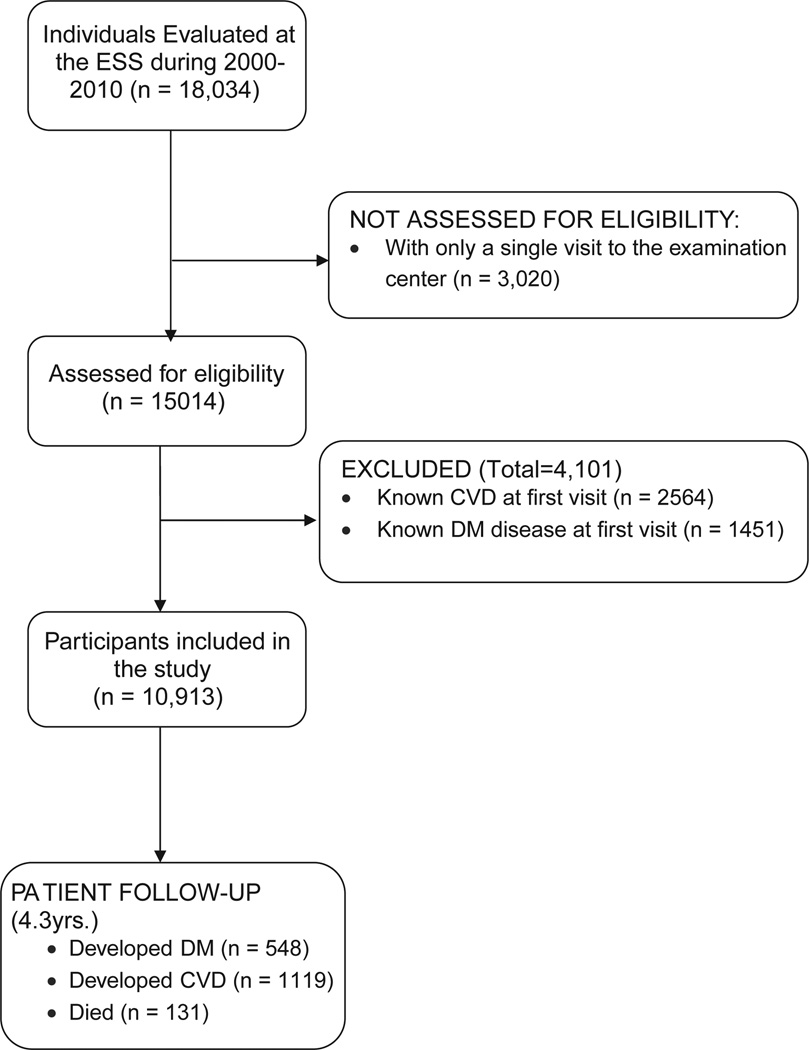
Men and women older than 34 years with FPG levels <126 mg/dL at their first annual visit at the ESS have been included. In addition, follow-up data must have been available from at least 1 subsequent visit, >2 years after the first visit (average no. of visits per person, 5; range, 2–9). We selected patients without confirmed type 1 or type 2 DM at the time of enrollment and without a current or past diagnosis of CVD (ischemic heart disease, acute coronary syndrome, acute myocardial infarction [MI], or ischemic stroke). Assessed for eligibility were 18,034 participants who visited the ESS between January 2000 and December 2009. Excluded were 4,101 with preexisting DM or CVD and 3,020 with only a single visit (Figure). Baseline characteristics of the 3,020 individuals who had a single visit to the ESS were not statistically different from the characteristics of the included participants (data not shown). Included for analysis were 10,913 participants, (7,940 men and 2,973 women).
Figure.
Flowchart of study participants. A total of 18,034 men and women were evaluated initially; among them, 4101 were excluded because of a medical history of DM or CVD, and 3020, because of having only a single visit to the ESS. Eventually, 10,913 participants, 7940 men and 2973 women, participated in the study. During follow-up, 548 developed DM, 1,119 developed CVDs, and 131 died.
Definitions of CVD outcomes
Diagnosis of CVD was the primary end point of the study. Cardiovascular disease was defined as a composite of coronary heart disease (CHD), MI, cerebral vascular events, or transient ischemic attacks (TIAs). Coronary outcome was defined by clinically significant CHD (angiography-proven stenosis of <50% in at least 1 coronary artery), MI, or a positive Bruce test. The diagnosis of MI was self-reported, and when available, medical records were reviewed. At each annual ESS visit, each participant had a treadmill exercise test (Bruce protocol15) in the presence of a board-certified cardiologist. End points for the exercise test were clinically significant ST-segment depression (>2 mm depression in 2 contiguous leads, measured 80 milliseconds after the J point), intolerable symptoms of angina and exhaustion, or achievement of the target heart rate without such findings. All cases with a pathologic stress test were referred for coronary angiography. For individuals with a positive Bruce stress test, for whom data on coronary angiography or single-photon emission computed tomography was not available, a positive Bruce test was considered as confirmation for a positive CHD. In participants with a borderline stress test, or when participants reported angina symptoms without diagnostic electrocardiographic changes, stress perfusion imaging with thallium-201 was performed. Those with a pathologic thallium-201 cardiac scan underwent coronary angiography. Cerebrovascular accident/TIA outcome included self-reported cerebral vascular events or TIAs. When available, medical records were reviewed.
Diagnosis of DM
The diagnosis of DM was defined as the secondary outcome of this study. Subjects with 2 fasting glucose tests ≥126 mg/dL (preformed on different days) were diagnosed with DM.
Laboratory methods
Biochemical analyses of blood were performed on fresh samples in a core laboratory facility. Tests were performed in the Sheba Medical Center, Tel Hashomer, using the Olympus AU2700TM Chemistry-Immuno Analyzer (Olympus; Shinjuku, Tokyo, Japan).
Statistical analysis
Included for analysis were 7,940 men and 2,973 women. For each participant, person-time of follow-up was calculated from the date of the first ESS visit until censoring, as detailed above. We used 1-way analysis of variance to compare the means and proportions of the population baseline characteristic across the FPG groups, which were arbitrarily divided to 5-mg/dL intervals for levels between 80 and 100 mg/dL. In addition, we have also included the 2 extreme groups, FPG <80 mg/dL and IFG (100–126 mg/dL). Levels below 100 were considered as “fasting normoglycemia.” Log transformation was used for triglyceride (TG) and high-density lipoprotein (HDL) cholesterol. χ2 test was used for categorical data. We conducted Cox proportional hazard analysis to estimate the hazard ratio (HR) and 95% CIs for developing CVD outcome. We controlled for age, gender, systolic blood pressure (continuous), TG (continuous), HDL and low-density lipoprotein (LDL) cholesterol (both continuous), smoking status (current vs past or never), BMI (continuous), pharmacologic treatment (grouped into β-blockers, cholesterol-lowering drugs, antiarrhythmics, antithrombotics, diuretics, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers), and family history for coronary disease (yes or no). Mortality rates, according to FPG levels, were assessed by Kaplan-Meier survival analysis. The Wald score was used to rank the strength of each independent risk factor for cardiovascular diseases. All statistical analyses were performed with SPSS statistical software version 15.0 (IBM, Armonk, NY).
This study was supported by a grant from the Shlavi foundation for medical research. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
Results
Data from 10,913 apparently healthy men and woman (mean age 50.4 years, range 34–91), 9,687 with FPG levels of <100 mg/dL at baseline and 1,226 with IFG values (100– 125 mg/dL), were analyzed. Participants were categorized based on glucose levels to 5 groups (50–79, 80–84, 85–89, 90–94, and 95–99 mg/dL). In addition, we have also analyzed the IFG group (100–125 mg/dL). Age, male gender, systolic blood pressure, family history of CVD, TG, and LDL cholesterol levels were more likely to increase across the entire range of fasting glucose level. The percentage of current smokers and HDL cholesterol levels inversely correlated with FPG levels Table I).
Table I.
Baseline characteristics of 10,913 men and women according to 5 groups of normal fasting plasma plus IFG levels*
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | IFG | P for trend | |
|---|---|---|---|---|---|---|---|
| FG values (Mg %) | 50–79 | 80–84 | 85–89 | 90–94 | 95–99 | 100–125 | |
| Mean FG (Mg %) | 74.4 | 82.1 | 87 | 92 | 97 | 106 | – |
| n | 2017 | 2065 | 2317 | 1837 | 1451 | 1226 | – |
| Mean follow-up (y) | 4.3 | 4.3 | 4.3 | 4.4 | 4.3 | 4.2 | – |
| Age | 48 | 49 | 50 | 50.7 | 52 | 54.7 | <.001 |
| Male gender | 61.1% | 67.2% | 74.1% | 77.3% | 79.3% | 83.1% | <.001 |
| BMI | 24.6 | 25.4 | 26 | 26.5 | 27 | 27.6 | .1 |
| SBP | 123 | 125 | 127 | 129 | 130 | 134 | <.001 |
| TG | 91 | 99 | 107 | 113 | 117 | 132 | <.001 |
| LDL | 121 | 123 | 124 | 126 | 125 | 126 | <.001 |
| HDL | 51 | 50 | 49 | 47 | 47 | 45 | <.001 |
| Smoking | 21.6% | 18.3% | 15.7% | 14.5% | 14.1% | 10.9% | <.001 |
| Family history | 30.1% | 32.5% | 32.8% | 33.0% | 33.7% | 34.4% | .02 |
A total of 10,913 men and women were divided according to glucose levels to 5 groups (50–79, 80–84, 85–89, 90–94, and 95–99 mg/dL) plus an IFG group (100–125 mg/dL). Cardiovascular disease risk factors (age, male gender, systolic blood pressure [SBP], and family history of CVD, TG, LDL cholesterol, and BMI levels) were more likely to increase across the entire range of fasting glucose level. The percentage of smokers (current) and HDL cholesterol levels inversely correlated with fasting glucose levels.
Triglyceride and HDL were log transformed; SBP, TG, LDL, HDL are adjusted for age.
During 46,991 person-years of follow-up (mean follow-up, 4.3 years), there were 1,119 documented incident cases of CVD end-points. Age-adjusted HRs for CVD outcomes have increased linearly across the entire range of FPG levels, with significant HR already observed for FPG levels of 85 to 89 mg/dL (1.34, 95% CI 1.08–1.65), reaching an HR of 1.53 (95% CI 1.2–1.9) for the 95-to-99-mg/dL group as compared with the lower FPG group (P for trend <.001) (Table II).
Table II.
Hazard ratios for CVD outcomes among 10,913 individuals according to increasing FPG levels
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | IFG |
P for trend |
|
|---|---|---|---|---|---|---|---|
| FPG values | 50–79 | 80–84 | 85–89 | 90–94 | 95–99 | 100–125 | |
| n | 2017 | 2065 | 2317 | 1837 | 1451 | 1226 | |
| Coronary outcomes | 124 (6.1%) | 160 (7.7%) | 213 (9.2%) | 196 (10.6%) | 173 (12%) | 159 (13%) | |
| Stroke/TIA | 14 (0.7%) | 17 (0.8%) | 19 (0.8%) | 13 (0.7%) | 8 (0.6%) | 23 (1.8%) | |
| Total outcomes | 138 | 177 | 232 | 209 | 181 | 182 | |
| % of patients with any outcome | 6.8% | 8.5% | 10.0% | 11.3% | 12.5% | 14.8% | |
| HR (adjusted to age) (95%CI) | 1 | 1.2 (0.95–1.52) | 1.34 (1.08–1.65) | 1.47 (1.18–1.82) | 1.53 (1.22–1.91) | 1.74 (1.39–2.18) | <.001 |
| HR (multivariate adjustment*) (95%CI) |
1 | 1.20 (0.96–1.51) | 1.28 (1.03–1.59) | 1.4 (1.12–1.75) | 1.40 (1.11–1.76) | 1.51 (1.19–1.91) | <.001 |
Incidence of cardiovascular (CVD) outcomes (divided to coronary and cerebral), across FPG levels. Age-adjusted HRs for CVD outcomes have increased linearly across the entire range of FPG levels (P for trend <.001).
Multivariate adjustment included age, sex, hypertension, TG, HDL, LDL, smoking status, BMI, pharmacologic treatment, and family history for coronary disease.
In a multivariate model adjusted for age, sex, BMI, systolic blood pressure, TGs, HDL and LDL-cholesterol levels, smoking status, pharmacologic treatment, and family history for CHD, we observed a significant and progressive increase in the risk of CVD events in individuals with FPG levels in the third, fourth, and fifth groups as compared with the low-glucose group (P for trend <.001) (Table II). The addition of the IFG group (100–125 mg/dL) further improved the prediction model (P < .001 on the basis of the likelihood-ratio test). In a multivariate model adjusted for the above parameters using FPG levels in the normoglycemic range as a continuous variable, we revealed a significant increase of 7.6% in the risk for CVD for every increment of 1 mg/dL of glucose. In a subgroup analysis, we excluded patients who had developed DM before having a cardiovascular event. The risk for CVD remained significant even after multivariant analysis controlling for the aforementioned risk factors. We further analyzed the interaction of sex and FPG in respect to cardiovascular risk, which was nonsignificant (P of interaction = .321).
When stratified for the various component of the composite CVD outcome, increased FPG in the normoglycemic range was associated with increased risk of coronary but not of cerebrovascular events, for which the risk had increased significantly only for the IFG group (Table III). To better assess whether the correlation between FPG and CVD is age related, we next stratified the study population based on the mean age of 50 years. Although both groups showed similar correlations between FPG levels and CVD outcomes, only the group of participants older than 50 years (n = 5072) had reached statistically significant HRs (P < .05 for all groups as compared with the reference group). The P value for interaction between FPG, age, and CVD outcomes was insignificant (P = .478). The importance of each risk factor for CVD was ranked independently by the Wald score: for FPG levels the score was 15.3, after age (171), gender (39) and smoking (17.9) similar to hypertension (15.2) and more significant than BMI (3.6), family history (10.6), cholesterol (12.4), and TG (7.2). We next assessed mortality rates at each FPG group. Mortality rates were not statistically different for groups 1 though 5 being 2.3, 2.1, 2.1, 2.3, and 3.3 per 1000 participants per year, respectively (P = .25). Mortality among participants of the IFG group was higher, reaching 5.7 per 1000 participants per year (P = .016).
Table III.
Coronary and cerebrovascular outcomes according to FPG levels*
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | IFG100- | P for trend | |
|---|---|---|---|---|---|---|---|
| FGP values | 50–79 | 80–84 | 85–89 | 90–94 | 95–99 | 126 | |
| n | 2017 | 2065 | 2317 | 1837 | 1451 | 1226 | |
| HR coronary events (95%CI) |
1 | 1.21 (0.96–1.52) | 1.31 (1.04–1.62) | 1.49 (1.13–1.85) | 1.52 (1.19–1.95) | 1.52 (1.19–1.95) | <.001 |
| HR cerebral events (95%CI) |
1 | 1.10 (0.52–2.13) | 1.02 (0.50–2.02) | 0.80 (0.31–1.71) | 0.50 (0.21–1.18) | 1.34 (1.65–2.69) | .2 |
Hazard ratios for cardiovascular outcomes divided to coronary or cerebrovascular diseases. The HR for coronary disease incidence increased across FPG levels (P < .001), when compared with the lower group (group 1, FPG 50–79 mg/dL). In contrast, HR for cerebrovascular incidence decreased across FPG levels and were significantly increased only in the IFG range.
Hazard ratios are adjusted for age, sex, hypertension, TG, HDL, LDL, smoking status, BMI, pharmacologic treatment, and family history for coronary disease.
To confirm previous observations that increased FPG in the normal range is associated with incident DM in young adults could also be observed in an older population, we next analyzed the cases of new-onset DM based on FPG at baseline. During the follow-up period, 22% of those in the IFG compared with only 0.2% of those in the lowest FPG group developed DM. After adjustment for age, sex, BMI, hypertension, TGs, LDL, HDL, smoking status, and family history for DM, the HR for the IFG group was 77 (95% CI 32–186), as compared with 18 (95% CI 7.5–46) in the 95-to-99-mg/dL group and with 3.9 (95% CI 1.48–10.36) observed for those with FPG of 80 to 84 mg/dL.
Discussion
In this study, 10,913 men and women without DM or apparent CVD were followed up for a mean of 4.3 years. The risk for coronary disease has linearly increased across FPG levels, already within the normoglycemic range (≤100 mg/dL). This increased risk was independent of other traditional risk factors for CVD and was not mediated by the higher risk of DM incidence. Cerebrovascular disease did not follow this pattern, and an increased risk was observed only among participants with IFG. Although the overall CVD risk for individuals with FPG levels <80 mg/dL was 7% in 5 years, as many as 12.5% of the high-normal glucose group (fasting levels of 95–99 m/dL) had developed CVD. As expected, the risk continued to increase linearly, reaching 17% in 5 years in the IFG group. Our findings suggest that elevated fasting glucose level, within the normal range, is an independent predictor of CVD in men and of type 2 DM in both women and men.
Although there is a wealth of epidemiological data assessing the cardiovascular risk associated with FPG in the prediabetic range (FPG ≥100 mg/dL), only few studies have analyzed the role of glycemia as a continuum, including the normoglycemic range as well. Past findings were inconsistent,14,15 whereas others demonstrated a J-curve.16 A recent meta-analysis of 102 prospective studies involving approximately 700,000 people showed that, in those without DM, there was a modest nonlinear increased risk for vascular disease, which was not significant after conventional risk factor adjustments.17 Another recent meta-analysis of 26 prospective studies in Western countries, involving over 300,000 people with approximately 17,000 incident CHD cases, has demonstrated that fasting glucose concentration is modestly associated with CHD risk in people without DM with an increased risk of 6% for every 1 mmol/L increment (18 mg/dL) in FPG.18 In a recent community-based study, glycated hemoglobin but not FPG was significantly associated with cardiovascular disease or all-cause mortality.19 However, many of these studies did not include specific analysis for the normal glucose range, and data were analyzed for the entire “nondiabetic” range (ie, normoglycemia and IFG). A very large study of >650,000 Korean men is one of the few studies that directly evaluated CVD risk based on glucose levels in both the normoglycemic and prediabetic/diabetic range.20 Although the increase in the risk for ischemic stroke was independently observed already at the upper range of normoglycemia, the increased risk for MI could be observed only for glucose levels in the diabetic range. The somewhat contradicting results in our study may be explained by the different outcome definitions and our inability to differentiate between ischemic and hemorrhagic strokes. Another recent study from Israel reported an increased risk for coronary revascularization among apparently healthy men and women with elevated FPG in the normoglycemic range.21 However, a marked attenuation of the risk was observed in a multivariate analysis adjusted for age, sex, lipids, BMI, and smoking. The profound difference in the population studied and the outcome definitions could explain the apparent discrepancy with our observations of an increased risk associated with FPG, independent from the “traditional” CVD risk factors. Moreover, by choosing revascularization as the primary outcome, a potential effect of glucose levels on early stages in the pathophysiology of cardiovascular disease could have been easily missed.
The linear increase in CVD risk across the entire FPG range is similar to results reported with other biomarkers of CVD risk such as LDL cholesterol, blood pressure, and TG levels. Although the definition of various glucose ranges (DM, IFG, and normoglycemia) has mainly been determined by epidemiological data evaluating the risk for DM and its late complications, the risk for macrovascular disease has been clearly shown to be elevated at FPG levels below that required for the development of significant microvascular disease. A potential explanation could be that elevated FPG, long before overt hyperglycemia, may be a strong marker for insulin resistance and its associated metabolic abnormalities, which have been reported to be key players in the pathophysiology of atherosclerosis. Several limitations of this study warrant consideration. First, the CARMA cohort may be considered representative of a subgroup of the Israeli population at higher than the average socioeconomic status, although we do not believe that this should significantly affect the interaction between FPG and incident CVD. However, this population may have less competing risk factors (eg, less smokers, lower rates of obesity) for cardiovascular outcomes, which may explain the higher HRs observed in this study as compared with previous reports (see above). In addition, the low percentage of women enrolled in our study, especially among the higher FPG groups, may have compromised the statistical power to detect the role of FPG in CVD risk prediction in women. Although all participants were actively screened for coronary disease, cerebrovascular diseases were mostly based on self-reporting, which may have decreased the accuracy of the outcome definitions, especially in the case of TIAs in which neurologic examination is unrevealing. In addition, given the inability to assess subclinical cerebrovascular disease, a potential contribution of elevated FPG to early pathophysiologic stages of the disease could have been missed. The strengths of our study include the large population included and the tight follow-up with repeated measurements of many confounders as well as the active screening for coronary disease (not currently indicated for routine screening of low-risk individuals), which most probably increased the sensitivity of detecting relatively early coronary disease, before ischemic symptoms develop.
In summary, in a large adult population, FPG well within the normoglycemic range was found as an independent risk factor for coronary disease. Including FPG in the risk stratification for cardiovascular morbidity may allow better detection of individuals at risk for disease who may benefit from a more aggressive modification of their CVD risk factors.
Acknowledgments
The authors thank Ms Ilana Gelernter from the Department of Statistics and Operations Research, in the School of Mathematical Sciences, at Tel Aviv University, Israel, for statistical analysis and Ms Yael Mintz from the Institute for Preventive Medicine of Sheba Medical Center, Israel, for her administrative and technical support. The study was funded by a grant from the Shlavi foundation for medical research. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript, and its final contents.
References
- 1.Genuth S. Lowering the criterion for impaired fasting glucose is in order. Diabetes Care. 2003;26:3331–3332. doi: 10.2337/diacare.26.12.3331. [DOI] [PubMed] [Google Scholar]
- 2.Tirosh A, Shai I, Tekes-Manova D, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–1462. doi: 10.1056/NEJMoa050080. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Vaccaro O, Neaton JD, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 4.Tanne D, Koren-Morag N, Goldbourt U. Fasting plasma glucose and risk of incident ischemic stroke or transient ischemic attacks: a prospective cohort study. Stroke. 2004;35:2351–2355. doi: 10.1161/01.STR.0000140738.94047.55. [DOI] [PubMed] [Google Scholar]
- 5.Wei M, Gaskill SP, Haffner SM, et al. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21:1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 6.Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 7.Balkau B, Bertrais S, Ducimetiere P, et al. Is there a glycemic threshold for mortality risk? Diabetes Care. 1999;22:696–699. doi: 10.2337/diacare.22.5.696. [DOI] [PubMed] [Google Scholar]
- 8.DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26:688–696. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 9.Levitzky YS, Pencina MJ, D'Agostino RB, et al. Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol. 2008;51:264–270. doi: 10.1016/j.jacc.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 10.Coutinho M, Gerstein HC, Wang Y, et al. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Gerstein HC. Is glucose a continuous risk factor for cardiovascular mortality? Diabetes Care. 1999;22:659–660. doi: 10.2337/diacare.22.5.659. [DOI] [PubMed] [Google Scholar]
- 12.Lawes CM, Parag V, Bennett DA, et al. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27:2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 13.Shaw JE, Zimmet PZ, Hodge AM, et al. Impaired fasting glucose: how low should it go? Diabetes Care. 2000;23:34–39. doi: 10.2337/diacare.23.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Preiss D, Welsh P, Murray HM, et al. Fasting plasma glucose in non-diabetic participants and the risk for incident cardiovascular events, diabetes, and mortality: results from WOSCOPS 15-year follow-up. Eur Heart J. 2010;31:1230–1236. doi: 10.1093/eurheartj/ehq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei M, Gibbons LW, Mitchell TL, et al. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation. 2000;101:2047–2052. doi: 10.1161/01.cir.101.17.2047. [DOI] [PubMed] [Google Scholar]
- 16.Barr EL, Boyko EJ, Zimmet PZ, et al. Continuous relationships between non-diabetic hyperglycaemia and both cardiovascular disease and all-cause mortality: the Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia. 2009;52:415–424. doi: 10.1007/s00125-008-1246-y. [DOI] [PubMed] [Google Scholar]
- 17.Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarwar N, Aspelund T, Eiriksdottir G, et al. Markers of dysglycaemia and risk of coronary heart disease in people without diabetes: Reykjavik prospective study and systematic review. PLoS Med. 2010;7:e1000278. doi: 10.1371/journal.pmed.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sung J, Song YM, Ebrahim S, et al. Fasting blood glucose and the risk of stroke and myocardial infarction. Circulation. 2009;119:812–819. doi: 10.1161/CIRCULATIONAHA.108.776989. [DOI] [PubMed] [Google Scholar]
- 21.Pereg D, Elis A, Neuman Y, et al. Cardiovascular risk in patients with fasting blood glucose levels within normal range. Am J Cardiol. 2010;106:1602–1605. doi: 10.1016/j.amjcard.2010.07.036. [DOI] [PubMed] [Google Scholar]