Abstract
Gamma-band (25–140 Hz) oscillations are ubiquitous in mammalian forebrain structures involved in sensory processing, attention, learning and memory. The optic tectum (OT) is the central structure in a midbrain network that participates critically in controlling spatial attention. In this review, we summarize recent advances in characterizing a neural circuit in this midbrain network that generates large amplitude, space-specific, gamma oscillations in the avian OT, both in vivo and in vitro. We describe key physiological and pharmacological mechanisms that produce and regulate the structure of these oscillations. The extensive similarities between midbrain gamma oscillations in birds and those in the neocortex and hippocampus of mammals, offer important insights into the functional significance of a midbrain gamma oscillatory code.
Introduction
Gamma-band (25–140 Hz) oscillations of network activity, measured as rhythmic fluctuations in the extra-cellular local field potential (LFP), are observed in many regions of the forebrain [1]. Gamma power is modulated during mnemonic and cognitive processes. In the hippocampus, for example, LFP gamma power is modulated during the encoding of novel information [2], and the strength of gamma power predicts the precision of subsequent recall [3]. In the neocortex, gamma power is modulated by attention: directing attention to a particular stimulus typically increases the amplitude of gamma oscillations and increases the synchronization of spikes to these oscillations within neocortical regions that encode the stimulus [4]. Attention also increases the synchronization of spikes and LFPs in the gamma band across distant regions of the neocortex [5].
Despite these well-established empirical observations, it is unknown whether gamma-band synchronization actually plays a role in neural information processing or if gamma oscillations are simply epiphenomenal manifestations of neural processing [6–9,10•,11]. Here we describe the recent discovery of a neural circuit in a midbrain network in birds that generates and broadcasts large amplitude gamma oscillations. Results from experiments in birds and mammals demonstrate that the specific mechanisms for generating and shaping gamma oscillations are strikingly similar in widely different parts of the brain and across species separated by >300 million years of evolution, arguing for a conserved, functional role of gamma oscillations [12].
The midbrain spatial attention network consists of the optic tectum (OT; superior colliculus, SC, in mammals) and a number of interconnected tegmental nuclei. The OT and each of the component nuclei contain a multimodal, topographic map of space. The OT is a multilayered structure that combines sensory spatial information with descending spatial information from the forebrain, including the goals of impending orienting movements, and encodes the highest priority location for the animal’s attention [13–16]. Each of the various tegmental nuclei receives focal, topographic input directly from the OT, and feeds back in an architecturally unique pattern to the OT (Figure 1a) [17,18]. The circuits formed by these tegmental nuclei play critical roles in generating the representation of the highest priority location in the OT space map.
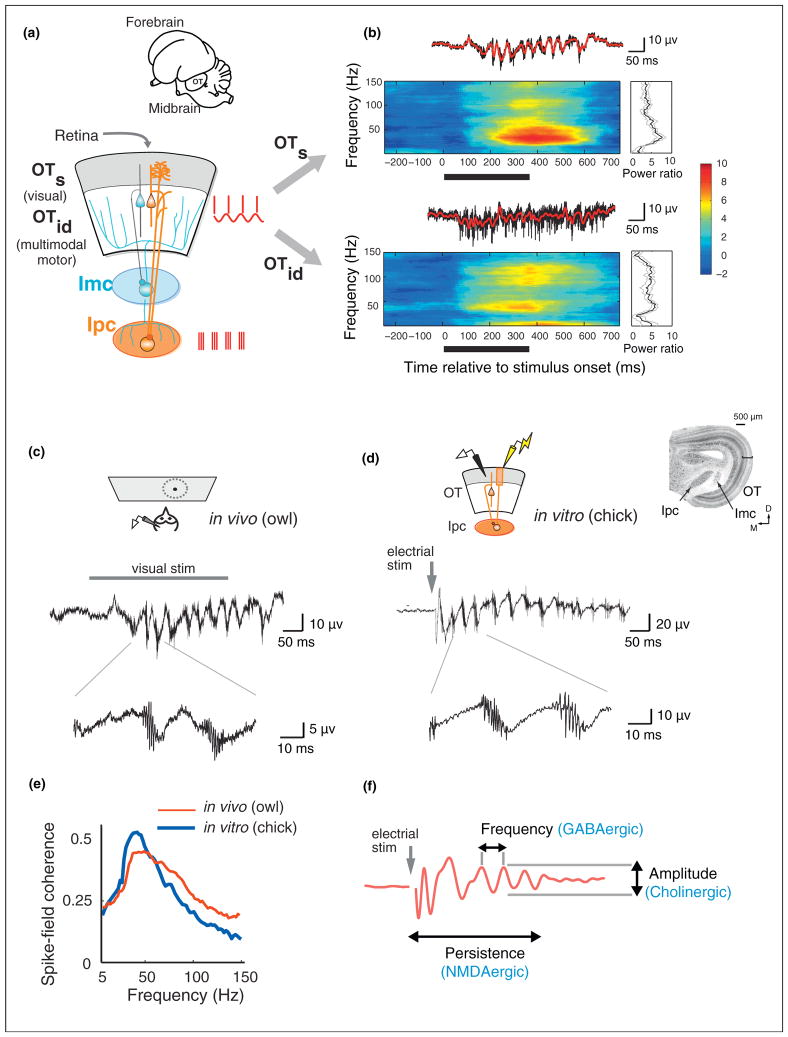
Figure 1.
Properties and mechanisms of midbrain gamma oscillations. (a) Schematic of the midbrain network showing the pattern of connectivity between the optic tectum (OT) and adjacent tegmental nuclei: the GABAergic Imc (blue circuit) and the cholinergic Ipc (orange circuit). Superficial OT (OTs) layers are shaded in gray. Intermediate/deep OT (OTi/d) layers are unshaded. Gray arrow: retinal input. The OT-Ipc circuit generates robust gamma oscillations in the midbrain network, depicted by the spike and LFP icons (red) shown alongside. (Inset) Outline sketch of the owl brain showing the forebrain and the midbrain including the optic tectum (OT). (b) (Top) LFP oscillation (above), induced spectrogram (center) and R-spectrum (right) recorded at a site in the superficial OT in response to a visual stimulus (15°/s vertically moving dot). Black bar: duration of visual stimulus. (Bottom) Same as top panel, but at a site recorded in the intermediate/deep OT. (c) (Top) Schematic of the in vivo preparation in the owl. Field recordings were made from the OTs in the owl midbrain. (Middle) Gamma oscillations evoked by a visual stimulus (same as in panel b). (Bottom) Expanded timescale showing a high frequency burst of spikes phase locked to a lower frequency (low-gamma) LFP. (d) (Inset) Slice of midbrain network (chick) showing the relative locations of the OT, Ipc and Imc. (Top) Schematic of the in vitro slice preparation of the chick midbrain. A stimulating electrode (yellow) was placed in the retinal afferent layer 1, and the field was recorded from OTs layer 5 (black). (Middle) Gamma oscillation evoked by a 10 μA, 0.1 ms electrical pulse (gray arrow) delivered to the retinal afferents. (Bottom) Expanded timescale showing a high frequency burst of spikes phase locked to a lower frequency (low-gamma) LFP. (e) Spike-field coherence (SFC) measured in the OTs from in vivo (red, thin line) and in vitro (blue, thick line) preparations (same recordings as in panels c and d). The SFC profiles in both preparations show closely aligned peaks in the low-gamma band. (f) Schematic showing pharmacological mechanisms in vitro that control distinct aspects of the gamma oscillation (red trace). GABAergic mechanisms control the temporal structure (frequency), cholinergic mechanisms control the amplitude, and glutamatergic (NMDA) mechanisms control the persistence of the oscillations. Panel (a) reproduced with permission from [68] panel (b) reproduced with permission from [21••], panels (c–f) reproduced with permission from [27••].
The midbrain attention network is most differentiated in birds, in which the OT consists of 15 distinct layers and the tegmental network components are differentiated into several distinct nuclei (Figure 1a,d inset) [15]. Superficial layers of the OT (OTs, layers 1–9) receive direct retinal input as well as input from primary and secondary visual areas in the forebrain, and project to visual nuclei in the thalamus. The intermediate/deep OT layers (OTi/d, layers 10–13) receive multimodal sensory inputs as well as movement-related information, and project via thalamus to higher order forebrain areas and to brainstem nuclei involved generating orienting movements [15].
Of particular significance in the OT is a distinctive cell-layer at an intermediate depth (layer 10). This layer receives input from both the superficial and deep layers, and projects to the adjacent tegmental nuclei, forming specialized circuits that participate in distinct neural computations [15]. One specialized circuit comprises a tegmental nucleus Imc (nucleus isthmi pars magnocellularis), a GABAergic nucleus that projects back broadly to the OT and mediates global competitive inhibition across the OT space map (Figure 1a, blue). This circuit is essential to the network’s computation of the highest priority location [19]. A second circuit comprises tegmental nucleus Ipc (nucleus isthmi pars parvocellularis), a cholinergic nucleus that receives focal input and feeds back precisely and topographically to the OT (Figure 1a, orange). As discussed below, the Ipc amplifies and distributes strong, space-specific, gamma-periodic activity to neurons in both the superficial and deep layers of the OT [20]. It is the functional properties of this OT-Ipc circuit that is the focus of this review.
We summarize the current knowledge and recent advances in characterizing the various elements of this gamma-generating circuit, and emphasize the remarkable commonalities between gamma mechanisms in the avian midbrain and those in the mammalian forebrain. We conclude by presenting plausible hypotheses regarding the functional role of a midbrain gamma oscillatory code, and motivate several important directions for immediate future research.
Properties of midbrain gamma oscillations recorded in vivo
Many properties of sensory stimuli that are encoded by unit activity in the OT are also encoded by LFP gamma power [21••]. In vivo LFP recordings in the barn owl OT have demonstrated that spatially localized visual or auditory stimuli induce robust increases in LFP gamma power with similar spectral characteristics. Moreover, induced gamma power varies systematically with stimulus location: like OT units, gamma power exhibits multimodal spatial receptive fields. The stimulus location that induces maximum gamma power coincides closely with the center of unit receptive fields, based on spike rates, recorded at the same location in the OT space map. Furthermore, the spatial extent (tuning width) of gamma power receptive fields is comparable to that of unit tuning curves in the superficial OT layers, and marginally narrower than unit tuning curves in the deep OT layers. In addition, gamma power increases systematically with the physical strength of the sensory stimulus (either light contrast or sound intensity), resembling the stimulus strength-response functions that have been reported for OT units.
Recent studies have emphasized the importance of distinguishing ‘true’ gamma oscillations from artifactual, broadband increases in LFP power induced by spike waveforms that ‘bleed’ into the gamma-band [7,22,23]. This distinction is crucial because of the concurrence of putative gamma oscillations with spiking activity and the overlap in the mechanisms that generate gamma oscillations and spikes [23]. Recently developed approaches, including spike subtraction techniques [24,25] and the matching-pursuit algorithm [22], facilitate making this distinction.
In the avian OT, induced gamma power following spike subtraction shows different spectral characteristics in the superficial versus deep layers [21••]. In the superficial layers, the LFP exhibits a conspicuous oscillation (Figure 1b, upper trace) and the spike-subtracted, induced power spectrum shows a distinct narrow-band peak in the low-gamma band (25–90 Hz; Figure 1b, upper spectrogram). In addition, spiking activity recorded in the superficial layers is dominated by periodic spike bursts that phase-lock with the LFP (spike-field coherence) in the low-gamma band (Figure 1c, bottom; e, red). This is clear evidence of a true gamma oscillation.
On the other hand, in the deep OT layers, the spike-subtracted, induced LFP power spectrum typically exhibits a broadband increase across the gamma band: At some sites, power increases show a clear peak in the ‘high-gamma’ band (90–140 Hz), and at others, power increases occur in two distinct spectral bands, one in the low-gamma and the other in the high-gamma band (e.g. Figure 1b, lower) [21••]. In addition, spike discharges in the deep layers are irregular and do not show clear gamma periodicity (Figure 1b, lower trace). Nevertheless, a large proportion of units in the deep OT layers (~50–60%) exhibit spike-field coherence, with a distinctive peak in the low-gamma band, again indicative of a network gamma rhythm [26].
Circuit mechanisms of midbrain gamma oscillations
What is the source of the low-gamma oscillations, so prominent in the superficial layers of the OT? Previously, descending inputs from sensory or attention-related forebrain regions were considered a likely source for the midbrain gamma oscillations. However, the capacity of the isolated midbrain network to generate low-gamma oscillations was recently demonstrated by recording the oscillations in slices of the midbrain that preserved the connectivity between the various network components in vitro [27••].
The largest amplitude gamma oscillations, both in vivo and in vitro, are recorded in superficial layer 5 (L5) of the OT. In this layer, the oscillations are associated with gamma-rhythmic bursts of spikes that are phase-locked to the LFP (Figure 1c,d). The distinctive temporal microstructure of the L5 oscillations observed in vivo is entirely preserved in vitro: a single pulse of electrical microstimulation applied to retinal afferents in L1 in midbrain slices, evokes a persistent (>100 ms) bout of gamma oscillations (Figure 1d), with strong spike-field coherence (Figure 1e, blue) [27••]. The ability to evoke in vivo like gamma oscillations in midbrain slices, isolated from the forebrain, provides clear evidence that the midbrain contains its own, independent circuit for generating gamma oscillations.
The midbrain slice preparation has enabled a detailed pharmacological investigation of the roles of different kinds of neurotransmitters in controlling various aspects of the gamma oscillation [27••]. GABAergic inhibitory transmission was shown to play a crucial role in regulating the temporal structure (frequency) of the oscillations (Figure 1f): blocking GABAergic transmission, by adding the GABA receptor blocker picrotoxin to the bath in the slice preparation, completely disrupted both the oscillations and the distinctive spike bursts in L5. Conversely, enhancing GABAergic transmission, by applying the GABA receptor agonist pentobarbital to the bath, slowed down the oscillation frequency. Cholinergic (ACh) transmission was shown to play a critical role in controlling oscillation amplitude: Simultaneously blocking both nicotinic and muscarinic receptors, by adding DHβE and atropine to the bath, substantially reduced the amplitude of the oscillations without altering their frequency. Finally, glutamatergic tansmission was shown to enable the persistence of the oscillations (timescale of ~100 ms): blocking NMDA receptors, by adding APV to the bath, eliminated sustained oscillations.
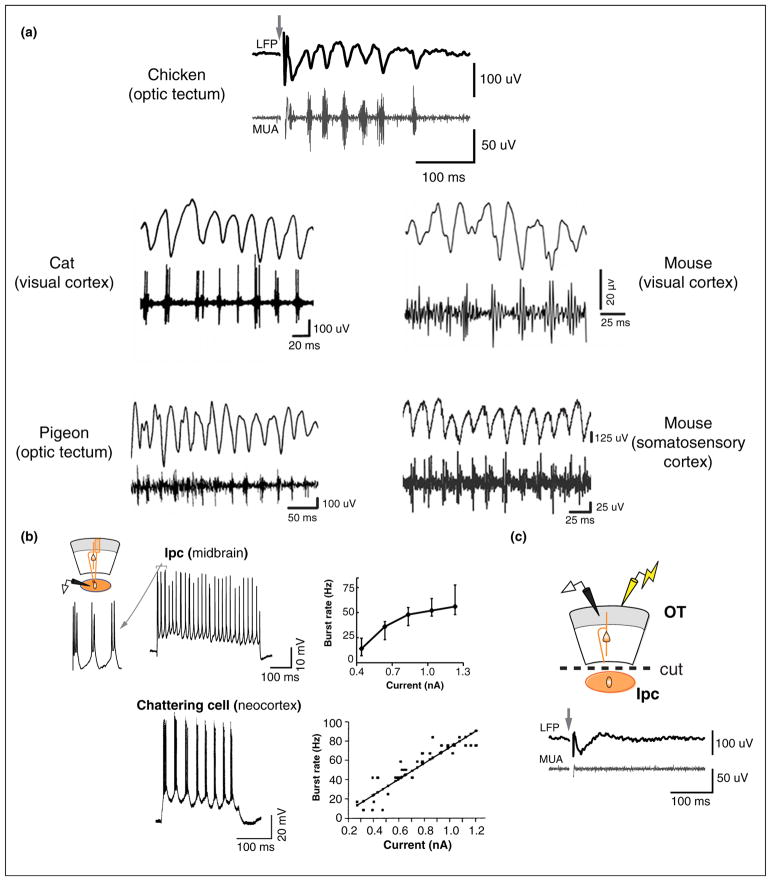
Spike bursts recorded in the superficial layers (L5) are generated by the discharges of large diameter, Ipc axons that arborize densely in the OT, including in L5 (Figure 1a, orange axons) [20,18]. Ipc neurons, recorded in vivo, show narrow spatial tuning, and respond to sensory stimuli with gamma periodic bursts of spikes [20,28]. Upon sustained step depolarization, Ipc neurons in vitro burst with a periodicity in the low-gamma range (~25–50 Hz) across a wide range of suprathreshold input current levels (Figure 2b, upper) [27••]. Thus, Ipc neurons have intrinsic cellular mechanisms that equip them to discharge at gamma frequencies. Removing Ipc input to the OT by surgically transecting the fiber bundle that connects the Ipc with the OT or by pharmacological blockade of the Ipc, completely abolishes the oscillation signature in superficial OT layers (Figure 2c). Thus, the Ipc is critically involved in expressing gamma oscillations recorded in the superficial OT.
Figure 2.
Similarities between gamma oscillations in diverse species and brain structures. (a) Gamma oscillations in diverse brain structures in various species. (Clockwise from top) Chicken optic tectum, mouse visual cortex, mouse somatosensory cortex, pigeon optic tectum, and cat visual cortex. For all but the somatosensory cortex recordings, the gamma-band filtered LFP is shown above and the high frequency multiunit activity (MUA) is shown below, temporally aligned to the LFP trace. For the somatosensory cortex recordings (mouse), the unfiltered field recording is shown above the MUA. Gamma oscillations were induced by electrical stimulation (chicken optic tectum), visual stimulation (cat visual cortex, mouse visual cortex, pigeon optic tectum) or optogenetic stimulation (mouse somatosensory cortex). Across species, brain structures and stimulation techniques, the signature phase-locking of bursts to the low-gamma LFP is apparent. (b) (Top) (Left) Schematic of the intracellular recording configuration for the chick Ipc. (Middle) Bursting pattern of responses recorded intracellularly from an Ipc neuron in the chick midbrain in vitro (1 nA step depolarization). (Right) Plot of burst rate versus depolarizing current (n = 5 cells). Data show medians with 25–75th percentile whiskers. (Bottom) (Left) Bursting pattern of responses recorded intracellularly from a chattering cell in cat visual cortex in vivo (0.9 nA step depolarization). (Right) Plot of burst rate versus depolarizing current for one cell. Data show mean burst rate for each depolarizing current pulse (n = 104). Black line: linear fit. (c) Eliminating Ipc input to the OT, by surgical transection of the Ipc-OT fiber bundle (dashed line), completely abolishes the oscillations recorded in the OTs. Other conventions are as in panel a and Figure 1d. Panel (a) reproduced with permission from [27••,30–33]; panel (b) reproduced with permission from [27••,52]; panel (c) reproduced with permission from [27••].
Nevertheless, a core circuit that is capable of generating gamma rhythms (independent of the Ipc) lies within the OT [27••]. Even after surgical transection of the Ipc-OT fibers, retinal afferent stimulation evokes narrow-band increases in gamma power in the intermediate OT layers, although the magnitude of the power increase is considerably lower relative to the preparation with the Ipc-OT connections intact. Moreover, focal blockade of inhibition (with picrotoxin) in the OT, but not in the Ipc, severely disrupts the temporal structure of the oscillations, indicating that a core gamma oscillator network lies within the OT.
These findings provide converging evidence for a specialized pathway that generates gamma oscillations starting in the intermediate OT layers, to the Ipc, and back to the OT (Figure 3b). Patch clamp recordings in vitro reveal that, during gamma oscillations, a majority of layer 10 (L10) neurons (intermediate layer) receive strong IPSCs and EPSCs that are coherent with extracellular LFP phase in the low-gamma band [27••]. Parvalbumin-positive, putatively inhibitory, local interneurons are concentrated in the upper half of L10. Pyramidal output neurons in the lower half of L10 project to the Ipc and provide gamma rhythmic input to the Ipc [27••]. Ipc neurons, that are themselves tuned to discharge with gamma periodicity, entrain to this gamma periodic input and broadcast an amplified signal back to neurons in all layers of the OT, as bursts of gamma-periodic spikes. The bursting activity in the Ipc synchronizes spikes recorded not only in the OT, but also spikes recorded in downstream target structures of the OT, in the thalamus and in higher forebrain areas [29••].
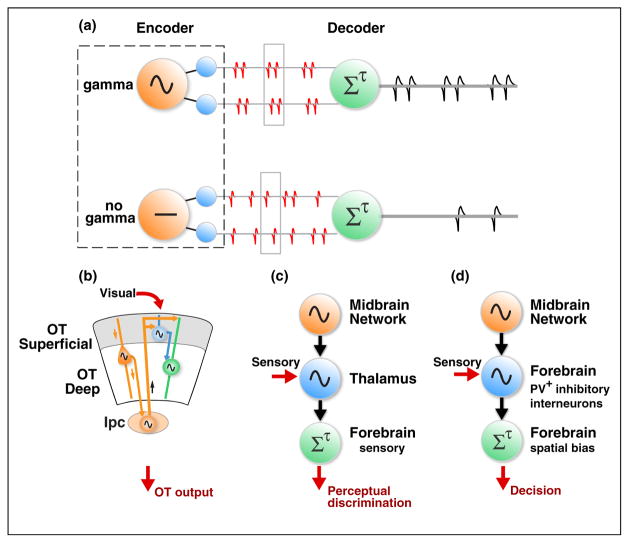
Figure 3.
Potential functional roles of midbrain gamma oscillations. (a) Schematic showing a core mechanism that underlies the three roles shown in panels (b–d). (Top) Gamma oscillations are generated by the oscillator node shown in orange. These oscillations entrain synchronized, gamma-rhythmic neural firing across neurons within a spatial channel of the sensory encoder (blue). These synchronized spikes from the encoder are transmitted to a downstream decoder (green). The decoder is most effectively driven by gamma-rhythmic, synchronized input because of (i) a short integration time window (gray outlined box) and high threshold (non-linear temporal integration of coincident input) and (ii) resonant intrinsic or circuit properties that produce a maximal response when driven at gamma-frequencies. (Bottom) Same as top panel, but when the oscillator node does not produce gamma oscillations. In this case encoder node spikes are incoherent, and not gamma synchronized across neurons within the spatial channel. These spikes are far less effective at driving the downstream decoder because the numbers of input spikes within the decoder’s integration window (gray outline) rarely suffice to drive the spiking output of the decoder. (b) Schematic of Role I (see Box 1 for details). Other conventions are as in panel a and Figure 1a. (c) Schematic of Role II (see Box 1 for details). Other conventions are as in panel a. (d) Schematic of Role III (see Box 1 for details). Other conventions are as in panel a.
Similarities between avian midbrain and mammalian forebrain gamma mechanisms
Gamma oscillations have been most thoroughly studied in the mammalian forebrain, particularly in the sensory neocortex and the hippocampus. To what extent do midbrain oscillations in birds share commonalities with forebrain oscillations in mammals?
Figure 2a depicts gamma oscillations recorded from four different species of animals, belonging to four different phylogenetic orders in two different classes, separated by at least three hundred million years of evolution. These oscillations were recorded in: the midbrain in birds (chicken and pigeon) [27••,30], the visual cortex in cats [31], and the visual and somatosensory cortex in mice [32,33]. Despite significant differences in the persistence of the oscillations, in part due to differences in recording and stimulation protocols employed in each case, the remarkable similarity in oscillation microstructure is apparent. The microstructure consists of bursting, high-frequency spike activity locked to a specific phase of the LFP oscillation (gamma-band ‘burst-LFP coherence’).
Similarly, gamma oscillations have also been observed in a variety of other species ranging from insects (locusts and honeybees) [34,35] to primates including humans [11,36•].
Both the avian OT and the mammalian neocortex exhibit a multi-layered cytoarchitecture. Although the cells in these layers may serve distinct functional roles in each structure, it is noteworthy that the dominance of gamma power in the superficial versus deeper layers of the avian OT parallels the stronger gamma power reported in the superficial versus deeper layers of the mammalian neo-cortex [37].
The striking physiological similarities across species and brain structures likely reflect underlying mechanistic similarities. As already discussed, L10 in the bird OT contains output neurons, which project to the Ipc, as well as a dense population of parvalbumin-positive (PV+) inhibitory interneurons that are ideally positioned to regulate L10 output neuron activity [27••]. The anatomical clustering of these circuit elements suggests the following mechanism for generating gamma oscillations in the OT: sensory and forebrain inputs to the L10 output neurons act via NMDA-receptor rich synapses to generate space-specific, persistent activity. This activity is, then, temporally sculpted into gamma oscillations by periodic inhibition from the local PV+ inhibitory circuitry. A similar interplay of excitatory and PV+ inhibitory mechanisms is thought to underlie the generation of gamma oscillations in the neocortex and hippocampus [38–42].
In addition, as in the avian midbrain, cholinergic and NMDAergic mechanisms in the mammalian hippocampus and neocortex regulate gamma oscillations by modulating network excitability [42–46]. Cholinergic agonists promote gamma oscillations, possibly by reducing adaptation currents onto principal neurons [47] or by directly deactivating inhibitory interneurons [48]. On the other hand, blocking NMDA receptors increases baseline gamma power in the hippocampus and neocortex in awake animals [49,50], possibly by reducing excitatory drive onto inhibitory interneurons [51•]. Further investigation is necessary to determine the sub-types of cholinergic and NMDAergic receptors that participate in the oscillations in the avian midbrain, as well as their specific sites of action within the gamma generating circuitry.
In the bird midbrain network, specialized neurons in the cholinergic nucleus Ipc produce periodic, gamma-rhythmic bursts of spikes that are transmitted to target neurons in the OT. A specialized type of neuron with similar physiological properties has been observed in the superficial layers of the visual cortex in cats [52,53]. These neurons, termed ‘chattering cells’ (or fast rhythmic bursting cells) also produce periodic, gamma-rhythmic bursting discharges in response to suprathreshold depolarization (Figure 2b, lower). Ipc neurons and chattering cells show remarkably similar input–output relationships (Figure 2b, right). Like Ipc neurons, these chattering cells have been suggested to play a key role in mediating gamma rhythms in the neocortex [54]. Whether chattering cells are also cholinergic remains to be determined.
Concluding remarks
The striking parallels between the functional properties of midbrain and forebrain gamma oscillations across distant species suggest the hypothesis that the brain employs universal mechanisms for generating and shaping gamma oscillations, as well as for encoding and decoding neural information with gamma rhythms. It seems unlikely that circuits and mechanisms for generating and transmitting gamma-rhythmic activity would be conserved through evolution if they did not play an essential role in information processing. Determining the functional role of gamma oscillations in information processing is, therefore, a crucial goal for systems neuroscience.
The midbrain attention network (Figure 1) plays a causal role in the selection of the next location for spatial attention, as reviewed elsewhere [15,55]. In addition, it generates robust, space-specific gamma oscillations, as reviewed here. Box 1 presents three plausible roles for midbrain gamma oscillations in facilitating neural computation during selective attention (Figure 3). These roles are: enhanced efficiency of input-output transformations within the OT, enhanced communication with target structures in the forebrain, and selective routing of sensory information in decision-making. None of these proposed roles depends critically on the frequency of gamma oscillations being constant (stationary) over time or for different stimuli [9]. Recent advances in technologies, which enable recording from and perturbing specific functional components of the midbrain network [41,51•,56], could pave the way towards validating or rejecting some of these roles, as well as answering other important outstanding questions regarding the midbrain oscillations. The spatial segregation and accessibility of component cell-types in the midbrain attention network, the ability to activate the network in a physiologically meaningful way in vitro, and the ability to interpret the functional significance of spatial patterns of activity within the network provide unique opportunities for discovering mechanisms controlling gamma oscillations and the role of these oscillations in information processing in the brain.
Box 1. Potential functional roles of midbrain gamma oscillations.
Role I. Enhancing sensitivity in the OT: selective modulation of input gain
Facts
Neurons in the superficial (visual) layers of the OT (OTs) receive direct retinal input and project to the thalamus and intermediate/deep OT layers [57]. Neurons in the intermediate/deep layers (OTi/d) project to the forebrain via the thalamus and to brainstem circuits that generate orienting movements, including saccadic eye movements. The OTs and OTi/d contain mutually aligned maps of space [15].
Ipc neurons broadcast an amplified gamma rhythm across the superficial layers [20,27••]. The dendrites of many OTi/d neurons arborize densely in these superficial layers. The spikes of a significant proportion (over 50%) of OTi/d neurons are coherent with LFP oscillations at low-gamma frequencies [21••].
Proposed role
Gamma synchrony alters visual input gain by modulating the effectiveness of information transmission within the OT (Figure 3a,b).
An attentional cue causes the midbrain circuit to generate gamma rhythmic activity at a specific locus in the OT and Ipc space maps that encodes the cued location (Figure 3b, orange). Gamma-rhythmic Ipc input induces synchronized, gamma-rhythmic spiking activity in OTs neurons encoding the cued location (Figure 3b, blue). The same Ipc input also induces periodic modulation of excitability of the OTi/d neurons, via their dendrites, within the same OT column (Figure 3b, green). Because the entire column receives a synchronized gamma rhythm from the Ipc, the windows of high excitability in the OTi/d neurons are temporally synchronized with the windows of spiking of the OTs neurons. This enables the rhythmic OTs spiking to drive activity in the OTi/d more effectively (Figure 1a, top). As a result, OTi/d neurons encoding a spatial location that is selected for attention would be more sensitive to rhythmically modulated spikes from OTs neurons encoding target stimuli at the same location. In contrast, OTi/d neurons encoding other (unattended) locations would receive asynchronous input (or no input) from the Ipc, and would be less sensitive to OTs spikes, as the windows of OTi/d excitability and OTs spiking are not temporally synchronized (Figure 1a, bottom).
Other mechanisms that could increase input gain as a result of periodic Ipc bursts are those that depend on cholinergic transmission. Acetylcholine is known to enhance gain or excitability through both presynaptic (e.g. enhancing vesicular release probability) [58,59] and post-synaptic (e.g. reducing neuronal leak currents) [60] mechanisms. Other mechanisms include enhancing excitability through focal disinhibition, as reported in the neocortex [61,62]. These alternative mechanisms could be activated in a manner dependent on gamma-rhythmic input (see Role III).
Role II. Enhancing communication through coherence: establishing privileged channels for prioritized information
Facts
Converging evidence indicates that the OT plays a critical role in generating a map of spatial priority [15,55]. Such a map could enable sensory information at particular ‘prioritized’ locations to be processed preferentially in forebrain regions involved in feature analysis [63].
Gamma-rhythmic discharges in the Ipc are synchronized with bursting activity recorded in the thalamic nucleus that receives input from the OTi/d, as well as in the subsequent, high-order forebrain areas [29••]. When multiple stimuli are presented, responses in the forebrain synchronize with the gamma activity of the Ipc neurons that encode the strongest (physically most salient) stimulus. Inactivation of the Ipc abolishes periodic bursting activity in both the thalamic and forebrain areas [29••].
Proposed role
Midbrain gamma oscillations create a channel for enhanced communication of spatially prioritized sensory information from the thalamus to the forebrain (Figure 3c).
An attentional cue causes the midbrain network to generate space-specific, synchronized gamma oscillations in the region that encodes the cued stimulus. The oscillations entrain rhythmic neural firing in the thalamus and, subsequently, in the forebrain that synchronize with the midbrain oscillations. The thalamic oscillations enable more effective communication between the thalamus and forebrain first, by creating temporally aligned windows of excitability between the thalamus and forebrain [64]; and second, by enhancing transmission efficacy by coincidence-dependent temporal integration, that is, coincident spikes are more effective at driving post-synaptic targets than incoherent spikes [65,66] (Figure 3a, top versus bottom).
Spikes encoding the attended target arrive at a gamma-synchronized region of the thalamus (a prioritized location in space ‘tagged’ by the midbrain oscillation). These spikes are transmitted more effectively to the corresponding gamma-synchronized region of the forebrain resulting in enhanced perceptual processing. In contrast, spikes encoding irrelevant, distracting stimuli arrive at an incoherently firing region of the thalamus. These spikes are transmitted less effectively to the forebrain. Thus, by ‘tagging’ a prioritized location with gamma oscillations, the midbrain network creates an enhanced transmission channel between the thalamus and forebrain for prioritized information.
Role III. Generating space-specific bias: routing information within the forebrain
Facts
Parvalbumin-positive (PV+) interneurons exhibit gamma frequency resonance: networks of PV+ neurons produce strongest modulations of LFP amplitude when activated periodically at low-gamma frequencies [42]. PV+ interneurons are ubiquitous in midbrain and cortical circuits that generate gamma oscillations [23].
The effect of the OT/SC on selective attention has been studied in attention tasks, in which two or more task-relevant stimuli are concurrently presented on each trial. In these tasks, the animal must attend to, and make decisions about, one of these stimuli (the ‘target’), but completely ignore the other stimuli (the ‘distracters’). Under these conditions, when the SC in monkeys is inactivated and the target stimulus is placed in the inactivated region of space, neural encoding of both the target and distracter stimuli remains intact in the sensory neocortex. Nevertheless, the animal makes significant errors by utilizing information from distracter stimuli (in non-inactivated regions of space) to guide its decisions [16].
Proposed role
In this role, space-specific gamma oscillations from the midbrain network selectively route information from forebrain sensory areas to forebrain decision-making areas (Figure 3d) [67]. This space-specific routing of information biases the sensory evidence that is used for decision-making. Typically, the bias heavily favors evidence from the cued location.
Gamma oscillations from the midbrain network, representing the cued location, are communicated to a forebrain decision-making region that integrates sensory evidence about the target. The oscillatory midbrain input generates enhanced gamma-frequency responses in PV+ inhibitory interneurons at the cued location in the decision-making area. The enhanced gamma-periodic activity of the PV+ neurons could produce a bias in favor of the cued location through two mechanisms. First, selective disinhibition: the PV+ interneurons inhibit a second class of interneurons that inhibit the output (pyramidal) neurons. This disinhibitory mechanism increases the gain of the sensory evidence being integrated for the behavioral decision. Second, coincidence dependent temporal integration: the gamma-periodic activity of the PV+ neurons synchronizes stimulus-related sensory activity, and the resultant pattern of coincident spiking is more effective at driving the evidence integrator (Figure 3a,d), thereby enhancing the gain of stimulus-related information in the final decision.
According to this role, when the SC is focally inactivated, gamma power from the midbrain network at the (inactivated) target location is eliminated, abolishing the bias for evidence from the cued location in the decision-making area. At the same time, gamma power from the non-inactivated portion of the SC, evoked by a distracter stimulus, biases information being routed to the decision-making area in favor of the distracter. This mechanism induces an erroneous bias for distracter-related information that results in incorrect decisions.
Acknowledgments
We would like to thank Phyllis Knudsen for help with figure preparation. This research was supported by grant NIH Grant EY024243 to EIK.
Footnotes
Conflict of interest
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Buzsaki G. Rhythms of the Brain. Oxford University Press; 2006. [Google Scholar]
- 2.Montgomery SM, Buzsaki G. Gamma oscillations dynamically couple hippocampal CA3 and CA1 regions during memory task performance. Proc Natl Acad Sci U S A. 2007;104:14495–14500. doi: 10.1073/pnas.0701826104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jutras MJ, Fries P, Buffalo EA. Gamma-band synchronization in the macaque hippocampus and memory formation. J Neurosci. 2009;29:12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 5.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ray S, Maunsell JH. Differences in gamma frequencies across visual cortex restrict their possible use in computation. Neuron. 2010;67:885–896. doi: 10.1016/j.neuron.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns SP, Xing D, Shapley RM. Is gamma-band activity in the local field potential of V1 cortex a ‘clock’ or filtered noise? J Neurosci. 2011;31:9658–9664. doi: 10.1523/JNEUROSCI.0660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing D, Shen Y, Burns S, Yeh CI, Shapley R, Li W. Stochastic generation of gamma-band activity in primary visual cortex of awake and anesthetized monkeys. J Neurosci. 2012;32:13873–13880. doi: 10.1523/JNEUROSCI.5644-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolic D, Fries P, Singer W. Gamma oscillations: precise temporal coordination without a metronome. Trends Cogn Sci. 2013;17:54–55. doi: 10.1016/j.tics.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 10•.Roberts MJ, Lowet E, Brunet NM, Ter Wal M, Tiesinga P, Fries P, De Weerd P. Robust gamma coherence between macaque V1 and V2 by dynamic frequency matching. Neuron. 2013;78:523–536. doi: 10.1016/j.neuron.2013.03.003. With simultaneous recordings from visual areas V1 and V2 in monkeys, the authors showed that gamma-band coherence manifests robustly between remote populations in the two areas despite significant non-stationarities in gamma frequency, thereby providing a mechanism for communication through oscillatory gamma coherence. [DOI] [PubMed] [Google Scholar]
- 11.Hermes D, Miller KJ, Wandell BA, Winawer J. Stimulus dependence of gamma oscillations in human visual cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu091. http://dx.doi.org/10.1093/cercor/bhu091. [DOI] [PMC free article] [PubMed]
- 12.Buzsaki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- 14.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen EI. Control from below: the role of a midbrain network in spatial attention. Eur J Neurosci. 2011;33:1961–1972. doi: 10.1111/j.1460-9568.2011.07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Major DE, Karten HJ. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus) J Comp Neurol. 2004;469:275–297. doi: 10.1002/cne.11007. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol. 2006;494:7–35. doi: 10.1002/cne.20821. [DOI] [PubMed] [Google Scholar]
- 19.Marin G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J Neurosci. 2007;27:8112–8121. doi: 10.1523/JNEUROSCI.1420-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marin G, Mpodozis J, Sentis E, Ossandon T, Letelier JC. Oscillatory bursts in the optic tectum of birds represent reentrant signals from the nucleus isthmi pars parvocellularis. J Neurosci. 2005;25:7081–7089. doi: 10.1523/JNEUROSCI.1379-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Sridharan D, Boahen K, Knudsen EI. Space coding by gamma oscillations in the barn owl optic tectum. J Neurophysiol. 2011;105:2005–2017. doi: 10.1152/jn.00965.2010. This study characterized the spatial tuning and strength encoding properties of gamma oscillations across the superficial and deep layers of the owl optic tectum, in vivo. The authors demonstrated that both visual and auditory stimuli induced space-specific gamma-band responses with robust spike-field coherence in the low-gamma band. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray S, Maunsell JH. Different origins of gamma rhythm and high-gamma activity in macaque visual cortex. PLoS Biol. 2011;9:e1000610. doi: 10.1371/journal.pbio.1000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pesaran B, Pezaris JS, Sahani M, Mitra PP, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nat Neurosci. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- 25.Zanos TP, Mineault PJ, Pack CC. Removal of spurious correlations between spikes and local field potentials. J Neurophysiol. 2010;105:474–486. doi: 10.1152/jn.00642.2010. [DOI] [PubMed] [Google Scholar]
- 26.Wang XJ. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol Rev. 2010;90:1195–1268. doi: 10.1152/physrev.00035.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Goddard CA, Sridharan D, Huguenard JR, Knudsen EI. Gamma oscillations are generated locally in an attention-related midbrain network. Neuron. 2012;73:567–580. doi: 10.1016/j.neuron.2011.11.028. This study showed that in vivo-like gamma oscillations can be evoked in acute slices of the avian midbrain that preserved the connectivity between the optic tectum and the cholinergic nucleus, Ipc, in vitro. The authors identified key circuit mechanisms that generate and regulate midbrain gamma oscillations. The findings confirmed that the midbrain contains its own, independent circuit for generating gamma oscillations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asadollahi A, Mysore SP, Knudsen EI. Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci. 2010;13:889–895. doi: 10.1038/nn.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Marin GJ, Duran E, Morales C, Gonzalez-Cabrera C, Sentis E, Mpodozis J, Letelier JC. Attentional capture? Synchronized feedback signals from the isthmi boost retinal signals to higher visual areas. J Neurosci. 2012;32:1110–1122. doi: 10.1523/JNEUROSCI.4151-11.2012. By recording from the avian Ipc, in vivo, this study showed that gamma-rhythmic, bursting discharges in the Ipc are synchronized with bursting activity recorded in the thalamic nucleus that receives projections from the optic tectum, as well as in subsequent, high-order forebrain areas. Inactivating the Ipc abolished gamma-rhythmic bursting in these thalamic and forebrain regions, indicating that the midbrain drives gamma-synchronization in these regions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neuenschwander S, Varela FJ. Visually triggered neuronal oscillations in the pigeon: an autocorrelation study of tectal activity. Eur J Neurosci. 1993;5:870–881. doi: 10.1111/j.1460-9568.1993.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 31.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989;86:1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nase G, Singer W, Monyer H, Engel AK. Features of neuronal synchrony in mouse visual cortex. J Neurophysiol. 2003;90:1115–1123. doi: 10.1152/jn.00480.2002. [DOI] [PubMed] [Google Scholar]
- 33.Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 35.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 36•.Brunet N, Bosman CA, Roberts M, Oostenveld R, Womelsdorf T, De Weerd P, Fries P. Visual cortical gamma-band activity during free viewing of natural images. Cereb Cortex. 2013 doi: 10.1093/cercor/bht280. http://dx.doi.org/10.1093/cercor/bht280 With electrocorticographic (ECoG) grid recordings in monkeys this study showed that gamma-band responses were induced during free-viewing of natural images. Induced responses exhibited a clear spectral peak in the 50–80 Hz range over much of visual area V1 indicative of a true network oscillation. [DOI] [PMC free article] [PubMed]
- 37.Buffalo EA, Fries P, Landman R, Buschman TJ, Desimone R. Laminar differences in gamma and alpha coherence in the ventral stream. Proc Natl Acad Sci U S A. 2011;108:11262–11267. doi: 10.1073/pnas.1011284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 39.Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 40.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, Rozov A, Whittington MA, Traub RD, Rawlins JN, Monyer H. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 41.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513(Pt 1):117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 45.Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 46.Deco G, Thiele A. Attention: oscillations and neuropharmacology. Eur J Neurosci. 2009;30:347–354. doi: 10.1111/j.1460-9568.2009.06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borgers C, Epstein S, Kopell NJ. Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc Natl Acad Sci U S A. 2005;102:7002–7007. doi: 10.1073/pnas.0502366102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- 49.Pinault D. N-methyl D-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry. 2008;63:730–735. doi: 10.1016/j.biopsych.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–1464. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 51•.Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D, Ruhlmann C, Jones SR, Deisseroth K, Sheng M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17:537–548. doi: 10.1038/mp.2011.31. This study showed that mutant mice lacking NMDA-R neurotransmission selectively in parvalbumin-positive interneurons exhibited enhanced baseline gamma rhythms and impaired gamma rhythm induction following optogenetic stimulation. In addition, the mutant mice showed pronounced behavioral deficits in cognitive tasks, indicating a specific role for NMDA-Rs on PV+ interneurons in associative learning and working memory function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray CM, McCormick DA. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science. 1996;274:109–113. doi: 10.1126/science.274.5284.109. [DOI] [PubMed] [Google Scholar]
- 53.Steriade M, Timofeev I, Grenier F, Durmuller N. Role of thalamic and cortical neurons in augmenting responses and self-sustained activity: dual intracellular recordings in vivo. J Neurosci. 1998;18:6425–6443. doi: 10.1523/JNEUROSCI.18-16-06425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cunningham MO, Whittington MA, Bibbig A, Roopun A, LeBeau FE, Vogt A, Monyer H, Buhl EH, Traub RD. A role for fast rhythmic bursting neurons in cortical gamma oscillations in vitro. Proc Natl Acad Sci U S A. 2004;101:7152–7157. doi: 10.1073/pnas.0402060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36:165–182. doi: 10.1146/annurev-neuro-062012-170249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Kerkoerle T, Self MW, Dagnino B, Gariel-Mathis MA, Poort J, van der Togt C, Roelfsema PR. Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex. Proc Natl Acad Sci U S A. 2014;111:14332–14341. doi: 10.1073/pnas.1402773111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gale SD, Murphy GJ. Distinct representation and distribution of visual information by specific cell types in mouse superficial superior colliculus. J Neurosci. 2014;34:13458–13471. doi: 10.1523/JNEUROSCI.2768-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.King WM. Nicotinic depolarization of optic nerve terminals augments synaptic transmission. Brain Res. 1990;527:150–154. doi: 10.1016/0006-8993(90)91074-q. [DOI] [PubMed] [Google Scholar]
- 59.Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- 60.Tang AC, Bartels AM, Sejnowski TJ. Effects of cholinergic modulation on responses of neocortical neurons to fluctuating input. Cereb Cortex. 1997;7:502–509. doi: 10.1093/cercor/7.6.502. [DOI] [PubMed] [Google Scholar]
- 61.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang S, Xu M, Kamigaki T, Hoang Do JP, Chang WC, Jenvay S, Miyamichi K, Luo L, Dan Y. Selective attention. Long-range and local circuits for top-down modulation of visual cortex processing. Science. 2014;345:660–665. doi: 10.1126/science.1254126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 2006;10:382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 64.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 65.Rossant C, Leijon S, Magnusson AK, Brette R. Sensitivity of noisy neurons to coincident inputs. J Neurosci. 2011;31:17193–17206. doi: 10.1523/JNEUROSCI.2482-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brette R. Computing with neural synchrony. PLoS Comput Biol. 2012;8:e1002561. doi: 10.1371/journal.pcbi.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hahn G, Bujan AF, Fregnac Y, Aertsen A, Kumar A. Communication through resonance in spiking neuronal networks. PLoS Comput Biol. 2014;10:e1003811. doi: 10.1371/journal.pcbi.1003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sridharan D, Schwarz JS, Knudsen EI. Selective attention in birds. Curr Biol. 2014;24:R510–R513. doi: 10.1016/j.cub.2013.12.046. [DOI] [PubMed] [Google Scholar]