Introduction
The term malnutrition can be defined as a condition when the body does not receive enough nutrition to maintain health. Malnutrition can also be defined as a loss of muscle and/or fat mass. In addition to skeletal muscle loss, another component of malnutrition is an alteration in energy metabolism with accelerated starvation1,2. Malnutrition is the most frequent complication in liver disease and cirrhosis, however, there are no standardized definitions which makes comparisons across studies difficult. It is necessary to accurately define nutritional status in alcoholic liver disease so that published data can be interpreted appropriately and the use of standardized terminology will allow comparisons of results from different studies3. The term malnutrition has been used to define loss of body weight, muscle mass, fat mass, muscle strength, visceral protein levels, immune function as well as poor oral intake of nutrients4-6. Each of these measures suffers from significant limitations. Visceral protein concentrations in blood including albumin, pre-albumin and retinol binding proteins have been used extensively but are truly measures of hepatic synthetic function4. Immune function is directly altered by alcohol and viral infection and cannot be truly considered measures of hepatic effects on the skeletal muscle or nutritional indices7. Anthropometric measures including skinfold thickness that measures fat mass and arm muscle area are used as nutritional indices but suffer from intra and inter-observer variability8. Nitrogen balance studies are difficult and suffer from imprecision in the clinical setting, long term accurate dietary intake studies are difficult and retrospective studies suffer from recall bias. The term malnutrition is increasingly used to refer to the phenotype of loss of skeletal muscle mass with/without fat loss and not dietary intake, digestion, and absorption of nutrients even though the latter contribute to muscle and fat loss and disordered energy metabolism.
The term sarcopenia refers to muscle loss in patients with chronic diseases 2,9-11. Even though micronutrient deficiencies are common in alcoholic hepatitis and cirrhosis12,13, the focus of this review will be on skeletal muscle loss and how it is integrated with the disordered energy metabolism in patients with alcoholic liver disease (ALD).
Most publications refer to malnutrition in cirrhosis and alcoholic liver disease without specifying if the effects are due to sarcopenia or loss of fat mass or a combination of both. Careful analyses of published studies show that sarcopenia is the major component of malnutrition4,5,14,15 and both liver injury and alcohol contribute to muscle loss. The role of nutritional deficiencies on progression of liver injury is not known. Myokines are proteins secreted by the skeletal muscle including interleukin 6 and 15 that may alter hepatic metabolism and fibrosis but a muscle-liver axis has not yet been established16,17. The present review will focus on prevalence and methods to quantify malnutrition, specifically sarcopenia, the mechanisms of muscle loss in alcoholic liver disease including the liver-muscle axis and potential therapies including novel, molecular targeted treatments. The impact of disordered energy metabolism will be discussed in the context of its contribution to sarcopenia. Micronutrient deficiencies including zinc are common in patients with ALD, and their impact on outcomes in alcoholic liver disease have been reviewed elsewhere and will not be discussed in detail here12,13,18. The effect of different nutrient supplements on hepatic histology and liver function in ALD will also not be discussed and the interested reader is referred to reviews published on this topic 19,20. For more information on nutritional therapy for acute alcoholic hepatitis, please see Paulina K. Phillips and Michael R. Lucey: Acute Alcoholic Hepatitis – Therapy, in this issue.
Prevalence and methods to quantify malnutrition in alcoholic liver disease
Malnutrition is present in 20-90% of patients with liver disease and sarcopenia in nearly 70% of these patients2,4,5,21,22. We have recently reported that the prevalence of alcoholic cirrhosis is likely to be the major form of liver disease in the United States23. Therefore, malnutrition, and its constituents, muscle loss and fat loss, are major clinical consequences that need to be managed. As mentioned earlier, different criteria have been used to define malnutrition but there is evolving consensus among hepatologists and transplant surgeons that the major determinant of outcome is skeletal muscle mass10,24. In addition to anthropometric measurements, lean body mass quantified by bioelectrical impedance analysis, dual energy X ray absorptiometry and impedance plethysmography have also been used as a measure of skeletal muscle mass despite the recognized limitations of these techniques (see table 1)25. Recently, imaging methods including ultrasonography, computed tomography and magnetic resonance imaging have been used to quantify skeletal muscle mass26,27. Skeletal muscle area determined from a single section at the 3rd or 4th lumbar vertebra on CT or MRI has been shown to reflect whole body muscle mass26. Using these methods, cirrhotic patients have lower muscle mass and fat mass compared to controls28,29. Even though most studies to date have included alcoholic cirrhosis as part of the cohort of cirrhotic patients studied, there are no published data on differences in prevalence of sarcopenia defined by direct muscle mass measurements stratified by etiology of cirrhosis.
Table 1.
Methods to quantify malnutrition in alcoholic liver disease
| Assessment tool | Advantages | Disadvantages |
|---|---|---|
|
Anthropometry Skin fold thickness Limb circumference |
Simple, inexpensive, easy to perform. | Overinterpretation- muscle mass is not directly measured, bone size is assumed. Inter and intraobserver variability makes reproducibility difficult. Fat mass is measured but accuracy and precision not certain. |
| Subjective global assessment | Simple, inexpensive, easy to use. | Long questionnaire, modifications limit precision. Main emphasis is on muscle mass Inter and intraobserver variability. Fat and muscle mass contribute to score but muscle loss major factor. |
| Grip strength | Objective, reproducible. | Measures contractile strength and not muscle mass. No measure of fat mass. |
|
Lean body mass Bioelecrical impedance analysis Dual energy Xray absorptiometry Impedance plethysmography |
Objective, reproducible. | Measures lean body/ fat free mass an indirect measure of muscle mass. DEXA has risk of radiation, especially if used for repeated measures. |
|
Imaging methods Ultrasound CT abdomen MRI abdomen |
Direct quantification of muscle and fat mass. | Expensive. Observer variability with ultrasound. Radiation risk with CT. Single slices are an estimate of whole body measurements. |
|
Indirect calorimetry Cart calorimeter Hand held calorimeter |
Measures resting energy expenditure, respiratory quotient. Hand-held calorimeter easy to use and portable, can be used in most clinical settings in cirrhotics. Can be used to determine metabolic response to nutrient interventions. |
Conventional cart based indirect calorimetry logistically difficult. Hand held calorimetry not as accurate as conventional cart calorimetry. |
Cirrhosis is a state of accelerated starvation that is defined not by appetite, rather by the low respiratory quotient, that is the ratio of the oxygen consumed and carbon dioxide released28,30. Unlike that observed in healthy subjects, post-prandial reduction in RQ occurs much earlier in cirrhotics suggesting a more rapid fatty acid and amino acid oxidation as a source of energy30,31. The use of indirect calorimetry to define starvation and nutritional needs are not universally used even though hand held, easy to use calorimeters are now available for clinical use28,32,33. Using these criteria, most patients with cirrhosis, at least in the hospitalized setting have low RQ and the reduction in RQ is associated with sarcopenia providing the critical link between muscle and fat loss as well as disordered energy metabolism. As mentioned before, even though the clinical consequences of sarcopenia, the major component of malnutrition is now well recognized, the other components of malnutrition, fat loss and altered whole body energy utilization are not as well understood and their contribution to clinical outcomes have not been evaluated but reduction in RQ has been reported to be accompanied by more severe muscle loss28. These observations suggest that accelerated utilization of fat is associated with muscle loss, but the mechanisms by which more rapid fatty acid oxidation results in sarcopenia are not well understood.
In the past, it was believed that patients with alcoholic liver disease have more severe malnutrition, using anthropometric and subjective global assessment criteria14,15,34. However, this has been questioned by others, who have reported no significant differences in the prevalence of malnutrition between alcoholic and non-alcoholic cirrhosis35,36. Concerns with interpretation of published data include the definition of and measures used to define malnutrition, the duration between the time alcohol was consumed last and the determination of nutritional indices, the severity of liver disease in different groups and the contribution of other factors that result in progression of muscle and fat loss (see box 2, and figure 1) 22,35,36.
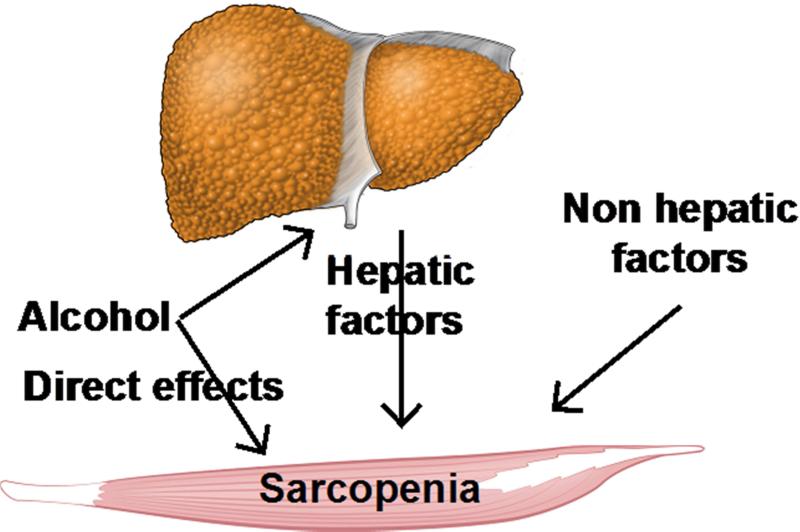
Figure 1. Schematic of mediators of sarcopenia in alcoholic liver disease.
A number of factors contribute to sarcopenia in ALD including the direct effects of ethanol or its metabolism in the muscle as well as ethanol-induced liver disease with consequent hyperammonemia as well as non-hepatic factors including gut-derived endotoxemia and cytokine-mediated effects.
Clinical consequences of malnutrition in alcoholic liver disease
Almost all patients with liver disease will have some evidence of malnutrition, specifically, sarcopenia21,37. A number of factors contribute to varying degrees to malnutrition and sarcopenia in cirrhosis including ALD (see boxes 3 and 4). Even though the adverse impact of malnutrition on survival, quality of life, and development of other complications of cirrhosis has been well recognized1,2,38, recent data on the impact of sarcopenia has been much more compelling. Sarcopenia in cirrhosis adversely affects long-term survival24, development of other complications of liver disease39,40 and post liver transplantation outcomes10. Because skeletal muscle is a major non-hepatic organ for ammonia disposal41,42, it is intuitively obvious that sarcopenia will be accompanied by more severe encephalopathy as was reported recently40. Other complications including hospitalizations and infections are also more frequent in malnourished/sarcopenic cirrhotic patients39,40,43. Whether the severity of malnutrition, specifically sarcopenia in alcoholic liver disease contributes to adverse clinical outcomes is not well studied. Published data, that are predominantly descriptive, do provide compelling evidence that malnutrition, specifically sarcopenia, is a major adverse consequence of alcoholic liver disease. Interestingly, malnutrition is severe as measured by anthropometric and visceral protein levels in acute alcoholic hepatitis but direct body composition measures and muscle mass by imaging have not been reported14,15,34.
Hypermetabolism is another fairly consistent observation in cirrhosis of any etiology and contributes to increased mortality44. The mechanisms of hypermetabolism and its regulation in cirrhosis are however not well understood45,46. Whether alcoholic cirrhotics are hypermetabolic to a greater degree than non-alcoholic cirrhotic is not known. Interestingly, despite ethanol contributing to “empty calories”, patients with alcoholic cirrhosis and alcohol abuse are not obese47. This has been suggested to be due to increased energy expenditure and lower respiratory quotient induced by ethanol that prevents fat accumulation and potentially promotes fat loss.
Micronutrient deficiencies have been extensively examined in alcoholic patients and their replacement should be part of routine clinical practice. Micronutrient deficiencies occur in patients with ALD because the major proportion of calories derived from alcohol lack minerals and vitamins. Specific emphasis is necessary for zinc, vitamin D, thiamine, folate, cyanocobalamin, and selenium13. Selenium is gaining interest because of the recognition of selenocysteine and selenoproteins as potential antioxidants in various tissues including skeletal muscle. Whether these mineral deficiencies, including zinc and selenium contribute to impaired ammonia disposal and protein synthesis are not known13,48,49.
In summary, there is clinical recognition of the contribution of malnutrition to poor outcomes but the focus of therapies has always been on reversing the hepatic injury. Lack of effective management strategies for malnutrition in alcoholic liver disease has been mainly due to the absence of an integrated approach to understand the molecular pathogenesis2. An in-depth analysis of the potential molecular mediators of sarcopenia50-52, the major component of malnutrition is beyond the scope of this review but a summary of recent advances is provided below that allows identification of novel therapeutic targets.
Molecular and metabolic perturbations contributing to malnutrition in alcoholic liver disease
There are two major contributors to sarcopenia in alcoholic liver disease: 1. consequences of liver disease, and 2. the direct effect of ethanol. Liver disease has two major components: 1. hepatic necroinflammation/fibrosis with hepatocyte dysfunction, and 2. vascular consequences resulting in hepatic bypass of portal circulation. One of the mediators of the liver muscle axis is hyperammonemia that occurs due to impaired hepatic ureagenesis 42,53-55. The effects of ethanol include lower skeletal muscle protein synthesis56-59, increased autophagy60, and mitochondrial dysfunction caused by ethanol or its metabolites including acetaldehyde61,62. In alcoholic cirrhosis and liver disease, both hyperammonemia and ethanol contribute to muscle loss that can explain more severe muscle loss in these patients. One of the questions is the relative contribution of ethanol versus hyperammonemia to sarcopenia. Importantly, acetaldehyde, the immediate oxidative product of ethanol impairs hepatic ureagenesis63, the main metabolic disposal pathway for cytotoxic ammonia. This is of immediate clinical and translational relevance because therapeutic targets may be different and the response to therapies may be affected by the impact of these divergent mechanisms. In addition to hyperammonemia and ethanol effects, other alterations including endotoxemia and cytokine alterations in alcoholic liver disease also contribute to sarcopenia by a number of mechanisms. Major advances in our understanding of maintenance of skeletal muscle mass and the impact of ethanol on these pathways allows for identifying novel molecular targets (see figure 2)50,64. The canonical signaling pathway for protein synthesis is mediated by the Akt/PKB dependent phosphorylation and activation of mTORC1 that in turn activates downstream molecules to promote protein synthesis. mTORC1 also inhibits autophagy and ethanol has been reported to inhibit mTORC1 phosphorylation in animal and human studies suggesting that this may be a common mechanism of molecular perturbations and sarcopenia60. Interestingly, hyperammonemia also inhibits mTORC1 but the mechanisms may be different. Expression of myostatin, a TGFβ superfamily member, is increased in response to hyperammonemia and alcohol42,59,65. Ethanol activates autophagy via its metabolite acetaldehyde60. Skeletal muscle expresses CYP2E1, a component of the microsomal ethanol oxidizing system (MEOS) that metabolizes ethanol and generates reactive oxygen species in addition to mitochondrial dysfunction and mitochondrial reactive oxygen species, targeting either ethanol metabolism or the consequent perturbations may provide novel targets to reverse sarcopenia of alcoholic liver disease.
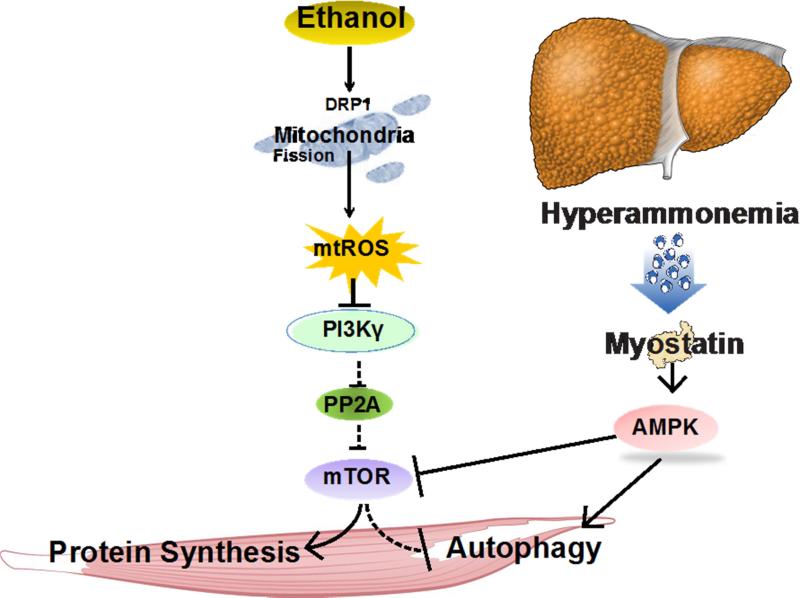
Figure 2. Schematic of putative molecular pathways contributing to sarcopenia of liver disease.
Ethanol or its metabolites (primarily acetaldehyde) activate a number of pathways either via myostatin or reactive oxygen species-mediated impairment of critical molecular signaling component, mammalian target of rapamycin complex 1 (mTORC1) with reduced protein synthesis and increased autophagy, both of which contribute to sarcopenia and loss of muscle mass. Dephosphorylation of critical regulatory proteins (mTORC1, DRP1) at specific sites causes perturbation of downstream signaling responses. AMPK AMP kinase; DRP1 dynamin related protein 1 (mitochondrial fission regulatory protein); PP2A protein phosphatase 2 A; PI3Kγ Phosphoinositide 3 kinase gamma isoform; ROS reactive oxygen species.
A number of metabolic perturbations also occur in skeletal muscle in response to ethanol exposure. Ethanol is metabolized primarily in the liver, but is also metabolized in non-hepatic (muscle) tissue66, there is increased oxidation via the mitochondrial and non-mitochondrial MEOS. Since MEOS oxidation does not generate much ATP and ethanol causes mitochondrial dysfunction, skeletal muscle ATP content is expected to be lower. Preliminary studies from our laboratory have shown that muscle mitochondrial dysfunction and lower ATP content in response to ethanol. Since protein synthesis is one of the most energy intense processes, it is possible that the additional contribution of impaired ATP synthesis also contributes to sarcopenia. Both molecular perturbations with low mTORC1 activation and low ATP content with increased reactive oxygen species activate autophagy, a protein breakdown mechanism that is additive with the impaired protein synthesis42,60. Finally, perturbations due to ethanol are compounded by ammonia induced metabolic alterations including lower tricarboxylic acid cycle intermediates in the skeletal muscle that also contribute to reduced muscle mass.
Ethanol induces loss of adipose tissue mass and oxidative stress as well as lipolysis all of which contribute to lower fat mass47 in addition to reduced muscle mass in patients with alcoholic liver disease.
Role of the gut in malnutrition in alcoholic liver disease
Gut microbial ammoniagenesis is well recognized and its contribution to hyperammonemia results in not only encephalopathy67, but to sarcopenia also42,68. As mentioned earlier, in addition to the consequences of cirrhosis, ethanol directly impairs absorption, results in defective gut permeability barrier and alters gut microbiota all of which contribute to decreased absorption and transport of essential nutrients and endotoxemia69-73. There is nascent data that the gut microbiome contributes to skeletal muscle responses but few studies have examined the role of disrupted gut permeability and consequent endotoxemia on nutritional consequences including sarcopenia74. Targeting the gut as a potential mechanism to reverse muscle loss and other components of malnutrition including lipopolysaccharide-induced hypermetabolism are novel areas that need investigation.
Management of malnutrition in alcoholic liver disease (see box 5)
The mainstay of the management of malnutrition in alcoholic liver disease has focused on nutrition therapy that has not been effective except in replacing micronutrients18,75. Malnutrition is a relatively broad term and response can depend on the specific component being targeted and the specificity of the measures used to quantify malnutrition. Additionally, treatments that do not target the mechanisms that contribute to malnutrition can also explain the lack of effective therapies. Increased protein intake is likely to be of benefit in patients with ALD and those with cirrhosis. However, just providing calories and proteins without altering the translational machinery responsible for peptide chain elongation, decreasing the expression of genes that impair protein synthesis or altering the mechanisms of protein breakdown via the autophagy pathway contribute to the absence of any definitive measures. Malnutrition and sarcopenia are nearly universal in patients with alcoholic liver disease. Thus, reversing sarcopenia should be an integral therapeutic strategy as this improves survival in cirrhosis76. It is however not known if improving malnutrition/sarcopenia will also improve other outcome measures including encephalopathy, ascites, infection, hospitalizations and quality of life.
Because cirrhosis is a state of accelerated starvation, strategies to lower respiratory quotient (RQ) may be helpful because low RQ has been correlated with sarcopenia28. Late evening snacks, decreasing periods of post prandial or fasting states by providing daytime snacks, preventing prolonged fasting in hospitalized patients for various medical procedures, and encouraging increased protein intake (specifically, low ammoniagenic proteins such as vegetable proteins) are simple strategies to increase RQ and avoid sarcopenia and its adverse consequences.
Abstinence
Ethanol and its metabolites contribute to malnutrition and sarcopenia by multiple mechanisms. Abstinence should therefore be the mainstay of treating these patients. Continued alcohol use in patients with ALD is likely to result in greater muscle metabolism of ethanol as well as increased toxicity due to circulating acetaldehyde-generated from the liver. Pharmacotherapy has been of limited success in ensuring abstinence, but a multidisciplinary approach should be part of improving the diet, calorie and protein intake and reversing sarcopenia.
Nutrition
A number of clinical trials and a meta-analysis have been published to date on nutritional support in cirrhosis in general and alcoholic liver disease in particular18,20,36,75,77,78. These studies have not been conclusive despite large numbers of patients studied. Reasons for inconclusive results include lack of benefit of oral, enteral and parenteral supplementation, inconsistency in the measures and definition of nutritional indices and malnutrition, heterogeneous population of patients, variable duration and composition of the supplements, and continued consequences of underlying liver disease and alcohol effects on tissues primarily, the skeletal muscle.
Evaluation of published literature suggests that a daily caloric intake of about 2000kcal with about 1.2-1.5g/kg/d protein distributed over the day with short intervals between meals to decrease the post-absorptive state is likely to be of benefit in patients with ALD and those with cirrhosis. Different routes of caloric administration can be utilized.
Oral supplementation may not be effective due to poor intake and compliance due to anorexia, dysguesia, impaired absorption, and continued hypermetabolic states. The non-aromatizable anabolic steroid, oxandrolone, showed benefit when added to oral nutritional supplementation34,79,80. Late evening snacks with high protein content have the potential to exploit the window of anabolic opportunity at night and prevent muscle loss31.
Enteral tube feedings can overcome the effects of anorexia and dysguesia but not poor enteral absorption and have been shown to be beneficial, well-tolerated, and may improve hepatic function but the impact on skeletal muscle and other nutritional parameters is not conclusive18.
Parenteral nutrition ensures delivery of nutrients and has been used in patients with ALD. In a comprehensive analysis of parenteral nutrition in alcoholic liver disease, short-term benefits on some nutritional parameters were observed but long-term consequences remain unknown. Parenteral nutrition is associated with significant risks including infections that preclude such supplementation as routine treatment in patients with ALD who have compromised immune function18.
Amino acid supplementation including branched-chain amino acids have been used in patients with a variety of liver diseases2,13,18,20,77. In acute alcoholic hepatitis there does not seem to be a benefit of branched-chain amino acid supplementation. Meta-analyses of amino acid supplementation for the treatment of cirrhosis and hepatic encephalopathy did not show consistent benefit in nutritional parameters2. There may be some benefit in improving visceral protein levels but there are no data on recovery or improvement in skeletal muscle mass in response to amino acid supplementation in cirrhotics with encephalopathy.
Of the essential amino acids, leucine has been investigated the most. In a randomized study in patients with alcoholic cirrhosis, signaling abnormalities in the skeletal muscle and the fractional muscle protein synthesis rate were reversed by a single dose of leucine-enriched branched chain amino acid mixture65. These data suggest that much higher doses of leucine may promote skeletal muscle protein synthesis and improve mitochondrial function by increased mitochondrial biogenesis. The use of high-dose leucine or isoleucine in patients with ALD warrants further evaluation.
Exercise in alcoholic liver disease
Increased physical activity improves both functional capacity and muscle mass, depending on the type of exercise performed81. Alcohol ingestion, either acutely or chronically, impairs exercise capacity, depletes skeletal muscle glycogen stores, and decreases circulating testosterone androgen receptor on the skeletal muscle, all of which impair muscle hypertrophy82,83. Exercise also increases muscle ammonia generation that may compound the adverse effects of ethanol42,68,84. Muscle strength is decreased due to both loss of as well as impaired function of contractile proteins. There are no data on the response of skeletal muscle to exercise in alcoholic liver disease specifically but data in cirrhosis in general suggests that increasing physical activity improves functional capacity81. Whether exercise in addition to nutrient supplementation increases muscle mass in the context of alcoholic liver disease is also not known. Studies on the effects of alcohol on muscle responses to exercise have been conducted only in the context of alcohol ingestion and muscle strength recovers rapidly after stopping alcohol, but it is not known if the hypertrophic responses recover after stopping alcohol83,85,86. This is of relevance since ammonia and ethanol potentially complement the adverse effects on molecular signaling pathways in the skeletal muscle42,60,68 and once abstinence is achieved, persistent hyperammonemia of chronic liver disease may continue to blunt the benefits of exercise. Based on current published data, one may however conclude that exercise in combination with abstinence and improved nutrient intake is likely to benefit both functional capacity and muscle mass in alcoholic liver disease.
Novel therapeutic targets
Myostatin
The initial report in rats that ethanol feeding increased myostatin expression that was reversed by administration of insulin-like growth factor 1 as a binary complex59 was followed by a report that myostatin expression was increased in alcoholic cirrhosis65. A number of strategies to block myostatin including monoclonal antibodies, follistatin, and antagomirs have the potential to reverse muscle loss in ALD50.
Muscle targeted antioxidants
Both mitochondrial and non-mitochondrial sites of reactive oxygen species with oxidative stress occur in the skeletal muscle. Mitochondrial targeted catalase, mitochondrial fission inhibitor, p110 are novel agents that may be helpful87. In prior studies, antioxidant supplementation has generally not been therapeutically effective48,88. However, novel methods to target specific sites of ROS generation may be beneficial and warrant investigation.
Autophagy regulators
Because increased skeletal muscle autophagy contributes to muscle loss, pharmacological regulators of autophagy may be a potential treatment option. Both inhibitors and activators of autophagy have been developed and can be used to modulate autophagy.. Another option is to identify the mechanism by which ethanol activates autophagy and control the initiation of dysregulated autophagy.
Mitoprotective agents
Mitochondrial fission and fragmentation occur in response to cellular stress and ethanol exposure in mice, and targeting mitochondrial fission machinery is a potential therapeutic approach87. Ethanol also causes a reduction in tricarboxylic acid cycle intermediates and providing cell permeable esters of these intermediates holds promise not only to provide anaplerotic substrates but also a novel method to remove ammonia by conversion to glutamine.
Summary
The lack of common data elements/standardized definitions of various terms that are included under the term “malnutrition” has resulted in difficulty in interpreting data from different groups. Because sarcopenia is the major component of “malnutrition”, future studies evaluating interventions should include sarcopenia as an outcome measure. Because muscle mass changes slowly over time, other more acute measures are needed including changes in resting energy expenditure and respiratory quotient using the relatively easily accessible hand held calorimeters. Increasing calories and high quality proteins alone are clearly insufficient and targeting molecular and metabolic perturbations is likely be a more successful strategy. The anticipated increase in proportion and number of patients with ALD in the next decades, limited therapeutic armamentarium to treat alcohol use, and continued organ shortage for transplantation make it imperative to develop novel and effective non-transplant treatments for ALD. Improving muscle mass is expected to increase survival, improve quality of life and lower the incidence of other complications in patients and should form a priority area for future clinical studies in patients with ALD.
Key Points.
Malnutrition is the most frequent complication in alcoholic liver disease (ALD) and adversely affects the clinical outcomes of the disease.
Malnutrition in liver disease has 2 components: loss of skeletal muscle mass or sarcopenia and perturbations in energy metabolism.
Among the different etiologies of liver disease, malnutrition and sarcopenia are believed to be most severe in alcoholic liver disease.
Very recent data show that alcohol is directly metabolized in the skeletal muscle and contributes to loss of muscle mass but the contribution of skeletal muscle ethanol metabolism to other organ injury is not known.
Other contributors include hyperammonemia of liver disease, endotoxemia and cytokine-mediated muscle loss.
Box 2. Factors affecting severity/measures of malnutrition in alcoholic liver disease.
Duration of alcohol use/abuse
Amount of alcohol consumed
Time of measurement from last alcohol consumed
Other underlying causes of liver disease
Severity of liver disease (Child-Pugh/MELD score)
Specific measure used (anthropometry, body composition, circulating proteins, immune function, muscle mass, indirect calorimetry)
Box 3. Factors contributing to sarcopenia in alcoholic liver disease.
Reduced oral intake due to inebriated state
Anorexia
Dysguesia
Low quality of diet
Hypermetabolism
Low sodium diet
Acute hepatitis- cytokines
Hyperammonemia of liver disease
Other complications that affect nutrient intake- gastrointestinal bleeding, encephalopathy
Diarrhea, portal hypertensive enteropathy with reduced nutrient absorption
Nausea, emesis
Box 4. Factors specific to alcohol induced sarcopenia.
Direct metabolism of ethanol by muscle with changes in redox ratio
Ethanol induced skeletal muscle mitochondrial dysfunction
Protein adducts with increased muscle autophagy
Impaired muscle protein synthesis
Box 5. Nutritional supplementation in alcoholic liver disease.
Step 1. Abstinence from alcohol
- Step 2. Nutritional support: increased protein/calorie intake
- Oral supplementation
- Enteral nutrition
- Parenteral nutrition
- Amino acid supplementation
- Micronutrient replacement
- Step 3
- Increase physical activity
- Step 4
- Novel targets
- Myostatin antagonists
- mTORC1 activators
- Muscle targeted antioxidants
- Autophagy regulators
- Mitoprotective agents
- Cell permeable TCA cycle intermediates
- Specific amino acid and analog supplementation
Acknowledgments
Dr. Dasarathy's research has been funded in part by NIH RO1 DK 83414, R21 AA 022742, UO1 AA021893 and UO1DK061732.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16(1):95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3(4):225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. https://www.nlm.nih.gov/cde/. Common Data Elements Portal at NIH.
- 4.Merli M, Romiti A, Riggio O, Capocaccia L. Optimal nutritional indexes in chronic liver disease. JPEN J Parenter Enteral Nutr. 1987;11(5 Suppl):130S–134S. doi: 10.1177/014860718701100521. [DOI] [PubMed] [Google Scholar]
- 5.Romiti A, Merli M, Martorano M, et al. Malabsorption and nutritional abnormalities in patients with liver cirrhosis. Ital J Gastroenterol. 1990;22(3):118–123. [PubMed] [Google Scholar]
- 6.Alberino F, Gatta A, Amodio P, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17(6):445–450. doi: 10.1016/s0899-9007(01)00521-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown LA, Cook RT, Jerrells TR, et al. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30(9):1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 8.Klipstein-Grobusch K, Georg T, Boeing H. Interviewer variability in anthropometric measurements and estimates of body composition. Int J Epidemiol. 1997;26(Suppl 1):S174–180. doi: 10.1093/ije/26.suppl_1.s174. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 10.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasarathy S, McCullough AJ, Muc S, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54(5):915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammad MK, Zhou Z, Cave M, Barve A, McClain CJ. Zinc and liver disease. Nutr Clin Pract. 2012;27(1):8–20. doi: 10.1177/0884533611433534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leevy CM, Moroianu SA. Nutritional aspects of alcoholic liver disease. Clin Liver Dis. 2005;9(1):67–81. doi: 10.1016/j.cld.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Mendenhall CL, Moritz TE, Roselle GA, et al. A study of oral nutritional support with oxandrolone in malnourished patients with alcoholic hepatitis: results of a Department of Veterans Affairs cooperative study. Hepatology. 1993;17(4):564–576. doi: 10.1002/hep.1840170407. [DOI] [PubMed] [Google Scholar]
- 15.Mendenhall CL, Moritz TE, Roselle GA, et al. Protein energy malnutrition in severe alcoholic hepatitis: diagnosis and response to treatment. The VA Cooperative Study Group #275. JPEN J Parenter Enteral Nutr. 1995;19(4):258–265. doi: 10.1177/0148607195019004258. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Reimers E, Fernandez-Rodriguez CM, Santolaria-Fernandez F, et al. Interleukin-15 and other myokines in chronic alcoholics. Alcohol Alcohol. 2011;46(5):529–533. doi: 10.1093/alcalc/agr064. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(Pt 2):337–346. doi: 10.1242/jeb.048074. [DOI] [PubMed] [Google Scholar]
- 18.Halsted CH. Nutrition and alcoholic liver disease. Semin Liver Dis. 2004;24(3):289–304. doi: 10.1055/s-2004-832941. [DOI] [PubMed] [Google Scholar]
- 19.Mullen KD, Dasarathy S. POTENTIAL NEW THERAPIES FOR ALCOHOLIC LIVER DISEASE. Clinics in Liver Disease. 2(4):851–881. [Google Scholar]
- 20.Stickel F, Hoehn B, Schuppan D, Seitz HK. Review article: Nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. 2003;18(4):357–373. doi: 10.1046/j.1365-2036.2003.01660.x. [DOI] [PubMed] [Google Scholar]
- 21.Guglielmi FW, Panella C, Buda A, et al. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2005;37(9):681–688. doi: 10.1016/j.dld.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Campillo B, Richardet JP, Scherman E, Bories PN. Evaluation of nutritional practice in hospitalized cirrhotic patients: results of a prospective study. Nutrition. 2003;19(6):515–521. doi: 10.1016/s0899-9007(02)01071-7. [DOI] [PubMed] [Google Scholar]
- 23.Guirguis J, Chhatwal J, Dasarathy J, et al. Clinical Impact of Alcohol-Related Cirrhosis in the Next Decade: Estimates Based on Current Epidemiological Trends in the United States. Alcohol Clin Exp Res. 2015;39(11):2085–2094. doi: 10.1111/acer.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montano-Loza AJ, Meza-Junco J, Prado CM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–173. e161. doi: 10.1016/j.cgh.2011.08.028. 173. [DOI] [PubMed] [Google Scholar]
- 25.Dasarathy J, Alkhouri N, Dasarathy S. Changes in body composition after transjugular intrahepatic portosystemic stent in cirrhosis: a critical review of literature. Liver Int. 2011;31(9):1250–1258. doi: 10.1111/j.1478-3231.2011.02498.x. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 27.Bemben MG. Use of diagnostic ultrasound for assessing muscle size. J Strength Cond Res. 2002;16(1):103–108. [PubMed] [Google Scholar]
- 28.Glass C, Hipskind P, Tsien C, et al. Sarcopenia and a physiologically low respiratory quotient in patients with cirrhosis: a prospective controlled study. J Appl Physiol (1985) 2013;114(5):559–565. doi: 10.1152/japplphysiol.01042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsien C, Garber A, Narayanan A, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29(6):1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plank LD, Gane EJ, Peng S, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- 31.Tsien CD, McCullough AJ, Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27(3):430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 32.Hipskind P, Glass C, Charlton D, Nowak D, Dasarathy S. Do handheld calorimeters have a role in assessment of nutrition needs in hospitalized patients? A systematic review of literature. Nutr Clin Pract. 2011;26(4):426–433. doi: 10.1177/0884533611411272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass C, Hipskind P, Cole D, Lopez R, Dasarathy S. Handheld calorimeter is a valid instrument to quantify resting energy expenditure in hospitalized cirrhotic patients: a prospective study. Nutr Clin Pract. 2012;27(5):677–688. doi: 10.1177/0884533612446195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendenhall C, Roselle GA, Gartside P, Moritz T. Relationship of protein calorie malnutrition to alcoholic liver disease: a reexamination of data from two Veterans Administration Cooperative Studies. Alcohol Clin Exp Res. 1995;19(3):635–641. doi: 10.1111/j.1530-0277.1995.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 35.Caregaro L, Alberino F, Amodio P, et al. Malnutrition in alcoholic and virus-related cirrhosis. Am J Clin Nutr. 1996;63(4):602–609. doi: 10.1093/ajcn/63.4.602. [DOI] [PubMed] [Google Scholar]
- 36.McClain CJ, Barve SS, Barve A, Marsano L. Alcoholic liver disease and malnutrition. Alcohol Clin Exp Res. 2011;35(5):815–820. doi: 10.1111/j.1530-0277.2010.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31(1):193–199. doi: 10.1016/j.nut.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23(11):982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- 39.Merli M, Nicolini G, Angeloni S, Riggio O. Malnutrition is a risk factor in cirrhotic patients undergoing surgery. Nutrition. 2002;18(11-12):978–986. doi: 10.1016/s0899-9007(02)00984-x. [DOI] [PubMed] [Google Scholar]
- 40.Merli M, Giusto M, Lucidi C, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28(2):281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 41.Lockwood AH, McDonald JM, Reiman RE, et al. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia. J Clin Invest. 1979;63(3):449–460. doi: 10.1172/JCI109322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110(45):18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merli M, Lucidi C, Giannelli V, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8(11):979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 44.Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85(5):1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 45.Muller MJ, Bottcher J, Selberg O, et al. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69(6):1194–1201. doi: 10.1093/ajcn/69.6.1194. [DOI] [PubMed] [Google Scholar]
- 46.Mathur S, Peng S, Gane EJ, McCall JL, Plank LD. Hypermetabolism predicts reduced transplant-free survival independent of MELD and Child-Pugh scores in liver cirrhosis. Nutrition. 2007;23(5):398–403. doi: 10.1016/j.nut.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Levine JA, Harris MM, Morgan MY. Energy expenditure in chronic alcohol abuse. Eur J Clin Invest. 2000;30(9):779–786. doi: 10.1046/j.1365-2362.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 48.Ward RJ, Peters TJ. The antioxidant status of patients with either alcohol-induced liver damage or myopathy. Alcohol Alcohol. 1992;27(4):359–365. [PubMed] [Google Scholar]
- 49.Rua RM, Ojeda ML, Nogales F, et al. Serum selenium levels and oxidative balance as differential markers in hepatic damage caused by alcohol. Life Sci. 2014;94(2):158–163. doi: 10.1016/j.lfs.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14(1):58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 51.Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005;37(10):2180–2195. doi: 10.1016/j.biocel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Sola J, Preedy VR, Lang CH, et al. Molecular and cellular events in alcohol-induced muscle disease. Alcohol Clin Exp Res. 2007;31(12):1953–1962. doi: 10.1111/j.1530-0277.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 53.Rudman D, DiFulco TJ, Galambos JT, Smith RB, 3rd, Salam AA, Warren WD. Maximal rates of excretion and synthesis of urea in normal and cirrhotic subjects. J Clin Invest. 1973;52(9):2241–2249. doi: 10.1172/JCI107410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomura F, Ohnishi K, Terabayashi H, et al. Effect of intrahepatic portal-systemic shunting on hepatic ammonia extraction in patients with cirrhosis. Hepatology. 1994;20(6):1478–1481. doi: 10.1002/hep.1840200616. [DOI] [PubMed] [Google Scholar]
- 55.Shangraw RE, Jahoor F. Effect of liver disease and transplantation on urea synthesis in humans: relationship to acid-base status. Am J Physiol. 1999;276(5 Pt 1):G1145–1152. doi: 10.1152/ajpgi.1999.276.5.G1145. [DOI] [PubMed] [Google Scholar]
- 56.Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (1985) 2014;117(10):1170–1179. doi: 10.1152/japplphysiol.00180.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(5):E917–928. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- 58.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res. 2004;28(11):1758–1767. doi: 10.1097/01.alc.0000145787.66405.59. [DOI] [PubMed] [Google Scholar]
- 59.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab. 2004;286(6):E916–926. doi: 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- 60.Thapaliya S, Runkana A, McMullen MR, et al. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy. 2014;10(4):677–690. doi: 10.4161/auto.27918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr. 2010;140(5):932–938. doi: 10.3945/jn.109.120501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298(2):737–743. [PubMed] [Google Scholar]
- 63.Holmuhamedov EL, Czerny C, Beeson CC, Lemasters JJ. Ethanol suppresses ureagenesis in rat hepatocytes: role of acetaldehyde. J Biol Chem. 2012;287(10):7692–7700. doi: 10.1074/jbc.M111.293399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiner JL, Lang CH. Dysregulation of skeletal muscle protein metabolism by alcohol. Am J Physiol Endocrinol Metab. 2015;308(9):E699–712. doi: 10.1152/ajpendo.00006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tsien C, Davuluri G, Singh D, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61(6):2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dam G, Sorensen M, Munk OL, Keiding S. Hepatic ethanol elimination kinetics in patients with cirrhosis. Scand J Gastroenterol. 2009;44(7):867–871. doi: 10.1080/00365520902929856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dasarathy S. Role of gut bacteria in the therapy of hepatic encephalopathy with lactulose and antibiotics. Indian J Gastroenterol. 2003;22(Suppl 2):S50–53. [PubMed] [Google Scholar]
- 68.Qiu J, Tsien C, Thapalaya S, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303(8):E983–993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.World MJ, Ryle PR, Thomson AD. Alcoholic malnutrition and the small intestine. Alcohol Alcohol. 1985;20(2):89–124. [PubMed] [Google Scholar]
- 70.Chaudhry KK, Shukla PK, Mir H, et al. Glutamine supplementation attenuates ethanol-induced disruption of apical junctional complexes in colonic epithelium and ameliorates gut barrier dysfunction and fatty liver in mice. J Nutr Biochem. 2015 doi: 10.1016/j.jnutbio.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhong W, Li Q, Sun Q, et al. Preventing Gut Leakiness and Endotoxemia Contributes to the Protective Effect of Zinc on Alcohol-Induced Steatohepatitis in Rats. J Nutr. 2015 doi: 10.3945/jn.115.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcohol Clin Exp Res. 2011;35(9):1635–1643. doi: 10.1111/j.1530-0277.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9(5):e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Norman K, Pirlich M, Schulzke JD, et al. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr. 2012;66(10):1116–1119. doi: 10.1038/ejcn.2012.104. [DOI] [PubMed] [Google Scholar]
- 75.Antar R, Wong P, Ghali P. A meta-analysis of nutritional supplementation for management of hospitalized alcoholic hepatitis. Can J Gastroenterol. 2012;26(7):463–467. doi: 10.1155/2012/945707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tsien C, Shah SN, McCullough AJ, Dasarathy S. Reversal of sarcopenia predicts survival after a transjugular intrahepatic portosystemic stent. Eur J Gastroenterol Hepatol. 2013;25(1):85–93. doi: 10.1097/MEG.0b013e328359a759. [DOI] [PubMed] [Google Scholar]
- 77.Nasrallah SM, Galambos JT. Aminoacid therapy of alcoholic hepatitis. Lancet. 1980;2(8207):1276–1277. doi: 10.1016/s0140-6736(80)92338-7. [DOI] [PubMed] [Google Scholar]
- 78.Calvey H, Davis M, Williams R. Controlled trial of nutritional supplementation, with and without branched chain amino acid enrichment, in treatment of acute alcoholic hepatitis. J Hepatol. 1985;1(2):141–151. doi: 10.1016/s0168-8278(85)80762-5. [DOI] [PubMed] [Google Scholar]
- 79.Bonkovsky HL, Singh RH, Jafri IH, et al. A randomized, controlled trial of treatment of alcoholic hepatitis with parenteral nutrition and oxandrolone. II. Short-term effects on nitrogen metabolism, metabolic balance, and nutrition. Am J Gastroenterol. 1991;86(9):1209–1218. [PubMed] [Google Scholar]
- 80.Orr R, Fiatarone Singh M. The anabolic androgenic steroid oxandrolone in the treatment of wasting and catabolic disorders: review of efficacy and safety. Drugs. 2004;64(7):725–750. doi: 10.2165/00003495-200464070-00004. [DOI] [PubMed] [Google Scholar]
- 81.Jones JC, Coombes JS, Macdonald GA. Exercise capacity and muscle strength in patients with cirrhosis. Liver Transpl. 2012;18(2):146–151. doi: 10.1002/lt.22472. [DOI] [PubMed] [Google Scholar]
- 82.Peters TJ, Nikolovski S, Raja GK, Palmer TN, Fournier PA. Ethanol acutely impairs glycogen repletion in skeletal muscle following high intensity short duration exercise in the rat. Addict Biol. 1996;1(3):289–295. doi: 10.1080/1355621961000124906. [DOI] [PubMed] [Google Scholar]
- 83.Vingren JL, Koziris LP, Gordon SE, Kraemer WJ, Turner RT, Westerlind KC. Chronic alcohol intake, resistance training, and muscle androgen receptor content. Med Sci Sports Exerc. 2005;37(11):1842–1848. doi: 10.1249/01.mss.0000176679.80555.cd. [DOI] [PubMed] [Google Scholar]
- 84.Graham TE, Turcotte LP, Kiens B, Richter EA. Effect of endurance training on ammonia and amino acid metabolism in humans. Med Sci Sports Exerc. 1997;29(5):646–653. doi: 10.1097/00005768-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 85.Barnes MJ, Mundel T, Stannard SR. A low dose of alcohol does not impact skeletal muscle performance after exercise-induced muscle damage. Eur J Appl Physiol. 2011;111(4):725–729. doi: 10.1007/s00421-010-1655-8. [DOI] [PubMed] [Google Scholar]
- 86.Haugvad A, Haugvad L, Hamarsland H, Paulsen G. Ethanol does not delay muscle recovery but decreases testosterone/cortisol ratio. Med Sci Sports Exerc. 2014;46(11):2175–2183. doi: 10.1249/MSS.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 87.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J Cell Sci. 2013;126(Pt 3):789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fernandez-Sola J, Villegas E, Nicolas JM, et al. Serum and muscle levels of alpha-tocopherol, ascorbic acid, and retinol are normal in chronic alcoholic myopathy. Alcohol Clin Exp Res. 1998;22(2):422–427. [PubMed] [Google Scholar]
- 89.Olde Damink SW, Jalan R, Deutz NE, et al. Isoleucine infusion during “simulated” upper gastrointestinal bleeding improves liver and muscle protein synthesis in cirrhotic patients. Hepatology. 2007;45(3):560–568. doi: 10.1002/hep.21463. [DOI] [PubMed] [Google Scholar]