Abstract
Background aims
PR1 is an HLA-A2 restricted leukemia-associated antigen derived from neutrophil elastase and proteinase 3, both of which are normally stored in the azurophil granules of myeloid cells but overexpressed in myeloid leukemic cells. PR1-specific cytotoxic lymphocytes (PR1-CTL) have activity against primary myeloid leukemia in vitro and in vivo and thus could have great potential in the setting of adoptive cellular therapy (ACT). Adult peripheral blood derived PR1-CTL are infrequent but preferentially lyse myeloid leukemia cells. We sought to examine PR1-CTL in umbilical cord blood (UCB) since UCB units provide a rapidly available cell source and a lower risk of graft-versus-host disease (GVHD), even in the setting of mismatched human leukocyte antigen (HLA) loci.
Methods
We first determined the frequency of PR1-CTL in HLA-A2+ UCB units and then successfully expanded them ex vivo using repeated stimulation with PR1 peptide-pulsed antigen-presenting cells (APCs). After expansion, we assessed the PR1-CTL phenotype (naïve, effector, memory) and function against PR1-expressing target cells.
Results
PR1-CTL are detected at an average frequency of 0.14% within the CD8+ population of fresh UCB units, which is 45 times higher than in healthy adult peripheral blood. UCB PR1-CTL are phenotypically naïve, consistent with the UCB CD8+ population as a whole. In addition, the cells can be expanded by stimulation with PR1 peptide-pulsed APCs. Expansion results in an increased frequency of PR1-CTL, up to 4.56%, with an average 20-fold increase in total number. After expansion, UCB PR1-CTL express markers consistent with effector memory T cells. Expanded UCB PR1-CTL are functional in vitro as they are able to produce cytokines and lyse PR1-expressing leukemia cell lines.
Conclusions
This study is the first report showing that T cells specific for a leukemia-associated antigen (LAA) are found at a significantly higher frequency in UCB than adult blood. Our results also demonstrate specific cytotoxicity of expanded UCB-derived PR1-CTL against PR1-expressing targets. Together, our data suggest that UCB PR1-CTL could be useful to prevent or treat leukemia relapse in myeloid leukemia patients.
Keywords: PR1, antigen specific cytotoxic T lymphocyte, umbilical cord blood, leukemia
Introduction
PR1 is an HLA-A*0201 restricted nonameric peptide derived from the serine proteases proteinase 3 and neutrophil elastase, which are both overexpressed in myeloid leukemia cells [1] [2]. PR1 has been effectively targeted in myeloid leukemia using PR1-CTL and an anti-PR1/HLA-A2 antibody [3–7]. PR1-specific memory T cells have been detected at very low frequencies in the peripheral blood (PB) of healthy donors, but are found at significantly higher frequencies in myeloid leukemia patients where their frequency is positively correlated with treatment response [5]. Previous studies have shown that PR1-CTL isolated from patients with myeloid leukemia or elicited from the PB of normal adults demonstrated preferential cytotoxicity against myeloid leukemia cells in vitro and in vivo [3, 8, 9]. Further, Rezvani et al. [10] showed that PR1-CTL can be transferred from adult donor to recipient, leading to an expansion of the CTL and resulting in a graft-versus-leukemia (GVL) effect in the recipient.
Due to the potential therapeutic value of PR1-CTL in the setting of cellular therapy and the advantages of umbilical cord blood (UCB), including off-the-shelf availability and less stringency in HLA matching, the isolation and/or expansion of UCB-derived PR1-CTL could provide cells capable of targeting, and possibly eliminating, myeloid leukemia. Herein, we investigated the frequency, phenotype, and anti-leukemia function of PR1-CTL derived from UCB. Despite the limited number of mononuclear cells in an average UCB unit (~5 ×108 cells) and the low frequency of PR1-CTL in adult PB (1/15,000 to 1/345,000 CD8+ T-cells) [5], we discovered that PR1-CTL precursors in UCB are 45-fold more frequent than in adult PB. In addition, we demonstrated the ex vivo expansion capacity of UCB-derived PR1-CTL and their specific cytotoxic function in vitro. The number of PR1-CTL expanded ex vivo is sufficient for adoptive immunotherapy. To our knowledge, this is the first report investigating the phenotype and function of UCB-derived CTLs targeting a myeloid leukemia-associated antigen (LAA).
Materials and Methods
Reagents
HLA-A2 positive fresh UCB units and healthy adult PB were obtained from the Cord Blood Bank of M.D. Anderson Cancer Center and Gulf Coast Regional Blood Center of Houston, respectively. HLA-A2 status was determined serologically by flow cytometry using the monoclonal antibody (mAb), BB7.2 (BioLegend). PR1 peptide (169–177VLQELNVTV) was synthesized by Bachem Company. Wild type U937 and K562 cell lines were obtained from American Type Culture Collection. HLA-A*0201 transduced U937 cells (U937-A2) were provided by Dr. Greg Lizee. Specialized K562 antigen-presenting cells (APCs) co-transfected with CD86, CD137L, CD64, CD32, and HLA-A*0201 were kindly provided by Dr. Laurence Cooper of M.D. Anderson Cancer Center. CD4, CD8, CD45RA, and CCR7 mAbs (BD Biosciences) were used for immunophenotyping. Aqua live/dead cell stain (BioLegend) as well as CD14, CD16, and CD19 mAbs were used to exclude monocytes, NK cells, and B cells in the dextramer analyses. PE-conjugated HLA-A*0201 PR1 dextramer as well as WT1, MART1, and Tyrosinase(368–376) dextramers were all from Immudex, Denmark.
Ex vivo expansion of PR1-CTL from UCB
Stimulation and expansion of UCB-derived PR1-CTL was performed according to the protocol by Hanley et al. with some modifications [11]. Briefly, UCB mononuclear cells (CBMCs) were isolated and co-cultured with irradiated PR1 peptide-pulsed K562-A2 cells at a ratio of 10:1 in the presence of soluble IL-7 (10 ng/ml), IL-12 (10 ng/ml), and IL-15 (5ng/ml) (R&D Systems, Inc.). CBMCs were stimulated every week for 3 weeks with PR1-pulsed K562-A2 cells in CTL medium (50% Click’s (Irvine Scientific) and 50% RPMI 1640 (HyClone) supplemented with 10% human AB serum and 2 mM L-glutamine). IL-15 was again added after the second stimulation. Cells were supplemented with 100 IU/ml recombinant human IL-2 (R&D Systems, Inc.) 3 days after the second and 1 day after the third stimulation. Medium was replenished biweekly. Cells were harvested on day 19 after the first stimulation. CD8+ cells were enriched from bulk culture using the MACS CD8+ T cell isolation kit (MIltenyi Biotec) for the intracellular cytokine detection and cytotoxicity assays.
Intracellular cytokine and cytotoxicity assays
CD8+ T cells from expanded UCB units were incubated with target cells at an E:T ratio of 5:1. Cells were incubated at 37°C, 5% CO2 for a total of 16 hours (brefeldin A was added at a final concentration of 10 µg/ml after the initial hour). The cells were fixed and permeabilized by FACS Lyse and FACS Perm (BD Biosciences). Cells were stained, washed, and resuspended in 1% PFA. Data were acquired on the LSRFortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software. For the cell-mediated cytotoxicity assay, target cells were stained with 5 µg/ml of Calcein AM (Thermo Fisher) for 15 minutes at 37°C, washed 4 times with RPMI 1640 and resuspended at 2×105/ml. Target cells (2,000) in 10 µl were incubated with antigen specific CTL at varying E:T ratios. To quench the reaction, 5 µl of 0.4% Trypan Blue was added after 4 hours [12].
Statistical analysis
GraphPad Prism 6 (GraphPad, San Diego, CA) was used to perform statistical analyses. Student’s t test was used to test for significance and P value < 0.05 was considered significant.
Results
PR1-CTL are found at a relatively high frequency in UCB
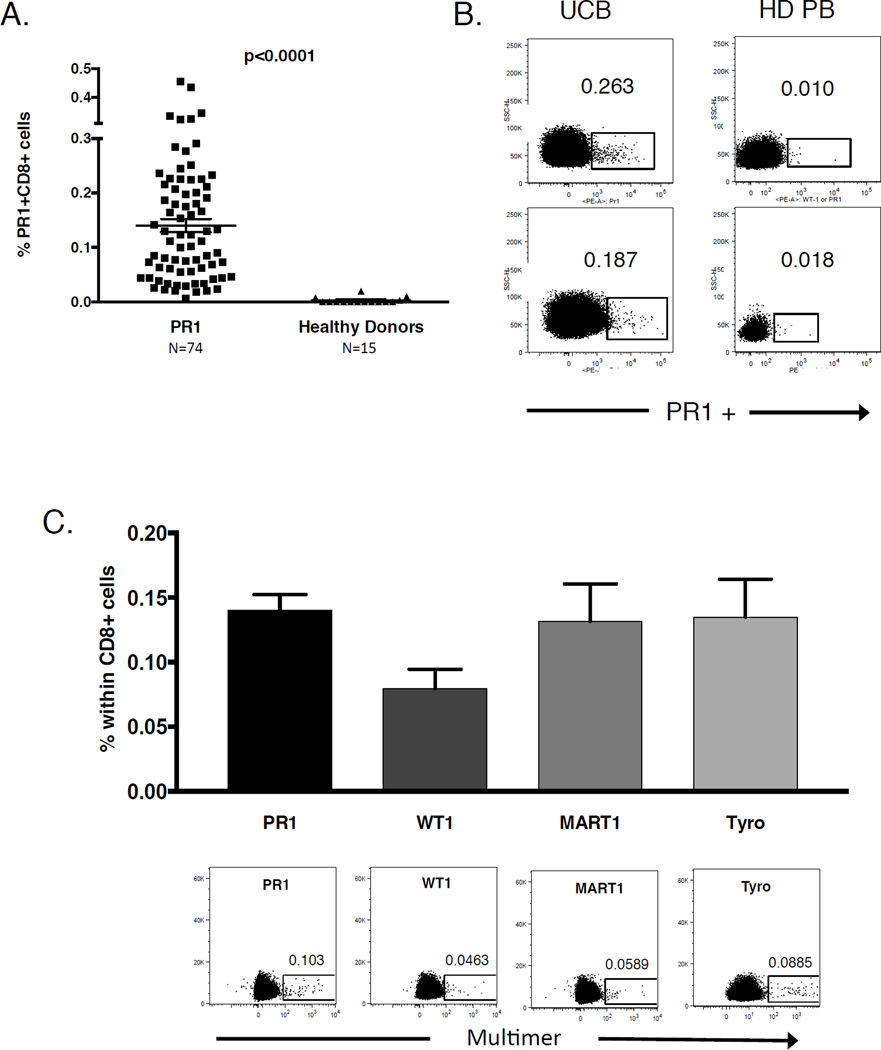
As shown in Figure 1A, the frequency of PR1-CTL in UCB was approximately 45 times higher than in healthy adult PB (mean 0.140±0.104% of CD8+ cells in UCB [n=74] vs. 0.0031% of CD8+ cells in adult PB [n=15], [p<0.0001]). PR1-CTL with low, intermediate, and high avidities were clearly visible within the UCB CD8+ population when stained with PR1-specific multimer followed by flow cytometric analysis (Figure 1B, left). In comparison, PR1-CTL were found at low, sometimes undetectable levels in healthy adult PB (Figure 1B, right) [4]. The relatively high PR1-CTL frequency in UCB was an unexpected finding as T cells specific for self-antigens, like PR1, are subject to central tolerance via deletion in the thymus. Given these data, we sought to examine other tumor antigen specific CTLs in UCB. As shown in Figure 1C, the frequency of other self-antigen specific T cells recognizing WT1- (~ 0.0796%), MART1- (~0.1316%) and Tyrosinase- (~0.1348%) was comparable to PR1-CTL and was significantly higher in UCB than has been reported in studies of healthy donor adult PB [13–15]. These data complement the results published by Su et al. in which detectable levels of self-antigen specific CD4+ T cells were seen in cord blood [16].
Figure 1. UCB contains a high frequency of PR1-CTL.
(A) Summary of the percentage of PR1-CTL in the CD8+ population in UCB (0.140%) and healthy adult donor PB (0.0031%). Horizontal bars indicate mean values ± SD. (B) Representative dextramer staining of PR1-CTL in UCB (left) compared to adult healthy donor PB (right). (C) The average frequency (upper panel) and representative multimer staining (lower panel) of other tumor self-antigen (WT1, MART1, Tyro) specific T cells in UCB (n=5 for non-PR1 antigens).
UCB PR1-CTL can be successfully expanded ex vivo
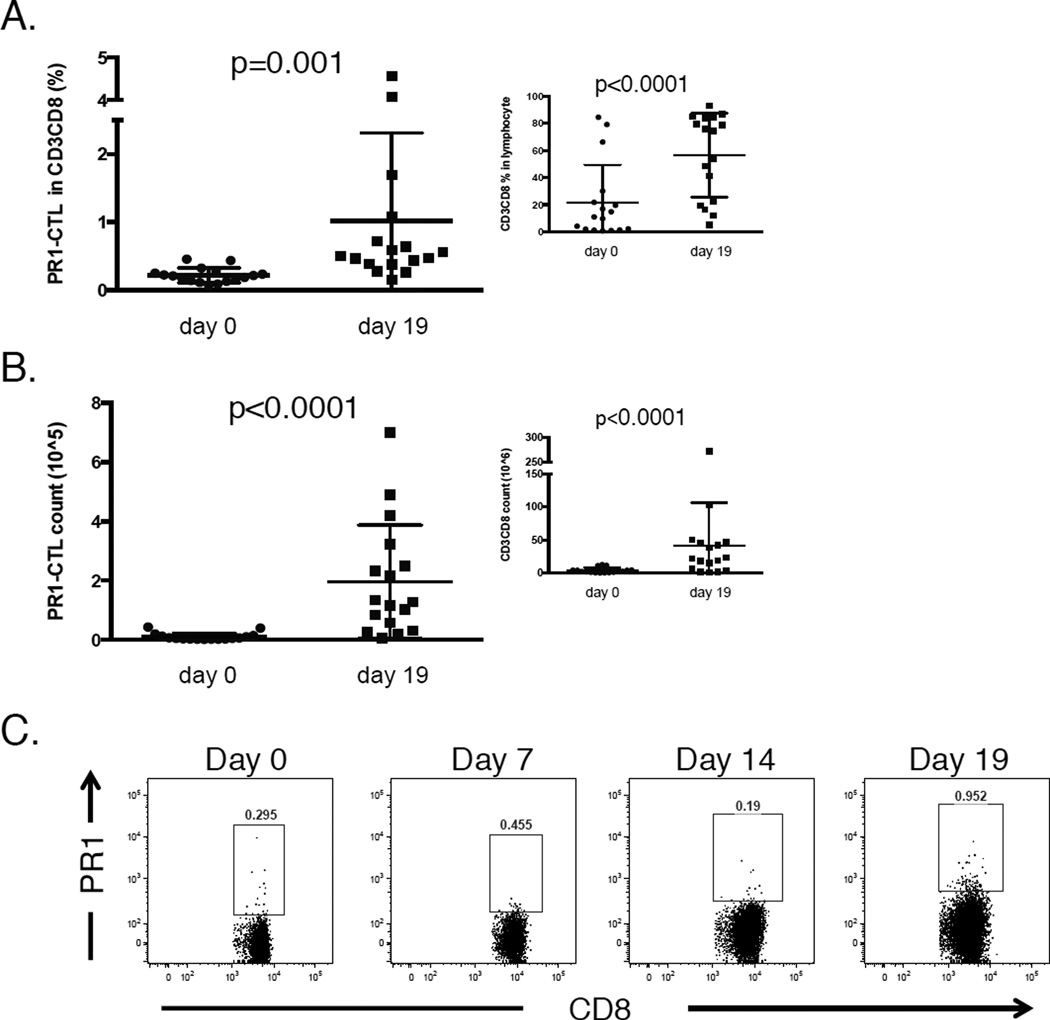
Although PR1-CTL are detectable in UCB, the absolute number of cells in an unmanipulated UCB unit is too low to be considered a viable option for ACT. We sought to expand PR1-CTL using PR1 peptide-pulsed K562-A2 cells as stimulators, along with a cytokine cocktail of IL-7, IL-12, IL-15, and IL-2 [11]. As shown in Figure 2A, the percentage of PR1-CTL within the CD8+ population increased significantly from an average of 0.219% (range: 0.074%–0.456%) prior to expansion to 1.014% (range: 0.155%–4.56%) after three rounds of stimulation (n=17; p=0.001). The bulk CD3+CD8+ subset also increased from a mean of 21.5% to 56.4% of the lymphocytes during the 19-day protocol (figure 2A, inset). Critically, the absolute number of PR1-CTL also significantly increased during the expansion by an average of 20-fold (95% CI 10.25~38.78) (p<0.001) (Figure 2B). This represents an average of 2×105 PR1-CTL per expanded UCB unit. Notably, the total number of CD3+CD8+ cells increased by only 7.5-fold (95% CI 3.92~14.19) (Figure 2B, inset) (p<0.001), indicating preferential expansion of PR1-CTL. Longitudinal analysis of a representative HLA-A2+ UCB unit during the expansion protocol revealed T cells with a range of apparent avidities for PR1/HLA-A2 on Day 19 (Figure 2C).
Figure 2. PR1-CTL from UCB units can be expanded 20-fold ex vivo.
(A) The mean percentage of PR1-CTL within the CD3+CD8+ subset increased from 0.219% to 1.014% following stimulation with PR1-peptide pulsed APCs over 19 days (n=17). Inset: The CD3+CD8+ lymphocyte subset also increased during the expansion (mean 21.5% pre- to 56.4% post-expansion). (B) The absolute number of PR1-CTL increased by an average of 20-fold (95% CI 10.25–38.78) during the expansion while the increase in CD3+CD8+ T cells was 7.5 fold (inset), indicating preferential expansion of PR1-CTL. (C) Longitudinal PR1 dextramer analyses of a representative HLA-A2+ UCB unit undergoing the 19-day in vitro PR1-CTL expansion protocol.
UCB PR1-CTL transition to an effector memory phenotype during expansion and demonstrate HLA-A2-dependent cytotoxicity
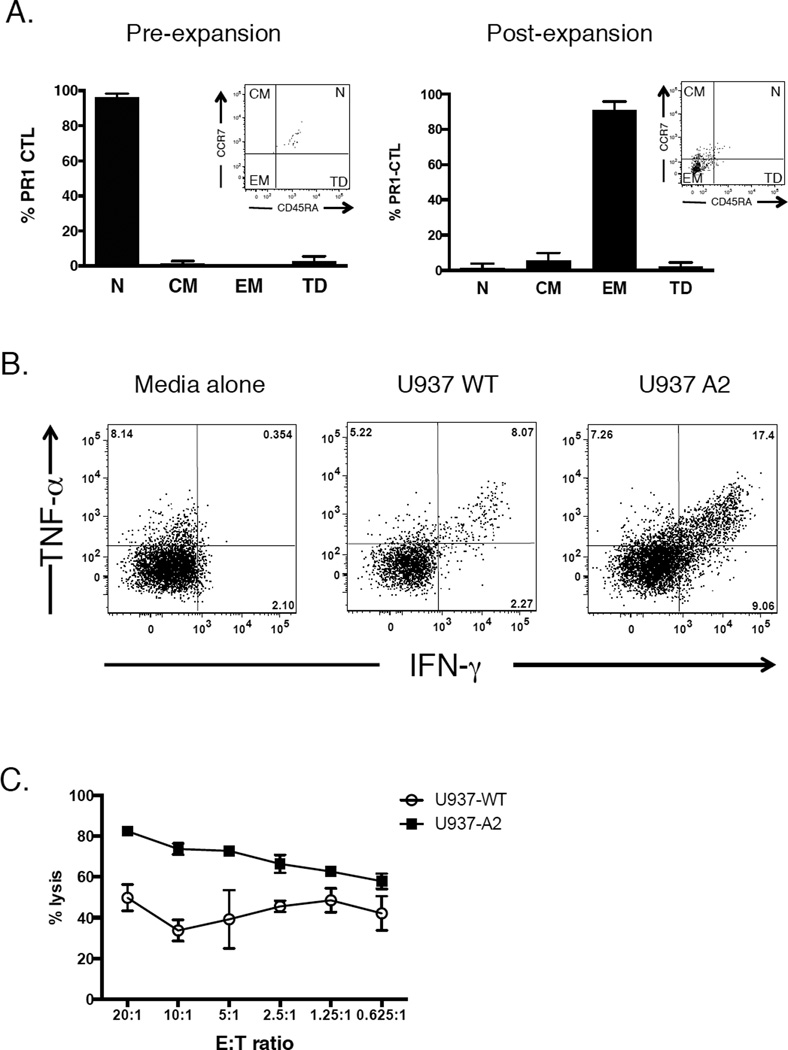
Surface phenotyping of lymphocytes from 8 HLA-A2+ UCB units indicated that 95% of PR1-CTL were CD45RA+CCR7+ prior to expansion (Figure 3A, left). Thus, PR1-CTL directly sorted from UCB were phenotypically naïve, consistent with previous reports of UCB CTL subsets [17–20]. While PR1-CTL directly sorted from UCB were phenotypically naïve, the expanded UCB PR1-CTL lost expression of CD45RA and CCR7, characteristic of effector memory T cells (Figure 3A, right) [21]. We next asked whether the PR1-CTL remained functional after the 19-day in vitro expansion protocol. After expansion, UCB PR1-CTL demonstrated a >2-fold increase in secretion of IFN-γ and TNF-α in response to the PR1-expressing target cells U937-A2 when compared to wild type, HLA-A2 negative U937 cells or PR1-CTL alone (Figure 3B). Finally, to measure the specific cytotoxic activity of expanded UCB PR1-CTL, U937 leukemia cells, HLA-A2+ and wild type, were used as targets in a CTL assay. As shown in Figure 3C, expanded UCB-derived PR1-CTL cells were capable of killing the U937 leukemia cells in an HLA-A2-dependent manner in vitro.
Figure 3. Expanded UCB PR1-CTL are memory CD8+ T cells that are functional against an HLA-A2+ leukemia cell line.
(A) Phenotype of UCB PR1-CTL pre- and post-expansion. Phenotype was determined using CCR7 and CD45RA cell surface markers (n=8). N=naïve (CD45RA+CCR7+), CM=central memory (CD45RA−CCR7+), EM=effector memory (CD45RA−CCR7−), TD=terminally differentiated (CD45RA+CCR7−). Inset: Representative flow cytometry plots of PR1-CTL before and after expansion. (B) Expanded UCB PR1-CTL secrete cytokines in response to a PR1-expressing, A2-transfected leukemia cell line, U937-A2. Expanded UCB cells were incubated either alone or with target cells (U937 WT and U937-A2) and the percentage of T cells positive for both intracellular IFN-γ+ and TNF-α+ was determined by flow cytometry. (C) Specific cytotoxic activity of expanded UCB PR1-CTL. Target cells U937-A2 and U937 WT were incubated with UCB PR1-CTL in a 4-hr CTL assay at the indicated E:T ratios. Data are presented as mean percent specific killing of target cells ± SD with experiments done in triplicate.
Discussion
In this study, we show that PR1-CTL are present at a significantly higher frequency in UCB than healthy adult PB. While detectable, the frequencies still represent very low overall cell numbers. Thus, in terms of clinical utility, we showed that PR1-CTL from UCB can be effectively expanded ex vivo. We were able to achieve, on average, a 20-fold expansion in PR1-CTL after repeated peptide stimulation with specialized APCs. Unlike expansion of PR1-CTL from healthy adult PB, expansion from UCB required the use of robust APCs, additional cytokines, and specific timing to ensure optimal cell proliferation [3]. This is likely due to the naïve nature of the cells, resulting in the need to prime cells before they are capable of extensive proliferation [17, 19]. Once expanded, PR1-CTL lose the phenotypic markers characteristic of naïve cells and take on both phenotypic and functional characteristics indicative of memory CTL. The expanded UCB PR1-CTL produced both IFN-γ and TNF-α in response to PR1-expressing target leukemia cells. In addition, the expanded PR1-CTL were capable of killing PR1-expressing leukemia cells in vitro in an HLA-A2 dependent manner.
We presumed that the majority of PR1-CTL undergo deletion in the fetal thymus prior to birth. However, our data suggest that central tolerance is incomplete for PR1-specific T cells, which is in agreement with the recent report by Yu et al. [22] that self-specific T cells can escape complete negative selection in the thymus, resulting in detectable levels of circulating self-specific T cells. We speculate that the presence of PR1-CTL in UCB may, at least in part, be explained by incomplete deletion in the thymus due to the lack of expression of PR1 by medullary thymic epithelial cells (mTECs) [23, 24]. Furthermore, the diminishing number of PR1-CTL in healthy adult PB suggests that these cells are deleted peripherally over time. These results agree with a report by Garderet et al. that the UCB T-cell repertoire is more comprehensively polyclonal than the adult PB repertoire [25].
Our finding of an increased frequency of PR1-CTL in UCB has important clinical implications. Surprisingly, the percentage of PR1-CTL observed in UCB was within one log of that found in leukemia patients post-immune therapy treatment [5]. Thus, UCB could conceivably represent an ideal source of PR1-CTL, and broadly, a source of CTL that target other tumor antigens in the context of ACT. Overall, our data indicate that PR1-CTL can be expanded from UCB and that the cells are functional against myeloid leukemia in vitro. Thus, the adoptive transfer of UCB-derived PR1-CTL could potentially treat myeloid leukemia or prevent leukemia relapse in HLA-A2 positive patients with high-risk myeloid leukemia.
Acknowledgments
This study was supported by research funding from NCI CA100632 (to JJM); NCI CA148600 (to JJM and EJS); NCI P30CA16672 (to KCD); NSFC 81500113 to (to LW); Leukemia and Lymphoma Society 6030-12 (to JJM); Leukemia and Lymphoma Society 7262-08 (to JJM).
Footnotes
Authorship statement: L.St J., L.W and H.H. performed experiments. L.St J., L.W, H.H. and K.C.D. analyzed data. L.St J., L.W, H.R.G., G.A., K.A., E.J.S. and C.M.B. wrote the paper. Q.M. and J.J.M. designed the research and wrote the paper.
We have no conflict of interests.
References
- 1.Dengler R, et al. Immunocytochemical and Flow Cytometric Detection of Proteinase-3 (Myeloblastin) in Normal and Leukemic Myeloid Cells. British Journal of Haematology. 1995;89(2):250–257. doi: 10.1111/j.1365-2141.1995.tb03297.x. [DOI] [PubMed] [Google Scholar]
- 2.Molldrem J, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88(7):2450–2457. [PubMed] [Google Scholar]
- 3.Ma Q, et al. Adoptive transfer of PR1 cytotoxic T lymphocytes associated with reduced leukemia burden in a mouse acute myeloid leukemia xenograft model. Cytotherapy. 2010;12(8):1056–1062. doi: 10.3109/14653249.2010.506506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molldrem JJ, et al. A PR1-human leukocyte antigen-A2 tetramer can be used to isolate low-frequency cytotoxic T lymphocytes from healthy donors that selectively lyse chronic myelogenous leukemia. Cancer Res. 1999;59(11):2675–2681. [PubMed] [Google Scholar]
- 5.Molldrem JJ, et al. Evidence that specific T lymphocytes may participate in the elimination of chronic myelogenous leukemia. Nat Med. 2000;6(9):1018–1023. doi: 10.1038/79526. [DOI] [PubMed] [Google Scholar]
- 6.Sergeeva A, et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011;117(16):4262–4272. doi: 10.1182/blood-2010-07-299248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sergeeva A, et al. Activity of 8F4, a T-cell receptor-like anti-PR1/HLA-A2 antibody, against primary human AML in vivo. Leukemia. 2016 doi: 10.1038/leu.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanodia S, et al. PR1-specific T cells are associated with unmaintained cytogenetic remission of chronic myelogenous leukemia after interferon withdrawal. PLoS One. 2010;5(7):e11770. doi: 10.1371/journal.pone.0011770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molldrem JJ, et al. Cytotoxic T lymphocytes specific for a nonpolymorphic proteinase 3 peptide preferentially inhibit chronic myeloid leukemia colony-forming units. Blood. 1997;90(7):2529–2534. [PubMed] [Google Scholar]
- 10.Rezvani K, et al. Transfer of PR1-specific T-cell clones from donor to recipient by stem cell transplantation and association with GvL activity. Cytotherapy. 2007;9(3):245–251. doi: 10.1080/14653240701218524. [DOI] [PubMed] [Google Scholar]
- 11.Hanley PJ, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alatrash G, et al. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. J Immunol. 2012;189(11):5476–5484. doi: 10.4049/jimmunol.1201221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho WY, et al. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310(1–2):40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Alanio C, et al. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood. 2010;115(18):3718–3725. doi: 10.1182/blood-2009-10-251124. [DOI] [PubMed] [Google Scholar]
- 15.Ogg GS, et al. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188(6):1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su LF, et al. Virus-Specific CD4(+) Memory-Phenotype T Cells Are Abundant in Unexposed Adults. Immunity. 2013;38(2):373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris DT, et al. Phenotypic and functional immaturity of human umbilical cord blood T lymphocytes. Proc Natl Acad Sci U S A. 1992;89(21):10006–10010. doi: 10.1073/pnas.89.21.10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beck R, Lam-Po-Tang PR. Comparison of cord blood and adult blood lymphocyte normal ranges: a possible explanation for decreased severity of graft versus host disease after cord blood transplantation. Immunol Cell Biol. 1994;72(5):440–444. doi: 10.1038/icb.1994.65. [DOI] [PubMed] [Google Scholar]
- 19.D'Arena G, et al. Flow cytometric characterization of human umbilical cord blood lymphocytes: immunophenotypic features. Haematologica. 1998;83(3):197–203. [PubMed] [Google Scholar]
- 20.Szabolcs P, et al. Coexistent naive phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Exp Hematol. 2003;31(8):708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 21.Mascher B, Schlenke P, Seyfarth N. Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. Journal of Immunological Methods. 1999;223(1):115–121. doi: 10.1016/s0022-1759(98)00200-2. [DOI] [PubMed] [Google Scholar]
- 22.Yu W, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific alpha beta CD8(+) T Lymphocytes. Immunity. 2015;42(5):929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotter J, et al. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199(2):155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derbinski J, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202(1):33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garderet L, et al. The umbilical cord blood alphabeta T-cell repertoire: characteristics of a polyclonal and naive but completely formed repertoire. Blood. 1998;91(1):340–346. [PubMed] [Google Scholar]