Abstract
Background and Aims Plant design refers to the construction of the plant body or its constituent parts in terms of form and function. Although neighbourhood structure is recognized as a factor that limits plant survival and species coexistence, its relative importance in plant design is not well understood. We conducted field research to analyse how the surrounding environment of neighbourhood structure and related effects on light availability are associated with changes in plant design in two understorey plants (Palicourea padifolia and Psychotria elata) within two successional stages of a cloud forest in Costa Rica.
Methods Features of plant neighbourhood physical structure and light availability, estimated using hemispherical photographs, were used as variables that reflect the surrounding environment. Measures of plant biomechanics, allometry, branching and plant slenderness were used as functional plant attributes that reflect plant design. We propose a framework using a partial least squares path model and used it to test this association.
Key Results The multidimensional response of plant design of these species suggests that decreases in the height-based factor of safety and increases in mechanical load and developmental stability are influenced by increases in maximum height of neighbours and a distance-dependence interference index more than neighbourhood plant density or neighbour aggregation. Changes in plant branching and slenderness are associated positively with light availability and negatively with canopy cover.
Conclusions Although it has been proposed that plant design varies according to plant density and light availability, we found that neighbour size and distance-dependence interference are associated with changes in biomechanics, allometry and branching, and they must be considered as key factors that contribute to the adaptation and coexistence of these plants in this highly diverse forest community.
Keywords: Economic plant design, Palicourea padifolia, plant allometry, plant biomechanics, Psychotria elata, plant competition
INTRODUCTION
‘Economic plant design’ refers to the construction of the plant body or its constituent parts in terms of form and function so as to minimize the allocation of energy and/or biomass of different genotypes (Givnish, 1987; Niklas, 1993a) and phenotypes. Any design is determined by the anatomical, developmental and mechanical properties of the tissues (Rowe and Speck, 2005), attributes that are important for plant growth, reproductive performance and fitness in different environments (Tremmel and Bazzaz, 1995; Henry and Aarssen, 1999; Weiner, 2004). In nature there is no optimal design (Niklas, 1986), because any form and function of the plant body depend directly on the cost–benefit of the investments, which may provide an advantage to survival and coexistence in different environments (McNickle and Dybzinski, 2013). In many cases, design responses are influenced by ontogenetic effects (Barthélémy and Caraglio, 2007) or environmental interactions in terms of abiotic stresses, like physical laws (Niklas and Hammond, 2013), light availability (Henry and Aarssen, 1999) and soil properties (Fitter and Stickland, 1991), and biotic stresses (Read and Stokes, 2006), like plant density (Mäkelä and Vanninen, 1998) and herbivory (Hambäck and Beckerman, 2003).
In the tropical understorey, where species experience great light heterogeneity (Chazdon and Fetcher 1984), plant design plays an important role in survival and energy capture (Valladares et al., 2002; Cooley et al., 2004). Many hypotheses have been proposed to explain how the designs of understorey species, especially shade-tolerant plants, survive and coexist in different environments (Valladares and Niinemets, 2008). For example, it is proposed that plants within shaded sites present greater leaf area, petiole length and bifurcation ratio, which maximizes light interception (Horn, 1971; Delagrange et al., 2004; Weijschedé et al., 2006), as well as lower plasticity, related to greater capacity to conserve resources (Valladares et al., 2000). The literature shows few examples of how understorey plant designs (e.g. allometry, biomechanics, branching) could be affected by neighbourhood structure and how these designs could allow coexistence in communities. It can be expected that plant design features that are under strong competition for light express the same patterns as those found in experiments with deep shade treatments. But what is the relative importance of neighbourhood structure in plant design? And what are the properties of neighbourhoods that most influence plant design patterns?
Because the interpretation of the effects of competition on plants is related to the way in which the competition is measured or defined (Freckleton and Watkinson, 1999; Weigelt and Jolliffe, 2003), in this study we use the practical definition of competition by Grime (1979) and Trinder et al. (2012) – ‘the capture of essential resources from a common, finite pool by neighbours’ – considering here light availability as a common limiting resource in the tropical understorey (Chazdon and Fetcher, 1984). Perspectives of how plant design changes as a function of neighbourhood structure or competition could be used to answer questions related to trait-based tests of survival and coexistence (e.g. Adler et al., 2013; Lasky and Uriarte, 2014; Kraft et al., 2015). We consider that changes in plant design associated with variations in neighbourhoods and light availability could be used as key factors that contribute to community structure in highly diverse tropical ecosystems.
The aim of this paper is to analyse the importance of neighbourhood structure and light availability on the plant designs of two understorey species (Palicourea padifolia and Psychotria elata) as traits that may explain the coexistence of these two species within two cloud forests with communities of different successional status in Costa Rica. We propose a new framework in which the ecological variables (neighbourhood structure and light availability) affect different sectors of plant design, specifically, allometry, biomechanics and plant attributes like bifurcation and slenderness (Fig. 1). We address the general hypothesis that adjustments in plant design are associated with variations in the neighbourhood structure and light availability of the surrounding environment. From this we show which ecological variable has the greatest contribution in the variation of plant design and in which direction. The conclusions obtained here allow an understanding of how changes in the ‘optimal plant design’ with respect to neighbourhood structure and light availability could provide an adaptive advantage in spatially heterogeneous environments and promote their coexistence in regenerating tropical cloud forest communities.
Fig. 1.
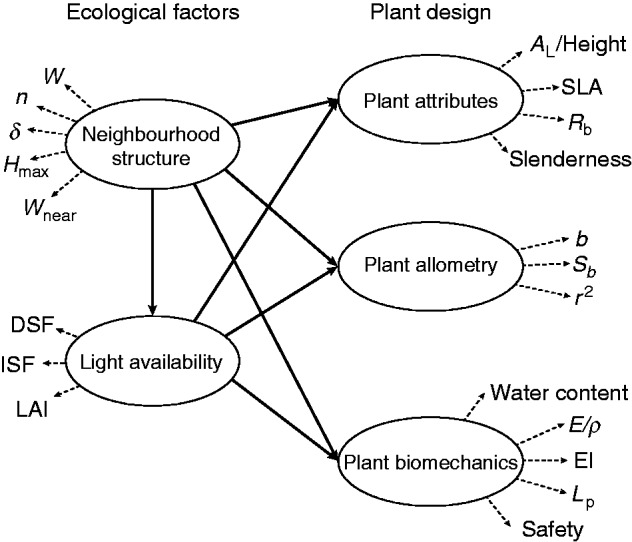
Partial least squares path model to test the effect of ecological factors on plant design. Solid and dashed lines represent the inner and outer model, respectively.
MATERIALS AND METHODS
Study site and plant species
We conducted this study at two sites in Río Macho, Cartago, Costa Rica. The first site is a secondary old forest (SOF) of 21·26 ha near Estación de Biología Tropical y Acuicultura (EBTARM) (9º45′52·64″ N, 83º51′44·13″ W, 1700 masl). Annual rainfall is 2416 mm and the monthly mean temperature is 15·8 °C (Pérez-Molina and Cordero, 2012). The most abundant tree species are Viburnum costaricanum, Lozania mutisiana, Symphonia globulifera, Ilex lamprophylla and Dendropanax arboreus. The second site is a secondary young forest (SYF) of 11·62 ha to the west of EBTARM (9º45′24·42″ N, 83º50′59·16″ W, 1700 masl). The average rainfall is similar to that of the SOF, but monthly mean temperature is 16·5 °C (Pérez-Molina and Cordero, 2012). Some of the most common plant species in this site are Syzygium jambos, Vismia baccifera, Miconia dodecandra, Myrsine coriacea and V. costaricanum. According to aerial photographs and interviews, the SOF and SYF had undergone natural regeneration for 53 and 20 years at the time of sampling, respectively, both from abandoned pastures and soils derived from Ultisol. Both sites are classified within the rainy tropical lower montane life zone (ITCR, 2008).
Two Rubiaceae plants were used in this study: Palicourea padifolia and Psychotria elata. P. padifolia is a common shrub (2–7 m in height) found in cloud forests from southern Mexico to Panama (Taylor, 1989). It can be recognized by its reddish-purple pyramidal, terminal inflorescences with yellow, 1-d flowers organized in cymose clusters (Taylor, 1989). Psychotria elata is an understorey shrub (5–8 m in height) of premontane and wet forests from southern Mexico to Ecuador, easy to recognize by its inflorescences surrounded by two ovate red involucral bracts.
Field design
At each study site we choose 27 plants of P. padifolia and 22 of P. elata as target plants to measure the physical structure and light availability of the neighbourhood and different plant design traits described below. We chose plants with no apparent mechanical damage, low herbivory, growing in sites with little slope, far from trails, and considering plant heights between 1 and 1 m. Each target plant was separated from other target plants by >5 m but including different areas along each site. For each target plant, we measured in the field the individual stem height (m) and basal diameter 5 cm above ground (Db, cm) and made schematic representations of the entire plant bifurcations. Later, plants were collected, cut at ground level, put into large bags and taken to the station. Thereafter, we extracted a segment of three mature and fully expanded leaves from the top crown to measure lamina thickness (mm) with a digital micrometer (293-349, Mitutoyo, Miyazaki, Japan). Likewise, total fresh leaf area (cm2) was measured with a leaf area meter (Li-3100, LI-COR, Lincoln, NE, USA). Then, the leaf segment was extracted and total leaf biomass was determined by drying at 58 °C for >72 h to calculate specific leaf area (SLA, cm2 g−1) and total dry leaf weight (g). We used the remaining plant material to estimate several allometric and biomechanical features, described later.
Neighbourhood structure
All neighbouring woody plants (excluding woody climbers and palms) within a 5-m radius that exceeded the horizontal height of the target plant were measured. For each neighbour plant, height (h, m), circumference at breast height (c, m), horizontal orientation with respect to magnetic north (α) and distance to the target plant (d, m) were measured. The value of c was measured with a tape (±0.05 cm), α with a digital compass (BG, Bushnell, Overland Park, KS, USA) and d using a laser range-finder (Disto D2, Leica Geosystems, Heerbrugg, Switzerland). Tree height was determined in two ways according to the visibility of the tree top crown: direct measurements of the tallest leaf by laser distance measurement and trigonometric height estimations with a digital clinometer (Forestry Pro, Shinagawa-ku, Tokyo, Japan) and a laser range-finder (Disto D2, Leica Geosystems AG, Heerbrugg, Switzerland).
We extracted five characteristics of the neighbourhood structure that influence plant features: (1) the number of neighbours (n) or the number of plants falling within the 5-m radius as a density-dependent factor; (2) maximum neighbour height (Hmax, m), which relates to neighbourhood size; (3) the tree aggregation index (δ), which is related to the neighbours’ dispersion; (4) near-tree interference (Wnear, m3 m−1), which is related to the nearest interaction factor; and (5) distance-dependent plant interference (W, m3 m−1), which is related to neighbourhood size distance pressure. W was calculated according to the Weiner model (1984), which defines W as:
| (1) |
where i is each neighbour plant, n is the total number of neighbours, and h, c and d are the height, circumference at breast height and distance described above. W varies between 0 and ∞, where higher values correspond to higher interference and competition. Wnear was calculated in the same way, except that we used the value of the nearest neighbour. We calculated δ according to Zar (2010) considering the horizontal orientation of the neighbours as follows:
| (2) |
where i and n are as described above, and α is the horizontal angle of orientation of each neighbour plant. The value of δ varies between 0 and 1, where 0 describes completely aggregated neighbourhoods and 1 completely dispersed neighbourhoods.
Understorey light environment measurement
We measured the neighbourhood light characteristics of each target plant using hemispherical photographs (Rich, 1989) after the harvest. Photographs were taken with a digital camera (EX SD14, Sigma, Setagaya-ku, Japan) equipped with a fisheye lens of 35 mm focal length. The camera was levelled at 1.50 m by a tripod and the photograph was taken oriented to the magnetic north. Five pictures with different shutter speeds were taken; however, for analysis we selected only the picture with the best contrast. Resulting images were analysed with HemiView 2·1 software (Delta-T Instruments, Cambridge, UK). Image processing was performed by creating 160 sky sectors (eight azimuth classes and 20 elevation angle classes) and, for a specific harvest day, the time series (1-min intervals) of openness along the solar track. From these analyses, the direct site factor (DSF, %), indirect site factor (ISF, %) and global site factor (GSF, %) were estimated. DSF and ISF estimate the fraction of direct and diffuse radiations, respectively, expected to reach the spot where the photograph was taken. GSF is the mean of DSF and ISF. Likewise, the leaf area index (LAI, amount of leaf surface area per unit ground area) was estimated as an indicator of forest cover above each plant.
Diameter–height allometry
For each species and site, the relationships between plant height and Db and between plant height and total leaf area were used to show the effect of the type of forest on the allometric scaling of the plants. Interspecific differences in slope and intercept reflect the trade-off between height growth and leaf area extension for understorey species across growth forms (King, 1990; Kohyama and Hotta, 1990).
Plant bifurcation
We studied the plant branching patterns according to the branch order method of Strahler (1957) and Shukla and Ramakrishnan (1986). This method assumes that the forking order of each branch is independent of age. For this, the last branch was designated as the first order; where two first-order branches came together the resulting proximal segment was assigned the second order and so forth. However, if two or more branches of unequal order were joined, the resulting branch maintained the higher order (for a schematic description see Supplementary Data Fig. S1). We calculated the natural antilogarithm of the slope of the linear regression between the natural base logarithm of the number of branches per order and the branch order per plant, as an indicator of the scale pattern of plant bifurcation (bifurcation ratio, RB). Even though there is great variability in branching ratios by species, growth form and light environment, a general expected trend for understorey shrubs is reducing bifurcation with increasing light (Valladares et al., 2012).
Plant allometry
We studied the relationships of segment length (L, cm) and diameter (D, cm) with segment order (N) as entities of pattern scale and stability of development on each plant. For this purpose, a plant segment was considered as the section between consecutive intersections of branches, stems or both. The same logic of branch ordering as that utilized for plant bifurcation was used to study segment order. From this, linear regression models between L and N and between D and N were inspected to calculate the scaling exponent (b) and interception (a) per target plant. Also, the accuracy of the regression was evaluated using the coefficient of determination (r2), as well as the standard error of the intersection (Sa) and slope (Sb). These parameters are good estimators of homeostasis disruption and the developmental stability of the plant (Alados et al., 1999), which help to describe the patterns of homogeneity of growth in plants in different ecosystems.
Biomechanical properties
Young's modulus of elasticity (E, MNm−2), flexural stiffness (EI, Nm2), tissue density (ρ, g cm–3) and the percentage of stem water content were measured in a segment of the main stem at the top of the crown of each harvested individual. E was calculated for each segment by bending experiments considering the segment of a cantilevered beam fixed at one end and measuring the deflections from the initial horizontal after putting a concentrated load at the free end. Ten discrete loads were used as bending forces (F) through the bending tests while increasing one by one and measuring the maximum deflection (Xmax) after 60 s (Speck 1994a, b). To remain in the linear part of the force–deflection curve, total deflection was <20 % of the segment length. To reduce shearing effects present during bending of anisotropic materials like stems, the ratio of stem length to stem radius was well above 24, which is the minimum recommended value (Cannell and Morgan, 1987; Niklas, 1992). We fitted a regression model using the linear part of the force–deflection curve (Hookean range), using the slope b to extract EI (Niklas, 1992) and E (Speck, 1994a):
| (3) |
| (4) |
| (5) |
where ls is stem length between the holder and its free end and Im is the mean second moment of area of the segment under test. Im was calculated from the mean diameter of the segment at three points as:
| (6) |
where r is the stem-over-bark radius. All segment tests in which the correlation coefficients of the force–deflection data were <0·95 were eliminated from the analyses. Calculations of E reported in this study are assumed to represent the biomechanical properties of the entire stem, which consists of several tissues. Thus, E is termed the apparent modulus Eapp (as in Gartner, 1991). The value of ρ was calculated as the ratio of stem segment dry weight and fresh volume displaced in a test tube filled with water, and percentage water content as the ratio of the difference of fresh weight less dry weight between the fresh weight multiplied by a hundred. In both cases, ρ and water content were estimated from wood pieces with bark of 3.43 ±1.56 cm3 sectioned from the segment in which we performed the bending experiments. Density-specific stiffness (E/ρ, MNm kg−1) was calculated as the ratio of Eapp and ρ, following Niklas (1993b).
From the above measurements, we determined the mechanical load parameter (Lp, dimensionless) and the factor of safety (S, dimensionless) by the critical height (hcr, m). Lp is the ratio of the dimensions of the static load at the end of the stem segment and its bending stiffness (Niklas 1990a). This was calculated as:
| (7) |
where Wt is the total fresh weight supported at the end of the stem segment, and ls, E and Im are as described above. Likewise, S was calculated as the ratio between the maximum height (critical height, hcr) that the main stem could sustain and actual plant height (h). When S =1 the plant has a height that it can maintain without breaking, when S =2 the plant has half of the hcr, which means that the plant is 2-fold safer biomechanically. We calculated hcr in two different ways, according to Holbrook and Putz (1989) (eqn 8), where the tree is described as a uniform column, and according to Niklas (1992) (eqn 9), where the tree stem tapers uniformly.
| (8) |
| (9) |
where E is the modulus of elasticity previously estimated for each individual stem, w is the ratio of fresh weight to volume (Nm−3) and Db is basal diameter. Two factors of safety according to Holbrook and Putz (1989) (SH&P) and Niklas (1992) (SN) were calculated.
Statistical analyses
We compared the light environment and neighbourhood characteristics for each species across sites using multivariate analysis of variance (MANOVA). Then we used univariate analysis of variance (ANOVA) to interpret the overall multivariate effects on each variable. For each species we compared plant attributes (allometric and biomechanical) across sites using MANOVA and extracted the univariate ANOVA in each comparison. We also applied one-way analysis of covariance (ANCOVA) to measure the effect of Db on plant height, plant height on leaf area, Im on Eapp, and Im on EI, using the site as predictor variables. We conducted the previous analysis using R software version 3.1.1 (R Development Core Team, 2014). We applied partial least squares (PLS) path modelling for each plant species to measure how ecological factors can affect plant design as a method for maximizing the amount of variance to predict variable relationships, rather than the statistical accuracy of the estimates. The latent variables consisted of neighbourhood characteristics and the light environment as ecological factors, and plant attributes, allometry and biomechanics as indicators of plant design (Fig. 1). From this, the variables for ecological factors were n, Hmax, δ, Wnear and W for neighbourhood characteristics and DSF, ISF and LAI for the light environment, while plant designs were AL/height, SLA, RB, and plant slenderness for plant attributes, b, Sb and r2 from the segment D–N regression for plant allometry and water content, and E/ρ, EI, Lp and SH&P for plant biomechanics. We used SmartPLS 3.1 software (Ringle et al., 2014) to conduct this analysis, in which we implemented a path weighting scheme to estimate the inner values of the standardized latent variable and a bootstrapping of 5000 iterations based on the individual changes to extract the variation of the inner and outer variables and their significance according to two-tailed t-tests.
RESULTS
Neighbourhood and light features
The ecological factors of neighbourhood and light environment differed between the SOF and SYF for each species (P. padifolia: Hotelling–Lawley(9,44) = 2·45, P > 0·001; P. elata: Hotelling–Lawley(9,34) = 3·37, P > 0·001) (Table 1). In general, neighbourhood parameters like n and Hmax were different between sites in both species, plants in the SOF showing higher values of Hmax and fewer neighbours than plants in the SYF. Neighbourhood characteristics like δ, W and Wnear did not differ between sites in either of the two species. All light parameters extracted were different in both species, the plants in the SYF having lower values of LAI and higher values of DSF, ISF and GSF than those in the SOF.
Table 4.
Biomechanical and tissue properties of stem sections of Palicourea padifolia and Psychotria elata in two cloud forests of different successional status in Río Macho, Costa Rica: secondary young forest (SYF) and secondary old forest (SOF). Values are mean and standard deviation. Significant differences between sites across species are represented by the univariate ANOVA extracted from MANOVA
| Property | Species |
|||||
|---|---|---|---|---|---|---|
|
P. padifolia |
P. elata |
|||||
| SYF | SOF | F | SYF | SOF | F | |
| Im (10−9) | 1.58 ± 0.10 | 1.34 ± 0.08 | 7.63** | 2.37 ± 0.17 | 1.61 ± 0.15 | 0.01 |
| Eapp (10+9) | 2.57 ± 0.97 | 2.70 ± 1.37 | 1.45 | 2.37 ± 1.52 | 2.84 ± 1.30 | 1.39 |
| EI | 0.38 ± 0.28 | 0.38 ± 0.29 | 0.77 | 0.48 ± 0.43 | 0.41 ± 0.40 | 0.27 |
| ρ | 302.40 ± 61.84 | 302.78 ± 91.99 | 2.86 | 335.12 ± 75.02 | 302.70 ± 73.68 | 0.08 |
| Water content | 65.72 ± 6.80 | 70.39 ± 4.96 | 10.61** | 72.14 ± 3.34 | 71.38 ± 6.04 | 0.49 |
| Eapp/ρ (10+6) | 9.00 ± 3.79 | 8.95 ± 4.51 | 3.01 | 7.60 ± 5.04 | 10.22 ± 5.80 | 0.45 |
| Lp (10−5) | 28.59 ± 38.56 | 24.48 ± 32.34 | 0.01 | 16.63 ± 19.21 | 28.65 ± 45.36 | 0.01 |
| SH&P | 2.21 ± 0.71 | 2.39 ± 0.62 | 1.25 | 2.68 ± 0.51 | 2.25 ± 0.50 | 6.70* |
| SN | 3.46 ± 1.12 | 3.74 ± 0.97 | 1.65 | 4.19 ± 0.80 | 3.52 ± 0.78 | 6.23* |
*P < 0.05; **P < 0.01.
Table 1.
Neighbourhood and light characteristics of all the studied plants as estimated by interference measures and hemispherical canopy photography in two cloud forests of different successional status in Río Macho, Costa Rica: secondary young forest (SYF) and secondary old forest (SOF). Values are mean and standard deviation. Significant differences between sites are represented by the univariate ANOVA extracted from MANOVA
| Characteristic | Species |
|||||
|---|---|---|---|---|---|---|
|
Palicourea padifolia |
Psychotria elata |
|||||
| SYF | SOF | F | SYF | SOF | F | |
| Neighbourhood characteristics | ||||||
| n | 36.04±14.31 | 28.54 ± 10.09 | 4.57* | 43.05 ± 9.32 | 32.05 ± 9.71 | 14.85*** |
| Hmax | 14.69 ± 3.37 | 22.86 ± 7.01 | 26.8*** | 13.76 ± 2.67 | 19.40 ± 5.36 | 23.92*** |
| Wnear | 1.67 ± 1.87 | 2.01 ± 4.38 | 0.60 | 4.66 ± 8.03 | 2.17 ± 2.93 | 1.04 |
| W | 25.05 ± 10.39 | 32.88 ± 16.31 | 3.28 | 29.27 ± 13.00 | 30.81 ± 14.96 | 0.10 |
| δ | 0.84 ± 0.08 | 0.80 ± 0.11 | 1.96 | 0.86 ± 0.07 | 0.85 ± 0.08 | 0.45 |
| Light characteristics | ||||||
| LAI | 2.32 ± 0.46 | 3.26 ± 0.64 | 48.99*** | 2.98 ± 0.43 | 2.98 ± 0.62 | 22.31*** |
| DSF | 0.20 ± 0.06 | 0.14 ± 0.04 | 18.26*** | 0.20 ± 0.07 | 0.15 ± 0.04 | 10.61** |
| ISF | 0.18 ± 0.04 | 0.11 ± 0.04 | 34.11*** | 0.17 ± 0.04 | 0.12 ± 0.03 | 26.19*** |
| GSF | 0.20 ± 0.06 | 0.13 ± 0.04 | 19.77*** | 0.19 ± 0.06 | 0.14 ± 0.04 | 11.66** |
*P <0.05; **P <0.01; ***P <0.001.
Plant attributes
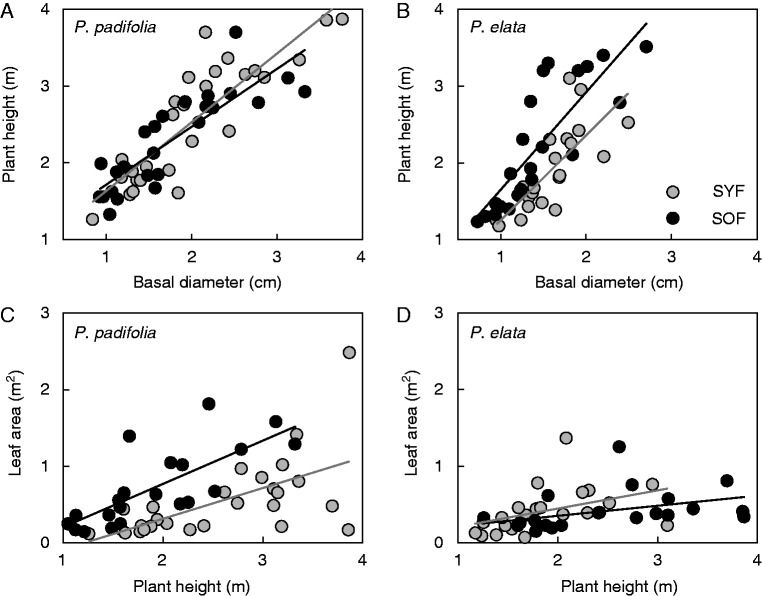
The multivariate response of the plant attributes within each species showed that plant attributes differed between sites (P. padifolia: Hotelling–Lawley(8,45) = 1·39, P < 0·001; P. elata: Hotelling–Lawley(8,35) = 2·88, P <0·001). These analyses suggest that the differences included leaf thickness, slenderness, SLA and RB in P. elata, but only SLA and RB in P. padifolia. In both species the values of SLA and plant slenderness were higher and leaf thickness was lower in the SOF than in the SYF, respectively. Surprisingly, plants of P. elata showed higher values of RB in the SYF than in the SOF, while in plants of P. padifolia the higher values of RB were found in the SOF. ANCOVA results showed that Db had a strong relationship with plant height in both species (Fig. 2, Supplementary Data Table S1), but this relationship did not differ between sites, or their site-Db interaction. Likewise, the relationship of plant height with total leaf area showed the same pattern in both species (Fig. 2), plant height having a strong effect on total leaf area, although variation between sites (except P. padifolia) and their site-plant height interaction were not significant (Table S1).
Fig. 2.
Relationship of basal stem diameter with plant height (A, B) and of plant height with total leaf area (C, D) in Palicourea padifolia (A, C) and Psychotria elata (B, D) in two cloud forests of different successional status in Río Macho, Costa Rica: secondary young forest (SYF) and secondary old forest (SOF). The two-way ANCOVA results are shown in Table S1.
Plant allometry
The multivariate comparisons of the allometric patterns across sites within each species showed that allometric plant design did not differ between sites (P. padifolia: Hotelling–Lawley(11,45) = 0·48, P = 0·08; P. elata: Hotelling–Lawley(8,35) = 0·63, P = 0·09). Despite this, the univariate responses extracted from these comparisons in P. padifolia showed that the parameters a, b and r2 from the L-N relationship were different between sites. None of the extracted variables from the D-N relationship in either species differed between sites.
Plant biomechanics
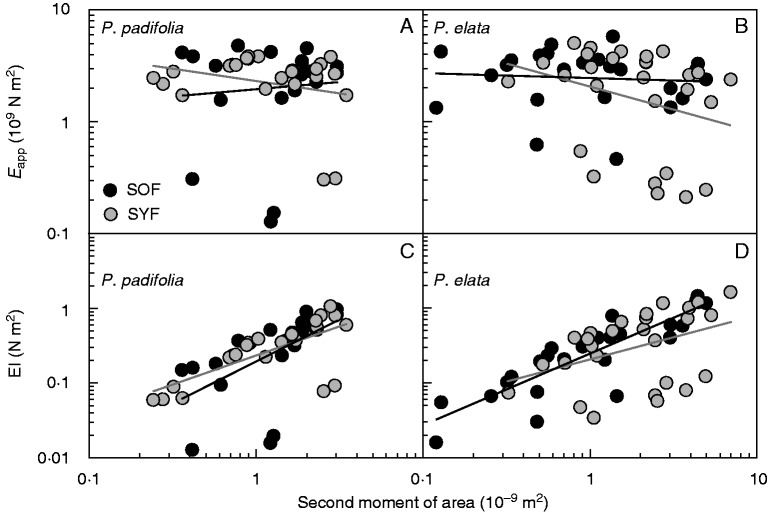
The multivariate response of the biomechanics across sites within each species showed that the biomechanical plant designs differed between sites in P. padifolia (Hotelling–Lawley(9,45) = 0·55, P = 0·02), but not in P. elata (Hotelling–Lawley(8,35) = 0·48, P = 0·13). Within P. padifolia, differences between sites were represented by changes in stem water content and Im, plants in the SOF presenting higher values of water content and lower values of Im than plants in the SYF. Differences across sites in P. elata were shown only in SH&P and SN, plants in the SYF presenting higher values than plants in the SOF. ANCOVA results showed in both species that Im, the site and their interaction did not have an effect on Eapp (Fig. 3A, B, Supplementary Data Table S2). However, there was a strong relationship between Im and EI in both species (Fig. 3C, D), but the EI pattern was not effected by site or the Im–site interaction.
Fig. 3.
Relationship of apparent Young’s modulus of elasticity (Eapp) (A, B) and flexural stiffness (EI) (C, D) with second moment of area of Palicourea padifolia (A, C) and Psychotria elata (B, D) stem in two cloud forests of different successional status in Río Macho, Costa Rica: secondary young forest (SYF) and secondary old forest (SOF). The two-way ANCOVA results are shown in Table S2.
Table 2.
Leaf and plant attributes of Palicourea padifolia and Psychotria elata in two cloud forests of different successional status in Río Macho, Costa Rica: secondary young forest (SYF) and secondary old forest (SOF). Values are mean and standard deviation. Significant differences between sites across species are represented by the univariate ANOVA extracted from MANOVA
| Attribute | Species |
|||||
|---|---|---|---|---|---|---|
|
Palicourea padifolia |
Psychotria elata |
|||||
| SYF | SOF | F | SYF | SOF | F | |
| Plant height | 2.55 ± 0.78 | 2.26 ± 0.62 | 1.71 | 1.90 ± 0.54 | 1.52 ± 0.80 | 1.79 |
| Plant diameter | 2.03 ± 0.75 | 1.73 ± 0.70 | 2.52 | 1.58 ± 0.37 | 1.32 ± 0.53 | 1.93 |
| Total leaf area | 5316.37 ± 5111.07 | 6173.81 ± 4906.46 | 0.78 | 4051.31 ± 2591.61 | 4176.60 ± 2997.81 | 0.29 |
| Total leaf biomass | 44.88 ± 19.39 | 29.81 ± 52.11 | 0.22 | 26.69 ± 55.50 | 35.07 ± 20.92 | 0.04 |
| SLA | 160.13 ± 32.18 | 237.72 ± 56.43 | 38.65*** | 197.34 ± 42.01 | 243.42 ± 22.18 | 30.40*** |
| Leaf thickness | 0.22 ± 0.08 | 0.20 ± 0.04 | 0.01 | 0.27 ± 0.11 | 0.18 ± 0.05 | 41.64*** |
| Slenderness | 130.15 ± 20.15 | 138.52 ± 28.92 | 1.16 | 120.36 ± 22.44 | 154.94 ± 27.80 | 23.19*** |
| RB | 6.84 ± 3.38 | 4.56 ± 1.87 | 9.83** | 3.33 ± 0.66 | 3.48 ± 1.81 | 5.41* |
*P <0.05; **P <0.01; ***P <0.001.
Structural equation model
The PLS path analysis showed that not all ecological factors affected plant design in both species (Fig. 4). The analysis suggested two ways in which plant designs of these species are affected by ecological factors: (1) the neighbourhood structure influences the allometric and biomechanical patterns; and (2) the changes in light availability associated with the neighbourhood structure influence the plant attributes. According to the outer loadings in both species (Table 5), Hmax and W were the variables that had a significant effect on the neighbourhood structure. The neighbourhood structure explained 29 and 10 % of light availability variance in P. padifolia and P. elata environment, respectively (Supplementary Data Table S3), increases in the neighbour’s structure leading to decreases in light availability. In this latter latent group in both species, all variables (ISF, DSF, LAI) had strong effect. In relation to plant design, neighbourhood and light characteristics together explained 30 and 52 % of the variance of plant attributes in P. padifolia and P. elata, respectively. In both species RB and SLA had an effect on plant attributes, but only in P. elata did plant slenderness also influence their design. Likewise, neighbourhood and light features explained 9 and 6 % of the variance of plant allometry in P. padifolia and P. elata, respectively; however only the neighbourhood structure significantly affected this latent group. In both species increases in the structure of the neighbours (Hmax and W mainly) led to increases in Sb and decreases in r2 of the plant allometric design, the latter being the only parameters that had a significant effect on the allometry latent group. The biomechanical plant design suggests that the neighbourhood structure influenced their properties; however, only in P. padifolia were these properties affected jointly with light availability. Together, neighbourhood and light availability explained 23 and 24 % of the variance in biomechanical design in P. padifolia and P. elata, respectively. In this last latent variable, the outer loadings showed that E/ρ was related to the changes in plant biomechanics in both species. In P. elata this change was also related to tissue water content and Lp, while in P. padifolia it was also related to SH&P.
Fig. 4.
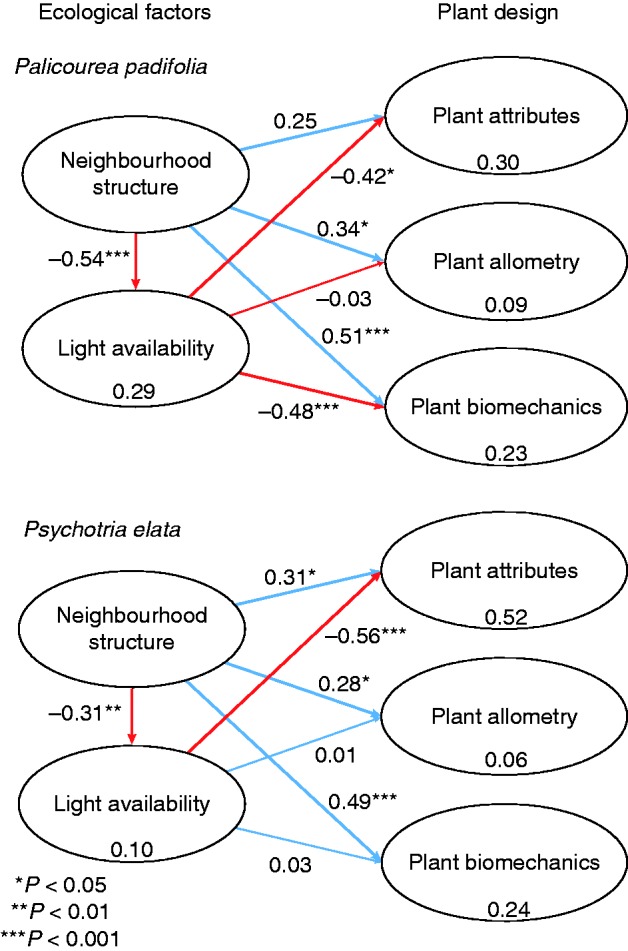
Partial least squares path model results showing the effect of ecological factors on plant design. Path coefficients and their significances extracted by bootstrapping are shown near the arrows; values within ellipses represent the proportion of variance explained by the model. Outer loadings are presented in Table 5.
Table 5.
Loadings of the outer model and bootstrapping results (mean ± standard error) and t-test of the partial least square path model for the effects of ecological factors on plant design. The inner variables are presented in bold
| Outer model | Species |
|||||
|---|---|---|---|---|---|---|
|
P.
padifolia |
P.
elata |
|||||
| Loadings | Mean ± s.e. | t-test | Loadings | Mean ± s.e. | t-test | |
| Neighbourhood structure | ||||||
| W | 0.81 | 0.89 ± 0.06 | 13.94*** | 0.86 | 0.69 ± 0.22 | 3.94*** |
| n | −0.03 | −0.25 ± 0.17 | 0.18 | −0.51 | −0.41 ± 0.23 | 2.17* |
| δ | 0.02 | 0.21 ± 0.18 | 0.12 | 0.03 | 0.25 ± 0.18 | 0.99 |
| Hmax | 0.77 | 0.72 ± 0.16 | 4.83*** | 0.60 | 0.55 ± 0.20 | 2.97** |
| Wnear | 0.01 | 0.23 ± 0.16 | 0.01 | 0.25 | 0.43 ± 0.23 | 1.06 |
| Light availability | ||||||
| DSF | 0.91 | 0.90 ± 0.06 | 17.38*** | 0.88 | 0.85 ± 0.10 | 9.33*** |
| ISF | 0.96 | 0.95 ± 0.03 | 28.01*** | 0.96 | 0.94 ± 0.08 | 13.20*** |
| LAI | −0.84 | −0.83 ± 0.06 | 13.65*** | −0.70 | −0.71 ± 0.12 | 5.56*** |
| Plant attributes | ||||||
| AL/height | 0.26 | 0.47 ± 0.26 | 0.98 | −0.10 | −0.23 ± 0.17 | 0.58 |
| RB | −0.60 | −0.51 ± 0.18 | 3.38** | 0.42 | 0.46 ± 0.18 | 2.35* |
| Plant slenderness | 0.10 | 0.41 ± 0.24 | 0.44 | 0.75 | 0.69 ± 0.16 | 4.76*** |
| SLA | 0.87 | 0.73 ± 0.18 | 4.60*** | 0.86 | 0.84 ± 0.10 | 8.30*** |
| Plant allometry | ||||||
| b | 0.02 | 0.35 ± 0.23 | 0.10 | 0.45 | 0.59 ± 0.30 | 1.50 |
| Sb | 0.90 | 0.81 ± 0.17 | 5.14*** | 0.96 | 0.67 ± 0.26 | 3.33*** |
| r2 | −0.89 | −0.81 ± 0.17 | 5.27*** | −0.56 | −0.60 ± 0.28 | 2.01* |
| Plant biomechanics | ||||||
| Water content | 0.20 | 0.36 ± 0.23 | 0.86 | 0.79 | 0.56 ± 0.25 | 3.13** |
| E/ρ | 0.50 | 0.49 ± 0.22 | 2.29* | 0.36 | 0.44 ± 0.21 | 2.33* |
| EI | 0.11 | 0.28 ± 0.20 | 0.53 | 0.27 | 0.51 ± 0.25 | 1.08 |
| Lp | −0.03 | −0.37 ± 0.23 | 0.14 | −0.63 | −0.48 ± 0.26 | 2.40* |
| Safety factor | −0.94 | −0.76 ± 0.18 | 5.21*** | −0.28 | −0.38 ± 0.23 | 1.18 |
*P < 0.05; **P < 0.01; ***P < 0.001.
Table 3.
Plant-scale properties of Palicourea padifolia and Psychotria elata in two cloud forests of different successional status in Río Macho, Costa Rica: secondary young forest (SYF) and secondary old forest (SOF).Values are mean and standard deviation. Significant differences between sites across species are represented by the univariate ANOVA extracted from MANOVA
| Property | Species |
|||||
|---|---|---|---|---|---|---|
|
P. padifolia |
P. elata |
|||||
| SYF | SOF | F | SYF | SOF | F | |
| Segments | 44.93 ± 42.52 | 36.50 ± 23.11 | 0.11 | 31.23 ± 25.73 | 37.05 ± 34.91 | 0.02 |
| Length–order relation | ||||||
| a | 19.74 ± 13.94 | 8.99 ± 11.94 | 9.15** | 12.96 ± 15.96 | 22.98 ± 23.48 | 2.74 |
| b | 4.20 ± 10.33 | 11.91 ± 12.18 | 7.29** | 7.44 ± 9.50 | 3.47 ± 13.90 | 1.55 |
| Sa | 10.84 ± 6.74 | 11.16 ± 7.91 | 0.30 | 10.98 ± 6.75 | 16.33 ± 15.07 | 0.89 |
| Sb | 6.88 ± 4.43 | 7.18 ± 5.91 | 0.18 | 6.43 ± 4.50 | 10.07 ± 10.44 | 0.63 |
| r2 | 0.06 ± 0.15 | 0.12 ± 0.13 | 11.16** | 0.13 ± 0.13 | 0.08 ± 0.11 | 2.48 |
| Diameter–order relation | ||||||
| a | −1.44 ± 1.33 | −1.07 ± 0.88 | 1.43 | −0.20 ± 0.74 | 0.19 ± 1.00 | 2.16 |
| b | 4.45 ± 1.08 | 3.98 ± 0.96 | 2.59 | 3.69 ± 0.72 | 3.30 ± 0.80 | 3.36 |
| Sa | 1.15 ± 0.56 | 0.94 ± 0.62 | 0.81 | 0.88 ± 0.42 | 0.92 ± 0.53 | 1.21 |
| Sb | 0.74 ± 0.42 | 0.60 ± 0.45 | 2.29 | 0.51 ± 0.28 | 0.55 ± 0.37 | 0.01 |
| r2 | 0.62 ± 0.14 | 0.68 ± 0.12 | 3.08 | 0.74 ± 0.10 | 0.71 ± 0.08 | 1.71 |
**P < 0.01.
DISCUSSION
Ecological factors
This study shows strong effects of neighbourhood structure on understorey plant design in highly diverse and heterogeneous ecosystems such as these tropical cloud forests. We show that these sites with different successional status have contrasting physical structure and light availability around these two understorey shrubs. Differences between sites in terms of neighbourhood structure were explained mainly by n and Hmax. In general, the highest value of n in the SYF corresponds to higher plant density, which is typical of early successional stages (Finegan, 1996; Guariguata et al., 1997), while the highest Hmax values in the SOF are indicative of forests during later successional states (Huston and Smith, 1987). The understorey light environments in the SYF had higher light availability than understories in the SOF, probably due to its forest structure, with small neighbours and low leaf cover. According to the PLS path model, increases in the parameters of neighbourhood structure (lower values of n) are associated with decreases in light availability, which suggests the capture of light by neighbouring plants, and perhaps plant competition. This analysis suggests also that W and Hmax were the neighbourhood characteristics that have the greater effects on the increases in LAI and decreases in ISF and DSF. In this sense, the tested model showed that neighbourhood features affected the light environment and plant design more by effects of distance and size-dependence than by nearness interference or density-dependent effects. Some studies have shown that distance-dependence indices like W could be used to determine the canopy light index (Grote et al., 2013); therefore, it would be expected that, in terms of size (Hmax) and distance (W), neighbour trees determine the light patterns of the surrounding plant environment.
Even though W did not show differences between sites, its calculation can compensate itself as a consequence of the number and size of neighbours within each site. In this sense, the light availability resulting from light interception by a distance-dependent neighbourhood structure could affect target plants through these variables. In addition, this problem may be a result of greater variation in W between sites than within sites. This suggests that observed variations in plant design of these species in relation to the light environment may depend not only on the differences in light availability between sites, but also on the properties of light generated by the heterogeneity of the neighbourhood structure between and within sites. The above statement may have strong implications for the quality of light that these and other species may experience in the understorey, which could determine the niche partitioning of the populations within this community. For example, studies in tropical forests suggest that the percentage of light transmitted below the adult crowns of late successional trees is much lower than the percentage of light transmitted through the tree crowns of early successional forest (Kabakoff and Chazdon, 1996; Kitajima et al., 2005). This would mean that the performance of these species within these kinds of forest depends not only on the ability to adapt their traits depending on the amount of light available in a distance-dependent environment, but also on their capacity to adapt their traits to the light quality generated by this same environment, although this latter aspect was not addressed in this study.
Plant attributes
In both species we found important changes in some attributes associated with forest structure. Changes in SLA, leaf thickness and plant slenderness between sites in both species strongly respond to the previously described ecological factors, as found elsewhere (Ackerly et al., 2002; Valladares et al., 2012). Plants within sites with higher environmental exposure (e.g. high solar radiation and low forest cover) had lower values of SLA and slenderness and higher values of leaf thickness. Changes in SLA and leaf thickness between sites could be associated with the energetic costs of leaf maintenance in understorey light intensities (Lusk and Warton, 2007). Likewise, the observed increase in plant slenderness in the SOF could be associated with increased long-term survival in the understorey for this strategy to be adaptive (Valladares et al., 2012). Although the species showed differences in RB across sites, the direction of change between species differed by site, indicating an adjustment in plant design according to forest succession. Some studies suggest that plant branching is an indicator of adaptation of design to different environments (Whitney, 1976); therefore, bifurcation traits respond to the needs of resource exploration by changing reiteration design and branch mortality. Within each species, plant height, diameter, total leaf area and total leaf biomass were not different between sites, although the allometric relationship showed a species–site interaction and an effect of plant size (diameter or height) on plant design. As previously found, these plants change their resource allocation to achieve an ‘optimal plant design’ as a product of dynamic interaction with the surrounding environment (King, 1990; Weiner and Thomas, 1992; Farnsworth and Niklas, 1995; Alados et al., 1999; Henry and Aarssen, 1999). According to the PLS path model by species, plant attributes may be more affected by light availability than by neighbourhood structure. This may be due to the great plastic phenotypic capacity of these understorey shade-tolerant species to change their branch bifurcation and SLA under different light regimens (Valladares and Niinemets, 2008).
Plant allometry
The plant-scale properties showed that the order–diameter relationship can represent the pattern of development of the plant better than the order–length relationship, due to the higher coefficient of determination and the lower standard error of the slope and intercept. Although increases in stem diameter have been associated with optimal plant design in a higher density-dependent structure (Weiner and Thomas, 1992; Escós et al., 1997), here we did not find evidence of the effects of neighbourhood structure on increases in stem diameter. According to the PLS path model, increases in neighbourhood structure (W and Hmax) have a direct effect on the allometric design in both species, specifically on the developmental stability described by Sb and r2 of the order–diameter relation. These results suggest that a surrounding environment with higher capture of the light resource produces plants with lower development stability.
Plant biomechanics
Even though some mechanical features (Eapp, EI and Lp) of stem segments in both species were not affected by the type of forest, some studies claim that changes in stem size (Im) are more than enough to achieve higher biomechanical stability than changes in tissue mechanical properties per se (Holbrook and Putz, 1989; Niklas, 1990a, b; Gartner, 1991). In ecological terms, biomechanical stability is a concept that describes the likelihood of damage and constrains the extension rate of plants by requiring an increment in diameter per unit of biomass or load (King, 1990). In this sense, changes in flexural stiffness dominated by stem size and decreases in plant slenderness in the SYF in both species are a viable strategy to achieve higher biomechanical stability in plant design. In many cases, studies have recognized that increases in mechanical load are associated with the loss of stability of the design (Niklas, 1990a), which may impact the production of different structures, such as branches, flowers and fruits (Gartner, 1991). Likewise, some studies have recognized that strategies to optimize biomechanical fitness, such as increases in stem and branch diameters, have been developed to avoid the effects of environmental stress like sites with low coverage or high wind exposure (Cordero, 1999; Henry and Thomas, 2002; Cooley et al., 2004). Although changes in the factor of safety suggest that height growth of P. elata in the SOF could affect its height stability, these effects only represent a 16 % reduction in plant safety and plants remain 2- to 3-fold safer in both environments. From a whole-plant perspective, plant slenderness and developmental and biomechanical stability in both species suggest that changes in diameter and their homeostasis between forests with different succession could be an adaptation to optimize biomechanical fitness in relation to neighbourhood structure. Moreover, studies suggest that increases in stem water content can increase Eapp (Cannell and Morgan, 1987), but we did not find any evidence of this effect between sites in both species.
Implication of the neighbourhood structure for plant design
Here the approach of defining whole-plant design responses by variations in neighbourhood structure could be related to the struggle of the species to find the ‘optimal plant design’ in different environments that could provide an adaptive advantage in spatial heterogeneous systems. We consider that changes in design in neighbourhoods in which there is high or low competitiveness may be related to an economic advantage in exploiting and using efficiently the light resource according to its availability. In this sense, it is likely that the observed variations in plant design are related to the resource-conservative and resource-acquisitive strategies (Adler et al., 2013), as proposed for different functional traits as ways that drive the coexistence in the plant communities. For example, in this study increases in plant slenderness in the SOF could be associated with a resource-conservative design for the exploration of resources in a low-light environment or a highly competitive neighbourhood. This last idea has been indirectly tested by King (1990, 1994), who described saplings that adapted their plant allometry as a strategy to make efficient use of biomass for height growth in the forest understorey. Likewise, increases in SLA in the SYF could be associated with a resource-acquisitive leaf economics strategy in high-light-gap environments or neighbourhoods with low competitiveness (Adler et al., 2013). If plant design responds to conservative-acquisitive strategies, it could prevent the environmental filtering of the species and promote coexistence in spatially heterogeneous environments. If the above is true, species coexistence based on adaptive plant design could predict the composition of communities in well-studied systems.
In summary, this study demonstrates that plant design, in terms of the allometric and biomechanical traits of two understorey species, varies concomitantly with the degree of neighbourhood structure in terms of size and distance, while plant bifurcation and SLA design respond more to light availability than to neighbourhood structure. Our results strongly suggest that changes in plant design by ecological factors may have important consequences for the coexistence of the understorey species in face to neighbourhood structure. Future works should demonstrate how plant design is associated with stabilizing processes (Chesson, 2000), and fitness equality (Hubbell, 2001) in the coexistence among communities and how this association allow the survivals and assembly of the species with different designs.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: schematic description of the method of measuring plant bifurcation proposed by Strahler (1957) and Shukla and Ramakrishnan (1986). Table S1: one-way ANCOVA results of the effect of basal plant diameter on plant height and plant height on total leaf area in Palicourea padifolia and Psychotria elata in two cloud forests of different successional status in Río Macho, Costa Rica. Table S2: one-way ANCOVA results on the effect of the second moment of area (Im) to the apparent Young’s modulus of elasticity (Eapp) and flexural stiffness (EI) of Palicourea padifolia and Psychotria elata plants in two cloud forests of different successional status in Río Macho, Costa Rica. Table S3: path coefficients of the inner model and bootstrapping results (mean ± standard error and t-test) of the partial least squares path model of the effect of ecological factors on plant design.
ACKNOWLEDGEMENTS
We greatly appreciate the field assistance of the LEFET laboratory team and many other colleagues. Thanks to EBTARM for facilities support. Warm thanks to Gerardo Ávalos, Elmer García, Ned Fetcher, John Herman and Jennifer Powers, who provided valuable comments and recommendations across this study. This study was supported by the Costa Rican Fondo Especial para la Educación Superior (FEES-CONARE 2012-2013) to UNA/UCR, a project of the Sistema de Información Académica of Universidad Nacional de Costa Rica (0555-12 to R.A.C.S.).
LITERATURE CITED
- Ackerly D, Knight C, Weiss S, Barton K, Starmer K. 2002. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species level and community level analyses. Oecologia 130: 449–457. [DOI] [PubMed] [Google Scholar]
- Adler PB, Fajardo A, Kleinhesselink AR, Kraft NJ. 2013. Trait-based tests of coexistence mechanisms. Ecology Letters 16: 1294–1306. [DOI] [PubMed] [Google Scholar]
- Alados C, Escos J, Emlen J, Freeman D. 1999. Characterization of branch complexity by fractal analyses. International Journal of Plant Sciences 160: S147–S155. [DOI] [PubMed] [Google Scholar]
- Barthélémy D, Caraglio Y. 2007. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99: 375–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M, Morgan J. 1987. Young’s modulus of sections of living branches and tree trunks. Tree Physiology 3: 355–364. [DOI] [PubMed] [Google Scholar]
- Chazdon R, Fetcher N. 1984. Photosynthetic light environments in a lowland tropical rain forest in Costa Rica. Journal of Ecology 72: 553–564. [Google Scholar]
- Chesson P. 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31: 343–366. [Google Scholar]
- Cooley A, Reich A, Rundel P. 2004. Leaf support biomechanics of neotropical understory herbs. American Journal of Botany 91: 573–581. [DOI] [PubMed] [Google Scholar]
- Cordero RA. 1999. Ecophysiology of Cecropia schreberiana saplings in two wind regimes in an elfin cloud forest: growth, gas exchange, architecture and stem biomechanics. Tree Physiology 19: 153–163. [DOI] [PubMed] [Google Scholar]
- Delagrange S, Messier C, Lechowicz MJ, Dizengremel P. 2004. Physiological, morphological and allocational plasticity in understory deciduous trees: importance of plant size and light availability. Tree Physiology 24: 775–784. [DOI] [PubMed] [Google Scholar]
- Escós J, Alados C, Emlen J. 1997. The impact of grazing on plant fractal architecture and fitness of a Mediterranean shrub Anthyllis cytisoides L. Functional Ecology 11: 66–78. [Google Scholar]
- Farnsworth K, Niklas K. 1995. Theories of optimization, form and function in branching architecture in plants. Functional Ecology 9: 355–363. [Google Scholar]
- Finegan B. 1996. Pattern and process in neotropical secondary rain forests: the first 100 years of succession. Trends in Ecology & Evolution 11: 119–124. [DOI] [PubMed] [Google Scholar]
- Fitter A, Stickland T. 1991. Architectural analysis of plant root systems 1. Architectural correlates of exploitation efficiency. New Phytologist 118: 375–382. [Google Scholar]
- Freckleton R, Watkinson A. 1999. The mis-measurement of plant competition. Functional Ecology 13: 285–287. [Google Scholar]
- Gartner B. 1991. Structural stability and architecture of vines vs. shrubs of poison oak, Toxicodendron diversilobum. Ecology 72: 2005–2015. [DOI] [PubMed] [Google Scholar]
- Givnish T. 1987. Comparative studies of leaf form: assessing the relative roles of selective pressures and phylogenetic constraints. New Phytologist 106: 131–160. [Google Scholar]
- Grime JP. 1979. Plant strategies and vegetation processes. Chichester: John Wiley & Sons. [Google Scholar]
- Grote S, Condit R, Hubbell S, Wirth C, Rüger N. 2013. Response of demographic rates of tropical trees to light availability: can position-based competition indices replace information from canopy census data? PLoS One 8: e81787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guariguata M, Chazdon R, Denslow J. 1997. Structure and floristics of secondary and old-growth forest stands in lowland Costa Rica. Plant Ecology 132: 107–120. [Google Scholar]
- Hambäck P, Beckerman A. 2003. Herbivory and plant resource competition: a review of two interacting interactions. Oikos 101: 26–37. [Google Scholar]
- Henry H, Aarssen L. 1999. The interpretation of stem diameter–height allometry in trees: biomechanical constraints, neighbour effects, or biased regressions? Ecology Letters 2: 89–97. [Google Scholar]
- Henry HAL, Thomas SC. 2002. Interactive effects of lateral shade and wind on stem allometry, biomass allocation, and mechanical stability in Abutilon theophrasti (Malvaceae). American Journal of Botany 89: 1609–1615. [DOI] [PubMed] [Google Scholar]
- Holbrook N, Putz F. 1989. Influence of neighbors on tree form: effects of lateral shade and prevention of sway on the allometry of Liquidambar styraciflua (sweet gum). American Journal of Botany 76: 1740–1749. [Google Scholar]
- Horn H. 1971. The adaptive geometry of trees. Princeton: Princeton University Press. [Google Scholar]
- Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton: Princeton University Press. [DOI] [PubMed] [Google Scholar]
- Huston M, Smith T. 1987. Plant succession: life history and competition. American Naturalist 130: 168–198. [Google Scholar]
- ITCR . 2008. Atlas digital de Costa Rica. Cartago, Costa Rica: Laboratorio de Sistemas de Información Geográfica, Escuela de Ingeniería Forestal, Instituto Tecnológico de Costa Rica. [Google Scholar]
- Kabakoff R, Chazdon R. 1996. Effects of canopy species dominance on understorey light availability in low-elevation secondary forest stands in Costa Rica. Journal of Tropical Ecology 12: 779–788. [Google Scholar]
- King D. 1990. Allometry of saplings and understorey trees of a Panamanian forest. Functional Ecology 4: 27–32. [Google Scholar]
- King D. 1994. Influence of light level on the growth and morphology of saplings in a Panamanian forest. American Journal of Botany 81: 948–957. [Google Scholar]
- Kitajima K, Mulkey S, Wright S. 2005. Variation in crown light utilization characteristics among tropical canopy trees. Annals of Botany 95: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama T, Hotta M. 1990. Significance of allometry in tropical saplings. Functional Ecology 4: 515–521. [Google Scholar]
- Kraft N, Godoy O, Levine J. 2015. Plant functional traits and the multidimensional nature of species coexistence. Proceedings of the National Academy of Sciences of the USA 112: 797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky J, Uriarte M- Trait-mediated assembly processes predict successional changes in community diversity of tropical forests. Proceedings of the National Academy of Sciences of the USA 111: 5616–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusk C, Warton D. 2007. Global meta‐analysis shows that relationships of leaf mass per area with species shade tolerance depend on leaf habit and ontogeny. New Phytologist 176: 764–774. [DOI] [PubMed] [Google Scholar]
- Mäkelä A, Vanninen P. 1998. Impacts of size and competition on tree form and distribution of aboveground biomass in Scots pine. Canadian Journal of Forest Research 28: 216–227. [Google Scholar]
- McNickle G, Dybzinski R. 2013. Game theory and plant ecology. Ecology Letters 16: 545–555. [DOI] [PubMed] [Google Scholar]
- Niklas K. 1986. Evolution of plant shape: design constraints. Trends in Ecology & Evolution 1: 67–72. [DOI] [PubMed] [Google Scholar]
- Niklas K. 1990a. Biomechanics of Psilotum nudum and some early Paleozoic vascular sporophytes. American Journal of Botany 77: 590–606. [Google Scholar]
- Niklas K. 1990b. Determinate growth of Allium sativum peduncles: evidence of determinate growth as a design factor for biomechanical safety. American Journal of Botany 77: 762–771. [Google Scholar]
- Niklas K. 1992. Plant biomechanics: an engineering approach to plant form and function. Chicago: University of Chicago Press. [Google Scholar]
- Niklas K. 1993a. Testing ‘economy in design’ in plants: are the petioles and rachises of leaves ‘designed’ according to the principle of uniform strength? Annals of Botany 71: 33–41. [Google Scholar]
- Niklas K. 1993b. Influence of tissue density-specific mechanical properties on the scaling of plant height. Annals of Botany 72: 173–179. [Google Scholar]
- Niklas K, Hammond S. 2013. Biophysical effects on plant competition and coexistence. Functional Ecology 27: 854–864. [Google Scholar]
- Pérez-Molina JP, Cordero RA. 2012. Recuperación de tres coberturas forestales de altura media en Costa Rica: análisis de los oligoquetos, el mantillo y suelo. Revista de Biología Tropical 60: 1431–1443. [PubMed] [Google Scholar]
- R Development Core Team. 2014. R: a language and environment for statistical computing. Vienna: http://www.R-project.org. [Google Scholar]
- Read J, Stokes A. 2006. Plant biomechanics in an ecological context. American Journal of Botany 93: 1546–1565. [DOI] [PubMed] [Google Scholar]
- Rich P. 1989. A manual for analysis of hemispherical canopy photography. New Mexico: Los Alamos National Laboratory. [Google Scholar]
- Ringle CM, Wende S, Becker J-M. 2014. SmartPLS 3. Hamburg: www.smartpls.com. [Google Scholar]
- Rowe N, Speck T. 2005. Plant growth forms: an ecological and evolutionary perspective. New Phytologist 166: 61–72. [DOI] [PubMed] [Google Scholar]
- Shukla R, Ramakrishnan P. 1986. Architecture and growth strategies of tropical trees in relation to successional status. Journal of Ecology 74: 33–46. [Google Scholar]
- Speck T. 1994a. A biomechanical method to distinguish between self-supporting and non self-supporting fossil plants. Review of Palaeobotany and Palynology 81: 65–82. [Google Scholar]
- Speck T. 1994b. Bending stability of plant stems: ontogenetical, ecological, and phylogenetical aspects. Biomimetics 2: 109–128. [Google Scholar]
- Strahler A. 1957. Quantitative analysis of watershed geomorphology. Transactions, American Geophysical Union 33: 913–920. [Google Scholar]
- Taylor C. 1989. Revision of Palicourea (Rubiaceae) in Mexico and Central America. Systematic Botany Monographs 26: 1–102. [Google Scholar]
- Tremmel D, Bazzaz F. 1995. Plant architecture and allocation in different neighborhoods: implications for competitive success. Ecology 76: 262–271. [Google Scholar]
- Trinder CJ, Brooker RW, Davidson H, Robinson D. 2012. A new hammer to crack an old nut: interspecific competitive resource capture by plants is regulated by nutrient supply, not climate. PLoS One 7: e29413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares F, Niinemets Ü. 2008. Shade tolerance, a key plant feature of complex nature and consequences. Annual Review of Ecology, Evolution, and Systematics 39: 237–257. [Google Scholar]
- Valladares F, Martinez-Ferri E, Balaguer L, Perez-Corona E, Manrique E. 2000. Low leaf-level response to light and nutrients in Mediterranean evergreen oaks: a conservative resource‐use strategy? New Phytologist 148: 79–91. [DOI] [PubMed] [Google Scholar]
- Valladares F, Skillman JB, Pearcy RW. 2002. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany 89: 1275–1284. [DOI] [PubMed] [Google Scholar]
- Valladares F, Saldana A, Gianoli E. 2012. Costs versus risks: architectural changes with changing light quantity and quality in saplings of temperate rainforest trees of different shade tolerance. Austral Ecology 37: 35–43. [Google Scholar]
- Weigelt A, Jolliffe P. 2003. Indices of plant competition. Journal of Ecology 91: 707–720. [Google Scholar]
- Weijschedé J, Martínková J, De Kroon H, Huber H. 2006. Shade avoidance in Trifolium repens: costs and benefits of plasticity in petiole length and leaf size. New Phytologist 172: 655–666. [DOI] [PubMed] [Google Scholar]
- Weiner J. 1984. Neighbourhood interference amongst Pinus rigida individuals. Journal of Ecology 72: 183–195. [Google Scholar]
- Weiner J. 2004. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology, Evolution and Systematics 6: 207–215. [Google Scholar]
- Weiner J, Thomas S. 1992. Competition and allometry in three species of annual plants. Ecology 73: 648–656. [Google Scholar]
- Whitney G. 1976. The bifurcation ratio as an indicator of adaptive strategy in woody plant species. Bulletin of the Torrey Botanical Club 103: 67–72. [Google Scholar]
- Zar J. 2010. Biostatistical analysis, 5th edn Upper Saddle River, NJ: Prentice-Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.