Abstract
Background and Aims Despite their significant capacity to propagate vegetatively, most orchids reproduce via seeds. Sexual reproduction via seed is commonly reported, in contrast to apomixis, whereby seeds are clones of the mother. Although insect pollination and autonomous self-pollination exist in mycoheterotrophic plants, the reproductive embryology of these plants remains under-studied. This paper provides evidence for the co-occurrence of both sexual and apomictic reproduction in a population of mycoheterotrophic plants – Epipogium aphyllum. We investigated seed formation via open pollination, induced autogamy, autogamy sensu stricto and autonomous apomixis.
Methods The study was performed on a population of E. aphyllum located in northern Poland. The research included studies of the micromorphology, histochemistry and embryology of four types of reproductive systems. Scanning, fluorescence and light microscopy accompanied by graphical and statistical analyses were employed.
Key Results We observed gametophyte development, from the one-nucleate stage to maturity, in unpollinated flower buds. The lack of zygotes in flower buds indicated that fertilization did not occur at this stage. Manual self-pollination led to a zygote, followed by embryo formation. Fertilization and embryo development derived from embryogenesis via open pollination is delayed compared with hand pollination. Isolation from external pollination resulted only in structures resembling zygotes that may originate either sexually or independent of fertilization. Parthenogenetic structures that resembled zygotes were observed in flowers that were emasculated and isolated from pollination. Zygotes formed at significantly higher frequencies via open pollination and induced autogamy in comparison to the parthenogenetic structures formed in other treatments.
Conclusions We showed the absence of pre-zygotic barriers for autogamy in E. aphyllum. Self-pollination and self-fertilization are possible; however, natural self-pollination is unlikely or rare due to the position of the pollinia. Incidental parthenogenesis in E. aphyllum is very likely, given the biology of ovule development of this mycoheterotrophic orchid. This species therefore has the potential to produce seeds via both sexual and asexual means, although the contribution of apomixis to this process appears largely negligible.
Keywords: reproduction by seeds, Orchidaceae, autogamy, apomixis, parthenogenesis, Epipogium aphyllum, mycoheterotrophic plant, ghost orchid
INTRODUCTION
During the reproductive phase of the life cycle in Orchidaceae, the developmental programme can switch from sexual (via fertilization) to asexual (vegetative or apomictic) reproduction, and different variations of morphogenesis are observed (Batygina et al., 2003). Seeds are formed during both sexual and apomictic reproduction. Depending on the species, habitat and environmental conditions, fertilization occurs mainly through pollinator visits to flowers, in the form of cross-pollination or geitonogamy. Infrequent pollinator visits to flowers allow the possibility of self-pollination (Barrett, 1985a, b; Arditti, 1992; Dressler, 1993; Jacquemyn et al., 2005), which has been reported in almost 31 % of orchid species (Arditti, 1992; Peter and Johnson, 2009). The wide range of autogamous mechanisms is a consequence of the modifications of the pollinarium and/or rostellum (Arditti, 1992; Johnson and Edwards, 2000). According to Arditti (1992) and Batygina et al. (2003), a few orchid species are cleistogamous or apomictic. In the sexual development of many orchids, only the egg cell is fertilized, forming a zygote that develops into the embryo. The central cell remains unfertilized, or endosperm development is arrested at a very early stage; thus, the seed contains only the embryo, surrounded by air space (Batygina et al., 2003; Merckx et al., 2013). A different type of seed production occurs during apomixis, in which the offspring produced are clones of the mother. In contrast to sexual reproduction, apomictic plants avoid meiosis and fertilization, leading to parthenogenetic development of an unreduced egg cell (gametophytic apomixis; Curtis and Grossniklaus, 2008). Although apomixis is not frequent in orchids due to the requirement for pollen to be present on the stigma to stimulate embryo sac formation (Neiland and Wilcock, 1998), gametophytic apomixis has been noted in a few tropical species (Batygina et al., 2003; Sorensen et al., 2009).
Ghost orchid (Epipogium aphyllum, Orchidaceae) is a mycoheterotrophic and very rare ephemeral plant. Although much is known about the relationship between mycoheterotrophic plants and fungi (e.g. Merckx et al., 2013), their reproductive biology remains empirically under-studied (Roy et al., 2009; Taylor and Roberts, 2011). Although many visitors to E. aphyllum flowers have been reported (Claessens and Kleynen, 2011; Jakubska-Busse et al., 2014; Święczkowska and Kowalkowska, 2015), confirming their function as pollinators has not been possible. The difficulty in determining whether species are pollinators, together with the low seed set and self-compatibility (Geitler, 1956), has led some authors to question the role of entomogamy in this species (Van der Pijl and Dodson, 1966) and to propose the possibility of self-fertilization or even cleistogamous pollination (Vöth, 1994). Moreover, self-pollination is known to be the dominant method of reproduction in E. roseum (Vöth, 1994); regardless, its flowers differ in structure from those of E. aphyllum. Additionally, Summerhayes (1951) claimed that self-fertilization is impossible in E. aphyllum, as the flowers are non-resupinate and the position of the pollinia is below the stigma. Instead, the latest results for scent and nectar composition and floral ultrastructure (Jakubska-Busse et al., 2014; Święczkowska and Kowalkowska, 2015) indicate that ghost orchid flowers are entomogamous.
In preliminary experiments, we observed spontaneous enlargement of the ovary/seed capsules in some emasculated flowers of E. aphyllum (E. Krawczyk et al., unpubl. res.). We consider apomixis to be one possible method of seed formation. This is supported by ongoing molecular studies using simple sequence repeat (SSR) markers (J. Minasiewicz et al., unpubl. res.), analyses that suggest genetic uniformity among the plants of the studied population, which is congruent with apomictic reproduction. However, this effect can also result from vegetative propagation by bulbils (see Roy et al., 2009). Thus far, there have been no reports of apomictic development in Epipogium (Geitler, 1956; Arekal and Karanth, 1981; Pridgeon et al., 1999). Therefore, we discuss autogamy and apomictic reproduction in ghost orchid in this paper. We investigated ovule and embryo development both before and after pollination and in cases when pollination does not occur.
The aims of our study were as follows: (1) to demonstrate the lack of prezygotic barriers to autogamy (in gynostemium micromorphology and in the process of pollen tube growth from the stigma to the embryo sac); and (2) to verify hypotheses regarding the possibility of both sexual and apomictic reproduction in E. aphyllum. Various pathways of embryo formation were investigated, including induced autogamy, autogamy sensu stricto and autonomous apomixis.
MATERIALS AND METHODS
Study species
Epipogium aphyllum Sw., Orchidaceae Juss. is a very rare Eurasian terrestrial orchid. It is listed on the European Red List of Vascular Plants (Bilz et al., 2011) and in the Polish Red Book of Plants as critically endangered and at risk of extinction [CR category (Hereźniak and Piękoś-Mirkowa, 2014)].
Study location
We conducted our study at an ecological site in Darżlubska Forest (northern Poland). This large population (with 300–1000 individuals, depending on the season) grows in a beech forest (Luzulo Pilosae-Fagetum) along with a few Pinus sylvestris. The soil contains a high concentration of calcium, which came from a cement plant situated nearby. The E. aphyllum population has been monitored since 2008. The study was carried out during two flowering seasons (2013 and 2014).
Gynostemium morphology and anatomy (LM, SEM and anatomical sections)
To verify the absence of prezygotic barriers to autogamy, we moved pollinia to the stigma in each examined flower (induced autogamy); hand-pollinated flowers were collected on the 2nd, 3rd, 4th and 8th days. Unpollinated flowers were collected as control samples. Plant material was fixed in 2·5 % glutaraldehyde in 0·05 m cacodylate buffer (pH 7·0) at room temperature. The samples for scanning electron microscopy (SEM), following dehydration in an ethanol series, were subjected to critical-point drying using liquid CO2, coated with gold and observed under a Philips XL-30 scanning electron microscope. After fixation and dehydration in ethanol, the plant material for light microscopy (LM) was embedded in methylmethacrylate-based resin (Technovit 7100) and stained with 0·05 % toluidine blue O in 1 % aqueous sodium tetraborate solution. Samples were prepared according to the procedures described previously (Kowalkowska et al., 2015; Święczkowska and Kowalkowska, 2015).
Histochemistry
Hand-pollinated flowers (induced autogamy) were collected on the 8th, 12th and 16th days to verify the absence of prezygotic barriers to autogamy. Flowers were isolated from any external pollination (e.g. cross-pollination, wind-pollination) using bags and were collected at the same time for checking autogamy sensu stricto. Unpollinated flowers were collected as control samples. Plant material was fixed in 8 % formaldehyde and 0·25 % glutaraldehyde in microtubule stabilizing buffer overnight at 4 °C, as described by Świerczyńska and Bohdanowicz (2003). After dehydration in a graded ethanol series, the material was infiltrated with Steedman’s wax: polyethylene glycol 400 distearate (Sigma Aldrich, St Louis, MO, USA) and cetyl alcohol (Sigma Aldrich) in a 9:1 (w/w) ratio (Vitha et al., 2000). Sections (10 μm) were dewaxed in ethanol and then rehydrated in the ethanol–phosphate-buffered saline series. To visualize chromatin in the nuclei and cell walls, respectively, the sections were stained with 7 μg ml–1 DAPI (4′,6′-diamidino–2-phenylindole dihydrochloride; Sigma) and treated with 0·01 % Calcofluor White [Sigma (Ruzin, 1999)]. The sections were coverslipped with Mowiol medium and viewed under a Nikon Eclipse E 800 microscope equipped with a Nikon DS-5Mc CCD camera.
Embryology of the reproductive system
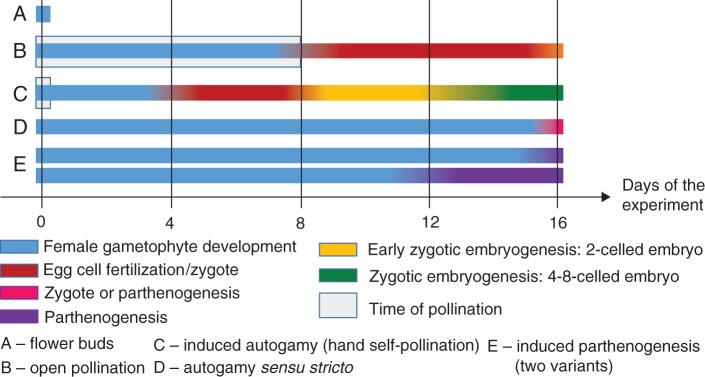
To assess the potential for the occurrence of sexual and apomictic reproduction in E. aphyllum, we planned four experiments. At the start point of experiments (day 0), buds were 24 h before anthesis (24 HBA). Such unpollinated flower buds of randomly selected individuals were marked and (1) allowed to remain on the stem for open pollination to evaluate the ability of seed formation, (2) emasculated and hand-pollinated by their own pollen to check autogamy, (3) isolated (using mesh bags) from external pollination to check autogamy sensu stricto or (4) emasculated and isolated from external pollination to check autonomous apomixis. A total of 12 flowers from different plants were used for each experiment (1–4) in each season. Ovaries from 11 flower buds were used as a control. The duration of the experiments (until the 16th day) was established based on the results of preliminary studies, in which seed capsule development was observed from artificial pollination through seed release (mean = 16, s.d. = 1·56; E. Krawczyk et al., unpubl. res.). For each experiment (1–4), flowers were excised and analysed on the 4th, 8th, 12th and 16th days of the experiment. Flowers were fixed in acetic alcohol (1^:3 dilution of acetic acid in 96 % ethanol), embedded in paraffin, sectioned and stained with Ehrlich’s haematoxylin combined with Alcian Blue as described by Rojek et al. (2005). Photomicrographs of the embryological slides were taken with a Nikon Eclipse E800 microscope.
For embryological analysis, we categorized ovule development into six stages: (1) meiosis and immature female gametophyte (FG) – from one- to four-nucleate; (2) mature female gametophyte with egg apparatus; (3) zygote; (4) two-cell embryo; (5) four-cell embryo; (6) and eight-cell embryo. To evaluate the appearance of individual developmental stages, the number of ovules in each stage was compared with the total number of ovules analysed. The number of ovules/embryos was counted from 30 randomly selected fields per ovary at 20× magnification and averaged across three repeats. Based on the average calculations, the percentage was calculated for each developmental stage on every tested day of ovary development.
To determine the contribution of different modes of reproduction (by seeds) in E. aphyllum, we compared the frequency (percentage) of zygotes or zygote-like structures (zls) among experiments 1–4 as the first sign of the seed production process. We also tested differences between the proportions of sexual and probable apomictic reproduction based on the ratio of zygotes/zls to ovules in the relevant experiments using the two-proportion z-test. We excluded autogamy sensu stricto experiments from these tests due to the uncertain origin (sexual or apomictic) of the resulting zls.
To document ovary growth, the three widest longitudinal sections of each of 16-d-old ovaries were chosen from every treatment. Their areas (mm2) were measured under 2× magnification using Lucia Image software. Differences in ovary size between treatments were evaluated for significance using Kruskal–Wallis non-parametric tests with Dunn’s post-test in STATISTICA v. 10 (StatSoft Inc., Tulsa, OK, USA).
Pollen and seed viability
To test whether the pollen of the analysed flowers is viable, the pollinia were harvested from nine plants at peak anthesis. Dead pollen samples were used as controls. In vitro pollen germination was carried out on BK medium (Brewbaker and Kwack, 1963) with 5 % sucrose. The results were scored after 48 h of incubation. Viability was assessed by placing pollen samples in fluorescein diacetate solution (Pritchard, 1985) and observing the samples on a fluorescence microscope. The results are presented as the mean value from three independent experiments. Mature E. aphyllum seed capsules, resulting from natural pollination, were collected just prior to seed release and used to test viability. The contents from 20 seed capsules were combined and thoroughly mixed. Approximately 300 seeds were tested in each of three replicates. Seed viability was assessed using a modified version of the tetrazolium method (Van Waes and Debergh, 1986). Orchid seeds were soaked for 8 h in 5 % Ca(OCl)2 and 1 % Tween 80 before being washed for 24 h in water and allowed to stain in 1 % 2,3,5-triphenyltetrazolium chloride (TTC) for 24 h. Completely stained embryos were scored as viable.
RESULTS
Gynostemium morphology and anatomy (LM, SEM and anatomical sections)
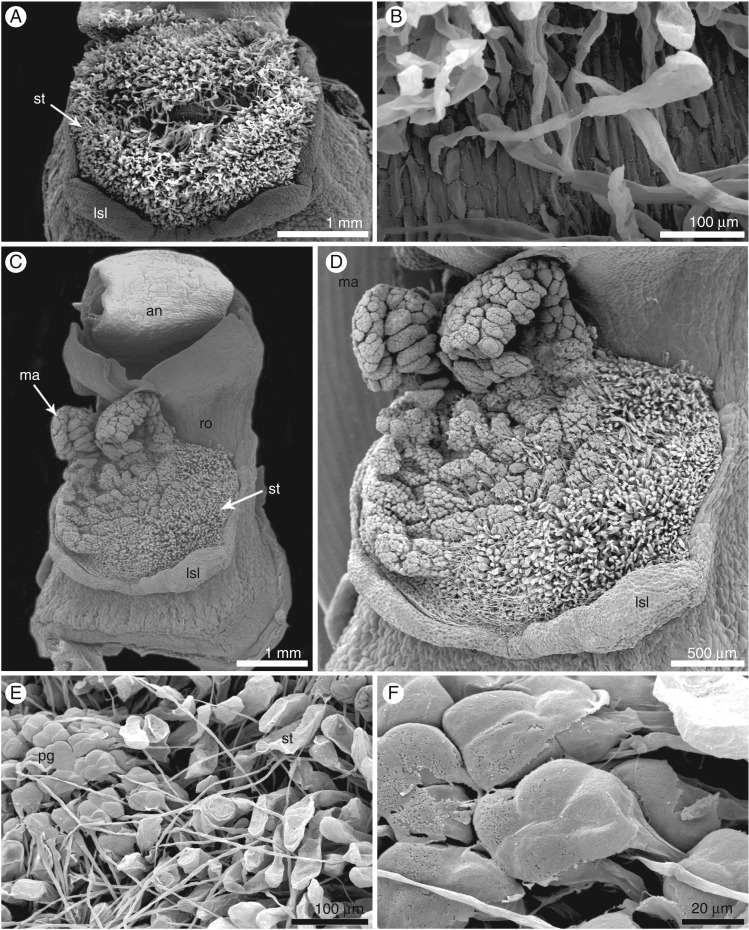
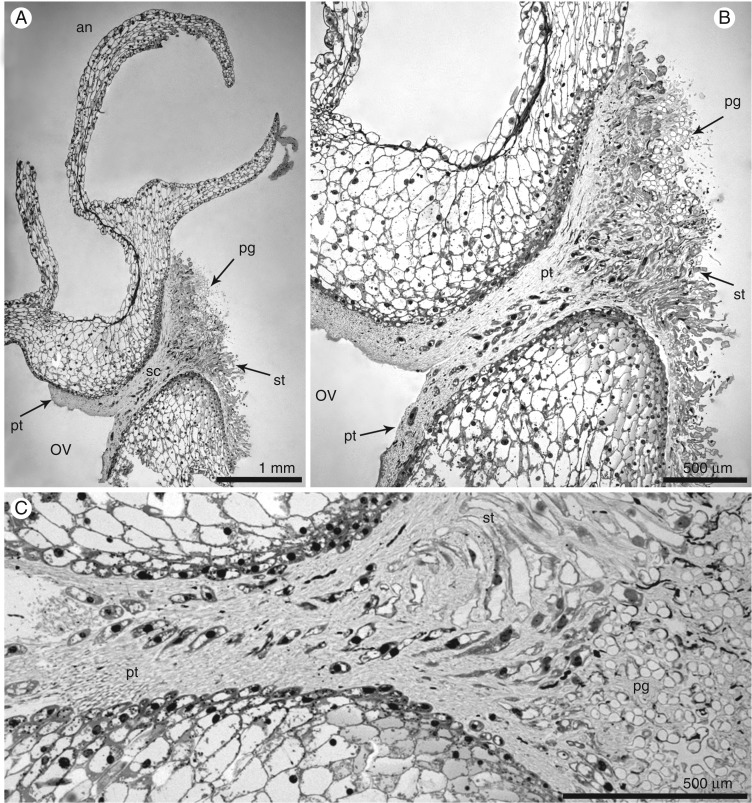
The gynostemium of E. aphyllum (Supplementary Data Fig. S1A–E) averaged 6 mm high and 3 mm wide and was massive, prominent and arcuate. The anther was incumbent, ovoid-ellipsoid to ellipsoid, with two parallel chambers. LM and SEM studies revealed two oblong-ovoid- to ovoid-shaped pollinia (Supplementary Data Figs S1E and S2A–C). Groups of pollen grains were broken away from pollen masses, and were visible on caudicles (Supplementary Data Fig. S2A, D–F). A single, relatively large viscidium was present in the middle and slightly to the right, whereas the caudicles were situated to the left (Supplementary Data Fig. S2D, E). The rostellum was ligulate, erect and rather short (Figs S1C–E and S2A, B). The stigma was three-lobed, flat, rather large and elliptical in shape, with a subhorizontal arrangement (Fig. 1A, Fig. S2B). The papillae comprise the basal part of the stigma (Fig. 1B). Two days after hand-pollination (Fig. 1C, D), we observed the germination of pollen grains grouped in tetrads (Fig. 1E, F). On the 4th (Fig. 2A, B) and 3rd (Fig. 2C) days, we observed that pollen tubes had massively germinated along the stylar canal and in the entrance to the ovary.
Fig. 1.
Micromorphology of a hand-pollinated flower of Epipogium aphyllum (SEM). (A,B) Stigma built by papillae. (C,D) Gynostemium with the massulae on the 2nd day after hand-pollination. (E,F) Germinated pollen grains grouped in tetrads on the stigma. Abbreviations: an – anther, lsl – lobe of stigma, ma – massulae, pg – pollen grains, ro – rostellum, st – stigma cells.
Fig. 2.
Pollen tube germination (LM). (A) Longitudinal section of the gynostemium of E. aphyllum with massively germinated pollen grains; pollen tubes are visible in the stylar canal and the ovary. (B,C) Details of stigma cells and stylar canal. (A,B) Fourth and (C) 3rd day after hand-pollination. Abbreviations: an – anther, ov – ovary, pg – pollen grains, pt – pollen tubes, sc – stylar canal, st – stigma cells.
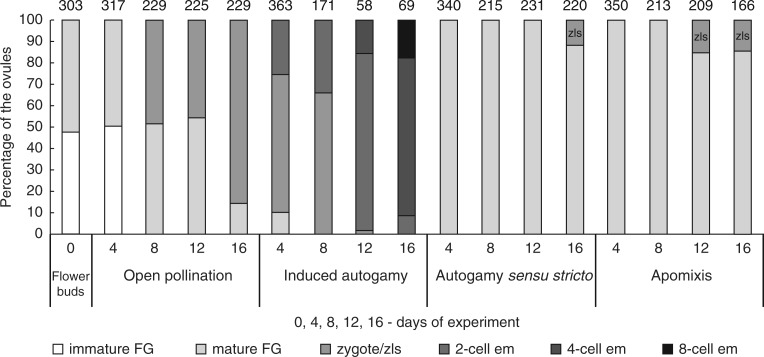
Fig. 3.
Graphical comparison of the developmental stages of ovules inside E. aphyllum ovaries. Summary data for two seasons. Experimental checkpoints (0, 4, 8, 12 and 16 d) for six categories: immature female gametophyte (FG), from one- to four-nucleate (immature FG); mature female gametophyte (mature FG); a zygote (zygote) or zygote-like structure (zls); two-cell embryo (2-cell em); four-cell embryo (4-cell em); eight-cell embryo (8-cell em). The value above each bar indicates the number of ovules counted.
Pollen and seed viability
Fresh pollen samples germinated with 74 % (± 0·1) efficiency. The viability of seeds obtained from open-pollinated flowers, measured via TTC staining, was 85 % (± 0·05).
Ovary size
The size of the ovaries between treatments differed significantly at the 16th day of their development (Kruskal–Wallis: H = 23·8, P < 0·001, d.f. = 4).). During the experiment, ovaries in induced autogamy and apomixis experiments doubled in average size compared with ovaries in flower buds: 20·3 mm2 (induced autogamy) and 20·7 mm2 (apomixis) vs. 9·5 mm2 (Supplementary Data Fig. S3). Only ovaries from autogamy sensu stricto remained roughly similar in size (10·8 mm2) to those in flower buds (9·5 mm2) on the 16th day.
Stage of ovules, male and female gametophyte development in unpollinated flower buds
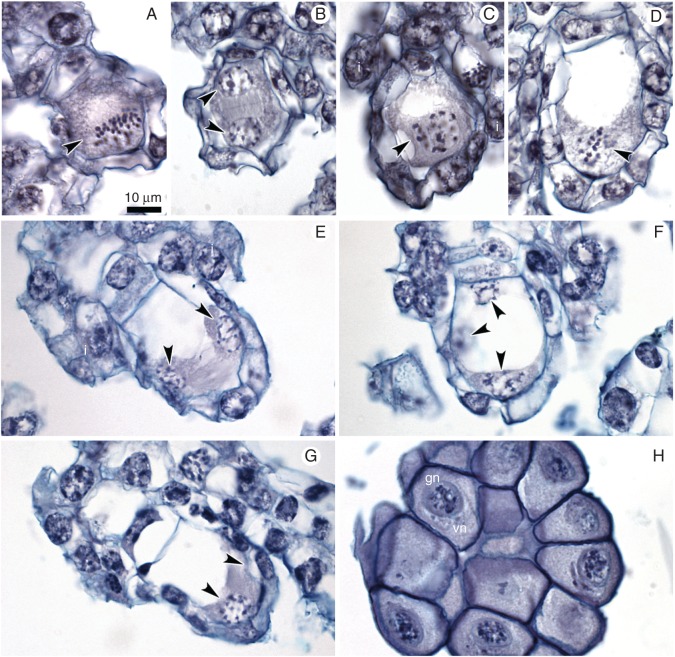
In ovules, at the bud stage of the flower, we observed substantial diversity in developmental stages (Fig. 3), from meiotic division in the megaspore mother cell up to the mature gametophyte. We noted stages of meiosis only in a very few ovules (metaphase I and late telophase I; Fig. 4A, B) for megasporogenesis; one-, two- or four-nucleate embryo sacs were often present (Fig. 4C–G, whereas archespore cells, divisions of dyads or tetrads were not present. Male and female gametogenesis proceeded with no disturbances. Most of the gametophytes were at the four-nucleate stage of FG development (Figs 3 and 4E–G). We did not observe embryos or endosperm in the analysed ovules. Gametophyte development was Polygonum type, with reduction of the nuclei number at the chalazal pole (Afzelius, 1954; Geitler, 1956). The pollinia mostly contained two-nucleate pollen grains, but one-nucleate microspores were also present (Fig. 4H).
Fig. 4.
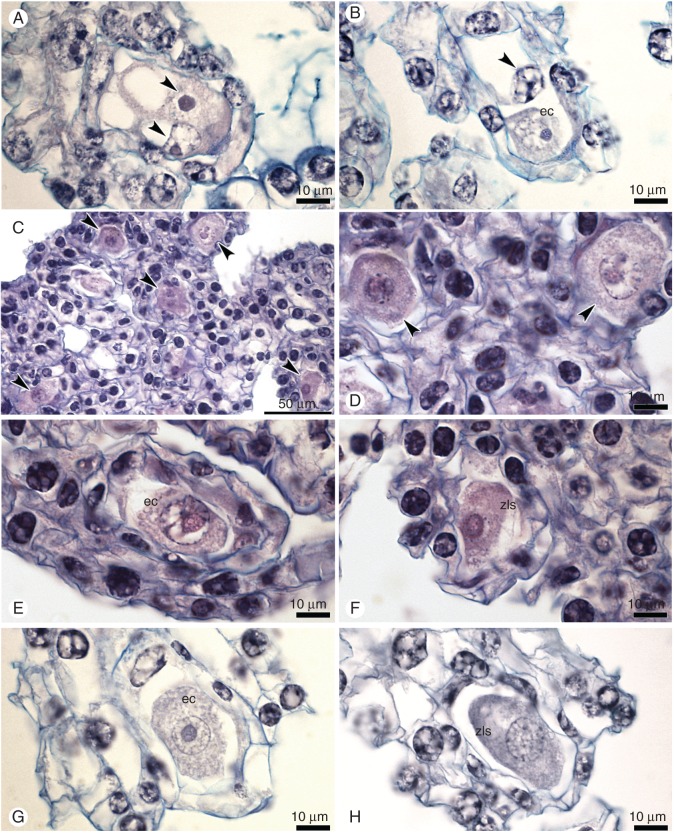
Embryological study of the ovules within unpollinated flower buds (control). (A,B) Different stages of meiosis: (A) metaphase I; a group of chromosomes is marked by an arrowhead; integuments (i) are just visible. (B) Late telophase I – two sister nuclei are marked by arrowheads. (C–G) Development of female gametophyte (FG). (C) One-nucleate FG (arrowhead); integuments are visible. (D) Two-nucleate FG; mitotic metaphase at micropylar end of FG (arrowhead). (E–G) Nuclei of FG are indicated by arrowheads; note the lack of cellularization and cell specification. (H) Two-nucleate pollen grains in pollinium; vn – vegetative nucleus, gn – generative nucleus. Scale bar = 10 μm.
Open pollination – observation of natural seed formation
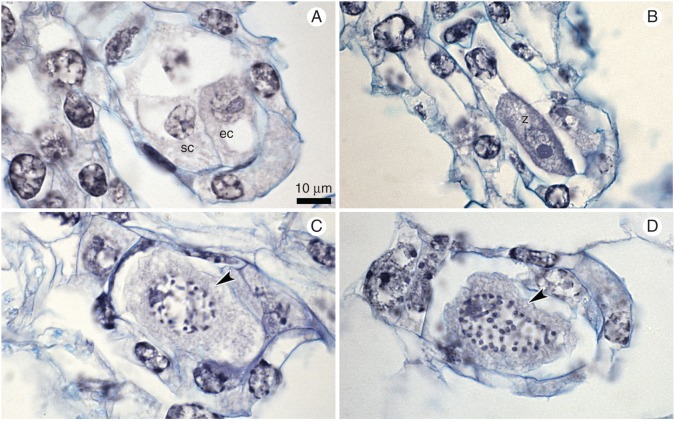
On the 4th day of open pollination, we discovered only unfertilized, but mature, FGs in most ovules (Fig. 3). All the observed FGs consisted of three cells of the egg apparatus and a chalazal nucleus (Fig. 5A). The micropylar walls of synergids were extended and created a filiform apparatus. The chalazal nucleus degenerated. Neither embryos nor endosperm was observed in the analysed ovules. On the 8th and 12th days, we detected young zygotes and mature female gametophytes in approximately equal proportions (Figs 3 and 5B, C). On the 16th day, we noted ovules with zygotes (86 %) at various stages of development [from young zygotes, just after fertilization, to zygotes in which the nucleus was dividing (Fig. 5D)]. No embryos were noted in the ovules.
Fig. 5.
Embryological study of the ovules after open pollination. (A) Mature female gametophyte on the 4th day, with egg cell (eg) and synergid (sc) and degenerating polar nucleus. (B) The zygote (z) on the 8th day. (C,D) Mitosis (arrowheads) in zygotes on the 12th (C) and 16th (D) days. Scale bar = 10 µm.
Hand-pollination – verification of the ability to undergo autogamy
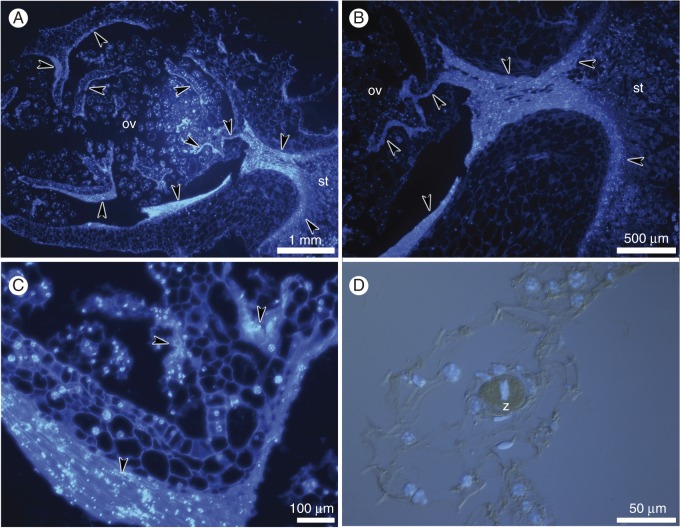
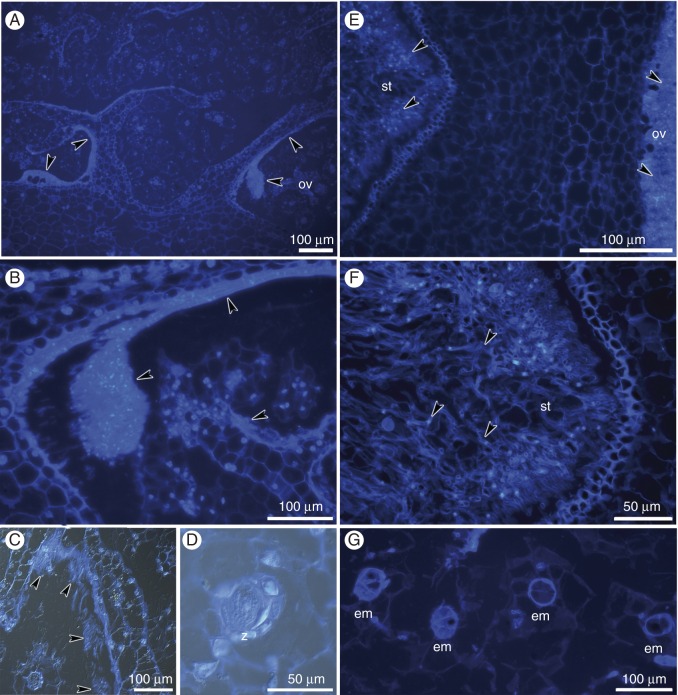
As early as the 4th day after hand-pollination, the results of induced autogamy were clearly visible (Figs 6–8). By this time, the pollen tubes, consisting of vegetative and generative sperm cells, have reached the ovules (Fig. 6A–C). Embryos grew in size over the next 8 d, until 16 d after hand-pollination, and the path of pollen tube growth was still detectable in the stigma, style and ovary locule (Figs 6D and 7A–G). The masses of empty pollen tubes showed only fluorescent callose plugs, and no generative or sperm cells were observed at this time (Fig. 7F).
Fig. 6.
Histochemical analysis of hand-pollinated pistils on 8th day after hand-pollination; longitudinal sections of the ovary. (A–C) Chromatin of the nuclei stained with DAPI and cell walls stained with calcofluor white. (D,E) Method combining fluorescence and differential interference contrast. (A,B) Masses of pollen tubes (arrowheads) penetrate the stigma and grow into the ovary. (C) Masses of pollen tubes (arrowheads) inside the ovary. (D) The zygote (z).
Fig. 7.
Histochemical analysis of hand-pollinated pistils on the 12th (A–D) and 16th (E–G) days after hand-pollination; longitudinal sections of the ovary. (A–G) Chromatin of the nuclei was stained with DAPI, and cell walls were stained with calcofluor white. (C,D) Method combining fluorescence and differential interference contrast. (A–C) Masses of pollen tubes within the ovary (arrowheads). (D) The zygote (z). (E,F) Masses of pollen tubes (arrowheads) within the stylar tissue and the ovary. (G) Two- and four-cell embryos (em).
Fig. 8.
Embryological study of the ovules after hand pollination (induced autogamy). (A) The zygote (z) on the 4th day. (B) Two-cell embryo on the 8th day; nuclei are indicated by arrowheads. (C) Four-cell embryo on the 12th day and (D) eight-cell embryo on the 16th day. Starch grains are clearly visible (C). Scale bar = 10 μm.
On the 4th day after hand-pollination, we observed both zygotes and two-cell embryos inside the ovary (Fig. 8A). Zygotes were accompanied by other, still-visible elements of FGs, while two large cells of the embryo filled the entire ovule. Similar numbers of zygotes and two-cell embryos (Fig. 8B) were observed on the 8th day (Fig. 3). On the 12th day, two- and four-celled embryos (Figs 3 and 8C) were arranged inside the tissue of the seed capsule, with the vast majority being two-cell embryos. We occasionally noted zygotes. Compared with the number of zygotes, a decrease in embryo number was clearly visible on the 4th and 8th days. The embryos reached maturity on the 16th day (Fig. 8D); eight cells of the embryo were arranged diagonally, according to Epipogoniinae-type embryogenesis, as described by Pridgeon et al. (1999).
Isolation from external pollination – verification of the existence of autogamy sensu stricto
In contrast to the hand-pollinated flowers, ovules mostly contained mature embryo sacs from the 4th day until the 12th day of the experiment (Fig. 9A–D). On the 8th, 12th and 16th days, we observed only increases in egg cell size (Fig. 9B–D). Structures that resembled zls were also noted, but only in a very few cases (the 16th day; Fig. 3). After 16 d, no pollen tubes were observed on the stigma, in the style or inside the ovary cavity in flowers isolated from pollination. Longitudinal sections of isolated flowers showed mainly undivided microspores in intact pollinia and no signs of growing pollen tubes inside ovaries. Only mature embryo sacs were present in the ovules (Supplementary Data Fig. S4A–D).
Fig. 9.
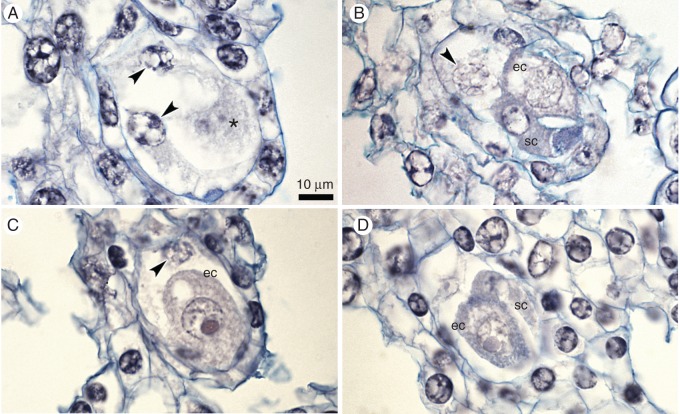
Embryological study of ovules from isolated flowers. (A) A few-nucleate female gametophyte on the 4th day; nuclei are indicated by arrowheads; the star indicates an invisible egg cell. (B) Mature embryo sac on the 8th day, with the egg apparatus (ec, sc) and one polar nucleus (arrowhead). (C) The enlarged egg cell (ec) and degenerated polar nucleus (arrowhead) on the 12th day. (D) Mature embryo sac with the egg apparatus (ec, sc) on the 16th day. Scale bar = 10 μm.
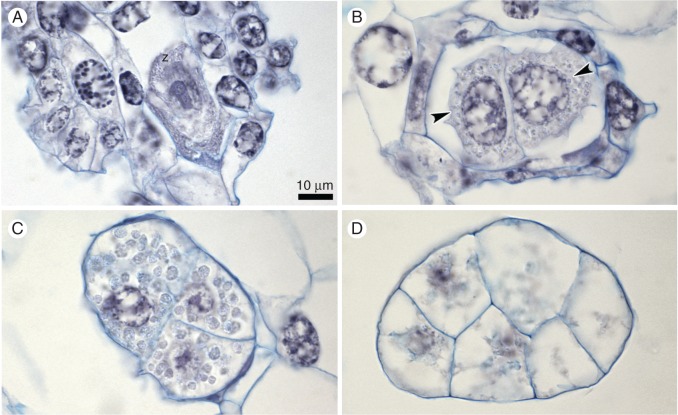
Emasculation and isolation from pollination – verification of the existence of apomixis
In this treatment, ovules contained mature female gametophytes until the 12th day (Fig. 10A, B). On the 8th and 12th days, we detected enlarged egg cells. We noted zls on the 12th and 16th days (Figs 3 and 10C–H). In one ovary, there were approx. 50 % zls (Fig. 10F). These fertilization-less structures differed from egg cells in several respects, including the cell shape, density of the cytoplasm, the lack of vacuoles and the lack of polarization (a centrally located nucleus) and were typical for the zygote.
Fig. 10.
Embryological study of ovules from flower buds that were emasculated and isolated from pollination. (A) A few-nucleate female gametophyte (FG) on the 4th day; nuclei are indicated by arrowheads. (B) The mature FG with the egg cell (ec) and polar nucleus on the 4th day. (C–F) A structure of ovary tissue on 12th day that contained equal numbers of unfertilized but enlarged egg cells (ec) (E) and of zygote-like structures (zls) (F). (G) Enlarged egg cell (ec) on the 12th day. (H) Zygote-like structure (zls) on the 16th day. Scale bar = 10 μm.
The highest frequencies of formed zygotes/zls were observed in induced autogamy (53 %) and open pollination (41 %). Apomixis and autogamy sensu stricto accounted for only 9 % of all observed zygotes/zls (Fig. 11). Despite the possibility of zls formation via apomixis, a significantly higher frequency of zygotes (z = 24, P < 0·001)) formed via sexual reproduction: open pollination and induced autogamy (0·058 vs. 0·456; apomictic vs. sexual).
Fig. 11.
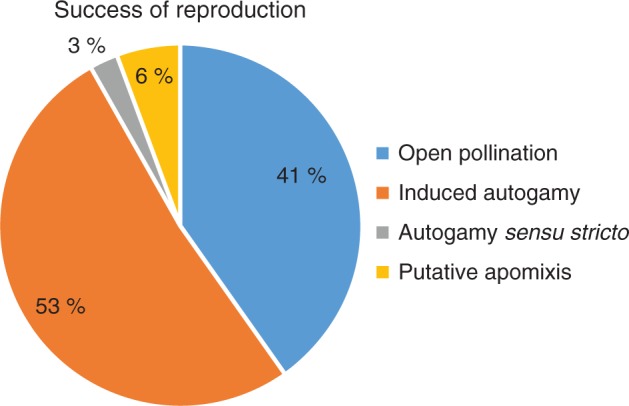
Success through various modes of reproduction in E. aphyllum. Because embryogenesis starts from zygotes or zygote-like structures, the proportions of these structures are shown for each individual experiment. A low appearance of autonomously formed structures (putative apomixis) per 938 analysed ovules from day 4 to 16 contrasts with the high percentage of zygotes from both induced autogamy and open pollination. The origin of the structures listed in natural autogamy (sensu stricto) may be either sexual or parthenogenetic.
DISCUSSION
Flowers of Epipogium aphyllum are capable of autogamy
Embryological experiments confirm the assumption that ghost orchid flowers may be self-compatible. The induced autogamy experiment, in which the ingrowth of pollen tubes was observed on the stigma, stylar tissue and the ovary cavity, indicates the absence of prezygotic barriers in flowers of E. aphyllum. The development of embryos after hand pollination, proceeding to full maturity, suggests the lack of early postzygotic barriers in the investigated species. Other authors have also considered autogamy to occur in this species (Afzelius, 1954; Geitler, 1956; Vöth, 1994). In his experiments, Geitler (1956) noted a similar percentage of seed capsules on flowering shoots that were manually self-pollinated or cross-pollinated. Some studies have suggested that autonomous self-pollination may be favourable for mycoheterotrophic plants, as they have limited access to carbon sources (Takahashi et al., 1993; Zhang and Saunders, 2000; Lehnebach et al., 2005) and are restricted to shaded forest understorey habitats with a paucity of pollinators (Dressler, 1993; Leake, 1994). However, the variety in flower morphology of many mycoheterotrophic plants instead suggests the diversity of breeding systems and pollinators, as has been confirmed by studies into the reproductive biology of Voyria (Hentrich et al., 2010) and Monotropoideae (Klooster and Culley, 2009). Embryogenesis proceeded much more quickly and with a higher frequency of embryo development within hand-pollinated flowers than in open pollination (Fig. 12). This difference was probably caused by a shift (by at least 4 d) in pollination time between the two treatments (Fig. 12), as the stigma may still be receptive in this time frame. Although almost all hand-pollinated flowers produced seed capsules, some plants whose flower(s) were left for open pollination seemed to disappear. The most probable explanation`n is that lack of pollination triggered rapid plant withering and decay. Pathogenic fungi and slugs may also lead to flower and seed capsule death (Taylor and Roberts, 2011), yet the high percentage of viable seeds formed via natural pollination, as shown in this study, indicates that sexual reproduction is stably maintained in E. aphyllum.
Fig. 12.
Time course of reproduction in E. aphyllum. The occurrence of subsequent developmental stages of the ovules during experiments. At day 0 (start), flower buds (A) were treated according to the experimental variants (B–E). The indicated stages of female gametophytes and the subsequent fate of fertilized or unfertilized egg cells (zygotic embryogenesis or parthenogenesis) are marked with different colours. The presence of two neighbouring stages on the same day of the experiment is marked as a mix of the corresponding colours.
Our study also demonstrated that, although the plant is fully self-compatible, no autogamy sensu stricto actually occurred. In flowers in unmanipulated and covered inflorescences, we did not observe pollinia adhering to the stigma and no pollen tubes were detected. The zls that we observed in the absence of pollination may develop autonomously. Spontaneous autogamy sensu stricto is, therefore, unlikely to occur in E. aphyllum because of the flower structure. Whether it can occur under stressful conditions, as in other orchids (e.g. Tałałaj and Brzosko, 2008) or other families (e.g. Peréz et al., 2009), remains to be tested. In other orchids, e.g. Dendrobium biflorum, adverse habitat conditions lead to autogamy. The pollen grains of D. biflorum germinated directly from the locules or after sliding slightly down onto the stigmatic surface (Kowalkowska and Margońska, 2012). In flowers of Epipactis microphylla (a cleistogamous and also chasmogamous species), pollen tubes grew inside the anther, bypassing the receptive stigma (Anderson, 1980; Caiola et al., 2000; Bonatti et al., 2006). In self-pollinated and cleistogamous flowers, emerging pollen tubes were retained in the locules and grew beneath the stigmatic surface layer (Bonatti et al., 2006). Geitonogamous fruit setting as a result of intraclonal pollination might be possible assuming that clonal propagation by bulbils occurs in the natural habitat (see Roy et al., 2009). However, this requires verification through ecological and genetic studies.
Lack of pollination and manipulation within flowers can induce parthenogenesis
The presence of structures resembling zygotes within unfertilized ovules in isolated and emasculated flowers led us to consider the possibility of parthenogenetic formation of embryos in this species. The parthenogenetic rather than sexual origin of zygote-like structures in flowers isolated from pollination also points to the lack of observed signs of spontaneous autogamy. The enlargement of ovaries observed in flowers that were emasculated and isolated from pollination resembles parthenocarpy (the formation of fruit without seeds). After pollination, enlargement of the ovary in Epipogium takes place due to the stretching and hydration of the cells (Geitler, 1956). Therefore, the enlargement of the ovary without pollination may be indicative of a programmed phenomenon that depends on mechanical, physiological and epigenetic factors, independent of the presence of pollination/fertilization. Mechanical manipulation during flower emasculation may also be the reason for the much higher frequency of zls compared with flowers isolated from pollination.
We cannot conclude with any certainty whether the observed zls resulted from apomixis. We noted no archesporial cells or late stages of meiosis (i.e. division in dyad, tetrad) in the ovules of unpollinated flower buds; instead, we observed only individual meiotic stages (metaphase I, telophase I) in very few ovules, as well as many very young stages of embryo sacs. However, ovule development was rarely initiated (as indicated by poorly developed integument) and was characteristic of the meiotic stage in Epipogium (Alfezius, 1954; Arekal and Karanth, 1981). Thus, meiosis might not occur in some ovules as in apomictic orchids (Sorensen et al., 2009; Kant and Verma, 2012), or it may be disturbed. Because megaspore formation is described as monosporic with typical meiosis in E. aphyllum (Afzelius, 1954), such meiosis disturbances must be verified across a wide spectrum. Complying with the requirements of apomixis for meiosis avoidance and fertilization-less embryogenesis, the occurrence of complete apomixis in E. aphyllum remains to be investigated. We plan to investigate the disappearance of meiotic stages in our observations via cytological studies on very young flower buds. We will also extend the duration of the experiment to explore parthenogenetic embryo formation and to assess the embryo ploidy.
Although apomixis restricts genetic diversity, it does not require the same level of plant resources for seed production and provides insurance against poor seed production from out-crossing flowers. In gametophytic apomixis, pollen grains and embryo sacs with egg cells and parthenogenetic embryos can develop correctly despite disturbances in meiosis and the lack of fertilization. The same developmental path can be used for sexual strategies in the presence of meiosis, pollination and fertilization. Studies of the apomictic gynodioecious orchid Satyrium ciliatum suggest an important role for sex in generating genotypic diversity in this apomictic orchid (Lu et al., 2010).
Epipogium aphyllum may exhibit mixed reproductive strategies that exist in parallel and provide the opportunity to switch from one to the other. Apomixis and sexuality are not mutually exclusive traits, as almost all apomictic plants exhibit facultative sexuality (Hojsgaard et al., 2014). Although angiosperms appear to be predisposed to shift from sexual reproduction to apomixis, the reversion to sexuality is much more likely. Such reversions may result from genetic or epigenetic destabilization events, which are accompanied by hybridization, polyploidy or other cytogenetic alterations (Hojsgaard et al., 2014). Studies of genetic diversity would be very helpful in elucidating the reproductive strategy of this orchid in natural populations.
In conclusion, E. aphyllum has the potential to produce seeds via both sexual and asexual means, although apomixis probably plays a very small part in reproductive success. Epipogium aphyllum belongs to a very small group of orchid species in which female gametes are ready to be fertilized before pollination can occur (Yeung and Law, 1997). Therefore, this species bypasses the requirement of pollination, and parthenogenetic development of the egg cell can occur. Mechanical factors may play an important role in the induction of this mode of reproduction. A future challenge is to determine whether apomictic reproduction occurs in E. aphyllum, from the failure of meiosis to parthenogenetic seed and plant formation. Despite the difficulties arising from the rarity of this protected species, which typically include small population sizes and failures in germinating seeds in vitro, future research would provide valuable information about the breeding systems of this species.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Figure S1: flower morphology of E. aphyllum (LM). Figure S2: flower micromorphology of E. aphyllum (SEM). Figure S3: boxplot showing the differences in the size of capsules of E. aphyllum on the 16th day of experiments in comparison with the size of ovary at flower bud stage. Figure S4: histochemical analysis of the isolated flowers on the 16th day; longitudinal sections.
ACKNOWLEDGEMENTS
We thank Prof. Marc-André Selosse (Muséum National d’Histoire Naturelle, France), Prof. Richard Bateman (c/o Royal Botanic Gardens Kew) and Prof. Jerzy Bohdanowicz (University of Gdańsk, Poland) for valuable comments on the manuscript. This work was supported by the National Fund for Environmental Protection and Water Management No. WFOŚ/D/210/131/2011, the National Science Centre grant No. DEC-2011/03/N/NZ8/02847 and the Young Scientists Grant from the University of Gdańsk no. 538-L150-B079-13. The research was done with permissions No. RDOŚ-Gd-PNII.6400.3.2013.MaK.1. and RDOŚ-Gd-PNII.6400.58.2014.MŚ.1.
LITERATURE CITED
- Afzelius K. 1954. Embryo-sac development in Epipogium aphyllum. Svensk Botanisk Tidskrift Utgifven af Svenska Botaniska Föreningen 48: 513–520. [Google Scholar]
- Anderson WR. 1980. Cryptic self-fertilization in the Malpighiaceae. Science 207: 892–893. [DOI] [PubMed] [Google Scholar]
- Arditti J. 1992. Fundamentals of orchid biology. New York: Wiley & Sons, Inc. [Google Scholar]
- Arekal GD, Karanth KA. 1981. The embryology of Epipogium roseum (Orchidaceae). Plant Systematics and Evolution 138: 1–7. [Google Scholar]
- Barrett SCH. 1985a. Floral trimorphism and monomorphism in continental and island populations of Eichhornia paniculata (Spreng.) Solms. (Pontederiaceae). Biological Journal of the Linnean Society 25: 41–60. [Google Scholar]
- Barrett SCH. 1985b. Ecological genetics of breakdown in tristyly In: Haeck J, Woldendorp JW, eds. Structure and functioning of plant populations 2. Phenotypic and genotypic variation in plant populations. Amsterdam: North-Holland Publishing Company, 267–275. [Google Scholar]
- Batygina TB, Bragina EA, Vasilyeva VE. 2003. The reproductive system and germination in orchids. Acta Biologica Cracoviensia Series Botanica 45: 21–34. [Google Scholar]
- Bilz M, Kell ShP, Maxted N, Lansdown RV. 2011. European red list of vascular plants. Brussels: European Commission. [Google Scholar]
- Bonatti PM, Sgarbi E, Del Prete C. 2006. Gynostemium micromorphology and pollination in Epipactis microphylla (Orchidaceae). Journal of Plant Research 119: 431–437. [DOI] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. American Journal of Botany 50: 859–865. [Google Scholar]
- Caiola MG, Brandizzi F, Canini A. 2000. Hermodactylus tuberosus L. (Iridaceae) pollen organisation before and after anther dehiscence. Plant Biosystems 134: 353–364. [Google Scholar]
- Claessens J, Kleynen J. 2011. The flower of the European orchid. Form and function, 1st edn. Geulle: Jean Claessens & Jacques Kleynen. [Google Scholar]
- Curtis MD, Grossniklaus U. 2008. Molecular control of autonomous embryo and endosperm development. Sexual Plant Reproduction 21: 79–88. [Google Scholar]
- Dressler RL. 1993. Phylogeny and classification of the orchid family. Cambridge, UK: University Press. [Google Scholar]
- Geitler L. 1956. Zur Fortpflanzungsbiologie, Embryologie und meehanistischen Deutung der Embryogenese von Epipogium aphyllum. Österreichische botanische Zeitschrift 103: 312–335. [Google Scholar]
- Hereźniak J, Piękoś-Mirkowa H. 2014. Epipogium aphyllum Swartz. Storzan bezlistny In: Każmierczakowa R, Zarzycki K, Mirek Z, eds. Polska Czerwona Księga Roślin. Kraków: Instytut Botaniki im. W. Szafera PAN, 749–752. [Google Scholar]
- Hentrich H, Kaiser R, Gottsberger G. 2010. The reproductive biology of Voyria (Gentianaceae) species in French Guiana. Taxon 59: 867–880. [DOI] [PubMed] [Google Scholar]
- Hojsgaard D, Klatt S, Baier R, Carman JG, Hörandl E. 2014. Taxonomy and biogeography of apomixis in angiosperms and associated biodiversity characteristics. Critical Reviews in Plant Sciences 33: 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemyn H, Micheneau C, Roberts DL, Pailler T. 2005. Elevational gradients of species diversity, breeding system and floral traits of orchid species on Reunion Island. Journal of Biogeography 32: 1751–1761. [Google Scholar]
- Jakubska-Busse A, Jasicka-Misiak I, Poliwoda A, Święczkowska E, Kafarski P. 2014. The chemical composition of the floral extract of Epipogium aphyllum Sw. (Orchidaceae): a clue for their pollination biology. Archives of Biological Sciences 66: 989–998. [Google Scholar]
- Johnson SD, Edwards TJ. 2000. The structure and function of orchid pollinaria. Plant Systematics and Evolution 222: 243–269. [Google Scholar]
- Kant R, Verma J. 2012. Obligate apomixis in Zeuxine strateumatica (Lindl.) Schltr. (Orchidaceae). Vegetos 25: 274–277. [Google Scholar]
- Klooster MR, Culley TM. 2009. Comparative analysis of the reproductive ecology of Monotropa and Monotropsis: two mycoheterotrophic genera in the Monotropoideae (Ericaceae). American Journal of Botany 96: 1337–1347. [DOI] [PubMed] [Google Scholar]
- Kowalkowska AK, Margońska HB. 2012. Notes on the self-pollination in Dendrobium biflorum (Orchidales, Dendrobiinae). Acta Societatis Botanicorum Poloniae 81: 223–228. [Google Scholar]
- Kowalkowska AK, Kostelecka J, Bohdanowixcz J, Kapusta M, Rojek J. 2015. Studies on floral nectary, tepals’ structure, and gynostemium morphology of Epipactis palustris (L.) Crantz (Orchidaceae). Protoplasma 252: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake JR. 1994. The biology of myco‐heterotrophic (‘saprophytic’) plants. New Phytologist 127: 171–216. [DOI] [PubMed] [Google Scholar]
- Lehnebach C, Robertson AW, Hedderley D. 2005. Pollination of four New Zealand terrestrial orchids. New Zealand Journal of Botany 43: 467–477. [Google Scholar]
- Lu Y, Luo YB, Huang SQ. 2010. Reduced recombination in gynodioecious populations of a facultative apomictic orchid. Plant Biology 12: 814–819. [DOI] [PubMed] [Google Scholar]
- Merckx VSFT, Mennes CB, Peay KG, Geml J. 2013. Evolution and diversification In: Merckx VSFT, ed. Mycoheterotrophy: the biology of plants living on fungi. Berlin: Springer Science + Business Media, 215–244. [Google Scholar]
- Neiland MRM, Wilcock CC. 1998. Fruit set, nectar reward, and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- Peréz F, Arroyo MTK, Armesto JJ. 2009. Evolution of autonomous selfing accompanies increased specialization in the pollination system of Schizanthus (Solanaceae). American Journal of Botany 96: 1168–1176. [DOI] [PubMed] [Google Scholar]
- Peter CI, Johnson SD. 2009. Autonomous self-pollination and pseudo-fruit set in South African species of Eulophia (Orchidaceae). South African Journal of Botany 75: 791–797. [Google Scholar]
- Pridgeon AM, Phillip JPJ, Chase MW, Rasmussen FN. 1999. Genera Orchidacearum: General Introduction, Apostasioideae, Cypripedioideae. Oxford: Oxford University Press. [Google Scholar]
- Pritchard HW. 1985. Determination of orchid seed viability using fluorescein diacetate. Plant, Cell and Environment 8: 727–730. [Google Scholar]
- Roy M, Yagame T, Yamato M, et al. 2009. Ectomycorrhizal Inocybe species associate with the mycoheterotrophic orchid Epipogium aphyllum but not its asexual propagules. Annals of Botany 104: 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojek J, Kuta E, Bohdanowicz J. 2005. In vitro culture promotes partial autonomous endosperm development in unfertilized ovules of wild-type Arabidopsis thaliana var. Columbia. Sexual Plant Reproduction 18: 29–36. [Google Scholar]
- Ruzin SE. 1999. Plant microtechnique and microscopy. New York: Oxford University Press. [Google Scholar]
- Sorensen AM, Rouse DT, Clements M, John P, Perotti E. 2009. Description of a fertilization-independent obligate apomictic species: Corunastylis apostasioides Fitzg. Sexual Plant Reproduction 22: 153–65. [DOI] [PubMed] [Google Scholar]
- Summerhayes VS. 1951. Wild orchids of Britain: with a key to the species, 1st edn. London: Collins. [Google Scholar]
- Świerczyńska J, Bohdanowicz J. 2003. Microfilament cytoskeleton of endosperm chalazal haustorium of Rhinanthus serotinus (Scrophulariaceae). Acta Biologica Cracoviensia Series Botanica 45: 143–148. [Google Scholar]
- Święczkowska E, Kowalkowska AK. 2015. Floral nectary anatomy and ultrastructure in mycoheterotrophic plant, Epipogium aphyllum Sw. (Orchidaceae). The Scientific World Journal 2015 ID: 201702. doi:10.1155/2015/201702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Roberts DL. 2011. Biological flora of the British Isles: Epipogium aphyllum Sw. Journal of Ecology 99: 878–890. [Google Scholar]
- Takahashi H, Nishio E, Hayashi H. 1993. Pollination biology of the saprophytic species Petrosavia sakurafi (Makino) van Steenis in Central Japan. Journal of Plant Research 106: 213–217. [Google Scholar]
- Tałałaj I, Brzosko E. 2008. Selfing potential in Epipactis palustris, E. helleborine and E. atrorubens (Orchidaceae). Plant Systematics and Evolution 276: 21–29. [Google Scholar]
- Van der Pijl L, Dodson CH. 1966. Orchid flowers: their pollination and evolution, 15th edn. Coral Gables, FL: Published jointly by the Fairchild Tropical Garden and the University of Miami Press. [Google Scholar]
- Van Waes JM, Debergh PC. 1986. In vitro germination of some Western European orchids. Physiolologia Plantarum 67: 253–261. [Google Scholar]
- Vöth W. 1994. Sind Blüten von Epipogium aphyllum Sw. entomogam oder autogam? Orchideen 45: 248–250. [Google Scholar]
- Vitha S, Jasik J, Volkmann D, Barlow P. 2000. Steedman’s wax for F-actin visualization In: Staiger CJ, Baluska F, Volkmann D, Barlow P, eds. Actin: a dynamic framework for multiple plant cell functions. Dordrecht: Kluwer, 619–636. [Google Scholar]
- Yeung EC, Law SK. 1997. Ovule and megagametophyte development in orchids In: Arditti J, Pridgeon AM, eds. Orchid biology: reviews and perspectives. Dordrecht: Kluwer, 31–73. [Google Scholar]
- Zhang DX, Saunders RMK. 2000. Reproductive biology of a mycoheterotrophic species, Burmannia wallichii (Burmanniaceae). Botanical Journal of the Linnean Society 132: 359–367. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.