Abstract
Compared to the animal kingdom, fertilization is particularly complex in flowering plants (angiosperms). Sperm cells of angiosperms have lost their motility and require transportation as a passive cargo by the pollen tube cell to the egg apparatus (egg cell and accessory synergid cells). Sperm cell release from the pollen tube occurs after intensive communication between the pollen tube cell and the receptive synergid, culminating in the lysis of both interaction partners. Following release of the two sperm cells they interact and fuse with two dimorphic female gametes (egg and central cell) forming the major seed components embryo and endosperm, respectively. This process is known as double fertilization. Here we review the current understanding of the processes of sperm cell reception, gamete interaction, their pre-fertilization activation and fusion as well as the mechanisms plants use to prevent the fusion of egg cells with multiple sperm cells. The role of Ca2+ is highlighted in these various processes and comparisons are drawn between fertilization mechanisms in flowering plants and other eukaryotes including mammals.
Keywords: micropyle, fertilization, calcium, egg, sperm, plasmogamy, polyspermy, pollen
Introduction
Fertilization generally describes the fusion of haploid gametes to initiate the development of a new diploid organism. In animals an ovum or egg fuses with a motile sperm generating the diploid zygote, which further develops into an embryo. Current representatives of early-branching eukaryotes, including protozoa and fungi, produce mating types of opposite sex that generate a zygote after fusion. In contrast, gametes of flowering plants are not the direct products of meiosis. Instead sperm cells are formed after two additional mitotic cell divisions creating a three-celled male gametophyte, the pollen and pollen tube (Figure 1A) [1]. The female gametophyte or embryo sac develops within the maternal organ called the ovule after three additional mitotic nuclear divisions of the functional megaspore. Following cellularization a seven-celled embryo sac is established in the majority of angiosperms containing two female gametes (egg and central cells) and accessory cells (synergids and antipodals, respectively) at both poles of the embryo sac [2, 3] (Figure 1B).
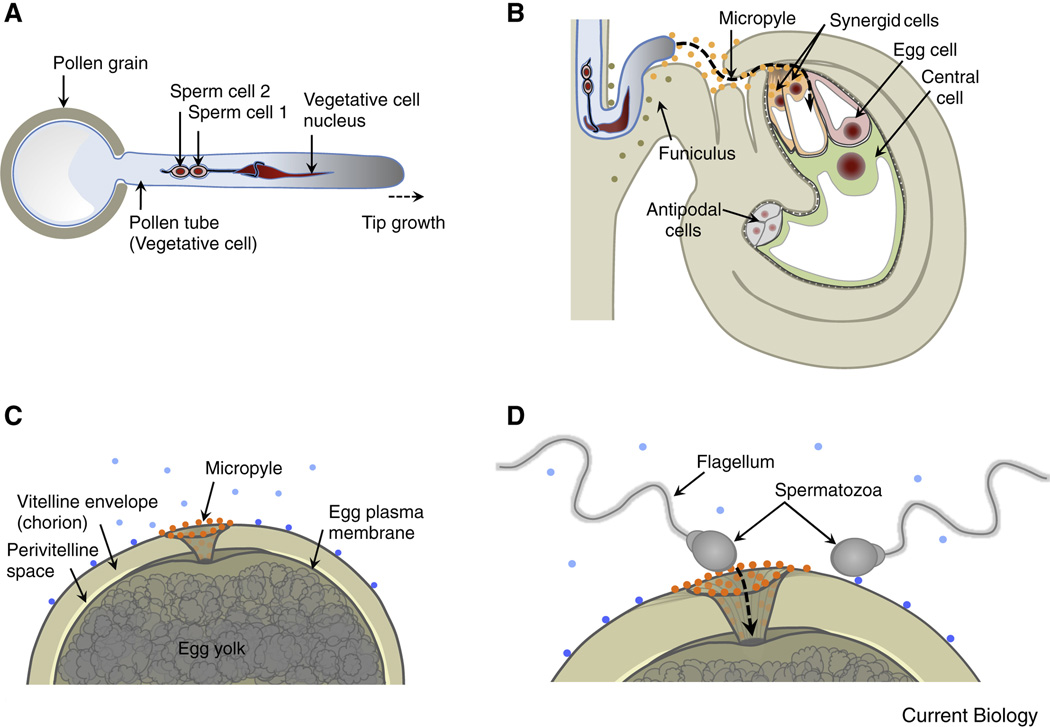
Figure 1. Comparison of gamete/gametophyte morphology between the model plant Arabidopsis thaliana and the model animal Zebrafish.
(A) Diagram of the haploid male gametophyte (pollen) of Arabidopsis comprising the vegetative cell (producing the growing pollen tube) and two non-motile sperm cells enclosed within the a membrane of the vegetative tube cell. The sperm cells are connected to each other and to the nucleus of the vegetative pollen tube cell forming the "male germ unit". Nuclei in red, vegetative cell membrane in blue, sperm cell membranes in black. (B) A pollen tube approaching the Arabidopsis ovule. The tube grows through the micropyle of the ovule along the funiculus towards the haploid female gametophyte that comprises the egg cell, central cell and accessory cells (synergid and antipodal cells). Secreted LURE peptides (orange dots) act as pollen tube attractants guiding the pollen tube through the micropyle. Other unknown ovule factors (olive dots) may be involved in guiding the pollen tube along the funiculus towards the micropyle. (C) Diagram of a fish egg (animal pole) covered by a thick glycoprotein coat (chorion). The sperm entry point toward the egg is restricted to the micropylar canal. Sperm attraction to the micropyle opening involves a “micropylar sperm guidance factor” (orange dots), a glycoprotein bound to the chorion immediately surrounding the opening of the micropyle and along the micropylar canal. Other secreted or surface-exposed factors (blue dots) may be involved in activating sperm movement or guiding sperm to the micropyle. (D) Highly active motile spermatozoa enters the micropyle. The diameter of the innermost region of the micropyle restricts sperm entry and fusion with the egg plasma membrane to the first sperm advancing to the lower portion of the micropyle.
To reach the two female reproductive cells, sperm cells in angiosperms have to conquer distances of a few millimeters in species such as Arabidopsis or up to 30 centimeters in maize. While mosses and ferns still possess motile sperm, sperm cells of angiosperms have lost their motility and the pollen tube cell acts as a vehicle to transport the sperm pair deep through the maternal reproductive tissues. The tube cell grows at its tip with a speed of up to 1 cm per hour with its cargo at a short distance from the tip. During their journey the sperm cells are connected to each other and to the nucleus of the tube cell [4], moving as a male germ unit (Figure 1A). Intensive communication takes place during the arduous pollen tube journey within the maternal tissues of the stigma, style, transmitting tract and ovule. These processes, collectively referred to as progamic phase, have been reviewed recently [5–9] and will not be further considered here. Upon its arrival at the ovule the directive communication continues with the female gametophyte (especially the synergid cells), guiding the pollen tube through the micropyle opening of the ovule and regulating the release of its cargo [7, 9–11].
The micropyle opening of flowering plant ovules enable sperm access and are reminiscent of the micropyle in many insect and fish eggshells, [12, 13]. In the funnel-shaped micropyle of herring and flounder eggs a yet unknown sperm attractant around the opening and inside of the micropyle directs the motile sperm (spermatozoa) into the micropyle and across the chorion to attach to the oocyte plasma membrane [14] (Figure 1C, D). The diameter of the inner aperture of the micropyle restricts the number of entering sperm. Attraction of sperm to the micropyle opening appears to be species-specific and dependent on extracellular Ca2+ [14] thus showing strong functional overlap with pollen tube guidance in plants (see details below).
During pollen tube reception in flowering plants the two sperm cells are released towards the cleft between the egg and central cell (Figure 2). Cell fusion (plasmogamy) then occurs, but only after successful sperm cell positioning, adhesion, and activation. With the exception of the Podostemaceae (Riverweed) family and some orchids where single fertilization occurs between the egg and a sperm (the second sperm cell is either not formed or disintegrates [15, 16]), in all other investigated angiosperm families one sperm cell fuses with the haploid egg cell generating the diploid embryo, while the second sperm cell fuses with the homo-diploid central cell forming the triploid endosperm [9]. Tremendous progress has been made during the past five years to understand the molecular mechanisms of fertilization in plants including the fusion of gamete nuclei (karyogamy) and the prevention of polyspermy (fusion of multiple sperm cells with female gametes) by avoiding the attraction of multiple pollen tubes (polytubey). Here we review fertilization mechanisms in flowering plants, highlight the role of Ca2+ in the various steps and compare the findings with the corresponding mechanisms described in other organisms.
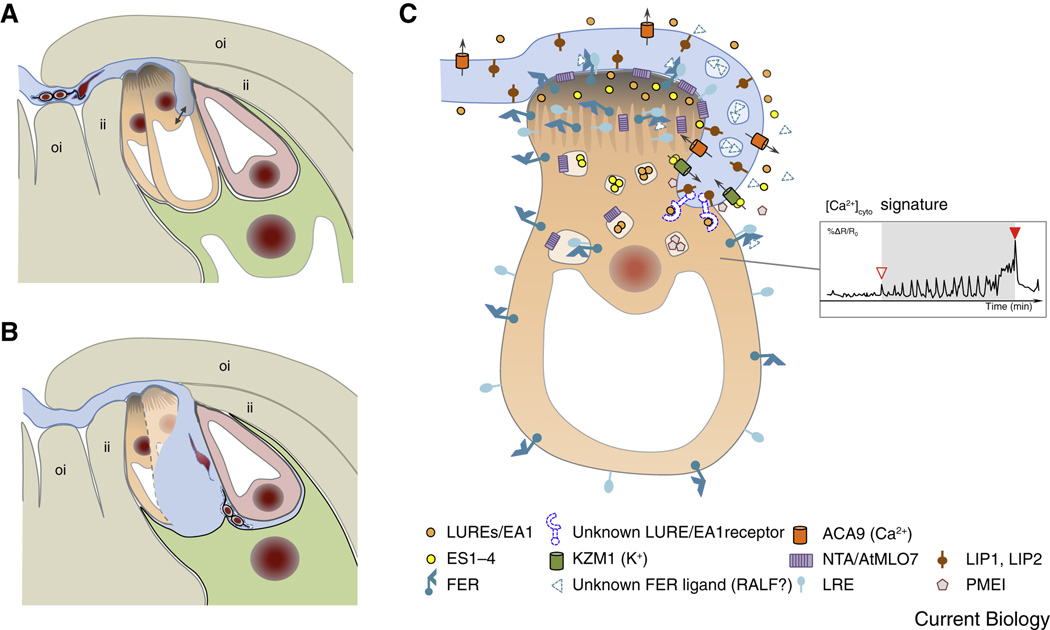
Figure 2. Pollen tube reception involves elaborate communication between the pollen and the receptive synergid cell.
(A) The arriving pollen tube enters the female gametophyte and grows beyond the filiform apparatus, which is formed at the micropylar ends of the synergid cells. The pollen tube continues to grow along or around the receptive synergid cell, extending toward the direction of the central cell. (B) The pollen tube bursts and explosively discharges its contents, including the two immotile sperm cells. Released sperm cells remain physically connected to each other and position between the membranes of the egg cell and the central cell, respectively. (C) Diagram of pollen tube-synergid interactions and the characteristic cytoplasmic (Ca2+)cyto signature induced in the receptive synergid cell. The open triangle in the inset in (C) indicates the moment of first physical interaction between the receptive synergid cell and the pollen tube membrane, the closed triangle marks the moment of pollen tube burst. For details on the molecular events and players of pollen tube-synergid interactions see text. An overview about the molecular players is listed in Suppl. Table S1. Abbreviations: ACA9, Arabidopsis auto-inhibited Ca2+-ATPase 9; EA1, maize EGG APPARATUS1; ES1-4, maize EMBRYO SAC 1-4; FER, Arabidopsis FERONIA; ii, inner integument; KZM1, maize K+ Shaker channel KZM1; LIP, Arabidopsis LOST IN POLLEN TUBE GUIDANCE; LRE, Arabidopsis LORELEI; LUREs, pollen tube attractants of Arabidopsis and Torenia; NTA, Arabidopsis NORTIA; oi, outer integument; PMEI, pectin methyl esterase inhibitor.
Pollen tube reception and sperm cell delivery
After arrival at the female gametophyte and upon interaction with the receptive synergid cell the pollen tube bursts and releases its contents, including the two sperm cells, while the receptive synergid cell degenerates. This highly coordinated lysis of the two gametophytic cells exposes the released sperm cell cargo to the egg and central cell (Figure 2A, B). Previously it had been assumed that the pollen tube enters the receptive synergid cell through the filiform apparatus, a unique structure of membrane invaginations and highly thickened cell wall generated at the micropylar region of both synergid cells. However, recently it became evident that in Arabidopsis the pollen tube does not invade into the filiform apparatus but enters the embryo sac at a more distant site by growing around or along the receptive synergid cell before it ceases growth and bursts [17, 18]. As the sperm cells are not motile, redirecting pollen tube growth at the filiform apparatus and the proximity of the bursting pollen tube tip to the female gametes may be necessary to support targeted sperm cell delivery and gamete attachment (Figure 2B).
When the pollen tube enters the female gametophyte, and before it bursts, intensive extracellular signaling takes place between the pollen tube and one or both of the two synergids [17, 18] (Figure 2C). The synergid cells are regarded as glandular-like cells of the female gametophyte and it is now well established that they regulate pollen tube attraction during the last step of its journey and its reception, comprising growth arrest and sperm release, as well as the prevention of polytubey upon successful fertilization [7, 11]. The identity of the signals for the reciprocally induced cellular lysis of pollen tube and receptive synergid cell are of great interest and have been sought in many ways [19, 20]. In Arabidopsis the search for mutants defective in pollen tube growth arrest and burst resulted in the identification of FERONIA/SIRÉNE (FER/SRN) encoding a member of the Catharanthus roseus subfamily of receptor-like kinases CrRLK1L-1 [21, 22]. FER is broadly expressed and the encoded protein localizes at the plasma membrane in diverse cell types. In the Arabidopsis ovule, FER-GFP appears most prominently at the filiform apparatus [21]. Its extracellular domain is likely glycosylated as mutants defective in the genes TURAN (TUN) and EVAN (EVN), encoding an uridine diphosphate (UDP)-glycosyltransferase superfamily protein and a dolichol kinase, respectively, that are both required for N-glycosylation in the endoplasmic reticulum (ER), display the same fer mutant phenotype [23].
Pollen tube reception and synergid degeneration is also affected in the Arabidopsis mutant LORELEI (LRE), encoding a GPI-anchored protein expressed predominantly in the synergid cells [24, 25]. Recent work on LRE and the closely related LORELEI-like-GPI-anchored protein 1 (LLG1; expressed in seedlings) suggest that both proteins are critical for the cell surface signaling capacity of FER. LRE and LLG1 interact with FER and seem to play a role in facilitating the localization of FER to the cell membrane or stabilizing the FER-receptor complex at the cell surface [26]. Comparing the extracellular domain of FER with that of corresponding proteins of other —species of the Brassicaceae (mustard and cabbage) family further revealed a high degree of amino acid diversification in this domain compared with the kinase domain indicating that this domain may be under selective pressure for species-specific interactions with the pollen tube and thus contribute to the reproductive isolation between species [21]. The complementary pollen tube-derived ligand that may interact with FER though, has not been identified. In roots, however, a small secreted cysteine-rich peptide (CRP) of the RAPID ALKANIZATION FACTOR (RALF) subfamily of CRPs was shown to directly interact with FER resulting in phosphorylation of plasma membrane-localized H+-ATPase 2 (AHA2) mediating the inhibition of proton transport [27]. RALF encoding genes are also strongly expressed in the pollen tube [28] and it now remains to be investigated whether one or some of these RALFs induce a FER-dependent signaling pathway in the synergid cell that triggers pollen tube growth arrest and the lysis of both interaction partners.
Progress has also been made to understand the FER-dependent intracellular signaling pathway in synergid cells [29]. NORTIA (NTA or AtMLO7), a member of the plant-specific Mildew resistance Locus O (MLO) family of 7-pass membrane proteins involved in susceptibility to the fungus powdery mildew, was found to be redistributed in a FER-dependent manner from endosomal compartments to the plasma membrane of synergids [30]. Although its role in pollen tube perception is unknown, NTA is also required for pollen tube growth arrest and burst, and might be required, for example, for pollen tube-synergid attachment. In addition it was recently shown that the FER/LRE signaling complex controls the production of reactive oxygen species (ROS), especially hydroxyl free radicals during pollen tube arrival [26, 31]. ROS themselves appear to induce bursting of the pollen tube in a Ca2+-dependent manner involving the activation of Ca2+-channels. During its growth around or along the synergid cell(s) [17] the pollen tube apex induces cytosolic Ca2+-oscillation in the receptive synergid (Figure 2C) culminating in the death of both interaction partners [18, 32]. The FER signaling pathway is required for induction and maintenance of the Ca2+ responses [33] and a RHO GTPase-based signaling mechanism likely acts directly downstream of FER/LRE activation [26]. However, the connection of the various signaling components is still unclear as the intracellular responses regulated by Ca2+ are multiple and complex [34].
Less is known about the mobile ligands involved in signaling pollen tube growth arrest and bursting. However, both pollen tubes and synergids express a diverse array of genes encoding CRPs at very high levels suggesting specific roles for some of these CRPs during pollen tube reception [28]. The defensin (DEF) and defensin-like (DEFL) subgroup of CRPs are prime candidates to mediate pollen tube bursting as they are closely related to the sequence of toxins leading to cell death in animals. The DEFLs ES1-4 (EMBRYO SAC1-4) were first discovered in the embryo sac of maize and were hypothesized to possess a dual role in pathogen defense and reproduction [11, 35]. Later they were shown to be secreted upon pollen tube arrival to induce tube burst at the very tip leading to explosive sperm release within seconds [36]. ES4 application in vitro leads to depolarization of the pollen tube membrane potential involving opening of the pollen tube potassium channel, KZM1. Other ion channels, including voltage-dependent channels, also may be activated upon the observed membrane-depolarization that culminate in an osmotic-based pollen tube burst, but these putative functions have yet to be tested. DEF/DEFL genes are also expressed by the pollen tube [28] suggesting that the male gametophyte may also release such toxin-like molecules to induce lysis of the receptive synergid cell. Furthermore, upon arrival at the synergid cells, the pollen tube cell wall likely is locally destabilized due to the activity of pectin methylesterase inhibitors (PMEIs) [37]. In support of this assumption the cognate genes are strongly up regulated in the maize female gametophyte.
These findings suggest that at least in maize, PMEI1 and ES1-4 act in concert to induce pollen tube burst. It remains to be shown how their release from synergid cells upon pollen tube interaction is regulated. Notably, a FER-homolog is also expressed in the maize egg apparatus and RALF-encoding genes are strongly expressed in maize pollen tubes (T. Dresselhaus and co-workers, unpublished data) suggesting conserved mechanisms to be involved. To what extent DEFs/DEFLs are required for cellular protection versus reproductive processes is not yet known. It has been reported recently that the pollen tube is capable of carrying a virus towards the embryo sac during fertilization [38], but it is unlikely that larger pathogens, such as bacteria or fungi, are delivered together with the sperm cell cargo because of the many defense levels against such invaders along the path of pollen tube growth. Moreover, DEFLs ES1-4, for example, possesses antifungal activity and the application of high concentrations of ES peptides or fragments thereof containing the predicted interaction domain(s) leads to fungal growth arrest, their swelling, and eventual bursting [39].
The only male factors known to be required for pollen tube growth arrest and sperm release are three transcription factors of the MYB family (MYB97, MYB101 and MYB120) localized to the pollen tube nucleus [10, 40, 41]. Together they regulate and activate a number of genes during pollen tube growth including those coding for secreted CRPs. Whether these CRPs are involved in pollen tube-synergid communication and induction of synergid degeneration via the FER/LRE or NTA signaling pathways remains to be seen.
In conclusion, although flowering plant and animal sperm delivery systems appear very different they do have common morphological features and mechanistic principles to guide spermatozoa or pollen tubes to the female gamete(s). This includes a micropyle and proteins secreted or exposed on the egg cell surface that act as short-range chemoattractants. However, among animal species there exists a great diversity of sexual organs and egg/oocyte morphologies that are difficult to compare as well as molecular pathways and principles that are largely unexplored in any species [42]. Future research hopefully will reveal to what extent, if any, the molecular components involved in sperm delivery are conserved between diverse taxa.
Gamete recognition and attachment
Compared with animals [43–46] little is known about direct gamete interactions in flowering plants. During the pollen tube journey a number of mechanisms have been established in plants to distinguish between self/non-self and alien pollen to prevent inbreeding and fertilization with foreign sperm cells [5]. Sperm cells are delivered towards the site of gamete fusion by the pollen tube and one would therefore expect that flowering plants might have lost specific gamete recognition systems. However, considering lessons from animal fertilization, we should not rush to this conclusion. In animals with internal fertilization, e.g. all mammals, many arthropods, some nematodes, and many others, one might think that genital compatibility, mating behaviors, and seasonal timing would obviate the need for species-specific gamete recognition mechanisms. Clearly this is not the case since in vitro studies using eggs and sperm of different species, e.g. mouse and human, clearly demonstrate that species-specific gamete recognition mechanisms are fully in play [47]. Rapidly evolving cell surface proteins play a key role during gamete recognition and adhesion in metazoans and reinforce barriers to interspecific hybridization [48, 49]. We are not equating a pollen tube to a penis here, but the fact that plants have many reproductive hurdles prior to gamete interaction should not be construed to mean that a plant has no species-specific gamete recognition mechanisms.
A unique mechanism in plant gamete recognition that remains to be understood is how one sperm attaches and fuses with the egg cell while the second sperm cell almost simultaneously fuses with the central cell. With the exception of a few plant species such as Plumbago zeylanica (leadwort) containing dimorphic sperm cells, where the larger sperm cell containing more mitochondria fuses preferentially with the central cell and the smaller sperm cell with the egg cell [50], sperm cells in most flowering plants are isomorphic and a preferential fusion with either of the female gamete has not been observed. In Arabidopsis it was shown that both sperm cells are able to fuse with either female gametes [51–54] thus preferential fertilization does not exist in this species. Live-cell imaging during double fertilization furthermore revealed that the two sperm cells remain close together and relatively immobile after being delivered to their future fusion sites. The beginning of sperm nuclei movement, which occurs around 7.4 min after the pollen tube bursts [51], suggests that plasmogamy has taken place. However, it remains to be explored to what extent gamete recognition and adhesion takes place during this short time period and how the male and female gametes communicate with each other.
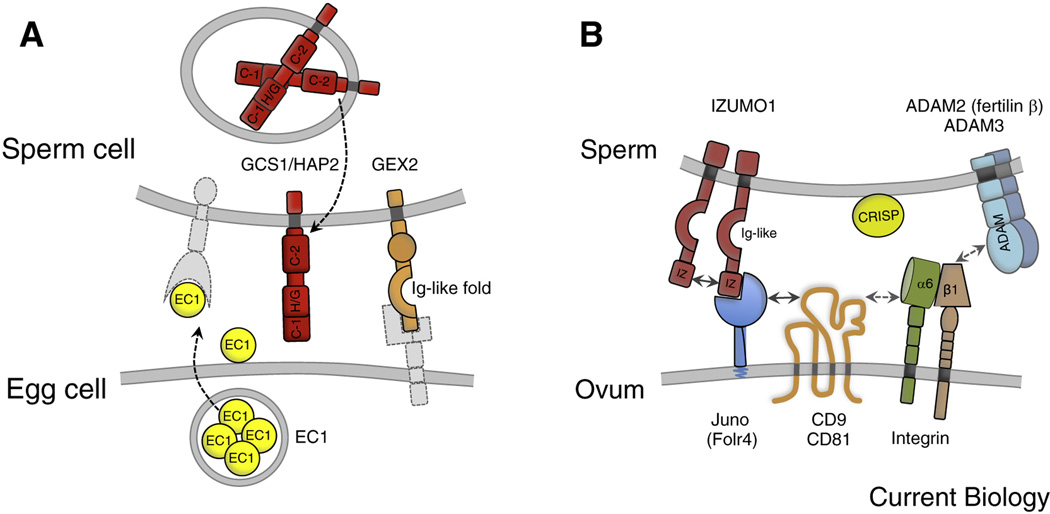
A recent study showed that Arabidopsis sperm cells become competent for fertilization after they are released from the pollen tube (see below), indicating that indeed communication takes place between gametes following sperm cell release from the pollen tube but before fusion is executed. The protein composition on the surface of plant gametes has not yet been determined, but lectin labeling experiments using isolated male and female maize gametes indicate the presence of surface carbohydrates, which might be of importance for cell adhesion as described in animals [55]. Moreover, the surface of isolated egg cells of Torenia fournieri (wishbone flower) was found to be positively charged [56]. Whether the surface of sperm cells is charged in a different manner and whether egg and central cell possess a different surface charge remains to be examined. A first indication that surface proteins involved in gamete attachment and recognition exist in flowering plants, similar to algae and metazoans, is given by the functional characterization of Arabidopsis GAMETE EXPRESSED 2 (GEX2), a protein localized to the sperm cell surface [57] (Figure 3A). GEX2 contains a single-pass transmembrane domain (TMD) at its C-terminal region and a extracellular localized filamin repeat domain similar to immunoglobulin-like (Ig-like) domains involved in gamete interaction in mammals and non-seed plants like algae [44] (Figure 3B). Using an in vivo assay it was shown that GEX2 is required both for sperm-egg cell and sperm-central cell attachment [57]. Investigating GEX2 of the closely related species A. thaliana and A. lyrata revealed that the filamin repeat domain is evolving much faster than other regions of the protein, making it an attractive domain to mediate species-specific gamete attachment. However, this remains to be tested.
Figure 3. Molecular players for direct gamete interactions in Arabidopsis thaliana and mammals.
(A) Arabidopsis cell surface proteins involved in gamete interaction. GAMETE EXPRESSED 2 (GEX2) on the sperm, containing extracellular immunoglobulin (Ig)-like filamin repeat domains, is required for gamete adhesion. Sperm cell-expressed GCS1/HAP2 is a single transmembrane domain (TMD) protein containing two cysteine-rich regions (C-1 and C-2) and a HAP2/GCS1-specific domain (H/G), separated by a less well-conserved region. GCS1/HAP2 is essential for gamete fusion and it´s relocation from the sperm endomembrane system to the plasma membrane is induced by EC1, a small cysteine-rich protein secreted by the egg cell upon sperm cell delivery. Putative interaction partners are depicted in light gray. (B) Mammalian cell surface proteins involved in gamete interaction. Fertilization-essential IZUMO1 of sperm contains one Izumo domain (IZ) at its N-terminus and one extracellular Ig-like domain. The binding partner of IZUMO1 on the oocyte is the GPI-anchored folate receptor 4 (Folr4) named Juno. Tetraspanin CD9 accumulates at the fusion site upon IZUMO1-Juno interaction and interacts laterally with other membrane proteins on the egg (e.g., integrins) through its larger extracellular loop. CD9/CD81 [137] double knock out mice are completely infertile, suggesting an overlapping role for these two tetraspanins during gamete fusion. Other proteins supporting sperm-egg interactions are integrin α6β1 (present on egg and sperm), the ADAM (A Disintegrin and A Metalloprotease) protein family on the sperm membrane and CRISPs (Cysteine-Rich Secretory Proteins) associating with the sperm surface when they transit the epididymis.
In summary our knowledge about molecules involved in gamete recognition and adhesion is scarce and this aspect of the double fertilization process urgently requires more attention from the research community. If one accepts the notion of positive selection on gamete recognition proteins as documented in animals [58] then one may be able to identify candidates based on computational analysis of dN/dS ratios in cell surface proteins. This method was already used to show, for example, that the extracellular domain of FER and the filiamin repeat domain of GEX2 are under positive selection [21, 57]. However, because of the differences in ratios of eggs and sperm between plants and animals, the sexual conflict experienced in a plant may be much less, and may not always drive positive selection mechanisms as robustly.
Gamete activation before fusion
A couple of minutes are required in Arabidopsis after pollen tube bursting before the sperm cells fuse with the female gametes [51]. Until recently it was unclear what was happening to the gametes during this time. A recent breakthrough was the identification of a family of small cysteine-rich proteins, named as EC1 (EGG CELL 1), accumulated in storage vesicles in the egg cell before fertilization. EC1-GFP fusion proteins were detected extracellularly only after sperm delivery, suggesting that exocytosis of EC1-GFP is actively triggered (Figure 3A). Both gamete fusion events are impaired in EC1 deficient ovules and EC1 peptides, exogenously applied to sperm cells, stimulate the relocation of the potential fusogen GENERATIVE CELL SPECIFIC1/HAPLESS2 (GCS1/HAP2) from the endomembrane system to the plasma membrane [59] (Figure 3A), indicating that the egg cell secretes EC1 proteins to achieve rapid sperm activation and gamete fusion. Later it was shown that some of the sperm cells in EC1 deficient ovules are able to accomplish markedly delayed fusion with the female gametes [60], suggesting that these sperm cells have very slowly acquired fusion competence. Moreover, EC1-related proteins are expressed in the synergid cells, which may also contribute to gamete activation during sperm cell delivery [4]. Altogether these findings indicate that first the egg cell becomes activated during or immediately after sperm cell release, in turn activating the sperm cells and enabling them to fuse quickly. Whether the central cell is involved in this process is unclear. However, plasmogamy of a sperm cell devoid of CYCLIN DEPENDENT KINASE A1 (CDKA;1) with the central cell is sufficient to trigger mitotic division despite failure of karyogamy (fusion of gametic nuclei) [54], suggesting the existence of signaling events associated with sperm entry into the central cell just as in most animal fertilizations. Notably, both plant female gametes display a Ca2+ transient during sperm cell discharge from the pollen tube [18, 61] prior to sperm-female gamete fusions, offering the possibility that a first level of female gamete activation is achieved by contents of the pollen tube and not by the sperm directly. Recent work in the fruit fly, Drosophila melanogaster (fruit fly), demonstrates that the Ca2+ rise that occurs in its oocytes also occurs independent of sperm fusion and instead relies on mechanical pressure on the egg provided normally during egg laying, or experimentally by an investigator [62]. Sperm-egg fusion in Drosophila occurs after the egg transits through the oviducts, but it is not the (only) stimulus for the Ca2+ wave that occurs in all animal eggs tested. Passage through the female tract at egg (zygote) laying then finalizes the activation event by the mechanically induced Ca2+ wave. Similarly, the bursting pollen tube causes a deformation on the female plant gametes, and thereby might stimulate mechanosensitive Ca2+ channels as seen in Drosophila [34]. This hypothesis is supported by the observation that both female gametes respond to pollen tube/synergid cell burst with a single Ca2+ transient, which is also observed in a gamete fusion mutant [18, 61]. Testing Ca2+ waves in the female plant gametes using mutants defective in mechanosensing or mechanotransduction and the use of Ca2+ chelators such as BAPTA and/or osmotic stress conditions may help focus future research on this critical activation step.
Gamete fusion (plasmogamy)
Very few cells of a plant or an animal are normally programmed to fuse with another. In fact, animal cells seem to pride themselves in an ability to tightly adhere to each other without fusing. This is particularly true in epithelial cells, where tight junctions, close appositions, and robust adhesions between cells are the norm but cells do not fuse. In a human, which contains about 200 different cells types, only a few cell types normally undergo fusion events: eggs and sperm (fertilization), skeletal myoblasts (myogenesis), osteoclasts (bone remodeling), and some placental cells (placenta formation in mammalian implantation) [63, 64]. As a consequence of these sparse examples, knowledge of cell fusion events is limited. It is evident that specific membrane proteins, called fusogens, are required to modulate lipid-bilayers and thereby facilitate membrane merging since lipid bilayers do not fuse spontaneously. The round worm C. elegans, for example, undergoes significant cell fusion in its outer, dorsal epithelium, and requires the type I membrane protein EFF-1 to do so. EFF-1 driven fusion entails trans-trimerization to bring TMDs anchored in the two opposite membranes in close contact [65]. It was also reported that adding EFF-1 and other fusogens to cells can stimulate cell fusion in cells that do not normally undergo fusion [66]. Thus, a major argument is that cells do not fuse because they do not possess a fusogen, and not because they are molecularly recalcitrant to fusing. Indeed, a plethora of membranous viruses are able to fuse with diverse animal cells, supporting this contention [67].
Surprisingly few and diverse proteins are known to play essential roles in gamete fusion, despite being such a fundamental and ancient process. Several excellent review articles on the fusion-essential proteins that were discovered so far in animal species such as mammals, C. elegans, Xenopus laevis, and Drosophila melanogaster (e.g. [44] which will therefore not be mentioned here in more detail). However, in mammals, for example, the only essential factors for fusion described to date are sperm IZUMO, and the ovum´s JUNO and CD9 (Figure 3B). IZUMO1 is a type I membrane glycoprotein with an extracellular Ig-like domain, one Izumo domain at its N-terminus and a short cytoplasmic tail [46]. Notably, IZUMO1 localizes on the inner and outer acrosomal membrane in mature spermatozoa and translocates to the equatorial segment (the region of the acrosome-reacted sperm which is important in initiating fusion with the egg plasma membrane) only during the acrosome reaction [68]. Recently, the egg-expressed receptor for IZUMO was identified as the GPI-anchored folate-receptor protein Juno, also known as Folr4 [45]. Upon IZUMO1-Juno interaction the fusion-essential tetraspanin CD9 [69, 70] is recruited to the adhesion site, suggesting that CD9 is a partner of Juno [71] (Figure 3B). Interestingly, the binding of monomeric IZUMO1 to Juno triggers the dimerization of IZUMO1 which, in turn, appears to abolish IZUMO1-Juno association as Juno became excluded from the adhesive surface in a cell-oocyte assay [72]. CD9 and other tetraspanins are able to form higher-order protein complexes, called ‘tetraspanin-enriched microdomains (TEMs)’, by direct or indirect lateral interactions with other membrane proteins such as immunglobulins, signaling proteins, integrins and other tetraspanins [73]. This explains why tetraspanins are involved in a range of complex interactions such as gamete fusion, endocytosis, exocytosis, and both entry and exit of viruses during host cell infection. Tetraspanins are conserved in multicellular organisms including flowering plants [74] and they may also be involved in plant reproduction as some tetraspanins accumulate in male and female reproductive tissues and gametes. So far, however, gene knock out studies in Arabidopsis did not reveal reproduction-related phenotypes [75, 76] suggesting functional redundancies.
Among other proteins reported to participate in mammalian sperm-egg interactions are members of the ADAM (A Disintegrin and A Metalloprotease) protein family, integrins and CRISPs (Cysteine-Rich Secretory Proteins) (Figure 3B), protein families that do not seem to have true orthologs in flowering plants. Mouse knockout studies have revealed that the testis/sperm-specific ADAM2 (fertilin β) and ADAM3 (cyritestin) are critical for fertilization because in vivo sperm migratory function in the female reproductive tract is affected and mature sperm cannot bind to the egg zona pellucida [77]. However, ADAM3 in human is a pseudogene [78] suggesting that another ADAM protein takes over ADAM3 function in human sperm. Integrins are obligate (αβ) heterodimers and represent a major family of cell-surface-adhesion receptors in metazoans [79]. Integrin α6β1 is present on egg and sperm and its participation in the process of sperm-egg interaction was supported by the fact that disintegrin-like domains of many ADAMs are capable of acting as integrin ligands. However, neither α6 nor the expression of the β1 integrin subunit by eggs is essential for female mice to be fertile [80], suggesting that integrins are beneficial for sperm adhesion but not essential for mammalian fertilization.
The vertebrate-specific secreted CRISPs (Cysteine-Rich Secretory Proteins) are modular proteins defined by the presence of 16 conserved cysteines. The N-terminal module is the CAP domain (CRISP, Antigen 5, PR-1), which is connected by a short hinge to the cysteine-rich domain (CRD) in the C-terminal region [81]. Although genes encoding CRISP-like proteins are not present in plants the CAP domain of CRISPs reveals sequence similarity to the plant pathogenesis-related protein 1 (PR-1) of Arabidopsis, which accumulates after infections with pathogens. Transcripts encoding a wheat PR-1-like protein are, however, abundant in the egg cell [82] and PR1 proteins may reveal a fertilization-related function. Members of the CRISP gene family are predominantly expressed in the mammalian male reproductive tract and in the venom of reptiles [83]. Rat epididymal CRISP1, for example, associates with the sperm surface during sperm maturation and locates to its equatorial segment (the fusogenic region of sperm) as the acrosome reaction occurs. In vitro experiments suggested that CRISP1 participates in the regulation of signaling pathways during capacitation. However, male and female CRISP1-null mice exhibited no differences in fertility [84], probably caused by functional redundancy between CRISPs. Reptile CRISPs have been reported to regulate a range of ion channel types and in vitro and in vivo methods identified mouse CRISP4 as an inhibitor of transient receptor potential M8 channel (TRPM8) activity [85]. Moreover, mouse CRISP1 was recently reported to be capable of modulating the principal sperm Ca2+ channel CatSper involved in hyperactivation [86], supporting the idea that CRISPs act as endogenous protein ion channel regulators.
Little is known about cell fusion in flowering plants, although the double fertilization process involving fusion of genetically identical male gametes (sperm cells) with two female gametes (egg and central cell) [87] is a major distinguishing and unique feature of flowering plants. Recently, even a third cell fusion event was reported in Arabidopsis [88] occurring between the fertilized central cell and the persisting synergid cell thereby preventing the attraction of multiple pollen tubes (see also below). Whether the occurrence of three cell fusion events is a general mechanism and exists also in plants with a different ovary and ovule morphology remains to be determined [89]. However, the sequence of fusion events and the precision by which fusion of both sperm cells with only one female gamete is accomplished are longstanding questions in plant reproduction research. Using fluorescent fusion proteins the simultaneous entry of sperm internal membrane components into the egg cell and central cell was reported [90] and adjustment and re-adhesion of one sperm cell may be required when the sperm cell pair is not properly positioned between the two female gametes [91].
More detailed studies have recently shown that fusion of one sperm cell with the egg cell occurs at about 4 min after their release and fusion between the second sperm cell with the central cell is on average a few minutes delayed [18, 61]. This sequence of events suggests that successful sperm cell-egg cell fusion is a prerequisite for sperm cell-central cell fusion. Further support is provided by studies of the female gametophytic mutant glauce (glc), where the second sperm cell fails to fuse with the central cell resulting in single fertilization [92] and the discovery that each female gamete contributes independently and synergistically in the block to polytubey upon fertilization [93]. These observations suggest that the central cell likely promotes its own fertilization by a yet unknown signaling mechanism.
The best characterized gamete interaction protein in plants is GENERATIVE CELL SPECIFIC1/HAPLESS2 (GCS1/HAP2) [94] (Figure 3A). Originally identified as a generative cell-specific single-pass TMD protein in pollen of lily (Lilium longiflorum) and Arabidopsis required to regulate gamete fusion and fertility [95–97], GCS1/HAP2 was later shown to function as a conserved protein involved in gamete fusion in many eukaryotes including unicellular protists and invertebrates [98]. Its function is best understood in the flagellated single cell algae, Chlamydomonas reinhardtii, where it is located at the fusion site of minus-type gametes and acts during gamete fusion, and thus after gamete attachment [99]. It is required in a similar manner as a male factor during fertilization in the malaria parasite, Plasmodium berghei [99, 100] and was recently shown to be required also for fertilization in a starlet sea anemone (Nematostella vectensis). Here the GCS1/HAP2 gene is expressed in the testis and the protein localizes to sperm where it may act in sperm-egg fusion, although this remains to be tested [101]. A very exciting observation was recently reported in the ciliated protozoan Tetrahymena thermophila, which generates seven sexes or mating types and bypasses the production of male gametes. It was shown that GCS1/HAP2 is expressed in all mating types and that fertility is only blocked when the gene is deleted from both fusion partners [102]. Interestingly, gametes of complementary mating types in this species can recognize each other, but GCS1/HAP2 is required for the stability of the interaction and membrane pore formation at the nuclear exchange junction. Plant egg cells in maize and Arabidopsis do not generate detectable GCS1/HAP2 amounts (T. Dresselhaus, S. Sprunck and co-workers, unpublished data) suggesting that presence of GCS1/HAP2 on sperm cells is sufficient for gamete fusion. It may therefore be possible that GCS1/HAP2 interacts with another protein and not with itself as indicated by above described findings in T. thermophila.
The extracellular N-terminal part of plant sperm cell GCS1/HAP2, containing a conserved GCS1/HAP2 domain, was shown to be critical for its function and was proposed to interact with proteins on the surface of female gametes, while the cytoplasmic C-terminus, which is enriched in positively charged amino acid residues, is dispensable for gamete fusion and might instead be involved in membrane interactions and/or downstream signaling events [103, 104]. These findings indicate that the molecular mechanisms of gamete fusion have elements shared between widely divergent organisms, more so than those mechanisms involved in gamete attraction and recognition. It will now be exciting to find out how widely throughout phylogeny GCS1/HAP2 is present, whether its protein domains are functionally interchangeable between species, and if the very few animal fusogens identified are capable of stimulating fusion also in plant cells.
Karyogamy
After plasmogamy microtubules and centrosomes direct the migration of gamete pronuclei for fusion (karyogamy) in animals. Oscar Hertwig described the process of karyogamy in 1876 for the first time [105]. This observation used the very accessible sea urchin eggs and sperm, and marked the definitive conclusion that both egg and sperm were involved in creating the embryo. Moreover, this finding formally ended the controversy of the spermists versus the ovists, that is, that the sperm contained a miniature individual that merely unfolded or grew in the highly nutritive source of the egg, or that the egg would normally be sufficient for complete development if only activated by the correct stimulus, a sperm. These results definitively documented that both gametes were involved in the developmental process.
Sea urchins use a paradigmatic mechanism of karyogamy – the sperm-donated centriole (the egg centriole is disassembled and lost in most animal species studied, rodents being a significant exception) nucleates extensive microtubular arrays that “capture” the female pronucleus, and draw each toward the center of the zygote, whereupon fusion of the two pronuclei ensues [106, 107] (Figure 4A). Yet other animals use a variety of variations on this theme. In the ctenophore Boroe, for example, multiple sperm pronuclei enter the large, yolk filled egg and “wait” to be selected [108]. The female pronucleus then proceeds to migrate throughout the egg cytoplasm, even bumping into some of the male pronuclei. At some point, the female pronucleus “chooses” one of the male pronuclei, fuses with it, and thereby stimulating degradation of the remaining male pronuclei. So too at least in some birds, multiple sperm appear to fuse with the egg and only later is one selected for karyogamy [109]. Between these extremes in mechanism lie most animal karyogamy examples [110].
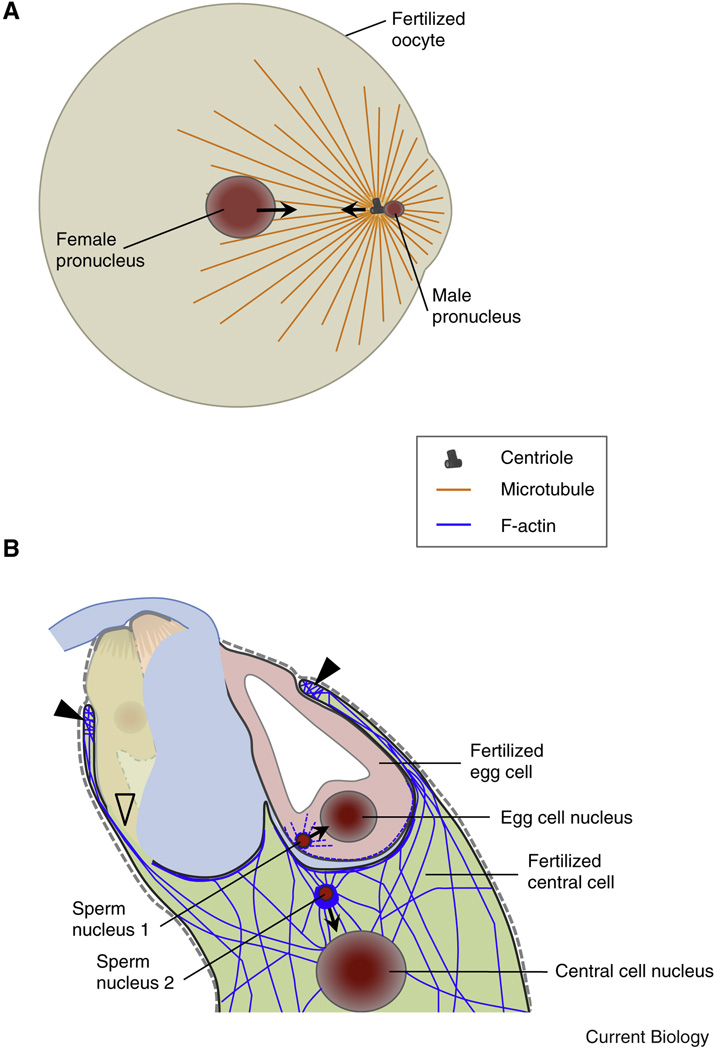
Figure 4. Following plasmogamy the migration of gamete nuclei is realized by microtubules in animals and actin in plants.
(A) Diagram of a fertilized fish oocyte. Sperm aster microtubules are established by the male centrosome. The sperm aster captures the female pronucleus and the two pronuclei rapidly move towards each other and to the center of the oocyte in a dynein-mediated process. (B) In Arabidopsis F-actin dynamics in the female gametes assist the migration of the male nuclei towards the female nuclei while microtubules are not required. After plasmogamy the sperm nucleus in the central cell becomes surrounded by a star-shaped structure of F-actin cables migrating together with the sperm nucleus towards the central cell nucleus. Closed triangles label the characteristic ring-shaped actin network at the micropylar end of the central cell surrounding the synergid cells and the egg cell. Centrioles are not present in plants.
While in animals gamete pronuclei approach each other and nuclear tracking along microtubules and centrosome-dependent nuclear positioning [111] governs their movement and positioning for karyogamy, the migration of nuclei following plasmogamy is considerably different in flowering plants, which lack centrosomes. Nevertheless, the two sperm cells of flowering plants migrate towards egg and central cell nuclei, respectively, and fuse, finalizing the process of double fertilization. It was recently reported that microtubules are dispensable for migration and fusion of male and female gamete nuclei of Arabidopsis [112, 113] and for nuclear positioning in the central cell [112, 113] while the presence of intact F-actin cables is necessary. After plasmogamy the sperm nuclei become surrounded by an aster-like structure formed by actin and this structure migrates together with the sperm nuclei (Figure 4B). Bundles of microfilaments are formed in a Rho-GTPase ROP8-dependent manner and appear to regulate sperm nuclei migration during fertilization [112, 113]. The finding that microtubules are dispensable in flowering plant karyogamy is surprising since microtubules in animals are involved at many levels [114]. The cytoplasm below the surface of plant female gametes appears also enriched in microfilaments [112, 113], which might be required for sperm cell incorporation similar to animals, but might also be required to establish the aster-like structure, similar to the microtubule-based aster associated with animal sperm pronuclei (Figure 4A). In vitro fertilization experiments with isolated plant gametes were performed to determine the timing between plasmogamy and karyogamy. While experiments in maize indicated that karyogamy occurred within 2 to 3h after gamete fusion [115], recent experiments in rice showed that movement of sperm nuclei adjacent to the egg nucleus takes only 5 to 10 min and occurs in an actin-dependent manner [116] thus confirming the above described findings in Arabidopsis.
The molecules required for karyogamy are not known. However, the membrane protein GEX1 containing three predicted TMDs and a large extracellular domain (ED) is an attractive candidate. GEX1 is expressed both in sperm and egg cells of Arabidopsis and was previously discussed as a candidate involved in gamete interaction [117]. More recent studies have shown that it is necessary for gametophyte development and early embryogenesis [118], which does not exclude that it is also required during fertilization. Its ortholog in Chlamydomonas, CrGEX1, localizes to the nuclear envelope of sexual stages and was recently shown to function in nuclear fusion similar to KAR5 (KARYOGAMY5), which is essential for nuclear fusion during sexual reproduction in yeast [119]. GEX1 thus appears as a prime candidate protein mediating fusion of plant gamete nuclei. However, this has to be tested by further experimentation.
Polytubey and prevention of polyspermy
Eggs in animals have significant investments of nutrition and resources (if not yolk as in chickens, sea urchins, flies etc. then in organelles and stored macromolecules) that are energetically costly for a female. It is evolutionarily advantageous to have this egg cell be successfully fertilized by one sperm only, since multiple sperm fusing with the egg (polyspermy) is usually lethal to the resultant embryo [42]. The lethality is caused usually from the multiple centrioles contributed by the multiple sperm that causes aberrant mitotic spindles, competition for the chromosomes, and cleavage abnormalities. So, the egg strategy is to emphasize a single sperm fusion to protect its investment. This is in contrast to the strategy in males in which sperm have relatively (compared to the egg) low nutritional investment in each sperm, and as a result the male makes large numbers of sperm that are selected based on their ability to get to and fuse with the egg first. The sperm onslaught is advantageous for the male in higher probability for passing on that male’s genes, even if sometimes killing the egg by polyspermy, because of the sometimes highly competitive conditions with other males. Getting there first, and outcompeting other males is often enhanced by increased sperm production, and hence the conflict of (self-interest) between the sexes. Indeed, mammals with high male competition for mating with promiscuous females, generally have larger testes for increased sperm production, whereas in species with low intraspecific male competition, the testes are smaller, and sperm production is lower [120]. Similarly in wind pollinated seed plants the number of sexual male organs (stamens containing anthers) is strongly increased compared to species pollinated by insects [121]. A way in which animal eggs counter sperm onslaught is by rapid diversification of receptors for sperm [48], and by rapid and permanent blocks to polyspermy. The investment made by the egg to blocks to polyspermy in some cases is enormous. In sea urchins for example, in which thousands of sperm may contact the egg within moments of each other, the egg has invested multiple mechanisms to block polyspermy. The sea urchin egg has devoted several percent of its mRNA, of its protein, and of its organelle volume to cortical granules, the most conserved egg organelle involved in the block to polyspermy [42, 122]. Notably, mammalian Juno (the binding partner of IZUMO1 on the egg; Figure 4B) appears to be rapidly shed from the membrane into the perivitelline space after fertilization [45], which may also play an essential role in the prevention of polyspermy in these species. Animals such as insects also use the micropyle as a limiting structure for sperm entry, such that the first sperm entering the micropyle may sterically hinder subsequent sperm candidates from reaching the egg (such as in the block to plant polytubey, see below). Remarkably, some insect eggs even close the micropyle prior to fertilization as a switch between sexual to asexual reproduction [123].
Similar to the mammalian Juno, which is a glycosylphosphatidylinositol-anchored protein (GAP), the plant-specific LORELEI (LRE) is also anchored to the membrane via a glycosylphosphatidylinositol-anchor. LRE is predicted as a co-receptor of FER at the surface of synergid cells [26] and it might also be cleared by GAP cleavage from the plasma membrane as a result of successful pollen tube reception and thereby contribute to the prevention of entry by subsequent pollen tubes into the same embryo sac, a condition known as polytubey [124].
However, the most prevalent mechanism for prevention of polyspermy in animal eggs is fertilization-dependent secretion such as the release of cortical granules, whose contents quickly change the egg extracellular matrix and the ability of sperm to reach the egg cell surface. The stimulus for the release of the contents of cortical granules is Ca2+ [122, 125, 126]. Ca2+ performs many roles in the activation of the egg, and fusion of the cortical granule membrane with the egg plasma membrane is Ca2+ dependent, and uses much the same molecular machinery as fusion of synaptic vesicles in a neuron e.g. SNAREs. During medaka and zebrafish fertilization, for example, a single self-propagating Ca2+ wave is initiated at the micropyle, the point of sperm/egg contact located at the animal pole, and then it propagates at about 10-12 µm/s to its antipode at the vegetal pole leading to the release of cortical granules [127]. This is different for animals such as mammals, ascidians, some amphibians and annelids where multiple, recurring Ca2+ waves, with species specific wave frequencies and amplitudes, are required for successful activation of development [128].
It is unclear, whether vesicles with functional analogy to cortical granules also exist in plant female gametes. However, vesicles containing the sperm cell activating EC1 proteins are released by the egg cell upon pollen tube reception and sperm cell arrival [59] (Figure 3A) shortly after a single strong Ca2+ transient is observed both in the egg and central cell [18, 61]. Plasmogamy between egg and sperm cell is associated with a 2nd extended Ca2+ elevation and release of cell wall material, which would prevent fusion of a fertilized egg cell with additional sperm cells, is visible within 30 sec after gamete fusion [129]. This observation points towards the possibility that vesicles containing cell wall material such as pectins are triggered in a Ca2+-dependent manner to fuse with the plasma membrane similar to the cortical reaction described in animals [130]. Once even a primitive cell wall is established, fusion with additional sperm cells is prevented. In this context two unique aspects of plant fertilization should be considered. First, even though only two sperm are deposited in the embryo sac for two female gametes, were random fusion occurring between sperm cells and the female gametes, this would result in polyspermy 50% of the time, unless plants indeed had a block to polyspermy. While no molecular evidence reveals a mechanism for blocks to polyspermy in plants with the exception of the above described quick appearance of cell wall material, the numbers suggest that female gametes do change upon fertilization in a way that limits polyspermy at least in the egg cell. The central cell is indeed able to fuse with more than one sperm cell, as demonstrated in the tetraspore (tes) mutant generating additional sperm cell pairs [131]. This finding supports the hypothesis that Ca2+ is involved in triggering this block as the 2nd elevated Ca2+ transient observed during egg-sperm cell fusion is absent during central cell fertilization [9, 18]. As a second consideration, while only two sperm cells are delivered inside the embryo sac, each of these sperm cells must function in fertilization. That is, 100% of the sperm must fuse in order for the embryo and endosperm to develop successfully. This is a very different kind of efficiency than in animal eggs in which tens to millions of sperm may confront a single egg within moments of each other. However, plants also possess mechanisms to achieve fertilization if gamete fusion fails. A mechanism called as fertilization recovery [132] associated with polytubey (attraction of supernumerary pollen tubes) [124] becomes active in Arabidopsis in case fusion-defective sperm cells are delivered by the first pollen tube. Secondary pollen tubes are attracted with a delay of a couple of hours by the remaining 2nd synergid cell and deliver their sperm cargo to the female gametes. This mechanism provides plants with an additional mechanism to maximize reproductive success [133]. The observation that secondary pollen tubes are not immediately attracted but only when sperm fusion with one or both female gametes fails furthermore indicates that pollen tube repellents must be released upon sperm cell discharge. In the grasses multiple pollen tubes compete for fertilization, but only a single tube enters the micropyle [134] providing further support for the existence of as yet unknown repellents. These have to be degraded or diluted until additional pollen tubes are capable to enter the micropyle and embryo sac. Moreover, to fully prevent polytubey, the 2nd persisting synergid cell generating the pollen tube attractant is quickly disintegrated by independent signals from both female gametes [93]. An EIN3-EIN2/EIL2-dependent ethylene-signaling cascade is predominately activated after fertilization of the egg cell, resulting in premature synergid degeneration and thus a subsequent block of supernumerary pollen tube attraction [88, 135]. Additionally, the persisting synergid cell fuses with the successfully fertilized central cell and thereby dilutes its content into a much larger cell causing rapid dilution of the pollen tube attractant [88].
Conclusions
Gamete fusion is a necessary pre-requisite for sexual reproduction in all organisms and one might think that the underlying mechanisms of such a prevalent event are also highly conserved. Instead, it is clear that organisms have evolved enormous diversity to accomplish the same goal. It appears that a unique reproductive niche is requisite to successful species competition and that organisms test new dimensions within which to occupy their niche more effectively. By judicious exploration of different organisms, researchers may eventually build a coherent mural of how fertilization has diversified, and what constraints reproductive niches might hold in species success. With a focus on flowering plant fertilization mechanisms, this review aimed to highlight similarities and differences in the morphology and molecular machinery involved in gamete interactions in plants and animals. While structures such as a micropyle are present both in plants and animal organisms to restrict the entry site of sperm to the egg, other processes such as the loss of sperm motility and the usage of the actin cytoskeleton instead of microtubules to achieve gamete nuclei fusion in plants are quite different. Research progress in flowering plants has recently revealed many molecular mechanisms common between plants and animals, and these windows of discovery cast new light into the reproductive process for all organisms. Pre-fertilization mechanisms such as sperm/pollen tube attraction, sperm cell reception and gamete recognition appear highly specific to species, and represent barriers to pre-zygotic hybridization through fusion of incompatible gametes. However, the subsequent steps of fertilization, including gamete adhesion, activation, and fusion appear more similar. As outlined above, GEX2 required for gamete adhesion in plants [57] contains extracellular protein domains previously described in animal gamete attachment [44] and GAPs such as LRE in plants and Juno in mammals as well as protein glycosylation have been shown to play critical roles in initiation of the fertilization process both in mammals and plants. Moreover, the putative gamete fusogen GSC1/HAP2 exists in protists, invertebrates, and plants and may function similarly in these organisms (see above). EC1, involved in sperm cell activation during double fertilization is conserved in all flowering plants including the most basal-branching extant flowering plant Amborella trichopoda [4, 59]. Whether cysteine-rich secretory peptides/proteins or other secreted ligands are also required for sperm activation in animals remains to be seen. However, molecules similar to plant CRPs of the β-defensin class have also been found in testis, within the epididymidal epithelium as well as in the seminal fluid of mammals, and does affect sperm motility and fertility [136]. These observations together with the finding that Ca2+ serves as an intracellular messenger in almost all fertilization mechanisms tested indicates a surprising number of similarities between plants and animals even though their reproductive organs and structures appear at first glance quite different. We hope that this review also reveals that researchers of plant and animal fertilization mechanisms are capable of speaking the same language, which will further accelerate future discoveries for basic, clinical, and agricultural applications.
Acknowledgments
Funding by the German Research Council DFG via collaborative research centers SFB924 and SFB960 to TD and SS as well as support from the NIH to GMW is gratefully acknowledged.
GLOSSARY OF TERMS
- Angiosperms
flowering plants, also known as Magnoliophyta. Vascular seed plants that differ from gymnosperms, for example, by a double fertilization process and seeds that develop enclosed within an ovary.
- Antipodals
haploid accessory cells at the chalazal pole of the angiosperm embryo sac neighboring the central cell; precise function is unknown.
- Central cell
homo-diploid 2nd female reproductive cell of flowering plants that develops into the triploid endosperm after successful fertilization.
- Egg cell or ovum
haploid female gamete (egg cell in plants, ovum or egg in animals) forming a zygote after fertilization, which gives rise to the diploid embryo.
- Egg apparatus
structure at the micropylar end of the embryo sac in seed plants consisting of the egg cell and usually two synergid cells.
- Embryo sac
the female gametophyte of a flowering plant, which develops within the ovule, protected by the nucellus and the integuments of the ovule. It typically consists of two female gametes (egg and central cell) and accessory cells (synergid and antipodal cells).
- Endosperm
a nutrient-rich tissue arising from the fertilized central cell. It nourishes the developing embryo in dicotyledonous plants and the germinating seedling in monocots.
- Filiform apparatus
highly thickened structure formed by the synergid cell wall at the micropylar end of the angiosperm embryo sac.
- Fusogen
any substance that enables membrane fusion, including the plasma membrane of cells. Fusogenic proteins are membrane proteins that remodel lipid bilayers to facilitate membrane merging.
- Gametophyte
The haploid generation of a plant´s life cycle. It develops from a spore produced by the sporophyte and produces gametes by mitotic division. This phase alternates with the sporophyte generation.
- Gymnosperm
“naked” seed producing plants, with distinct fertilization process from angiosperms (flowering plants) and include conifers and Ginkgo.
- Integuments
generally the covering of an organism or an organ, such as skin, shell, husk or rind. In plant sexual organs it describes the outermost layers of an ovule, enveloping the female gametophyte or embryo sac.
- Karyogamy
nuclear fusion, including fusion of gamete nuclei in fertilization.
- Micropyle
a minute opening in the ovule of a seed plant through which the pollen tube enters and a funnel-shaped opening in many animal eggshells, respectively, enabling sperm access.
- Oocyte
the cell in animals that develops into an egg. Once it is fertilizable, it is referred to as an egg.
- Ovule
the structure in seed plants that develops into a seed after fertilization. It consists of one or two integuments forming its outer layers, the nucellus and the female gametophyte.
- Plasmogamy
fusion of the cytoplasm of two parent cells including fusion of egg and sperm cells.
- Pollen
the highly reduced haploid male gametophyte generation in flowering plants, consisting of the tube cell as well as one generative and two sperm cells, respectively.
- Pronuclei
the haploid nuclei of animal gametes during the process of fertilization, after the sperm enters the ovum, but before they fuse.
- Sporophyte
The diploid generation of a plant´s life cycle; develops from the diploid zygote after fertilization; produces spores through meiosis.
- Synergids or synergid cells
haploid accessory cells at the micropylar pole of the angiosperm embryo sac; glandular-like cells involved in pollen tube attraction and reception.
- Syngamy
The fusion of two gametes in fertilization forming a zygote involving both plasmogamy and karyogamy.
REFERENCES
- 1.Twell D. Male gametogenesis and germline specification in flowering plants. Sex. Plant Reprod. 2011;24:149–160. doi: 10.1007/s00497-010-0157-5. [DOI] [PubMed] [Google Scholar]
- 2.Drews GN, Koltunow AM. The female gametophyte. Arabidopsis Book. 2011;9:e0155. doi: 10.1199/tab.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprunck S, Gross-Hardt R. Nuclear behavior, cell polarity, and cell specification in the female gametophyte. Sex. Plant Reprod. 2011;24:123–136. doi: 10.1007/s00497-011-0161-4. [DOI] [PubMed] [Google Scholar]
- 4.Sprunck S, Hackenberg T, Englhart M, Vogler F. Same same but different: sperm-activating EC1 and ECA1 gametogenesis-related family proteins. Biochem. Soc. Trans. 2014;42:401–407. doi: 10.1042/BST20140039. [DOI] [PubMed] [Google Scholar]
- 5.Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol. Plant. 2013;6:1018–1036. doi: 10.1093/mp/sst061. [DOI] [PubMed] [Google Scholar]
- 6.Palanivelu R, Tsukamoto T. Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. Wiley Interdiscip. Rev. Dev. Biol. 2012;1:96–113. doi: 10.1002/wdev.6. [DOI] [PubMed] [Google Scholar]
- 7.Higashiyama T, Takeuchi H. The mechanism and key molecules involved in pollen tube guidance. Annu. Rev. Plant. Biol. 2015;66:393–413. doi: 10.1146/annurev-arplant-043014-115635. [DOI] [PubMed] [Google Scholar]
- 8.Qu LJ, Li L, Lan Z, Dresselhaus T. Peptide signalling during the pollen tube journey and double fertilization. J. Exp. Bot. 2015;66:5139–5150. doi: 10.1093/jxb/erv275. [DOI] [PubMed] [Google Scholar]
- 9.Bleckmann A, Alter S, Dresselhaus T. The beginning of a seed: regulatory mechanisms of double fertilization. Front. Plant Sci. 2014;5:452. doi: 10.3389/fpls.2014.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leydon AR, Chaibang A, Johnson MA. Interactions between pollen tube and pistil control pollen tube identity and sperm release in the Arabidopsis female gametophyte. Biochem. Soc. Trans. 2014;42:340–345. doi: 10.1042/BST20130223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dresselhaus T, Marton ML. Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr. Opin. Plant Biol. 2009;12:773–780. doi: 10.1016/j.pbi.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Amanze D, Iyengar A. The micropyle: a sperm guidance system in teleost fertilization. Development. 1990;109:495–500. doi: 10.1242/dev.109.2.495. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi H, N Y. Formation and ultrastructure of the micropylar apparatus in Bombyx mori ovarian follicles. J. Morphology. 1984;179:47–58. doi: 10.1002/jmor.1051790106. [DOI] [PubMed] [Google Scholar]
- 14.Yanagimachi R, Cherr G, Matsubara T, Andoh T, Harumi T, Vines C, Pillai M, Griffin F, Matsubara H, Weatherby T, et al. Sperm attractant in the micropyle region of fish and insect eggs. Biol. Reprod. 2013;88:47. doi: 10.1095/biolreprod.112.105072. [DOI] [PubMed] [Google Scholar]
- 15.Sehgal A, Mann N, Mohan Ram HY. Structural and developmental variability in the female gametophyte of Griffithella hookeriana, Polypleurum stylosum, and Zeylanidium lichenoides and its bearing on the occurrence of single fertilization in Podostemaceae. Plant Reprod. 2014;27:205–223. doi: 10.1007/s00497-014-0252-0. [DOI] [PubMed] [Google Scholar]
- 16.Terasaka O, Nitsu T, Tanaka R. Single fertilization in Spiranthes sinensis. Bot. Mag. Tokyo. 1979;92:59–67. [Google Scholar]
- 17.Leshem Y, Johnson C, Sundaresan V. Pollen tube entry into the synergid cell of Arabidopsis is observed at a site distinct from the filiform apparatus. Plant Reprod. 2013;26:93–99. doi: 10.1007/s00497-013-0211-1. [DOI] [PubMed] [Google Scholar]
- 18.Denninger P, Bleckmann A, Lausser A, Vogler F, Ott T, Ehrhardt DW, Frommer WB, Sprunck S, Dresselhaus T, Grossmann G. Male-female communication triggers calcium signatures during fertilization in Ara/bidopsis. Nat. Commun. 2014;5:4645. doi: 10.1038/ncomms5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leydon AR, Tsukamoto T, Dunatunga D, Qin Y, Johnson MA, Palanivelu R. Pollen tube discharge completes the process of synergid degeneration that is initiated by pollen tube - synergid interaction in Arabidopsis. Plant Physiol. 2015;169:485–496. doi: 10.1104/pp.15.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandaklie-Nikolova L, Palanivelu R, King EJ, Copenhaver GP, Drews GN. Synergid cell death in Arabidopsis is triggered following direct interaction with the pollen tube. Plant Physiol. 2007;144:1753–1762. doi: 10.1104/pp.107.098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escobar-Restrepo JM, Huck N, Kessler S, Gagliardini V, Gheyselinck J, Yang WC, Grossniklaus U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656–660. doi: 10.1126/science.1143562. [DOI] [PubMed] [Google Scholar]
- 22.Rotman N, Rozier F, Boavida L, Dumas C, Berger F, Faure JE. Female control of male gamete delivery during fertilization in Arabidopsis thaliana . Curr. Biol. 2003;13:432–436. doi: 10.1016/s0960-9822(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 23.Lindner H, Kessler SA, Müller LM, Shimosato-Asano H, Boisson-Dernier A, Grossniklaus U. TURAN and EVAN mediate pollen tube reception in Arabidopsis synergids through protein glycosylation. PLoS Biol. 2015;13:e1002139. doi: 10.1371/journal.pbio.1002139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capron A, Gourgues M, Neiva LS, Faure JE, Berger F, Pagnussat G, Krishnan A, Alvarez-Mejia C, Vielle-Calzada JP, Lee YR, et al. Maternal control of male-gamete delivery in Arabidopsis involves a putative GPI-anchored protein encoded by the LORELEI gene. Plant Cell. 2008;20:3038–3049. doi: 10.1105/tpc.108.061713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsukamoto T, Qin Y, Huang Y, Dunatunga D, Palanivelu R. A role for LORELEI, a putative glycosylphosphatidylinositol-anchored protein, in Arabidopsis thaliana double fertilization and early seed development. Plant J. 2010;62:571–588. doi: 10.1111/j.1365-313X.2010.04177.x. [DOI] [PubMed] [Google Scholar]
- 26.Li C, Yeh FL, Cheung AY, Duan Q, Kita D, Liu MC, Maman J, Luu EJ, Wu BW, Gates L, et al. Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. Elife. 2015;4:e06587. doi: 10.7554/eLife.06587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR. A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science. 2014;343:408–411. doi: 10.1126/science.1244454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang Q, Dresselhaus T, Gu H, Qu LJ. Active role of small peptides in Arabidopsis reproduction: Expression evidence. J. Integr. Plant Biol. 2015;57:518–521. doi: 10.1111/jipb.12356. [DOI] [PubMed] [Google Scholar]
- 29.Kessler SA, Grossniklaus U. She's the boss: signaling in pollen tube reception. Curr. Opin. Plant Biol. 2011;14:622–627. doi: 10.1016/j.pbi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Kessler SA, Shimosato-Asano H, Keinath NF, Wuest SE, Ingram G, Panstruga R, Grossniklaus U. Conserved molecular components for pollen tube reception and fungal invasion. Science. 2010;330:968–971. doi: 10.1126/science.1195211. [DOI] [PubMed] [Google Scholar]
- 31.Duan Q, Kita D, Johnson EA, Aggarwal M, Gates L, Wu HM, Cheung AY. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014;5:3129. doi: 10.1038/ncomms4129. [DOI] [PubMed] [Google Scholar]
- 32.Iwano M, Ngo QA, Entani T, Shiba H, Nagai T, Miyawaki A, Isogai A, Grossniklaus U, Takayama S. Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development. 2012;139:4202–4209. doi: 10.1242/dev.081208. [DOI] [PubMed] [Google Scholar]
- 33.Ngo QA, Vogler H, Lituiev DS, Nestorova A, Grossniklaus U. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev. Cell. 2014;29:491–500. doi: 10.1016/j.devcel.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Gutjahr C, Bleckmann A, Dresselhaus T. Calcium signaling during reproduction and biotrophic fungal interactions in plants. Mol. Plant. 2015;8:595–611. doi: 10.1016/j.molp.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 35.Cordts S, Bantin J, Wittich PE, Kranz E, Lörz H, Dresselhaus T. ZmES genes encode peptides with structural homology to defensins and are specifically expressed in the female gametophyte of maize. Plant J. 2001;25:103–114. doi: 10.1046/j.0960-7412.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- 36.Amien S, Kliwer I, Marton ML, Debener T, Geiger D, Becker D, Dresselhaus T. Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol. 2010;8:e1000388. doi: 10.1371/journal.pbio.1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woriedh M, Wolf S, Marton ML, Hinze A, Gahrtz M, Becker D, Dresselhaus T. External application of gametophyte-specific ZmPMEI1 induces pollen tube burst in maize. Plant Reprod. 2013;26:255–266. doi: 10.1007/s00497-013-0221-z. [DOI] [PubMed] [Google Scholar]
- 38.Isogai M, Yoshida T, Shimura T, Yoshikawa N. Pollen tubes introduce Raspberry bushy dwarf virus into embryo sacs during fertilization processes. Virology. 2015;484:341–345. doi: 10.1016/j.virol.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 39.Woriedh M, Merkl R, Dresselhaus T. Maize EMBRYO SAC family peptides interact differentially with pollen tubes and fungal cells. J. Exp. Bot. 2015;66:5205–5216. doi: 10.1093/jxb/erv268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leydon AR, Beale KM, Woroniecka K, Castner E, Chen J, Horgan C, Palanivelu R, Johnson MA. Three MYB transcription factors control pollen tube differentiation required for sperm release. Curr. Biol. 2013;23:1209–1214. doi: 10.1016/j.cub.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y, Tan ZM, Zhu L, Niu QK, Zhou JJ, Li M, Chen LQ, Zhang XQ, Ye D. MYB97, MYB101 and MYB120 function as male factors that control pollen tube-synergid interaction in Arabidopsis thaliana fertilization. PLoS Genet. 2013;9:e1003933. doi: 10.1371/journal.pgen.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong JL, Wessel GM. Defending the zygote: search for the ancestral animal block to polyspermy. Curr. Top. Dev. Biol. 2006;72:1–151. doi: 10.1016/S0070-2153(05)72001-9. [DOI] [PubMed] [Google Scholar]
- 43.Hirohashi N, Kamei N, Kubo H, Sawada H, Matsumoto M, Hoshi M. Egg and sperm recognition systems during fertilization. Dev. Growth Differ. 2008;50(Suppl 1):S221–S238. doi: 10.1111/j.1440-169X.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 44.Evans JP. Sperm-egg interaction. Annu. Rev. Physiol. 2012;74:477–502. doi: 10.1146/annurev-physiol-020911-153339. [DOI] [PubMed] [Google Scholar]
- 45.Bianchi E, Doe B, Goulding D, Wright GJ. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature. 2014;508:483–487. doi: 10.1038/nature13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- 47.Avella MA, Xiong B, Dean J. The molecular basis of gamete recognition in mice and humans. Mol. Hum. Reprod. 2013;19:279–289. doi: 10.1093/molehr/gat004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilburn DB, Swanson WJ. From molecules to mating: Rapid evolution and biochemical studies of reproductive proteins. J. Proteomics. 2015 doi: 10.1016/j.jprot.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harada Y, Sawada H. Self-incompatibilty in gamete recognition: single self-recognizing determinants and multiple, non-self-recognizing ones function in the same individual. Mol. Reprod. Dev. 2013;80:2–7. doi: 10.1002/mrd.22134. [DOI] [PubMed] [Google Scholar]
- 50.Russell SD. Preferential fertilization in Plumbago: Ultrastructural evidence for gamete-level recognition in an angiosperm. Proc. Natl. Acad. Sci. USA. 1985;82:6129–6132. doi: 10.1073/pnas.82.18.6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hamamura Y, Saito C, Awai C, Kurihara D, Miyawaki A, Nakagawa T, Kanaoka MM, Sasaki N, Nakano A, Berger F, et al. Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana . Curr. Biol. 2011;21:497–502. doi: 10.1016/j.cub.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 52.Ingouff M, Sakata T, Li J, Sprunck S, Dresselhaus T, Berger F. The two male gametes share equal ability to fertilize the egg cell in Arabidopsis thaliana . Curr. Biol. 2009;19:R19–R20. doi: 10.1016/j.cub.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 53.Kong J, Lau S, Jürgens G. Twin plants from supernumerary egg cells in Arabidopsis. Curr. Biol. 2015;25:225–230. doi: 10.1016/j.cub.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 54.Aw SJ, Hamamura Y, Chen Z, Schnittger A, Berger F. Sperm entry is sufficient to trigger division of the central cell but the paternal genome is required for endosperm development in Arabidopsis. Development. 2010;137:2683–2690. doi: 10.1242/dev.052928. [DOI] [PubMed] [Google Scholar]
- 55.Sun MX, Kranz E, Yang HY, Lorz H, Moscatelli A, Cresti M. Fluorophore-conjugated lectin labeling of the cell surface of isolated male and female gametes, central cells and synergids before and after fertilization in maize. Sex. Plant Reprod. 2002;15:159–166. [Google Scholar]
- 56.Chen SH, Yang YH, Liao JP, Kuang AX, Tian HQ. Isolation of egg cells and zygotes of Torenia fournieri L. and determination of their surface charge. Zygote. 2008;16:179–186. doi: 10.1017/S0967199408004693. [DOI] [PubMed] [Google Scholar]
- 57.Mori T, Igawa T, Tamiya G, Miyagishima SY, Berger F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr. Biol. 2014;24:170–175. doi: 10.1016/j.cub.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 58.Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- 59.Sprunck S, Rademacher S, Vogler F, Gheyselinck J, Grossniklaus U, Dresselhaus T. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science. 2012;338:1093–1097. doi: 10.1126/science.1223944. [DOI] [PubMed] [Google Scholar]
- 60.Rademacher S, Sprunck S. Downregulation of egg cell-secreted EC1 is accompanied with delayed gamete fusion and polytubey. Plant Signal. Behav. 2013;8:e27377. doi: 10.4161/psb.27377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamamura Y, Nishimaki M, Takeuchi H, Geitmann A, Kurihara D, Higashiyama T. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat. Commun. 2014;5:4722. doi: 10.1038/ncomms5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, Wolfner MF. Calcium waves occur as Drosophila oocytes activate. Proc. Natl. Acad. Sci. USA. 2015;112:791–796. doi: 10.1073/pnas.1420589112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Primakoff P, Myles DG. Cell-cell membrane fusion during mammalian fertilization. FEBS Lett. 2007;581:2174–2180. doi: 10.1016/j.febslet.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, Wang H, Wong M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29:427–437. doi: 10.1016/j.tig.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perez-Vargas J, Krey T, Valansi C, Avinoam O, Haouz A, Jamin M, Raveh-Barak H, Podbilewicz B, Rey FA. Structural basis of eukaryotic cell-cell fusion. Cell. 2014;157:407–419. doi: 10.1016/j.cell.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Shilagardi K, Li S, Luo F, Marikar F, Duan R, Jin P, Kim JH, Murnen K, Chen EH. Actin-propelled invasive membrane protrusions promote fusogenic protein engagement during cell-cell fusion. Science. 2013;340:359–363. doi: 10.1126/science.1234781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vigant F, Santos NC, Lee B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015;13:426–437. doi: 10.1038/nrmicro3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Satouh Y, Inoue N, Ikawa M, Okabe M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012;125:4985–4990. doi: 10.1242/jcs.100867. [DOI] [PubMed] [Google Scholar]
- 69.Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 2000;24:279–282. doi: 10.1038/73502. [DOI] [PubMed] [Google Scholar]
- 70.Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- 71.Chalbi M, Barraud-Lange V, Ravaux B, Howan K, Rodriguez N, Soule P, Ndzoudi A, Boucheix C, Rubinstein E, Wolf JP, et al. Binding of sperm protein Izumo1 and its egg receptor Juno drives Cd9 accumulation in the intercellular contact area prior to fusion during mammalian fertilization. Development. 2014;141:3732–3739. doi: 10.1242/dev.111534. [DOI] [PubMed] [Google Scholar]
- 72.Inoue N, Hagihara Y, Wright D, Suzuki T, Wada I. Oocyte-triggered dimerization of sperm IZUMO1 promotes sperm-egg fusion in mice. Nat. Commun. 2015;6:8858. doi: 10.1038/ncomms9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 2005;5:136–148. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 74.Olmos E, Reiss B, Dekker K. The ekeko mutant demonstrates a role for tetraspanin-like protein in plant development. Biochem. Biophys. Res. Commun. 2003;310:1054–1061. doi: 10.1016/j.bbrc.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 75.Wang F, Muto A, Van de Velde J, Neyt P, Himanen K, Vandepoele K, Van Lijsebettens M. Functional analysis of the Arabidopsis TETRASPANIN gene family in plant growth and development. Plant Physiol. 2015;169:2200–2214. doi: 10.1104/pp.15.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boavida LC, Qin P, Broz M, Becker JD, McCormick S. Arabidopsis tetraspanins are confined to discrete expression domains and cell types in reproductive tissues and form homo- and heterodimers when expressed in yeast. Plant Physiol. 2013;163:696–712. doi: 10.1104/pp.113.216598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ikawa M, Inoue N, Benham AM, Okabe M. Fertilization: a sperm's journey to and interaction with the oocyte. J. Clin. Invest. 2010;120:984–994. doi: 10.1172/JCI41585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grzmil P, Kim Y, Shamsadin R, Neesen J, Adham IM, Heinlein UA, Schwarzer UJ, Engel W. Human cyritestin genes (CYRN1 and CYRN2) are non-functional. Biochem. J. 2001;357:551–556. doi: 10.1042/0264-6021:3570551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He ZY, Brakebusch C, Fassler R, Kreidberg JA, Primakoff P, Myles DG. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Dev. Biol. 2003;254:226–237. doi: 10.1016/s0012-1606(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 81.Guo M, Teng M, Niu L, Liu Q, Huang Q, Hao Q. Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K+ channel inhibitor-like fold. J. Biol. Chem. 2005;280:12405–12412. doi: 10.1074/jbc.M413566200. [DOI] [PubMed] [Google Scholar]
- 82.Sprunck S, Baumann U, Edwards K, Langridge P, Dresselhaus T. The transcript composition of egg cells changes significantly following fertilization in wheat (Triticum aestivum L.) Plant J. 2005;41:660–672. doi: 10.1111/j.1365-313X.2005.02332.x. [DOI] [PubMed] [Google Scholar]
- 83.Cohen DJ, Maldera JA, Weigel Munoz M, Ernesto JI, Vasen G, Cuasnicu PS. Cysteine-rich secretory proteins (CRISP) and their role in mammalian fertilization. Biol. Res. 2011;44:135–138. [PubMed] [Google Scholar]
- 84.Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS. Impaired sperm fertilizing ability in mice lacking Cysteine-Rich Secretory Protein 1 (CRISP1) Dev. Biol. 2008;320:12–18. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gibbs GM, Orta G, Reddy T, Koppers AJ, Martinez-Lopez P, de la Vega-Beltran JL, Lo JC, Veldhuis N, Jamsai D, McIntyre P, et al. Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc. Natl. Acad. Sci. USA. 2011;108:7034–7039. doi: 10.1073/pnas.1015935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ernesto JI, Weigel Munoz M, Battistone MA, Vasen G, Martinez-Lopez P, Orta G, Figueiras-Fierro D, De la Vega-Beltran JL, Moreno IA, Guidobaldi HA, et al. CRISP1 as a novel CatSper regulator that modulates sperm motility and orientation during fertilization. J. Cell. Biol. 2015;210:1213–1224. doi: 10.1083/jcb.201412041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sprunck S. Let's get physical: gamete interaction in flowering plants. Biochem. Soc. Trans. 2010;38:635–640. doi: 10.1042/BST0380635. [DOI] [PubMed] [Google Scholar]
- 88.Maruyama D, Völz R, Takeuchi H, Mori T, Igawa T, Kurihara D, Kawashima T, Ueda M, Ito M, Umeda M, et al. Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell. 2015;161:907–918. doi: 10.1016/j.cell.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 89.Sprunck S, Dresselhaus T. Three cell fusions during double fertilization. Cell. 2015;161:708–709. doi: 10.1016/j.cell.2015.04.032. [DOI] [PubMed] [Google Scholar]
- 90.Igawa T, Yanagawa Y, Miyagishima SY, Mori T. Analysis of gamete membrane dynamics during double fertilization of Arabidopsis. J. Plant Res. 2013;126:387–394. doi: 10.1007/s10265-012-0528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang J, Ju Y, Wang X, Zhang Q, Sodmergen A one-step rectification of sperm cell targeting ensures the success of double fertilization. J. Integr. Plant Biol. 2015;57:496–503. doi: 10.1111/jipb.12322. [DOI] [PubMed] [Google Scholar]
- 92.Leshem Y, Johnson C, Wuest SE, Song X, Ngo QA, Grossniklaus U, Sundaresan V. Molecular characterization of the glauce mutant: a central cell-specific function is required for double fertilization in Arabidopsis. Plant Cell. 2012;24:3264–3277. doi: 10.1105/tpc.112.096420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maruyama D, Hamamura Y, Takeuchi H, Susaki D, Nishimaki M, Kurihara D, Kasahara RD, Higashiyama T. Independent control by each female gamete prevents the attraction of multiple pollen tubes. Dev. Cell. 2013;25:317–323. doi: 10.1016/j.devcel.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 94.Wong JL, Johnson MA. Is HAP2-GCS1 an ancestral gamete fusogen? Trends Cell Biol. 2010;20:134–141. doi: 10.1016/j.tcb.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 95.Mori T, Kuroiwa H, Higashiyama T, Kuroiwa T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat. Cell Biol. 2006;8:64–71. doi: 10.1038/ncb1345. [DOI] [PubMed] [Google Scholar]
- 96.von Besser K, Frank AC, Johnson MA, Preuss D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development. 2006;133:4761–4769. doi: 10.1242/dev.02683. [DOI] [PubMed] [Google Scholar]
- 97.Nagahara S, Takeuchi H, Higashiyama T. Generation of a homozygous fertilization-defective gcs1 mutant by heat-inducible removal of a rescue gene. Plant Reprod. 2015;28:33–46. doi: 10.1007/s00497-015-0256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mori T, Kawai-Toyooka H, Igawa T, Nozaki H. Gamete dialogs in green lineages. Mol. Plant. 2015;8:1442–1454. doi: 10.1016/j.molp.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirai M, Arai M, Mori T, Miyagishima SY, Kawai S, Kita K, Kuroiwa T, Terenius O, Matsuoka H. Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr. Biol. 2008;18:607–613. doi: 10.1016/j.cub.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 101.Ebchuqin E, Yokota N, Yamada L, Yasuoka Y, Akasaka M, Arakawa M, Deguchi R, Mori T, Sawada H. Evidence for participation of GCS1 in fertilization of the starlet sea anemone Nematostella vectensis: implication of a common mechanism of sperm-egg fusion in plants and animals. Biochem. Biophys. Res. Commun. 2014;451:522–528. doi: 10.1016/j.bbrc.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 102.Cole ES, Cassidy-Hanley D, Fricke Pinello J, Zeng H, Hsueh M, Kolbin D, Ozzello C, Giddings T, Jr, Winey M, Clark TG. Function of the male-gamete-specific fusion protein HAP2 in a seven-sexed ciliate. Curr. Biol. 2014;24:2168–2173. doi: 10.1016/j.cub.2014.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong JL, Leydon AR, Johnson MA. HAP2(GCS1)-dependent gamete fusion requires a positively charged carboxy-terminal domain. PLoS Genet. 2010;6:e1000882. doi: 10.1371/journal.pgen.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mori T, Hirai M, Kuroiwa T, Miyagishima SY. The functional domain of GCS1-based gamete fusion resides in the amino terminus in plant and parasite species. PLoS One. 2010;5:e15957. doi: 10.1371/journal.pone.0015957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hertwig O. Beiträge zur Kenntniss der Bildung, Befruchtung und Theilung des thierischen Eies. Morphology. 1876;1:347–452. [Google Scholar]
- 106.Schatten H, Schatten G, Mazia D, Balczon R, Simerly C. Behavior of centrosomes during fertilization and cell division in mouse oocytes and in sea urchin eggs. Proc. Natl. Acad. Sci. USA. 1986;83:105–109. doi: 10.1073/pnas.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Longo FJ, Anderson E. The fine structure of pronuclear development and fusion in the sea urchin, Arbacia punctulata . J. Cell Biol. 1968;39:339–368. doi: 10.1083/jcb.39.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rouviere C, Houliston E, Carre D, Chang P, Sardet C. Characteristics of pronuclear migration in Beroe ovata. Cell Motil. Cytoskeleton. 1994;29:301–311. doi: 10.1002/cm.970290403. [DOI] [PubMed] [Google Scholar]
- 109.Hemmings N, Birkhead TR. Polyspermy in birds: sperm numbers and embryo survival. Proc. Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Snook RR, Hosken DJ, Karr TL. The biology and evolution of polyspermy: insights from cellular and functional studies of sperm and centrosomal behavior in the fertilized egg. Reproduction. 2011;142:779–792. doi: 10.1530/REP-11-0255. [DOI] [PubMed] [Google Scholar]
- 111.Reinsch S, Gonczy P. Mechanisms of nuclear positioning. J. Cell Sci. 1998;111(Pt 16):2283–2295. doi: 10.1242/jcs.111.16.2283. [DOI] [PubMed] [Google Scholar]
- 112.Kawashima T, Maruyama D, Shagirov M, Li J, Hamamura Y, Yelagandula R, Toyama Y, Berger F. Dynamic F-actin movement is essential for fertilization in Arabidopsis thaliana . Elife. 2014;3:e04501. doi: 10.7554/eLife.04501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kawashima T, Berger F. The central cell nuclear position at the micropylar end is maintained by the balance of F-actin dynamics, but dispensable for karyogamy in Arabidopsis. Plant Reprod. 2015;28:103–110. doi: 10.1007/s00497-015-0259-1. [DOI] [PubMed] [Google Scholar]
- 114.Sun QY, Schatten H. Regulation of dynamic events by microfilaments during oocyte maturation and fertilization. Reproduction. 2006;131:193–205. doi: 10.1530/rep.1.00847. [DOI] [PubMed] [Google Scholar]
- 115.Faure JE, Mogensen HL, Dumas C, Lörz H, Kranz E. Karyogamy after electrofusion of single egg and sperm cell protoplasts from maize: Cytological evidence and time course. Plant Cell. 1993;5:747–755. doi: 10.1105/tpc.5.7.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ohnishi Y, Hoshino R, Okamoto T. Dynamics of male and female chromatin during karyogamy in rice zygotes. Plant Physiol. 2014;165:1533–1543. doi: 10.1104/pp.114.236059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Engel ML, Holmes-Davis R, McCormick S. Green sperm. Identification of male gamete promoters in Arabidopsis. Plant Physiol. 2005;138:2124–2133. doi: 10.1104/pp.104.054213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alandete-Saez M, Ron M, Leiboff S, McCormick S. Arabidopsis thaliana GEX1 has dual functions in gametophyte development and early embryogenesis. Plant J. 2011;68:620–632. doi: 10.1111/j.1365-313X.2011.04713.x. [DOI] [PubMed] [Google Scholar]
- 119.Ning J, Otto TD, Pfander C, Schwach F, Brochet M, Bushell E, Goulding D, Sanders M, Lefebvre PA, Pei J, et al. Comparative genomics in Chlamydomonas and Plasmodium identifies an ancient nuclear envelope protein family essential for sexual reproduction in protists, fungi, plants, and vertebrates. Genes Dev. 2013;27:1198–1215. doi: 10.1101/gad.212746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pitcher TE, Dunn PO, Whittingham LA. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 2005;18:557–567. doi: 10.1111/j.1420-9101.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 121.Friedman J, Barrett SCH. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals Bot. 2009;103:1515–1527. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vogel SS, Delaney K, Zimmerberg J. The sea urchin cortical reaction. A model system for studying the final steps of calcium-triggered vesicle fusion. Ann. NY Acad. Sci. 1991;635:35–44. doi: 10.1111/j.1749-6632.1991.tb36479.x. [DOI] [PubMed] [Google Scholar]
- 123.Yashiro T, Matsuura K. Termite queens close the sperm gates of eggs to switch from sexual to asexual reproduction. Proc. Natl. Acad. Sci. USA. 2014;111:17212–17217. doi: 10.1073/pnas.1412481111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beale KM, Leydon AR, Johnson MA. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr. Biol. 2012;22:1090–1094. doi: 10.1016/j.cub.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abbott AL, Ducibella T. Calcium and the control of mammalian cortical granule exocytosis. Front. Biosci. 2001;6:D792–D806. doi: 10.2741/abbott. [DOI] [PubMed] [Google Scholar]
- 126.Kashir J, Nomikos M, Lai FA, Swann K. Sperm-induced Ca2+ release during egg activation in mammals. Biochem. Biophys. Res. Commun. 2014;450:1204–1211. doi: 10.1016/j.bbrc.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 127.Webb SE, Miller AL. Ca(2+) signaling during activation and fertilization in the eggs of teleost fish. Cell Calcium. 2013;53:24–31. doi: 10.1016/j.ceca.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 128.Stricker SA. Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev. Biol. 1999;211:157–176. doi: 10.1006/dbio.1999.9340. [DOI] [PubMed] [Google Scholar]
- 129.Kranz E, Wiegen P, Lörz H. Early cytological events after induction of cell division in egg cells and zygote development following in vitro fertilization with angiosperm gametes. Plant J. 1995;8:9–23. [Google Scholar]
- 130.Bosch M, Hepler PK. Pectin methylesterases and pectin dynamics in pollen tubes. Plant Cell. 2005;17:3219–3226. doi: 10.1105/tpc.105.037473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scott RJ, Armstrong SJ, Doughty J, Spielman M. Double fertilization in Arabidopsis thaliana involves a polyspermy block on the egg but not the central cell. Mol. Plant. 2008;1:611–619. doi: 10.1093/mp/ssn016. [DOI] [PubMed] [Google Scholar]
- 132.Kasahara RD, Maruyama D, Hamamura Y, Sakakibara T, Twell D, Higashiyama T. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr. Biol. 2012;22:1084–1089. doi: 10.1016/j.cub.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 133.Dresselhaus T, Sprunck S. Plant fertilization: maximizing reproductive success. Curr. Biol. 2012;22:R487–R489. doi: 10.1016/j.cub.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 134.Lausser A, Kliwer I, Srilunchang KO, Dresselhaus T. Sporophytic control of pollen tube growth and guidance in maize. J. Exp. Bot. 2010;61:673–682. doi: 10.1093/jxb/erp330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Völz R, Heydlauff J, Ripper D, von Lyncker L, Gross-Hardt R. Ethylene signaling is required for synergid degeneration and the establishment of a pollen tube block. Dev. Cell. 2013;25:310–316. doi: 10.1016/j.devcel.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 136.Narciandi F, Lloyd A, Meade KG, O'Farrelly C. A novel subclass of bovine beta-defensins links reproduction and immunology. Reprod. Fertil. Dev. 2014;26:769–777. doi: 10.1071/RD13153. [DOI] [PubMed] [Google Scholar]
- 137.Rubinstein E, Ziyyat A, Prenant M, Wrobel E, Wolf JP, Levy S, Le Naour F, Boucheix C. Reduced fertility of female mice lacking CD81. Dev. Biol. 2006;290:351–358. doi: 10.1016/j.ydbio.2005.11.031. [DOI] [PubMed] [Google Scholar]