Abstract
♦ Background:
In general, efforts to standardize care based on group consensus practice guidelines have resulted in lower morbidity and mortality. Although there are published guidelines regarding insertion and perioperative management of peritoneal dialysis (PD) catheters, variation in practice patterns between centers may exist. The objective of this study is to understand variation in PD catheter insertion practices in preparation for conducting future studies.
♦ Methods:
An electronic survey was developed by the research committee of the International Society for Peritoneal Dialysis – North American Research Consortium (ISPD-NARC) to be completed by physicians and nurses involved in PD programs across North America. It consisted of 45 questions related to 1) organizational characteristics; 2) PD catheter insertion practices; 3) current quality-improvement initiatives; and 4) interest in participation in PD studies. Invitation to participate in the survey was given to nephrologists and nurses in centers across Canada and the United States (US) identified by participation in the inaugural meeting of the ISPD-NARC. Descriptive statistics were applied to analyze the data.
♦ Results:
Fifty-one ISPD-NARC sites were identified (45% in Canada and 55% in the US) of which 42 responded (82%). Center size varied significantly, with prevalent PD population ranging from 6 – 300 (median: 60) and incident PD patients in the year prior to survey administration ranging from 3 – 180 (median: 20). The majority of centers placed fewer than 19 PD catheters/year, with a range of 0 – 50. Availability of insertion techniques varied significantly, with 83% of centers employing more than 1 insertion technique. Seventy-one percent performed laparoscopic insertion with advanced techniques (omentectomy, omentopexy, and lysis of adhesions), 62% of sites performed open surgical dissection, 10% performed blind insertion via trocar, and 29% performed blind placement with the Seldinger technique. Use of double-cuff catheters was nearly universal, with a near even distribution of catheters with pre-formed bend versus straight inter-cuff segments. There was also variation in the choice of perioperative antibiotics and perioperative flushing practices. Although 86% of centers had quality-improvement initiatives, there was little consensus as to appropriate targets.
♦ Conclusions:
There is marked variability in PD catheter insertion techniques and perioperative management. Large multicenter studies are needed to determine associations between these practices and catheter and patient outcomes. This research could inform future trials and guidelines and improve practice. The ISPD-NARC is a network of PD units that has been formed to conduct multicenter studies in PD.
Keywords: Peritoneal dialysis, peritoneal dialysis catheter, peritoneal dialysis access, Tenckhoff catheter
There are initiatives in the United States and elsewhere aimed at maximizing the use of peritoneal dialysis (PD). Peritoneal dialysis is promoted because it offers comparable clinical outcomes to hemodialysis but is much less expensive to provide (1–3). However, there is a lack of consensus regarding the optimal care of PD patients and a lack of evidence on which to base clinical guidelines. As a consequence, the development of quality measures for PD care has lagged behind other therapies.
The International Society of Peritoneal Dialysis – North American Chapter formed the North American Research Consortium (ISPD-NARC) to address knowledge gaps and improve PD practice. The ISPD-NARC selected PD catheter practices and outcomes as an initial area of focus. Safe, reliable access to the peritoneal cavity is a cornerstone of successful PD therapy, and while the ISPD has provided practice guidelines, the recommendations are largely based on low-quality evidence and do not appear to have been adopted in everyday practice (4).
We surveyed sites participating in the ISPD-NARC to describe current PD catheter practices and characterize variability in practice. This information will focus research agenda and facilitate the design of future prospective studies.
Methods
Settings and Participants
The PD Catheter Practices Survey was developed by the Research Committee of the ISPD-NARC. A total of 51 potential sites were identified both by participation at the inaugural meeting of the ISPD-NARC and through self-identification. Sites included academic and non-academic sites, private and not-for-profit sites, and urban and community sites in Canada and the United States (US).
Survey Instrument
The PD Catheter Practices Survey consisted of 45 questions organized into 4 sections: 1) organizational characteristics; 2) PD catheter insertion practices; 3) current quality-improvement initiatives; and 4) interest in participation in future studies regarding PD. The survey was pilot-tested and then distributed in an email that contained an electronic link (SurveyMonkey, Palo Alto, California) (5). To maximize the response rate, email reminders to nonresponders were sent in August and September 2014. Responses were reviewed for completeness and consistency. Site investigators were contacted to clarify any missing or inconsistent data.
Statistical Analysis
Continuous variables are presented with their mean and standard deviation, median, and interquartile range as appropriate. Categorical variables are presented with number and percent prevalence. All statistics for this survey were descriptive.
Results
Organizational Characteristics
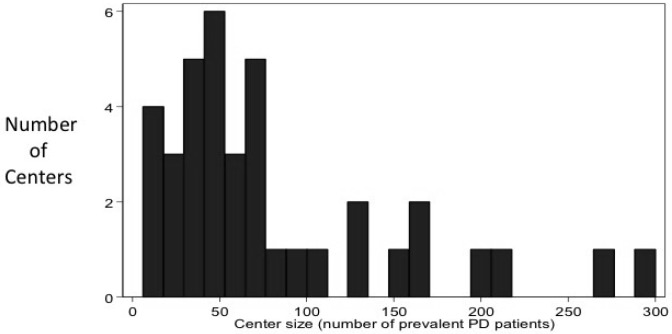
Of the 51 centers invited to participate, 82% completed the survey. Canadian centers accounted for 45% of the respondents while centers from the United States accounted for 55% (Figure 1). The majority of centers were academic centers (62%). Prevalent total dialysis patient population ranged from 7 to 1,500 (median: 317) while the prevalent PD population ranged from 6 – 300 (median: 60) (Figure 2). The number of incident PD patients in the year prior to survey administration varied from 3 – 180 (median: 20). Seventy-six percent of centers performed the majority of PD catheter insertions, and 93% provided PD catheter care following insertion.
Figure 1 —
Geographical representation of centers participating in the survey. Black dots represent the city in which the center is located. Some points may represent more than 1 center.
Figure 2 —
Distribution of the size of centers by prevalent PD patients. The median number of patients was 60. PD = peritoneal dialysis.
The number of nephrologists caring for PD patients ranged from 2 – 22 (mean: 6) indicating that some centers concentrated care between relatively few physicians, while others distributed care among most physicians in the practice. The frequency of routine follow-up of PD patients varied among centers: 62% followed patients monthly, 2.4% of sites saw patients every 6 weeks, 11.9% every 2 months, and 24% every 3 months. However, 100% of US sites saw patients monthly while in Canada, 16% saw patients every month, 5% every 6 weeks, 26% every 2 months, and 53% every 3 months.
Peritoneal Dialysis Catheter Insertion Practices
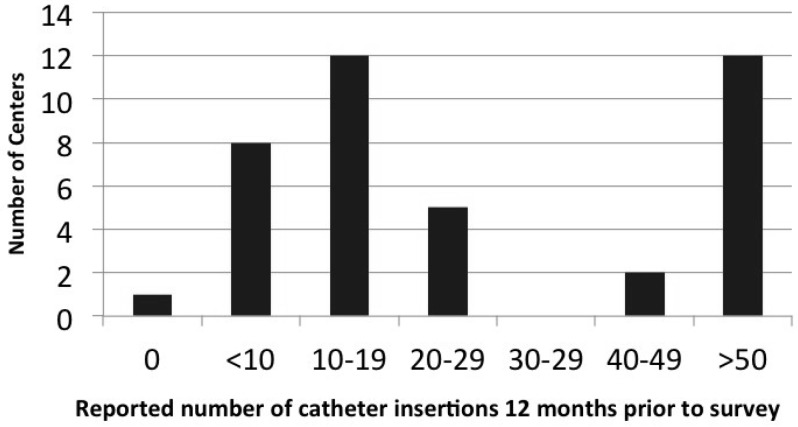
Peritoneal dialysis catheter insertion experience and practices varied considerably. The number of PD catheter insertions performed ranged from 0 to more than 50 per year, but the majority of centers placed fewer than 19 catheters per year (Figure 3).
Figure 3 —
Distribution of the centers by the number of PD catheter insertions in the 12 months prior to survey administration. PD = peritoneal dialysis.
In terms of the different techniques available for placement of PD catheters at each center, 71% had the availability of laparoscopic insertion with advanced techniques (omentectomy, omentopexy, lysis of adhesions), 62% of sites had open surgical dissection, 10% had blind insertion via trocar, 29% had blind placement with the Seldinger technique, and 80% of sites had at least 3 of these techniques available. At most centers (60%), general surgeons placed PD catheters. Nephrologists, interventional radiologists, and vascular surgeons each were available at 20% of sites, while rarely, urology and transplant surgeons were used for placing PD access. Buried catheters, upper abdominal catheters, and pre-sternal catheters were available in 36%, 43%, and 41% of sites, respectively.
Nearly all (95%) sites utilized double-cuff catheters. Fifty-six percent of sites preferred catheters that had a pre-formed bend in the inter-cuff segment, while 44% used a straight inter-cuff segment (44%). Entry-site preference varied as well. The majority of sites (81%) routinely used a downward facing exit site.
Perioperative and Postoperative Management
Pre-surgical care varied across sites. Only 58% of centers marked the PD catheter exit site in both a sitting and standing position pre-operatively. Sixty-six percent of centers routinely prescribed laxatives prior to surgery. There was near uniformity (98%) in the routine administration of prophylactic antibiotics prior to PD catheter insertion. Prophylactic antibiotic choices at the time of surgery also varied significantly: 78% of centers used cefazolin; 12% of centers used vancomycin; 10% used cefazolin and an aminoglycoside.
Post-operative care was more consistent across centers. Over 80% of sites used a non-occlusive dressing post-operatively, immobilized the catheter, and reported dressing changes done using sterile technique by a nurse. Likewise, over 80% of centers instructed patients not to shower or bathe for at least 1 week or do heavy lifting.
However, flushing practices varied among centers. Most centers (76%) flushed catheters weekly, 7% of centers flushed once after placement, 2% flushed catheters only twice, 2% flushed thrice weekly, and 2 % flushed biweekly. Some centers (10%) did not flush catheters at all. Eight percent of these were due to either using buried catheters exclusively or using the majority of catheters within 2 weeks of placement. In centers that flushed catheters post-operatively, 20% flushed with 0.9% NaCl while 81% flushed with PD solution. Two centers reported using heparinized 0.9% NaCl.
Current Quality-Improvement Initiatives
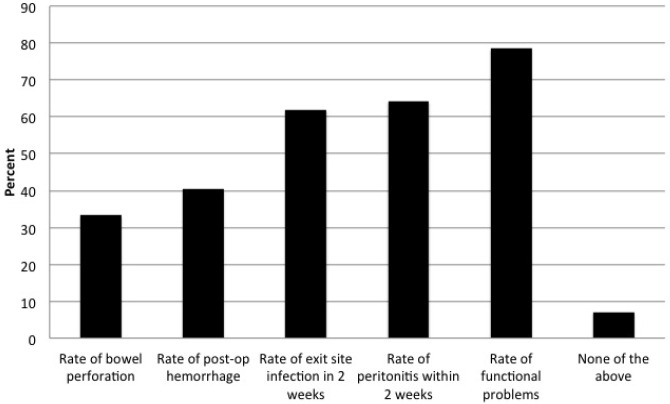
Of note, 86% of centers had quality-improvement initiatives in place at their center (Figure 4). This was similar among Canadian and US sites. Of these sites, the majority tracked rates of exit-site infection following PD catheter insertion (62%), peritonitis (64%), and functional problems leading to the need for manipulation or replacement or to technique failure (79%). When asked about acceptable rates of PD catheter non-function (a PD catheter never being able to be used for PD), there was little consensus as to an appropriate target. Twenty-five percent of centers felt that < 1% was acceptable, 42% thought 5% was acceptable, 32% believed 10% was acceptable, and 2% thought that a 15% non-function rate was acceptable. The 10 units who chose < 1% of catheter non-function as an acceptable target relied solely on surgeons to place catheters, with 80% of these units having the availability of laparoscopic surgery with advanced techniques. In contrast, of the 31 units willing to accept 5% or higher rates of non-function, 26% had nephrologists and 26% had interventional radiologists as available operators to place catheters.
Figure 4 —
Percentage of centers polled having a current quality-improvement initiative related to the shown PD catheter problems. As shown, 86% of centers had current quality-improvement initiatives. PD = peritoneal dialysis.
Discussion
We conducted a detailed survey of PD catheter practices in sites participating in the ISPD-NARC in order to identify potential targets for future prospective studies and to facilitate planning. The availability of multiple insertion techniques, the use of double-cuff catheters, and most postoperative practices were fairly consistent across sites. However, substantial variation in practice was found in the number of patients cared for, volume of PD catheters placed on an annual basis, perioperative care, and acceptable rates of PD catheter non-function.
Center size and the volume of catheters placed per year can affect outcomes, with larger units having been shown to be associated with improved mortality and technique survival (6–10). We found large variation in both the size of units and the number of catheters placed per year. Although small units may have difficulty maintaining proficiency, very large units may have difficulties managing details of patient care. Just as size of units may affect patient outcomes, the number of PD catheters placed in a year may also affect catheter outcomes. Most centers placed fewer than 19 PD catheters a year. Many specialties have adopted minimum procedural numbers needed to maintain proficiency. This is largely due to research showing improved outcomes of surgical procedures with high volumes, as well as simulation data showing loss of procedural skill without repetitive use or training (11–12). Whether the volume of PD catheters placed per year by surgeons, radiologists, or nephrologists is associated with catheter outcomes remains to be determined.
Most sites used double-cuff catheters, but we observed heterogeneity in method of PD catheter insertion and the configuration of the catheter used. The most commonly available PD catheter insertion techniques were both laparoscopic and open surgical, while almost 30% of sites placed catheters using a blind Seldinger technique. Only 4.8% of units had only 1 technique available to place PD catheters. This appears to be in contrast to a report from the 2013 United Kingdom Renal Registry, where 26% of units relied on only 1 technique to place PD catheters (13). The number of available techniques, however, may not correlate with the number of techniques actually used at a center. While there have been single-center studies comparing different insertion techniques, there are insufficient data to recommend one approach over another (14,15). The even split between a preference for straight vs preformed, bent inter-cuff segments in our sample likely reflects local preferences as well as a lack of clear benefit to straight or Swan-neck catheters over the others (16,17). The availability of buried catheters was limited to only 36% of centers, with 2 sites using buried catheters exclusively, despite data showing no clear benefit to this approach (18).
Interestingly, there appeared to be variation in perioperative care in terms of prophylactic antibiotics, frequency of catheter flushing after catheter placement, and the solution used for flushing. Most centers used cefazolin for preinsertion prophylaxis, while very few used vancomycin. Gaddallah et al. showed that single-dose vancomycin was superior to cefazolin in preventing postoperative peritonitis in a single-center randomized controlled trial (RCT) (19). However, application of this to multiple centers where microbiology and resistance patterns may vary remains to be determined. Furthermore, some centers may restrict the routine use of vancomycin for prophylaxis due to concerns for development of vancomycin-resistant infections (20). Similarly, there appears to be no consensus as to the frequency of flushing or what solution to use when flushing PD catheters following placement. There are no RCTs evaluating flushing practices and the impact on PD catheter outcomes and rates of complications, such as iatrogenic peritonitis.
It was reassuring that quality-improvement programs exist in most of the centers surveyed. Quality-improvement strategies have been shown to improve PD outcomes and are recommended by the ISPD (21,22). However, more direction regarding the measurement and reporting of quality-improvement metrics, as well as reasonable targets for these metrics are needed. For example, there was little to no agreement on the expected or acceptable rate of primary PD catheter non-function. Clinical guidelines have suggested that greater than 80% catheter survival at 1 year is a reasonable target (20,23). However, targets inferred from single-center studies done by surgeons dedicated to PD catheter placement may not be attainable targets for the average PD unit. There is a need to establish benchmark targets informed by data collected across the spectrum of PD practices and incorporate them into the quality-improvement initiatives in PD programs.
Our study has limitations. First, this is not a true cross-section of PD programs but a convenience sample of units dedicated to PD and may not accurately reflect practice in other sites. Second, this study did not observe practice at the participating sites, but relied on self-reported answers to questions regarding practice, which may reduce the accuracy of responses to certain questions and may not predict how an individual patient is treated.
In conclusion, our study shows wide variation in practice with regard to PD catheter placement and perioperative management in Canada and the US. It is interesting that such variation in practice patterns exists, even within this self-selected sample of units dedicated to improving PD quality, and who therefore presumably are familiar with the most recent literature. This variation may reflect knowledge gaps, the availability of resources locally, or other factors. It is unclear if this variation in practice is associated with differences in outcomes as it is possible that different practices may provide similar and acceptable outcomes. Future studies of the ISPD-NARC should be aimed at prospectively evaluating catheter insertion and perioperative practices on catheter outcomes. The ISPD-NARC is hopeful that it can sustain a coalition of PD programs to complete such future observational and interventional trials in an effort to improve the care of PD patients.
Disclosures
ELW, RBF, PGB, AML, TAG, and RRQ have nothing to disclose. MJO is the inventor of the Dialysis Measurement Analysis and Reporting (DMAR) quality-improvement system and had support for a new ISPD – North American Chapter PD Catheter Quality Improvement Registry from Baxter Corporation.
Acknowledgments
The authors would like to acknowledge the contributions of both Baxter Healthcare Corporation and the ISPD for their support.
REFERENCES
- 1. Perl J, Wald R, McFarlane P, Bargman JM, Vonesh E, Na Y, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival. J Am Soc Nephrol 2011; 22:1113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 2011; 171:110–8. [DOI] [PubMed] [Google Scholar]
- 3. USRDS 2012 Atlas of CKD and ESRD. Volume 2, Chapter 11, Figure 11.7–Total Medicare ESRD expenditures per person per year, by modality: 332 Available at http://www.usrds.org/atlas.aspx.
- 4. Figueiredo A, Goh B-L, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30(4):424–9. [DOI] [PubMed] [Google Scholar]
- 5. SurveyMonkey Inc www.surveymonkey.com Palo Alto, California, USA. [Google Scholar]
- 6. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009; 29(3):292–6. [PubMed] [Google Scholar]
- 7. Huisman RM, Nieuwenhuizen MGM, de Charro FT. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in The Netherlands. Nephrol Dial Transplant 2002; 17(9):1655–60. [DOI] [PubMed] [Google Scholar]
- 8. Mehrotra R, Khawar O, Duong U, Fried L, Norris K, Nissenson A, et al. Ownership patterns of dialysis units and peritoneal dialysis in the United States: utilization and outcomes. Am J Kidney Dis 2009; 54(2):289–98. [DOI] [PubMed] [Google Scholar]
- 9. Mehrotra R, Chiu Y, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int 2009; 76:97–107. [DOI] [PubMed] [Google Scholar]
- 10. Schaubel D, Blake P, Fenton S. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60:1517–24. [DOI] [PubMed] [Google Scholar]
- 11. Birkmeyer JD, Siewers AE, Finlayson EVA, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; 346(15):1128–37. [DOI] [PubMed] [Google Scholar]
- 12. Stefanidis D, Korndorffer JR, Sierra R, Touchard C, Dunne JB, Scott DJ. Skill retention following proficiency-based laparoscopic simulator training. Surgery 2005; 138(2):165–70. [DOI] [PubMed] [Google Scholar]
- 13. Rao A, Pitcher D, Fluck R, Kumwenda M. UK Renal Registry 17th Annual Report: Chapter 10. 2013 Multisite dialysis access audit in England, Northern Ireland and Wales and 2012 PD one year follow-up: national and centre-specific analyses. Nephron 2015; 129(Suppl 1):223–45. [DOI] [PubMed] [Google Scholar]
- 14. Crabtree JH. Fluoroscopic placement of peritoneal dialysis catheters: a harvest of the low-hanging fruits. Perit Dial Int 2008; 28(2):134–7. [PubMed] [Google Scholar]
- 15. CARI : Technique of insertion of peritoneal dialysis catheter. Available at http://www.cari.org.au.
- 16. CARI : Type of peritoneal dialysis catheter. Available at http://www.cari.org.au.
- 17. Lo W-K. Peritoneal dialysis utilization and outcome: what are we facing? Perit Dial Int 2007; 27(Suppl 2):S42–7. [PubMed] [Google Scholar]
- 18. Danielsson A, Blohme L, Tranaeus A, Hylander B. A prospective randomized study of the effect of a subcutaneously “buried” peritoneal dialysis catheter technique versus standard technique on the incidence of peritonitis and exit-site infection. Perit Dial Int 2002; 22(2):211–19. [PubMed] [Google Scholar]
- 19. Gadallah MF, Ramdeen G, Mignone J, Patel D, Mitchell L, Tatro S. Role of preoperative antibiotic prophylaxis in preventing postoperative peritonitis in newly placed peritoneal dialysis catheters. Am J Kidney Dis 2000; 36(5):1014–9. [DOI] [PubMed] [Google Scholar]
- 20. Flanigan M, Gokal R. Peritoneal catheters and exit-site practices toward optimum peritoneal access: A review of current developments. Perit Dial Int 2005; 25(2):132–9. [PubMed] [Google Scholar]
- 21. Ersoy FF. Improving technique survival in peritoneal dialysis: what is modifiable? Perit Dial Int 2009; 29(Suppl 2):S74–7. [PubMed] [Google Scholar]
- 22. Yu Y, Zhou Y, Wang H, Zhou T, Li Q, Li T, et al. Impact of continuous quality improvement initiatives on clinical outcomes in peritoneal dialysis. Perit Dial Int 2014; 34(Suppl 2):S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Figueiredo A, Goh B, Jenkins S, Johnson D, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30(4):424–9. [DOI] [PubMed] [Google Scholar]