Abstract
♦ Introduction:
Intraperitoneal tigecycline is a potential option for the treatment of peritoneal dialysis (PD)-associated peritonitis caused by microorganisms resistant to commonly used antibiotics. However, the stability of tigecycline must be assessed in the PD solution before evaluating its safety and therapeutic efficacy in PD-associated peritonitis. The objective of this study was to investigate the stability of tigecycline in 3 types of PD solutions at different temperatures for various time points.
♦ Methods:
A total of 27 PD bags (9 PD bags for each type of PD solution; 1.5% glucose, 7.5% icodextrin, and 1.5% glucose pH neutral) containing 2 μg/mL of tigecycline were prepared and stored at either 4, 25, or 37°C. An aliquot was withdrawn immediately before (0 hour) and after pre-defined time points. Each sample was analyzed in duplicate for the concentration of tigecycline using a stability-indicating high-performance liquid chromatography (HPLC) technique. Samples were also assessed for pH, color changes, and evidence of precipitation immediately after preparation and on each day of analysis.
♦ Results:
Tigecycline in all 3 types of PD solutions retained more than 90% of its initial concentration for at least 216, 72, and 8 hours at 4, 25, and 37°C, respectively. There was no evidence of precipitation at any time under the tested storage conditions. The pH and color of tigecycline admixed PD solutions stored at 4, 25, and 37°C remained essentially unchanged for 336, 96, and 48 hours respectively.
♦ Conclusion:
The results obtained from the study provide a platform for future clinical studies aiming to determine the safety and therapeutic efficacy of intraperitoneally administered tigecycline for the treatment of PD-associated peritonitis.
Keywords: Tigecycline, glucose, icodextrin, pH neutral, peritoneal dialysis, stability, high-performance liquid chromatography
Peritoneal dialysis (PD)-associated bacterial peritonitis represents one of the most detrimental complications of peritonitis (1). Peritoneal dialysis-associated peritonitis is not only difficult to treat but often leads to removal of the dialysis catheter and in some cases necessitates shifting patients to hemodialysis (1–3). Increasing rates of recurrence, treatment failure, and relapse are a growing concern in PD patients (2,4). Around 14% of all PD-associated peritonitis reported to the Australia and New Zealand (ANZ) registry from 2003 to 2007 were relapsed peritonitis, whereas around 6% of all cases were recurrent peritonitis (4). Similar rates of recurrences have been reported in Scotland, where the national incidence of recurrent peritonitis was around 10% over an 8-year study period (2). Although gram-positive bacteria have been the most common microbial etiology of PD-associated peritonitis, recent studies have observed a rise in gram-negative bacteria (1,2,5). Around 10% of all PD-associated peritonitis cases reported to the ANZ registry were polymicrobial, with a combination of gram-negative and gram-positive bacterial infection being the most common (6). Polymicrobial peritonitis not only resulted in higher hospitalization rates but was also associated with higher rates of catheter removal, transfer of patients to hemodialysis, and mortality compared with non-polymicrobial cases (6).
Irrespective of bacterial etiology, a major emerging concern is the increasing resistance of bacteria to first-line antibiotics (3,7–9). A single-centered 10-year observational study of Staphylococcal-associated peritonitis found that more than 25% of patients failed to achieve a complete cure (8). Similarly, another study reported that more than 40% of patients with Enterobacteriaceae-associated peritonitis failed to achieve a complete cure (9). This increasing trend of resistance has translated into an increased use of broad-spectrum antibiotics as an empiric choice for PD-associated peritonitis (10). This practice has increased the incidence of resistant gram-positive and gram-negative bacterial infections. For example, vancomycin-resistant enterococci (VRE)- and extended-spectrum beta lactamases (ESBL)-producing gram-negative bacteria are now resistant to commonly used antibiotics such as vancomycin, ceftazidime, and gentamicin (9,11).
Tigecycline is the first drug in the glycylcycline class of antibiotics, which resembles tetracyclines (12). Tigecycline has been successfully used to treat complicated intra-abdominal infections including infections caused by VRE- and ESBL-producing gram-negative bacteria (13,14). Limited reports are available on the usefulness of intravenously administered tigecycline for the treatment of PD-associated peritonitis (15,16). Given the lower cure rates of PD-associated peritonitis (6,8,9), together with significant morphological changes limiting the penetration of antibiotics in the peritoneal cavity via the systemic route (17), intraperitoneal administration of tigecycline can ensure rapid attainment of antibacterial concentration in peritoneal fluid, maximizing its therapeutic effect. In intraperitoneal-based treatment, an antibiotic is added to the PD solution before its intraperitoneal administration. However, clinicians need to know the stability of tigecycline when admixed with the PD solution before it can be used intraperitoneally for the treatment of PD-associated peritonitis. Failure to know the stability in PD solution can result in the administration of possible degradation products of tigecycline as well as sub-therapeutic dosing. To the best of the authors' knowledge, no study has investigated the stability of tigecycline in commercially available PD solutions. Therefore, the aim of this study was to investigate the physical and chemical stability of tigecycline in 3 types of PD solutions at different temperatures for various time points.
Materials and Methods
Sample Preparation
Tigecycline powder for injection (50 mg; Pfizer Pty Ltd, New South Wales, Australia) was reconstituted with 5 mL of water for injection BP to obtain 10 mg/mL of tigecycline solution. A suitable volume of the reconstituted solution was injected either into the non-glucose compartment (1.25 L) of the 1.5% glucose Balance (Fresenius Medical Care, Sydney, New South Wales, Australia) 2-compartment pH neutral PD bag (2.5 L), into the 1.5% glucose-based Dianeal (Baxter Pty Ltd, New South Wales, Australia) 1-compartment PD-4 bag (2 L), or into the 7.5% icodextrin-based Extraneal (Baxter Pty Ltd, New South Wales, Australia) 1-compartment PD bag (2 L) to obtain a final tigecycline concentration of 4 μg/mL in the non-glucose compartment of the Balance and 2 μg/mL in the Dianeal and Extraneal PD bags. This concentration was chosen because the minimum inhibitory concentration (MIC) for tigecycline is reported to be less than 1 μg/mL for all susceptible bacteria (18). After addition of tigecycline, each PD bag was shaken for approximately 1 minute to ensure proper mixing. A total of 27 PD bags (9 PD bags for each type of PD solution) were prepared. Three bags of each type were stored at 3 different temperatures: 4°C in a domestic refrigerator; 25°C (room temperature) in an incubator, and 37°C (body temperature) in an incubator. In clinical practice, immediately before the instillation of solution present in a dual compartment PD bag, the glucose-based solution from 1 compartment is mixed with the non-glucose solution present in another compartment. Then the mixed solution is warmed to 37°C on a heating plate. Therefore, immediately prior to the storage of a pH-neutral PD bag at 37°C, the glucose-based solution (1.25 L, pH 3.0, 0 μg/mL of tigecycline) from 1 compartment was mixed with the non-glucose (buffer) solution (1.25 L, pH 8.0, 4 μg/mL of tigecycline) present in another compartment. Control samples (n = 3) were also prepared in a similar way except that tigecycline was omitted. An aliquot (1.5 mL) from each PD bag was withdrawn and the concentration of tigecycline before (0 hour) and after storage was determined using stability-indicating high-performance liquid chromatography (HPLC). All samples stored at 4°C were analyzed at 0, 24, 48, 72, 168, 216, 240, and 336 hours. Samples stored at 25°C were analyzed at 0, 8, 12, 24, 48, 72, and 96 hours, and those stored at 37 °C were analyzed at 0, 8, 12, 24, and 48 hours. Baseline samples (0 hour) were kept at room temperature and analyzed within 30 minutes of their preparation. Samples were also assessed for pH (with a pH meter), color change and evidence of precipitation (visually) immediately after preparation and on each day of analysis.
HPLC Instrumentation
Chromatographic analyses were performed on a HPLC system (Thermo Fisher Scientific, New South Wales, Australia) consisting of a HPG-3400RS binary separation pump, WPS-3000TRS auto-sampler, TCC-3000RS column thermal compartment and VWD-3000RS detector. Instrument control and data acquisition were performed using Chromeleon (Thermo Fisher Scientific, Sunnyvale, CA, USA) software.
HPLC Assay
HPLC separation of tigecycline was performed using a Kinetex 5μ C18 column (100 mm × 4.6 mm × 2.6 μm) with a 100-Å pore size (Phenomenex, Victoria, Australia). The mobile phase was composed of 0.1% trifluoroacetic acid (TFA) in Milli-Q water (mobile phase A) and 0.1% TFA in acetonitrile (mobile phase B) with a flow rate of 1.0 mL/minute. The linear gradient for mobile phase A and B was set as follows: 95% (A) and 5% (B) for an initial 4 minutes, 90% (A) and 10% (B) for 1 minute, 85% (A) and 15% (B) for 4 minutes, 75% (A) and 25% (B) for 1 minute, and 95% (A) and 5% (B) for 3 minutes. The temperatures of the column and sample compartments were set at 40°C and 5°C respectively. The injection volume was 20 μL and the detector was set at 250 nm.
HPLC Assay Performance
Linearity: Calibration standards containing 0.5, 1, 2, 4, 6, and 8 μg/mL of tigecycline were prepared by diluting tigecycline reference standard (Sigma-Aldrich, New South Wales, Australia) in mobile phase A. The relationship (over 5 consecutive days) between tigecycline concentration and detector response was determined by analyzing calibration standards of tigecycline. Calibration graphs were generated by plotting peak areas versus tigecycline concentrations and used for the calculations of linear regression coefficient (r2). Each sample was prepared in triplicate and analyzed in triplicate.
Accuracy, precision and reproducibility: Three different concentrations of tigecycline standards (0.5, 2, and 8 μg/mL) were used for the determination of accuracy and precision. Mean intra- and inter-day (5 consecutive days) accuracy values for the tigecycline peak (n = 6) were determined using the following regression equation: (observed concentration - expected concentration)/expected concentration × 100. Intra- and inter-day (5 consecutive days) precision values were investigated using peak areas with repeat analysis (n = 6) of tigecycline. Reproducibility was investigated by determining mean intra- and inter-day (5 consecutive days) peak retention time of tigecycline (2 μg/mL, n = 6). Each sample was analyzed in triplicate.
Forced Degradation
The stability-indicating capability of the developed HPLC method was determined by subjecting tigecycline to an acidic, basic, or oxidative stress in capped glass vials. The tigecycline solution (n = 3, 5 μg/mL) was mixed with an equal volume of 1 M hydrochloric acid, 1 M sodium hydroxide, or 0.5% hydrogen peroxide and then heated at 50°C for 30 minutes. The HPLC analysis was performed in triplicate on stressed (n = 3) as well as on unstressed samples (n = 3).
Sample Preparation for HPLC Analysis
A standard stock solution of tigecycline (500 μg/mL) was prepared by dissolving 5 mg of tigecycline reference standard in 10 mL of mobile phase A. The standard stock solution was then diluted with mobile phase A to obtain a series of standard solutions (over the range of 0.5 to 8 μg/mL). A standard curve (n = 2) was generated on each day of analysis by linear regression of the peak area of tigecycline against its concentration. An aliquot (1.5 mL) withdrawn from each PD bag was transferred to a glass autosampler vial and subjected to HPLC analysis within 30 minutes. Each sample was analyzed in triplicate.
Data Analysis
The stability of tigecycline was determined by calculating the percentage of its initial concentration remaining at each time period. Tigecycline was considered chemically stable if it retained more than 90% of its initial concentration.
Results
Stability Indicating Capability of HPLC Method
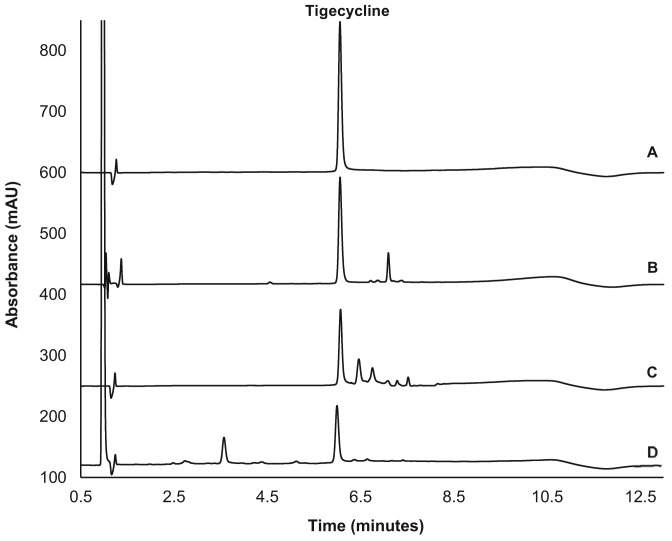
To determine whether the developed HPLC method was stability indicating, tigecycline was stressed under various conditions. Different chromatographic profiles were obtained for tigecycline when stressed under acidic, basic, or oxidative conditions (Figure 1). With the unstressed sample, the peak of tigecycline eluted at 6.0 minutes. Exposure to hydrochloric acid 0.5 M, sodium hydroxide 0.5 M, and hydrogen peroxide 0.25% for 30 minutes resulted in the loss of 16, 35, and 57% of tigecycline, respectively. Importantly, the observed degradation products did not interfere with the peak of tigecycline, suggesting the developed HPLC method is suitable for the stability determination of tigecycline.
Figure 1 —
High-performance liquid chromatography of tigecycline before (A) and after stressing under acidic (B), basic (C), and oxidative (D) conditions.
HPLC Assay Performance
Linearity estimated by r2 was greater than 0.999 with each of the 6 concentrations of tigecycline over 5 days. The intra- and inter-day precision relative standard deviation (RSD) was less than 1.5% (n = 6) and 2.1% (n = 6), respectively, at the level of 0.5, 2, and 8 μg/mL. The intra- and inter-day accuracy RSD at the level of 0.5, 2, and 8 μg/mL was less than 1.8% (n = 6) and 2.5% (n = 6), respectively. Intra- and inter-day retention time RSDs for tigecycline (2 μg/mL, n = 6) were less than 0.23% and 0.37%, respectively.
Chemical Stability of Tigecycline
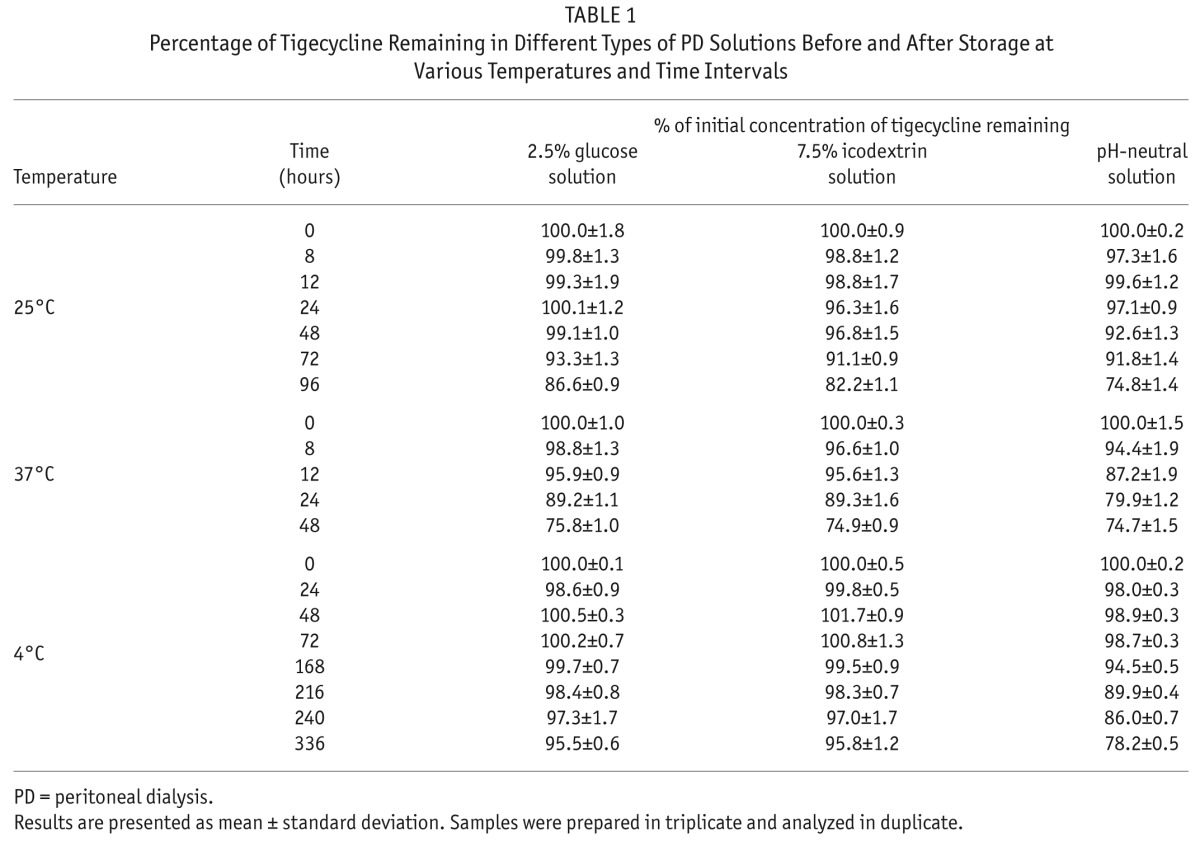
The mean concentration of tigecycline at time 0 (before storage) in 1.5% glucose or 7.5% icodextrin PD bags was found to be 2.18 μg/mL. In pH-neutral PD bags, the mean concentration of tigecycline at time 0 was found to be 4.08 μg/mL and 2.16 μg/mL in the non-glucose and mixed solution, respectively. These initial tigecycline concentrations were considered as 100%. The percentages of initial tigecycline concentration remaining in glucose, icodextrin, and pH-neutral PD solutions before and after storage at various time points are shown in Table 1. At 25°C (room temperature), tigecycline retained more than 90% of its initial concentration for at least 72 hours in 3 different types of PD solutions. At 37°C (body temperature), tigecycline was found to be stable for 8 hours when admixed with a pH-neutral PD solution and for 12 hours in glucose or icodextrin solutions. When stored at 4°C, tigecycline was stable for 216 hours (9 days) in pH-neutral PD solution and for 336 hours (14 days) in glucose and icodextrin PD solutions. No chromatographic changes were observed between the control samples analyzed before and after storage for up to 336 hours, suggesting the used PD solution did not undergo degradation when stored under the tested conditions.
TABLE 1.
Percentage of Tigecycline Remaining in Different Types of PD Solutions Before and After Storage at Various Temperatures and Time Intervals
Physical Stability of Tigecycline
All solutions were clear in appearance and there was no evidence of precipitation or color change throughout the study period. The baseline pH of glucose and icodextrin PD solution admixed with tigecycline was 4.84 ± 0.02 (mean ± SD) and 5.23 ± 0.01, respectively. The baseline pH of non-glucose and glucose containing solution admixed with tigecycline in pH-neutral PD bags was 7.03 ± 0.01 and 8.79 ± 0.01, respectively. The pH of glucose, icodextrin, and pH-neutral PD solution stored at 4, 25, and 37°C remained essentially unchanged for at least 336, 96, and 48 hours, respectively.
Discussion
This is the first study that investigated the stability of tigecycline in different types of PD solutions. Intraperitoneal administration of antibiotics is a standard practice in the treatment of PD-associated peritonitis and is recommended by the International Society for Peritoneal Dialysis practice guidelines (10). The intraperitoneal route also offers rapid attainment of bactericidal concentration in the peritoneal cavity compared with intravenous or oral routes of administration (19). Additionally, intraperitoneal administration of antibiotics can be easily managed by patients at home compared with an intravenous route, which requires additional healthcare resources, making it an inconvenient option. Scheetz et al. reported the use of tigecycline for the treatment of peritonitis caused by VRE in a critically ill patient (16). Despite administering twice the recommended dose intravenously, the peritoneal fluid concentration of tigecycline on day 5 of the treatment was 0.074 μg/mL (16). This concentration is at least 5 times smaller than the MIC reported for the most susceptible bacteria for tigecycline (18). This case report further highlights the importance of intraperitoneal administration of tigecycline for the treatment of PD-associated peritonitis, where rapid attainment of bactericidal concentration can be achieved.
Increasingly resistant gram-positive and gram-negative bacteria are being implicated in PD-associated infections (3,4,7,9,11). At least 10% of PD-associated peritonitis are recurrent or relapses (2,4) and the risk of resistant organisms causing PD-associated peritonitis is therefore expected to increase with time (3,7). Tigecycline is a broad-spectrum antibiotic that is effective against a variety of infections including complicated intra-abdominal infections such as peritonitis caused by gram-positive, anaerobic, or gram-negative bacteria (14). Tubau et al. studied the susceptibility of 600 clinical strains of gram-positive, gram-negative, and anaerobic bacteria obtained from hospitalized patients admitted due to secondary peritonitis. The study found that among all the isolates of E. coli, most commonly implicated gram-negative bacteria in PD-associated peritonitis, ESBL-producing isolates were susceptible to tigecycline (20). Intraperitoneal tigecycline is therefore an attractive therapeutic option for PD-associated peritonitis where resistant gram-positive and gram-negative bacteria are implicated.
Tigecycline was found to be stable for at least 216 hours at 4°C and 72 hours at 25°C when admixed with 1.5% glucose, 7.5% icodextrin, or pH-neutral PD solutions. In clinical practice, a PD bag is normally pre-warmed to body temperature prior to its intraperitoneal administration. Tigecycline was found to be stable for at least 8 hours at 37°C when admixed with the tested PD solutions, permitting pre-warming of a PD bag prior to its administration through an intraperitoneal route. The results obtained from the current study provide a platform for future clinical studies aiming to determine the safety, therapeutic efficacy, and pharmacokinetics of intraperitoneal tigecycline when used for the treatment of PD-associated peritonitis.
Conclusion
The present study for the first time reported the stability of tigecycline in commonly used PD solutions. Intraperitoneal administration of tigecycline appears to be a feasible option for the treatment of PD-associated peritonitis in case of treatment failure with first-line antibiotics or where resistant organisms are implicated as the source of infection.
Disclosures
The authors have no financial conflicts of interest to declare.
Acknowledgments
Funding for the study was provided by a Starter Grant from Royal Hobart Hospital Research Foundation, Australia, awarded to STW, RPP, and MDJ. The authors are grateful to Andrea Whittle, O'Dell Clark, and Andrew Reynolds (Home therapies nurses, Karingal Renal Education Centre, Department of Nephrology, Royal Hobart Hospital, Tasmania, Australia) for providing consumables and helpful information including the Royal Hobart Hospital standard protocols to prepare antibiotics admixed PD bags.
REFERENCES
- 1. Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002–2003. Perit Dial Int 2009; 29:297–302. [PubMed] [Google Scholar]
- 2. Brown MC, Simpson K, Kerssens JJ, Mactier RA. Peritoneal dialysis-associated peritonitis rates and outcomes in a national cohort are not improving in the post-millennium (2000–2007). Perit Dial Int 2011; 31:639–50. [DOI] [PubMed] [Google Scholar]
- 3. Kim DK, Yoo TH, Ryu DR, Xu ZG, Kim HJ, Choi KH, et al. Changes in causative organisms and their antimicrobial susceptibilities in CAPD peritonitis: a single center's experience over one decade. Perit Dial Int 2004; 24:424–32. [PubMed] [Google Scholar]
- 4. Burke M, Hawley CM, Badve SV, McDonald SP, Brown FG, Boudville N, et al. Relapsing and recurrent peritoneal dialysis-associated peritonitis: a multicenter registry study. Am J Kidney Dis 2011; 58(3):429–36. [DOI] [PubMed] [Google Scholar]
- 5. Cho Y, Badve SV, Hawley CM, McDonald SP, Brown FG, Boudville N, et al. Seasonal variation in peritoneal dialysis-associated peritonitis: a multicentre registry study. Nephrol Dial Transplant 2012; 5:2028–36. [DOI] [PubMed] [Google Scholar]
- 6. Barraclough K, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Polymicrobial peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes. Am J Kidney Dis 2010; 55:121–31. [DOI] [PubMed] [Google Scholar]
- 7. Borras M. Antibiotic resistance in gram-negative peritonitis. Perit Dial Int 2009; 29:274–6. [PubMed] [Google Scholar]
- 8. Szeto CC, Chow KM, Kwan BC, Law MC, Chung KY, Yu S, et al. Staphylococcus aureus peritonitis complicates peritoneal dialysis: review of 245 consecutive cases. Clin J Am Soc Nephrol 2007; 2:245–51. [DOI] [PubMed] [Google Scholar]
- 9. Szeto CC, Chow VC, Chow KM, Lai RW, Chung KY, Leung CB, et al. Enterobacteriaceae peritonitis complicating peritoneal dialysis: a review of 210 consecutive cases. Kidney Int 2006; 69:1245–52. [DOI] [PubMed] [Google Scholar]
- 10. Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. [DOI] [PubMed] [Google Scholar]
- 11. Chang CM, Liang CC, Yeh HC, Yang YF, Wang IK, Lin HH, et al. Vancomycin-resistant Enterococci peritonitis development after shifting from hemodialysis to peritoneal dialysis. Ren Fail 2010; 32:1121–2. [DOI] [PubMed] [Google Scholar]
- 12. Rubinstein E, Vaughan D. Tigecycline: a novel glycylcycline. Drugs 2005; 65:1317–36. [DOI] [PubMed] [Google Scholar]
- 13. Heizmann WR, Loschmann PA, Eckmann C, von Eiff C, Bodmann KF, Petrik C. Clinical efficacy of tigecycline used as monotherapy or in combination regimens for complicated infections with documented involvement of multiresistant bacteria. Infection 2015; 43:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antony S, Dominguez DC. Use of a novel antibiotic (tigecycline) in the treatment of peritoneal dialysis-associated MRSA peritonitis. Dial Transplant 2008; 37:30. [Google Scholar]
- 16. Scheetz MH, Reddy P, Nicolau DP, Noskin GA, Postelnick MJ, Stosor V, et al. Peritoneal fluid penetration of tigecycline. Ann Pharmacother 2006; 40:2064–7. [DOI] [PubMed] [Google Scholar]
- 17. Shimaoka T, Hamada C, Kaneko K, Io H, Sekiguchi Y, Aruga S, et al. Quantitative evaluation and assessment of peritoneal morphologic changes in peritoneal dialysis patients. Nephrol Dial Transplant 2010; 25:3379–85. [DOI] [PubMed] [Google Scholar]
- 18. EUCAST technical note on tigecycline. Clin Microbiol Infect 2006; 12:1147–9. [DOI] [PubMed] [Google Scholar]
- 19. Keller E, Reetze P, Schollmeyer P. Drug therapy in patients undergoing continuous ambulatory peritoneal dialysis. Clinical pharmacokinetic considerations. Clin Pharmacokinet 1990; 18:104–17. [DOI] [PubMed] [Google Scholar]
- 20. Tubau F, Linares J, Rodriguez MD, Cercenado E, Aldea MJ, Gonzalez-Romo F, et al. Susceptibility to tigecycline of isolates from samples collected in hospitalized patients with secondary peritonitis undergoing surgery. Diagn Microbiol Infect Dis 2010; 66:308–13. [DOI] [PubMed] [Google Scholar]