Abstract
♦ Background:
Recent investigations indicated that nonalcoholic fatty liver disease (NAFLD), a hepatic component of metabolic syndrome (MS), is associated with an increased risk of cardiovascular disease (CVD). Accordingly, we were interested in exploring the frequency of NAFLD in peritoneal dialysis (PD) patients and analyzing factors in PD patients associated with NAFLD occurrence. In addition, we were interested in investigating whether NAFLD is associated with higher CVD risk in our PD patients.
♦ Methods:
In the present cross-sectional study, we analyzed 58 PD patients. The controlled attenuation parameter (CAP) was used to detect and quantify liver steatosis with the help of transient elastography (TE) (FibroScan, Echosense SA, Paris, France). A carotid ultrasound was performed in all patients to measure carotid intimae media thickness (IMT) and plaque as surrogate measures of increased CVD risk, and we investigated their association with NAFLD.
♦ Results:
Nonalcoholic fatty liver disease was present in 74.1% of PD patients. Peritoneal dialysis/nonalcoholic fatty liver disease patients had statistically greater daily (136.5 ± 62.6 vs 93.6 ± 36.1; p = 0.02) and monthly (4,095.3 ± 1,877.7 vs 2,806.6 ± 1,083.2; p = 0.02) glucose load in comparison to the non-NAFLD/PD patients. In the next step, we were interested in analyzing what demographic and clinical characteristics in our PD patients are associated with a higher NAFLD occurrence. Presence of diabetes mellitus (DM), arterial hypertension (AH), dyslipidemia, body mass index > 25 kg/m2, and daily glucose load > 100 g were associated with NAFLD occurrence. Peritoneal dialysis patients with NAFLD showed more carotid atherosclerosis than PD patients without NAFLD. In addition, CAP values (as indicator of liver steatosis) showed strong positive association with IMT (r = 0.801; p < 0.0001). Nonalcoholic fatty liver disease was a strong predictor of carotid atherosclerosis in PD patients.
♦ Conclusion:
Nonalcoholic fatty liver disease is highly prevalent in PD patients. Peritoneal dialysis patients with NAFLD are at high risk of atherosclerosis. Assessment of NAFLD in PD patients may be helpful for CVD risk stratification.
Keywords: Cardiovascular risk, peritoneal dialysis, nonalcoholic fatty liver disease
Patients with chronic kidney disease (CKD) are at high risk of developing cardiovascular disease (CVD), with contributions from both “traditional” and “nontraditional” CVD risk factors. According to the literature, traditional risk factors such as hypertension, dyslipidemia, diabetes, and obesity have a higher prevalence in CKD patients and dialysis patients compared to non-CKD patients. In recent years, it is assumed that a number of non-traditional risk factors (endothelial dysfunction, chronic inflammation, increased oxidative stress, insulin resistance, etc.) could also play an important role in increased CVD morbidity and mortality in peritoneal dialysis (PD) patients. The interplay of many pathways, traditional risk factors, uremia-specific risk factors and novel, non-traditional risk factors may be responsible for the accelerated atherosclerosis that was observed in these patients. During the last decade, PD has become an increasingly popular treatment option for patients suffering from end-stage renal disease (ESRD). Despite significant improvement in the reduction of infectious complications in the past years, CVD mortality in PD patients remains unchanged. Therefore, searching for new causes of increased CVD risk in PD patients has attracted further research interest (1,2).
On the other hand, until recently, the presence of fatty liver was considered as a trivial finding. Nowadays, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of alternated liver enzymes and the most common liver disease in Western countries. Currently, the importance of NAFLD and its relationship to metabolic syndrome (MS) is increasingly recognized. There is growing evidence suggesting that CVDs are the leading cause of death in patients with advanced NAFLD and that NAFLD is associated with an increased risk of CVD (3–7). In recent years, NAFLD was recognized as an important factor in CKD pathogenesis. A growing numbers of studies as well as a recent meta-analysis by Musso et al. have shown that the presence and severity of NAFLD are associated with an increased risk and severity of CKD (8–12). Moreover, NAFLD is now recognized as a common condition that markedly increases the risk of ESRD (13). Results from the United Network Organ Sharing (UNOS) database during the time period from 2002 to 2011 show that cirrhosis related to nonalcoholic steatohepatitis is an increasing indication for simultaneous liver-kidney transplantation (14). In view of these preliminary observations, we had recently investigated whether the presence of transient elastography (TE)-defined NAFLD is associated with higher CVD risk in our hemodialysis (HD) patients. We have found that HD patients with NAFLD showed more carotid atherosclerosis and higher incidence of adverse CVD events in comparison to HD patients without NAFLD and the control subjects (15). Our results were in accordance with recent studies that investigated the association of NAFLD with the risk of CVD in the general population (3–7) as well as with 2 other studies that investigated the association of NAFLD with the risk of CVD in HD patients (16,17).
We were interested in exploring the frequency of NAFLD in PD patients and analyzing what factors in PD patients are associated with NAFLD occurrence. In addition, we were interested in investigating whether NAFLD was associated with higher cardiovascular risk in our PD patients.
Patients and Methods
In the present cross-sectional study, we analyzed 61 ESRD patients treated with PD for at least 3 months. The exclusion criteria were: serological evidence of chronic hepatitis B and/or C virus infection, alcohol abuse, presence of other autoimmune or cholestatic liver disease, malignancy, use of potentially hepato-toxic medications, uncontrolled secondary hyperparathyroidism, and technical reasons (failed transient elastography measurement). In the study group, there were no patients with infections at the time of TE measurements. Considering the above, 58 patients were enrolled in the further analysis.
Patients' demographic characteristics, medical history, and laboratory data were obtained by medical record. Co-morbid conditions included the presence of diabetes mellitus (DM), dyslipidemia, and arterial hypertension (AH), as well as obesity. This data was obtained using a standard questionnaire. Body fat (FAT) was assessed using skinfold caliper measurements. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Obesity was defined as a BMI > 25 kg/m2. Diabetes mellitus was defined by fasting glucose ≥ 5.6 mmol/L or drug treatment, dyslipidemia by triglycerides ≥ 1.7 mmol/L (150 mg/dL) or by drug treatment as well as high-density lipoprotein (HDL) < 0.9 mmol/L (< 40 mg/dL) in men and < 1.1 mmol/L (< 50 mg/dL) in women. Blood pressure was measured with a standard mercury sphygmomanometer. The criterion used for defining AH was systolic blood pressure (SP) > 140 mmHg and diastolic pressure (DP) > 90 mmHg or the routine use of anti-hypertensive medications.
Laboratory data included hemoglobin, serum iron, ferritin, aspartate aminotransferase (AST), alanin aminotransferase (ALT), γ-glutamyltransferase (GGT), alkaline phosphatase (ALP), C-reactive protein (CRP), calcium, phosphorus, and parathyroid hormone (PTH), which were obtained by standard clinical chemistry techniques. The clinical and laboratory data were collected within 2 weeks of TE measurements.
We calculated glucose weight as the sum of the products of the volume and glucose concentration for each exchange. We calculated glucose load as the average dialysate glucose concentration:total glucose weight/total volume of PD solution prescribed (18).
Liver stiffness was selected as the parameter to quantify liver fibrosis. Furthermore, the controlled attenuation parameter (CAP) was used to detect and quantify liver steatosis with the help of TE (FibroScan, Echosense SA, Paris, France). The cutoff value for defining liver steatosis was CAP ≥ 238 dB/m and cut-off value for defining the presence of fibrosis was liver stiffness of > 7.1 kPa. The diagnosis of NAFLD was defined according to the TE findings with the CAP ≥ 238 dB/m, with or without any stage of fibrosis and after exclusion of other secondary causes of chronic liver disease. Measurements were performed using the M and XL probe on the right lobe of the liver through intercostal spaces with the patients lying in dorsal decubitus with the right arm in maximal abduction. Ten successful measurements were performed on each patient and only cases with 10 successful acquisitions were taken into account for this study. With CAP being implemented on TE, both steatosis and fibrosis could be evaluated simultaneously (19).
A carotid ultrasound with a 9-MHz multifrequency transducer was performed in all patients to measure carotid intimae media thickness (IMT) and plaque as surrogate measures of increased CVD risk. The present analysis used the average of 10 electronic caliper IMT measurements from the far wall of the distal 10 mm of left and right common carotid arteries at a site free from any discrete plaque. A plaque was defined as a focal thickening of ≥ 1.2 mm in any of 12 carotid segments (near and far walls of right and left common carotid artery, bifurcation and internal carotid artery). These results were obtained at the time of TE measurements and were correlated with the presence of NAFLD.
In this explorative analysis we tried to explore the frequency of NAFLD in PD patients. In addition, we analyzed which factors in PD patients are associated with NAFLD occurrence. Also, we tried to investigate whether NAFLD is associated with higher cardiovascular risk in our PD patients.
Patients were informed of the purpose and methods of the research, and the study was done in accordance with the Declaration of Helsinki.
Statistical analysis of data was performed using descriptive statistics (mean and standard deviation). Categorical variables were tested by chi-square test or Fisher's exact test. Testing the importance of the difference of 2 independent groups was performed using t-test or ANOVA. The Pearson or Spearman correlation coefficient was used to express correlations between variables. Variables found significantly predicting NAFLD and carotid atherosclerosis were assessed by logistic regression analysis. P values < 0.05 were considered statistically significant. Statistical analysis was performed using the MedCalc statistical software package, version 10 (MedCalc, Mariakerke, Belgium).
Results
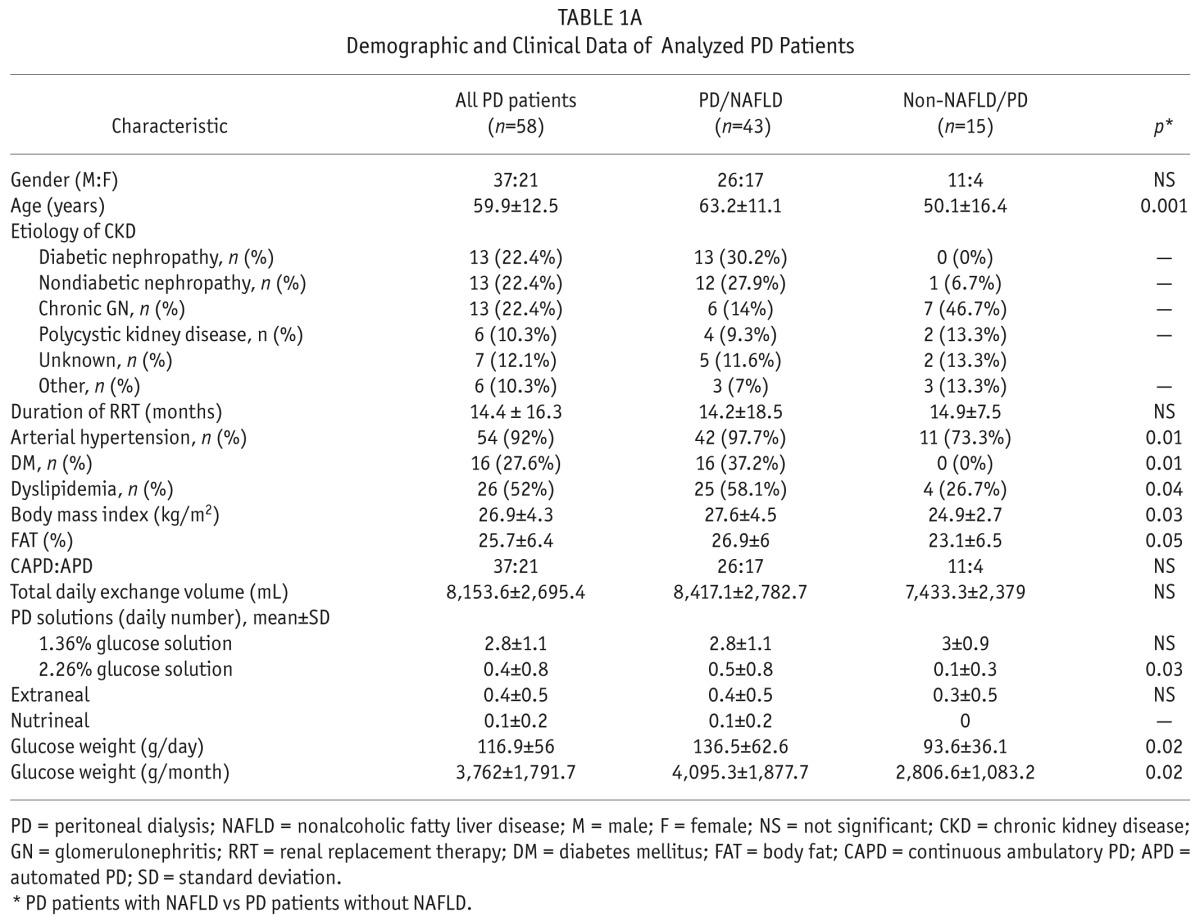
Demographic and clinical characteristics of the remaining 58 PD patients are shown in Table 1A. The average age of our patients (37 male and 21 female) was 59.9 ± 12.5 (range 23 to 83 years) years and the average duration of renal replacement therapy (RRT) was 14.4 ± 16.3 months. The most common primary kidney diseases that led to the development of ESRD were non-diabetic nephropathy of vascular origin (22.4%), diabetic nephropathy (22.4%), and chronic glomerulonephritis (22.4%).
TABLE 1A.
Demographic and Clinical Data of Analyzed PD Patients
We analyzed the prevalence of NAFLD in PD patients and found that NAFLD is highly prevalent in our PD patients. According to the TE findings, NAFLD was present in 43 of 58 patients (74.1%). Twenty (34.5%) patients with CAP ≥ 238 dB/m had liver stiffness of more than 7.1 kPa.
Therefore, for further analysis, PD patients were stratified into 2 subgroups according to the TE finding, i.e. the presence or absence of NAFLD.
The most common cause of ESRD in the PD/NAFLD subgroup was diabetic nephropathy (30.2%), while chronic glomerulonephritis was the most common cause of CKD in non-NAFLD/PD patients (46.7%). There was no significant difference in duration of RRT or PD modality between the 2 subgroups of patients. On the other hand, the daily number of 2.26% glucose solutions was higher in the PD/NAFLD subgroup of patients, while there was no significant difference in the use of other PD solutions (1.36% glucose solution, Extraneal and Nutrineal; Baxter Healthcare Corporation, Deerfield, IL, USA).
When analyzing comorbid conditions, we found all components of MS (DM, AH, dyslipidemia, and obesity) highly present in PD/NAFLD patients. We found a statistically significant difference between the PD/NAFLD patients and non-NAFLD/PD patients when comparing BMI values (p = 0.03) and the presence of DM (p = 0.01), hypertension (p = 0.01), and dyslipidemia (p = 0.04). Additionally, PD/NAFLD patients had a higher percentage of fat mass obtained by caliper in comparison to the non-NAFLD/PD patients (p = 0.05), table 1A.
Also, PD/NAFLD patients had statistically greater daily (136.5 ± 62.6 vs 93.6 ± 36.1; p = 0.02) and monthly (4,095.3 ± 1,877.7 vs 2,806.6 ± 1,083.2; p = 0.02) glucose load in comparison to the non-NAFLD/PD patients.
When analyzing biochemical parameters, we found statistically significant differences between the PD/NAFLD and non-NAFLD/PD patients in hemoglobin, serum iron, ferritin, and hs-CRP values. Peritoneal dialysis/nonalcoholic fatty liver disease patients had significantly lower hemoglobin and serum iron values in comparison to the non-NAFLD/PD patients. Furthermore, PD/NAFLD patients had significantly higher values of hs-CRP and ferritin than PD patients without NAFLD. On the other hand, we found no significant difference in liver tests, markers of bone metabolism and serum albumin levels between the 2 subgroups of patients (Table 1B).
TABLE 1B.
Laboratory Characteristics of Analyzed Groups of Patients
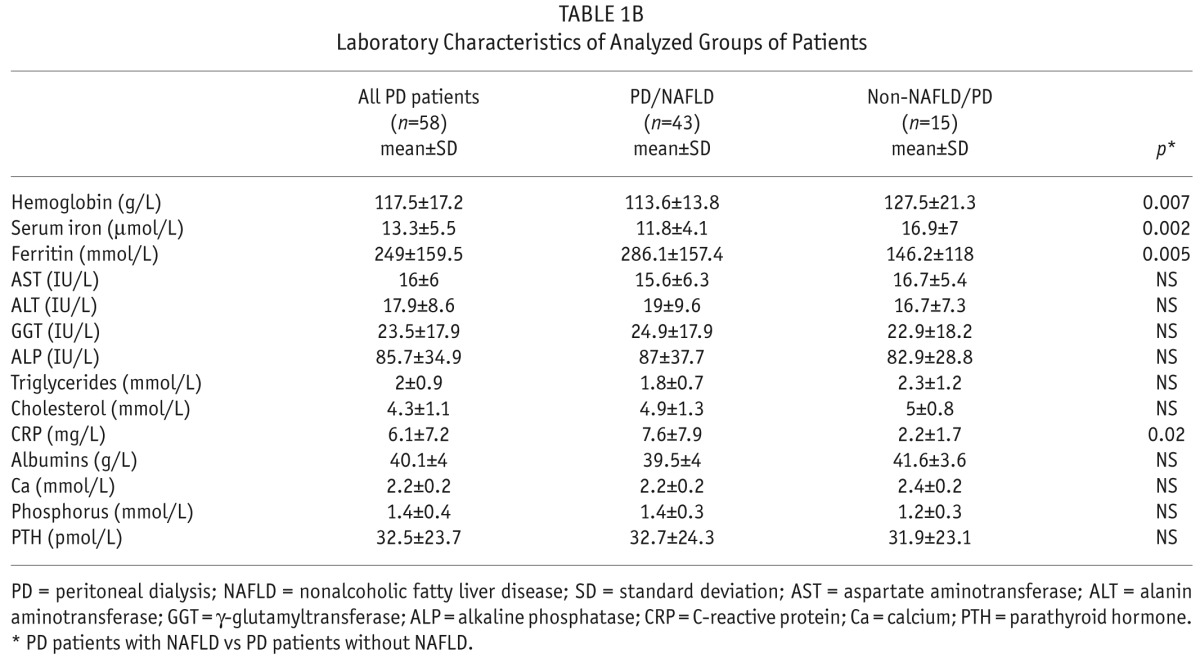
We were interested in exploring the association between liver tests and the presence of NAFLD. We did not find any significant correlation between CAP values and values of investigated liver tests: AST, ALT, GGT, and ALP (p = NS), (Table 1B).
In the next step, we were interested in analyzing what demographic and clinical characteristics in our PD patients are associated with a higher NAFLD occurrence. The presence of DM, AH, dyslipidemia, BMI > 25 kg/m2, and daily glucose load > 110 (g/day) were associated with NAFLD occurrence (Table 2). Moreover, there was a significant positive correlation between CAP and BMI values (r = 0.491; p = 0.0001). In addition, when analyzing the association between caliper measurements and the presence of NAFLD, we found that FAT showed a significant positive correlation with CAP values (r = 0.353; p = 0.01).
TABLE 2.
Predictors of NAFLD Occurrence in PD Patients
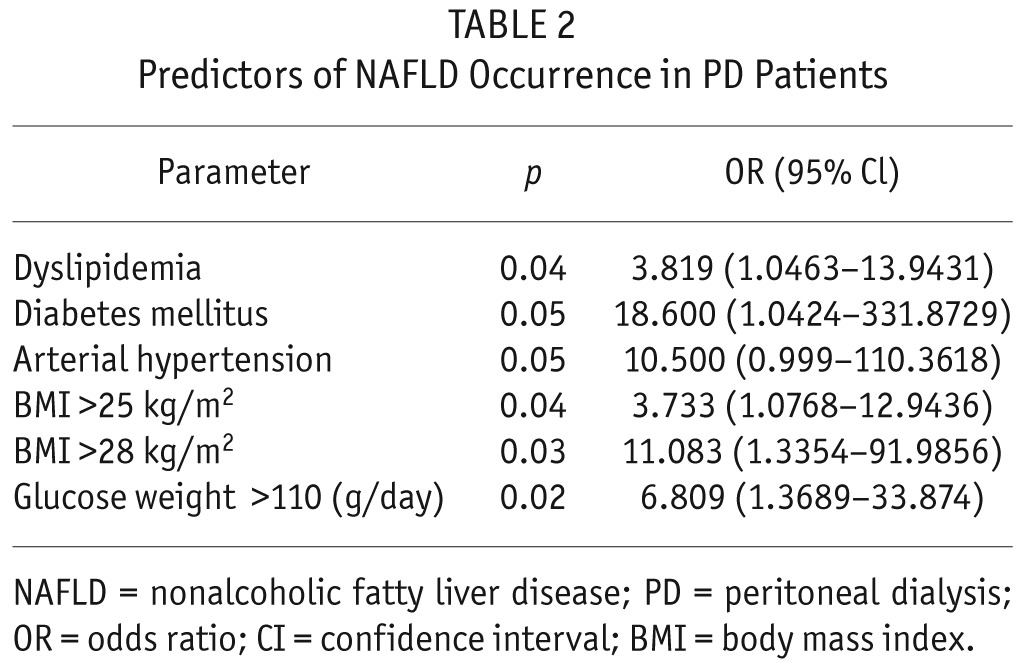
Next, we were interested in exploring if NAFLD in our PD patients is associated with advanced carotid atherosclerosis. As it is shown in Table 3, our PD patients with NAFLD show more carotid atherosclerosis compared to non-NAFLD/PD patients. In the next step, we investigated the association between CAP and IMT measurements. There was a highly significant positive correlation between CAP and IMT measurements (r = 0.801; p < 0.0001) (Figure 1).
TABLE 3.
Carotid IMT and Plaque in PD Patients With and Without NAFLD
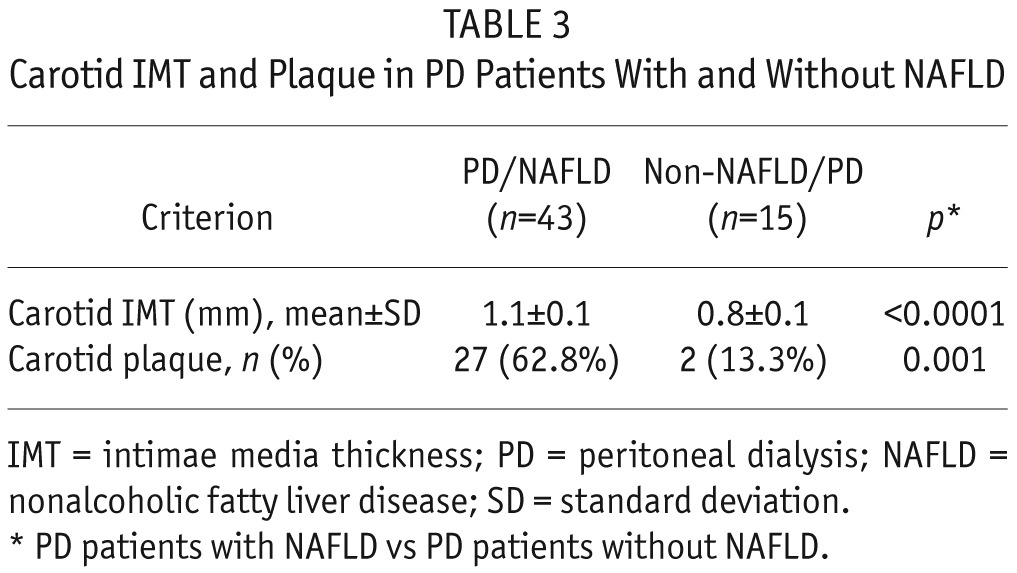
Figure 1 —
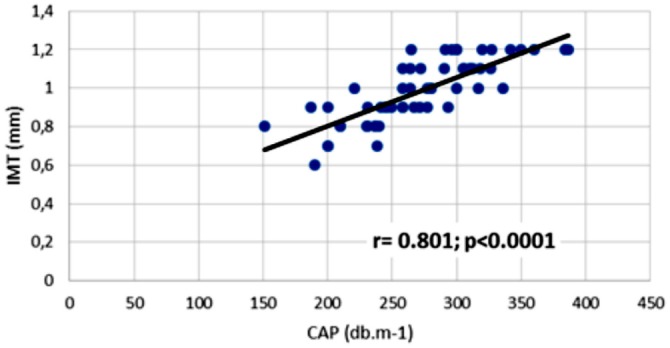
The correlation between CAP values and carotid IMT in PD patients. IMT = intimae media thickness; CAP = controlled attenuation parameter; PD = peritoneal dialysis.
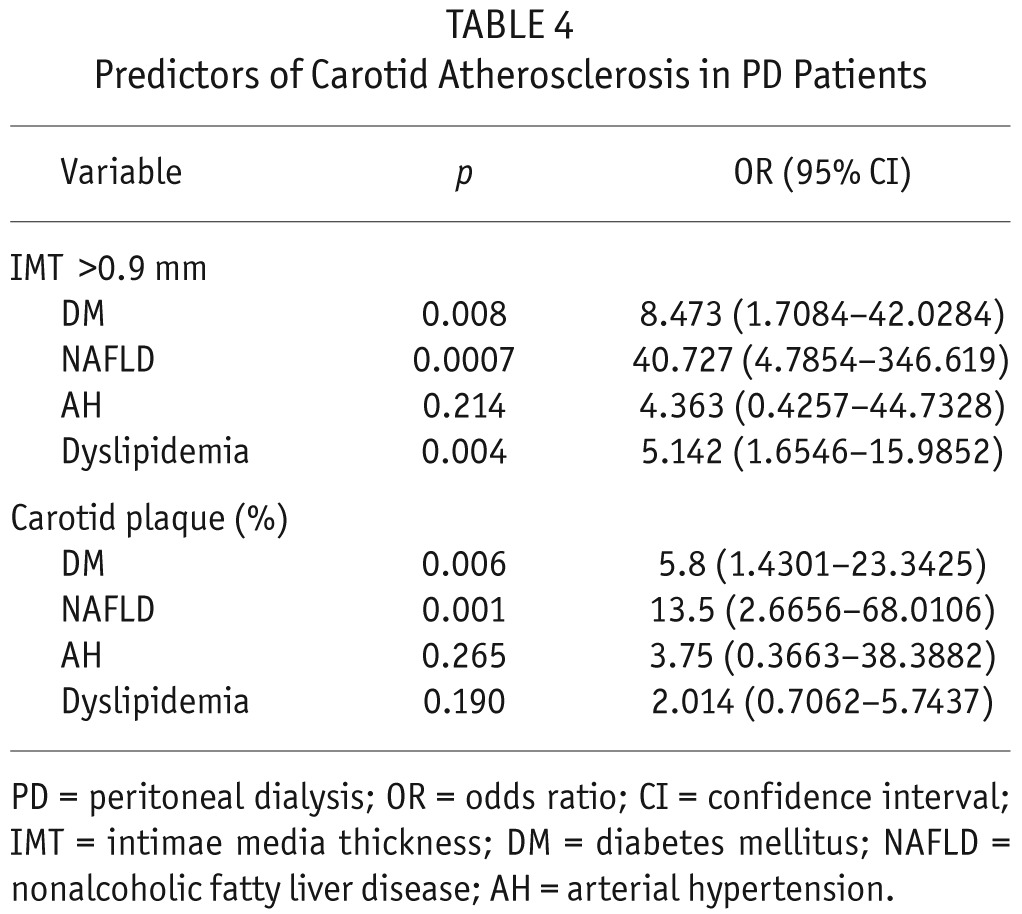
Finally, we were interested in exploring which demographic and clinical characteristics are associated with advanced carotid atherosclerosis in our PD patients. Presence of NAFLD, DM, and dyslipidemia were found to be significantly associated with higher IMT. Additionally, presence of NAFLD and DM were factors that contribute to plaque occurrence. Nonalcoholic fatty liver disease was the strongest predictor of carotid atherosclerosis (Table 4).
TABLE 4.
Predictors of Carotid Atherosclerosis in PD Patients
Discussion
While the incidence of infectious complications in PD patients has decreased, CVD mortality remains unchanged or has even slightly increased (20). According to the European Renal Association-European Dialysis and Transplantation Association Registry, at least half of all deaths in this population of patients are attributable to CVD (21). The interplay of many pathways, traditional risk factors, uremia-specific risk factors and novel non-traditional risk factors are responsible for the accelerated atherosclerosis seen in PD patients (1,2). Searching for new causes of CVD in PD patients has attracted further research interest.
To the best of our knowledge, this is the first study investigating the association between NAFLD and CVD risk in PD patients. Our results demonstrate that NAFLD is highly prevalent in PD patients. These results can be explained by the fact that the main risk factors responsible for development of NAFLD such as diabetes, hypertension, dyslipidemia and obesity are highly present in PD patients. It is believed that almost all NAFLD patients have more than 1 component of MS and 35 – 75% of patients meet all of the diagnostic criteria. These components of MS were highly present in our PD patients with NAFLD. Moreover, in our study, the daily number of glucose solutions, obesity, and presence of AH, DM, and dyslipidemia were found to be predictors of NAFLD occurrence. In addition, fat mass showed a significant positive correlation with the severity of liver steatosis. Also, PD/NAFLD patients had statistically greater daily and monthly glucose load in comparison to the non-NAFLD/PD patients. Therefore, it could be expected that PD patients have a high prevalence of NAFLD which was confirmed in our study. Insulin resistance is recognized as the pathophysiological hallmark of NAFLD. According to the literature, insulin resistance is prevalent in CKD patients. Glucose is the most commonly utilized osmotic agent in PD patients. On the other hand, absorption of glucose in PD patients can also lead to several cardiometabolic complications, such as hyperinsulinemia, hyperglycemia, hyperlipidemia and weight gain. Some authors have postulated that glucose loading in PD patients is associated with deterioration in insulin sensitivity (22). These observations can explain the association of NAFLD and applied glucose solutions in our PD patients, as well as a high prevalence of NAFLD in our PD patients, but further studies are needed.
The results of our study are important for 2 reasons. Nonalcoholic fatty liver disease patients have a higher risk of CVD due to the underlying metabolic disorders mentioned above, even without MS, as has been shown in recent studies (3–7). According to the literature, there is a strong association between the severity of liver histopathology in NAFLD patients and greater carotid IMT and plaque, and lower endothelial flow-mediated vasodilatation (as markers of subclinical atherosclerosis) even independently of MS (3,7,23). Recently, Targher found that NAFLD is not merely a marker of CVD, but it may be actively involved in its pathogenesis (24). Nonalcoholic fatty liver disease per se contributes to higher adverse CVD events due to subchronic inflammation and endothelial dysfunction, as has been observed in recent investigations (3–7,23,24). Our study showed that PD patients with NAFLD show more carotid atherosclerosis than PD patients without NAFLD. In addition, CAP values (as an indicator of liver steatosis) showed strong positive association with IMT. Nonalcoholic fatty liver disease was an independent predictor of carotid atherosclerosis in our PD patients. Our results in the present study are in accordance with recent studies investigating the association between NAFLD and the risk of CVD in the general population (3–7) and with our previous study (15) as well as 2 recent studies (16,17) regarding the association between NAFLD and CVD in HD patients. The exact mechanism linking NAFLD and increased CVD risk is not fully understood, but there is growing evidence supporting the idea that hepatic steatosis is associated with atherogenic dyslipidemia, inflammation, and endothelial dysfunction. In the last few years, many investigations have shown an increased production and release of various proinflammatory cytokines in patients with NAFLD. Accordingly, enhanced oxidation and inflammation with the release of inflammatory cytokines may account for the proatherogenic effect of NAFLD (3–7,23,24). We believe that the presence of NAFLD in PD patients could be a new risk factor or a new marker of increased CVD risk in this population of patients. Due to our relatively small number of patients, we can only speculate about these observations. Further, prospective, and randomized trials are needed.
In our study, none of the investigated liver tests showed any significant association with the presence of NAFLD in PD patients. We have observed similar results in our HD patients (15). On the other hand, NAFLD is the most common cause of liver enzyme abnormalities in the general population. These data indicate that standard reference liver test values cannot be used as markers of NAFLD in PD patients. These results can be explained by previous findings, which have shown that ESRD patients have decreased serum aminotransferase activity compared to the general population (25). To this day, there is still no clear recommendation that liver biopsy is required to confirm a diagnosis of NAFLD (26). Therefore, the evaluation of NAFLD using non-invasive methods such as TE-CAP may represent a potential future approach in the assessment of NAFLD in PD patients.
Our study has several limitations. The design is cross-sectional and the number of patients is relatively small. The cross-sectional format of our study does not allow conclusions as to whether the link between the NAFLD and greater CVD risk in PD patients is causal. In other words, due to the cross-sectional design it is not possible to conclude whether NAFLD is simply a reflection of MS or whether NAFLD adds additional risk. Therefore, further prospective investigations should be conducted to answer this question. In addition, the observational nature of our study makes it impossible to assess the impact of PD per se on the prevalence of NAFLD because there was no control group. On the other hand, our study has several strengths. It shows, for the first time, that PD patients with NAFLD show advanced carotid atherosclerosis. Therefore, we assume that NAFLD should be considered a new important risk factor or a new marker of increased CVD risk in PD patients. Further studies are needed that will investigate and confirm these observations. The clinical implication of this finding is that the presence of NAFLD in PD patients may help in cardiovascular risk stratification and assessment. The use of CAP as a screening method for NAFLD detection in PD patients could be beneficial since it is a non-invasive and quick method that is easy to perform and may be repeated.
Disclosures
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1. Wang AJM. Cardiovascular risk factors in peritoneal dialysis patients revisited. Perit Dial Int 2007; 27(S2):S223–7. [PubMed] [Google Scholar]
- 2. Garcia-Lopez E, Carrero JJ, Suliman ME, Lindholm B, Stenvinkel P. Risk factor for cardiovascular disease in patients undergoing peritoneal dialysis. Perit Dial Int 2007; 27(S2):S205–9. [PubMed] [Google Scholar]
- 3. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010; 363:1341–50. [DOI] [PubMed] [Google Scholar]
- 4. Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arterioscler Thromb Vasc Biol 2005; 25:1045–50. [DOI] [PubMed] [Google Scholar]
- 5. Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care 2006; 29:132–30. [DOI] [PubMed] [Google Scholar]
- 6. Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 2005; 42:473–80. [DOI] [PubMed] [Google Scholar]
- 7. Assy N, Dijbre A, Farah R, Grosovski M, Marmor A. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology 2010; 254:393–400. [DOI] [PubMed] [Google Scholar]
- 8. Yilmaz Y, Alahdab YO, Yonal O, Kurt R, Kedrah AE, Celikel CA, et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: association with liver fibrosis. Metabolism 2010; 59:1327–30. [DOI] [PubMed] [Google Scholar]
- 9. Manco M, Ciampalini P, DeVito R, Vania A, Cappa M, Nobili V. Albuminuria and insulin resistance in children with biopsy proven non-alcoholic fatty liver disease. Pediatr Nephrol 2009; 24:1211–7. [DOI] [PubMed] [Google Scholar]
- 10. Targher G, Bertolini L, Rodella S, Lippi G, Zoppini G, Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 2010; 5:2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mikolasevic I, Racki S, Bubic I, Jelic I, Stimac D, Orlic L. Chronic kidney disease and nonalcoholic fatty liver disease proven by transient elastography. Kidney Blood Press Res 2013; 37:305–10. [DOI] [PubMed] [Google Scholar]
- 12. Musso G, Gambino R, Tabibian JH, Ekstedt M, Kechagias S, Hamaguchi M, et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: a systematic review and meta-analysis. PLoS Med 2014; 11(7):e1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Targher G, Mantovani A, Pichiri I, Mingolla L, Cavalieri V, Mantovani W, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of chronic kidney disease in patients with type 1 diabetes. Diabetes Care 2014; 37:1729–36. [DOI] [PubMed] [Google Scholar]
- 14. Singal AK, Salameh H, Kuo YF, Wiesner RH. Evolving frequency and outcomes of simultaneous liver kidney transplants based on liver disease etiology. Transplantation 2014; 98:216–21. [DOI] [PubMed] [Google Scholar]
- 15. Mikolasevic I, Orlic L, Milic S, Zaputovic L, Lukenda V, Racki S. Nonalcoholic fatty liver disease (NAFLD) proven by transient elastography in hemodialysis patients; is it a new risk factor for adverse cardiovascular events? Blood Purif 2014; 37:259–65. [DOI] [PubMed] [Google Scholar]
- 16. Lai YC, Cheng BC, Hwang JC, Lee YT, Chiu CH, Kuo LC, et al. Association of fatty liver disease with nonfatal cardiovascular events in patients undergoing maintenance hemodialysis. Nephron Clin Pract 2013; 124(3-4):218–23. [DOI] [PubMed] [Google Scholar]
- 17. Neri S, Signorelli SS, Scuderi R, Bruno M, Bertino G, Clementi A, et al. Carotid intima-media thickness and liver histology in hemodialysis patients with nonalcoholic fatty liver disease. Int J Angiol 2011; 20:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu HY, Hung KY, Hu FC, Chen YM, Chu TS, Huang JW, et al. Risk factors for high dialysate glucose use in PD patients – a retrospective 5-year cohort study. Perit Dial Int 2010; 30:448–55. [DOI] [PubMed] [Google Scholar]
- 19. Sasso M, Beaugrand M, de Ledinghen V, Douvin C, Marcellin P, Poupon R, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010; 36(11):1825–35. [DOI] [PubMed] [Google Scholar]
- 20. Han SS, Ahn JM, Chin HJ, Chae DW, Oh KH, Joo KW, et al. Impact of C-reactive protein and pulse pressure evaluated at the start of peritoneal dialysis on cardiovascular events in the course of treatment with peritoneal dialysis. Perit Dial Int 2010; 30:300–10. [DOI] [PubMed] [Google Scholar]
- 21. Stel VS, Kramer A, Zoccali C, Jager KJ. The 2006 ERA-EDTA registry annual report: a précis. J Nephrol 2009; 22:1–12. [PubMed] [Google Scholar]
- 22. Van Biesen W, Verbeke F, Vanholder R. Cardiovascular disease in hemodialysis and peritoneal dialysis: arguments pro peritoneal dialysis. Nephrol Dial Transplant 2007; 22:53–8. [DOI] [PubMed] [Google Scholar]
- 23. Lizardi-Cervera J, Zapata-Aguilar D. Nonalcoholic fatty liver disease and its association with cardiovascular disease. Ann Hepatol 2009; 8:S40–3. [PubMed] [Google Scholar]
- 24. Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med 2007; 24:1–6. [DOI] [PubMed] [Google Scholar]
- 25. Ramos de Oliveira Liberato I, Pessoa de Almeida Lopes E, Gomes de Mattos Cavalcante MA, Costa Pinto T, Fernades Moura I, Loureiro Júnior L. Liver enzymes in patients with chronic kidney disease undergoing peritoneal dialysis and hemodialysis. Clinics 2012; 67(2)131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dowman JK, Tomlinson JW, Newsome N. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2011; 33:525–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


