Abstract
Purpose
In lung cancer, randomized trials assessing hyperfractionated or accelerated radiotherapy seem to yield conflicting results regarding the effects on overall (OS) or progression-free survival (PFS). The Meta-Analysis of Radiotherapy in Lung Cancer Collaborative Group decided to address the role of modified radiotherapy fractionation.
Material and Methods
We performed an individual patient data meta-analysis in patients with nonmetastatic lung cancer, which included trials comparing modified radiotherapy with conventional radiotherapy.
Results
In non–small-cell lung cancer (NSCLC; 10 trials, 2,000 patients), modified fractionation improved OS as compared with conventional schedules (hazard ratio [HR] = 0.88, 95% CI, 0.80 to 0.97; P = .009), resulting in an absolute benefit of 2.5% (8.3% to 10.8%) at 5 years. No evidence of heterogeneity between trials was found. There was no evidence of a benefit on PFS (HR = 0.94; 95% CI, 0.86 to 1.03; P = .19). Modified radiotherapy reduced deaths resulting from lung cancer (HR = 0.89; 95% CI, 0.81 to 0.98; P = .02), and there was a nonsignificant reduction of non–lung cancer deaths (HR = 0.87; 95% CI, 0.66 to 1.15; P = .33). In small-cell lung cancer (SCLC; two trials, 685 patients), similar results were found: OS, HR = 0.87, 95% CI, 0.74 to 1.02, P = .08; PFS, HR = 0.88, 95% CI, 0.75 to 1.03, P = .11. In both NSCLC and SCLC, the use of modified radiotherapy increased the risk of acute esophageal toxicity (odds ratio [OR] = 2.44 in NSCLC and OR = 2.41 in SCLC; P < .001) but did not have an impact on the risk of other acute toxicities.
Conclusion
Patients with nonmetastatic NSCLC derived a significant OS benefit from accelerated or hyperfractionated radiotherapy; a similar but nonsignificant trend was observed for SCLC. As expected, there was increased acute esophageal toxicity.
INTRODUCTION
Worldwide, lung cancer accounts now for the largest number of new cancer cases, with approximately 85% non–small-cell lung cancer (NSCLC) and 15% small-cell lung cancers (SCLC); poor survival rates are common even in patients with nonmetastatic disease.1–3 In recent years, considerable interest has been raised about modified fractionation radiotherapy (RT) regimens for head and neck and lung cancers.4–7 Two types of altered fractionation have been studied7: hyperfractionation in which the dose per fraction was decreased, with two or three fractions given per day instead of one; and acceleration, consisting of reducing the overall treatment time, thus delivering to the tumor a higher dose in a shorter overall time. Accelerated radiotherapy is often combined with hyperfractionation. In head and neck cancer, an individual patient data meta-analysis on altered-fractionated RT has demonstrated a significant benefit on overall survival (OS) of hyperfractionated and/or accelerated over conventionally fractionated RT.8 The randomized trials assessing hyperfractionated and/or accelerated RT in lung cancer seem to give conflicting results about the benefit on locoregional control and OS. The Meta-Analysis of Radiotherapy in Lung Cancer (MAR-LC) collaborative group decided to perform an individual patient data meta-analysis to accurately estimate the effect of modified RT on survival outcomes and toxicity and to distinguish between ineffective treatment and moderate treatment effects, which may be clinically relevant.8–13
MATERIALS AND METHODS
This meta-analysis was performed in accordance with a prespecified protocol, available on request.
Selection Criteria and Search Strategy
To be eligible, trials were to include patients with nonmetastatic lung cancer, randomly assigned in a way that precluded prior knowledge of treatment assignment. They had to compare modified radiotherapy (accelerated, hyperfractionated, or both) with conventional radiotherapy (five daily 1.8- to 2-Gy fractions per week and a minimum total dose of 40 Gy for SCLC and 60 Gy for NSCLC). Trials must have accrued between January 1, 1970, and December 31, 2005, and not be confounded by additional therapeutic differences between the two arms. Trials combining chemotherapy (CT) with radiotherapy were included only if CT doses and schedule were the same in the two arms. The searching strategy is available (Data Supplement).
Individual Patient Data
For each eligible trial, individual patient data were requested for all randomly assigned patients and comprised characteristics of both patient and tumor, date of randomization and treatment arm allocated, and details on treatment actually received. Acute and late toxicities (hematologic, esophageal, pulmonary, and cardiac) were collected. Data on the type of pulmonary and cardiac toxicities were not collected. Follow-up information was as updated as possible.
Each trial database was checked according to a standard procedure for missing data, inconsistencies, and for ensuring a suitable quality of follow-up in the two treatment arms. Randomization integrity was assessed through search of unusual patterns in the sequencing of allocation or imbalances between treatment arms. Queries were solved and final database validated by the responsible trial investigator or statistician.
Statistical Considerations
Definition of outcomes measures.
The primary outcome was OS, defined as the time from randomization until death resulting from any cause. Living patients were censored at their date of last follow-up. Progression-free survival (PFS) was defined as time from randomization to first event among locoregional or distant progression and death. Living patients without progression were censored at their date of last follow-up. Locoregional and distant failures were studied within the framework of competing risks as follows. Time to locoregional failure was defined as time from randomization until date of locoregional failure as first event. Patients having distant failure before locoregional failure were censored at the time of distant failure, and patients dying without recurrence were censored at date of death. Time to distant failure was similarly defined. Patients having both locoregional and distant failures occurring at the same time were considered as event for distant failure analysis only.
Non–lung cancer deaths were defined as deaths resulting from causes other than cancer and not occurring after disease progression. All other deaths, including deaths resulting from unknown cause, were considered as lung cancer deaths.9
Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC), WHO criteria, or Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC) criteria depending on the trials. Severe toxicity was defined as grade 3 to 4 toxicity.
Analysis
Analysis was done according to the intent-to-treat principle, including patients excluded from previous trials analyses. Two separate analyses were performed for SCLC and NSCLC. Median follow-up was estimated using the reverse Kaplan-Meier method.14 Analyses were stratified on trial. Individual and pooled hazard ratios (HRs) and 95% CIs were estimated through a fixed-effects model using log-rank expected number of events and its variance.15 A similar model was used to estimate odds ratios (OR) for comparison of toxicity between arms. Heterogeneity among trials was investigated through χ2 heterogeneity tests11 and was quantified through calculation of I2 index.16 Lung cancer and non–lung cancer mortality were analyzed according to Peto's method to take into account the competing risk framework.9,13 Peto's curves for survival and absolute benefits were calculated using OS rate in the control group and estimated HR at each time of interest. Rates of toxicity in the experimental arm were calculated using rate in the control arm and the OR.17 In the NSCLC trials, four prespecified and mutually exclusive subsets of trials were constituted according to number of daily fractions, total dose, and total duration: very accelerated RT, defined as shortening of the total duration of 50% or more compared with that of the control arm; moderately accelerated RT, defined as shortening of the total duration of more than 15%, but less than 50% as compared with the control arm; hyperfractionated RT with identical total dose; and hyperfractionated RT with increased total dose. HRs were computed by subset, and interaction with treatment effect was investigated through χ2 tests.11 As prespecified, influence of administration of CT on patient outcomes was also analyzed. Interaction between treatment effect and patients subgroups was also examined through χ2 test comparing effect between subgroups to study whether subgroups of patients benefit more or less from modified radiotherapy. Studied characteristics were age, sex, and performance status, and, in NSCLC trials only, histologic subtype and stage. If significant interaction was found, the result was to be confirmed with a second method that pools interactions between treatment effect and subgroups computed in each trial, minimizing the risk of bias related to indirect comparison.18 As exploratory analyses, survival and toxicity analyses adjusted on covariates were done through multivariate Cox models for survival end points and logistic models for toxicity end points. Results were similar with the nonadjusted analyses, thus only log-rank or χ2 ones are presented.
Individual patient data on treatment actually received were used to study the biologic effective dose (BED) corrected for the overall treatment time, 19,20 which allows the comparison of various dose-fractionation regimens. The BED was here defined as follows:
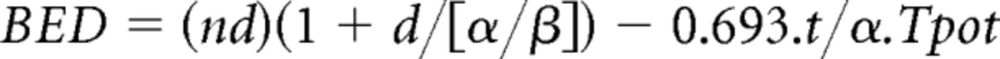 |
where n is the total number of fractions delivered, d is the dose per fraction (Gy), and t is the total duration of radiotherapy (days). The following assumptions were made on radiobiologic parameters for tumors: α/β = 10 Gy (for tumor and most acute effects); α = 0.3 Gy; and Tpot (the potential doubling time) equals 5.6 days. Exploratory analyses studied the impact on OS of the value of the equivalent BED received by patients through Cox model as well as the impact on the risk of acute esophageal toxicity through logistic model, stratified on trial and adjusted for age, sex, performance status, histologic type, and disease stage. The BED was initially studied in four categories defined by the quartiles, containing one fourth of the patients each. However, results of three categories were very similar, and as this was an exploratory analysis, these categories were aggregated to simplify the results. Consequently, the BED is presented in two categories, first quartile versus other quartiles. The equivalent dose using a fraction size of 2 Gy corrected for time, EQD2t, was similarly studied.21
All P values were two-sided. Analyses were performed using SAS software, version 9.1 (SAS Institute, Cary, NC).
RESULTS
Trials and Patients Description
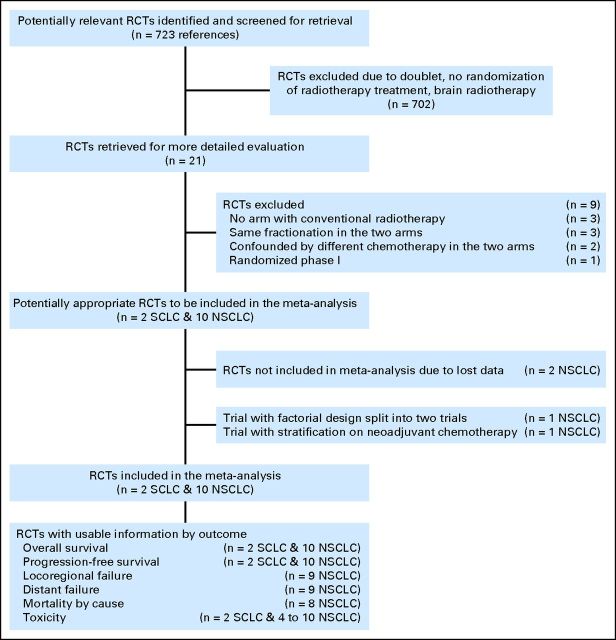
The different steps of the trial selection are presented in Figure 1. Twelve eligible trials were identified, two in SCLC and 10 in NSCLC. Excluded trials are listed (Data Supplement). Data were no longer available for two NSCLC trials,22,23 so that 10 trials were analyzed, two SCLC trials24,25 and eight NSCLC trials.26–33 One trial had a factorial design: patients were also randomly assigned to receive or not concomitant CT27; one trial had a randomization stratified on administration of induction chemotherapy.31 Each of these two trials were split into two separate trials, with and without chemotherapy (PMCI 88C091 and PMCI 88C091 CT; CHARTWEL and CHARTWEL CT). Therefore, 12 trials and 2,685 patients were analyzed (Table 1). In the two SCLC trials, patients received cisplatin and etoposide concomitantly with RT, plus induction and consolidation in one. In the 10 NSCLC trials, CT was administered concomitantly with RT in two trials27,30 (carboplatin alone or cisplatin plus etoposide) and as induction chemotherapy in two trials31,32 (according to the center practice or based on carboplatin plus paclitaxel). The NSCLC trials were divided into four categories as follows: six trials in the very accelerated RT subset,27,28,31,32 one trial in the moderately accelerated RT subset,33 two trials in the hyperfractionated RT with identical dose subset,29,30 and one trial in the hyperfractionated RT with increased total dose26 (Data Supplement).
Fig 1.
Flowchart of the trial selection and contribution to analyses. NSCLC, non–small-cell lung cancer; RCTs, randomized controlled trials; SCLC, small-cell lung cancer.
Table 1.
Description of Included Trials
| Trial | No. of Patients Randomly Assigned | Inclusion Period | Median Follow-Up (years) | Histology | RT Total Dose (Gy) | No. of Fractions | Duration (weeks) | BED St/EXP | CT Dose | Patient Characteristic |
|---|---|---|---|---|---|---|---|---|---|---|
| ECOG 358824 | 417 | 1989-1992 | 13.0 | SCLC | Standard: 45 | 25 | 5 | 39.5 | Cisplatin 60 mg/m2 day 1 | PS 0-2 |
| Experimental: 45 | 30 | 3 BID | 43.9 | Etoposide 120 mg/m2 days 1-3 | ||||||
| 4 cycles (3 weeks) | ||||||||||
| NCCTG 89205225 | 268 | 1990-1996 | 9.3 | SCLC | Standard: 50.4 | 28 | 5.5 | 43.8 | Cisplatin 30 mg/m2 days 1-3 | PS 0-2 |
| Experimental: 48 | 32 | 5.5 SC* BID | 39.5 | Etoposide 130 mg/m2 days 1-3 | ||||||
| 6 cycles† (4 weeks) | ||||||||||
| RTOG 8808-ECOG 458826 | 326 | 1989-1992 | 6.8 | NSCLC | Standard: 60 Gy | 30 | 6 | 55.5 | None | KPS ≥ 70 |
| Experimental: 69.6 | 58 | 6 BID | 61.9 | Stage II-III | ||||||
| PMCI 88C09127 | 101 | 1989-1995 | Not reached | NSCLC | Standard: 60 | 30 | 6 | 55.5 | None | PS 0-1 |
| Experimental: 60 | 30 | 3 BID | 64.2 | Stage I-III | ||||||
| PMCI 88C091 CT27 | 107 | 1989-1995 | Not reached | NSCLC | Standard: 60 | 30 | 6 | 55.5 | Carboplatin 70 mg/m2 days 1-5 | PS 0-1 |
| Experimental: 60 | 30 | 3 BID | 64.2 | + Carboplatin 70 mg/m2 days 29-33 in standard arm | Stage I-III | |||||
| CHART28 | 563‡ | 1990-1995 | 6.9 | NSCLC | Standard: 60 | 30 | 6 | 55.5 | None | PS 0-1 |
| Experimental: 54 | 36 | 1.5 TID | 57.2 | Stage I-III | ||||||
| NCCTG 90245129 | 74 | 1992-1993 | 8.1 | NSCLC | Standard: 60 | 30 | 6 | 55.5 | None | PS 0-2 |
| Experimental: 60 | 40 | 6 SC§ BID | 52.5 | Stage III | ||||||
| NCCTG 94245230 | 246 | 1994-1999 | 7.3 | NSCLC | Standard: 60 | 30 | 6 | 55.5 | Cisplatin 30 mg/m2 days 1-3, 28-30 | PS 0-1 |
| Experimental: 60 | 40 | 6 SC§ BID | 52.5 | Etoposide 100 mg/m2 days 1-3, 28-30 | Stage III | |||||
| CHARTWEL31 | 300 | 1997-2005 | 4.9 | NSCLC | Standard: 66 | 33 | 6.5 | 60.6 | None | PS 0-1 |
| Experimental: 60 | 40 | 2.5 TID | 61.6 | Stage I-III | ||||||
| CHARTWEL CT31 | 106 | 1997-2005 | 3.5 | NSCLC | Standard: 66 | 33 | 6.5 | 60.6 | Induction CT—dependent on institution's choice | PS 0-1 |
| Experimental: 60 | 40 | 2.5 TID | 61.6 | Stage I-III | ||||||
| ECOG 259732 | 119 | 1998-2001 | 6.7 | NSCLC | Standard: 64 | 32 | 6.5 | 58.7 | Carboplatin AUC 6 day 1 | PS 0-1 |
| Experimental: 57.6 | 36 | 2.5 TID | 60.2 | Paclitaxel 225 mg/m2 day 1 | Stage III | |||||
| 2 cycles‖ (3 weeks) | ||||||||||
| Gliwice 200133 | 58 | 2001-2006 | 5.3 | NSCLC | Standard: 72 | 40 | 8 | 62.7 | None | PS 0-1 |
| Experimental: 72 | 40 | 5.5 | 68.5 | Stage II-III |
Abbreviations: BED, biologic effective dose; BID, RT given twice a day; CT, chemotherapy; if not specified, the chemotherapy is concomitant to the radiotherapy; CHART, Continuous Hyperfractionated Accelerated Radiation Therapy; CHARTWEL, CHART Week-End Less; ECOG, Eastern Cooperative Oncology Group; Exp, experimental; (K) PS, (Karnofsky) performance status; NCCTG, North Central Cancer Treatment Group; NSCLC, non–small-cell lung cancer; PCMI, Peter MacCallum Institute; RT, Radiotherapy; RTOG, Radiation Therapy Oncology Group; SC, split course; SCLC, small-cell lung cancer; St, standard; TID, RT given three times a day.
Two series of 8 days with a break of 2.5 weeks.
Three cycles induction, two cycles concomitant, and one after RT; etoposide dose was reduced to 100 mg/m2 for cycles 4 to 6.
Patients were randomly allocated in a 3:2 ratio to CHART or conventional radiotherapy.
Two series of 2 weeks with a break of 2 weeks.
Induction chemotherapy.
Patients with NSCLC were mainly men (75%) and younger than 70 years (71%), with a performance status (PS) of 0 to 1 (> 99%); more than 60% had squamous cell cancer, and more than 80% had stage III cancer. All patients with SCLC had a limited stage, 58% were men, 6% had PS of 2, and 83% were younger than 70 years (Data Supplement). As compared with published trials, 63 additional patients were analyzed (+3%), allowing us to analyze all patients known to be randomly assigned in the included trials. Characteristics of patients were well balanced between the two randomization arms (Data Supplement).
NSCLC Trials
OS.
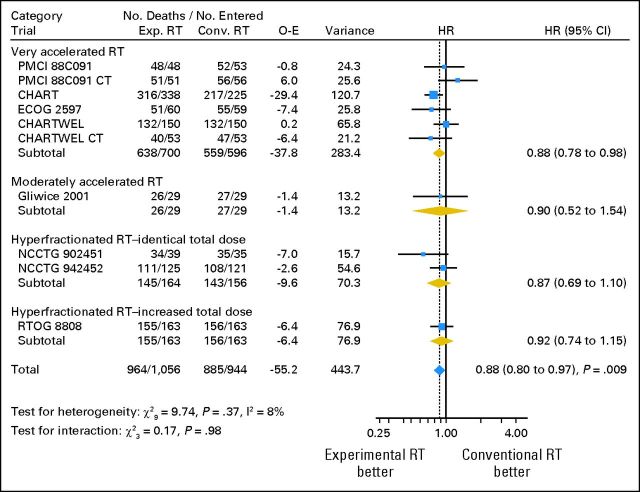
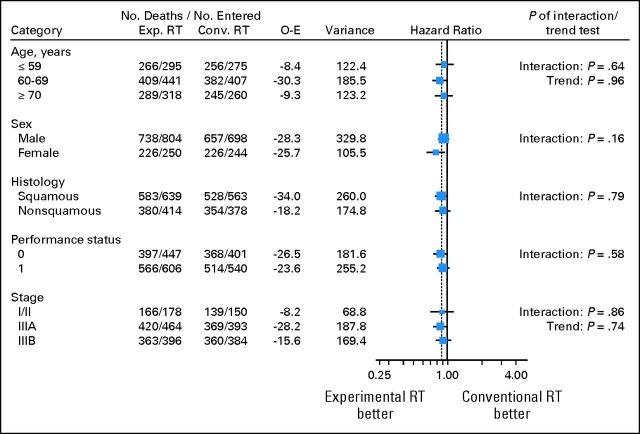
Overall survival results are based on 2,000 patients with a median follow-up of 6.9 years and 1,849 deaths. Across trials the risk of death was significantly reduced by 12% with the use of modified RT (HR = 0.88; 95% CI, 0.80 to 0.97; P = .009; Fig 2). The absolute benefit was 3.8% at 3 years and 2.5% at 5 years, increasing the survival rate from 15.9% to 19.7% at 3 years and from 8.3% to 10.8% at 5 years (Fig 3A). As shown in Figure 2, there was no evidence of heterogeneity in treatment effect between the trials (heterogeneity test, P = .37, I2 = 8%), and the effect of modified RT did not seem to differ between the trials subsets (interaction test, P = .98). Impact on overall survival seemed similar according to CT: HR = 0.92 (95% CI, 0.77 to 1.10) and HR = 0.87 (95% CI, 0.78 to 0.97) in trials with and without CT, respectively (interaction test, P = .57). There was no evidence that any subgroup of patients benefited more or less from modified RT, as shown on Figure 4.
Fig 2.
Effect of modified radiotherapy (RT) versus conventional RT on overall survival, by RT types in non–small-cell lung cancer trials. Each trial is represented by a blue square, the center of which denotes the hazard ratio (HR) for that trial comparison, with the horizontal lines showing the 95% CIs. The size of the square is directly proportional to the amount of information contributed by the trial. The gold diamonds represent pooled HRs for the trial groups and the blue diamond the overall HRs, with the center denoting the HR and the extremities the 95% CI. The fixed effect model was used. CHART, Continuous Hyperfractionated Accelerated Radiation Therapy; CHARTWEL, CHART Week-End Less; CT, chemotherapy; Conv., conventional; ECOG, Eastern Cooperative Oncology Group; Exp., experimental; NCCTG, North Central Cancer Treatment Group; O-E, observed-expected; PCMI, Peter MacCallum Institute; RTOG, Radiation Therapy Oncology Group.
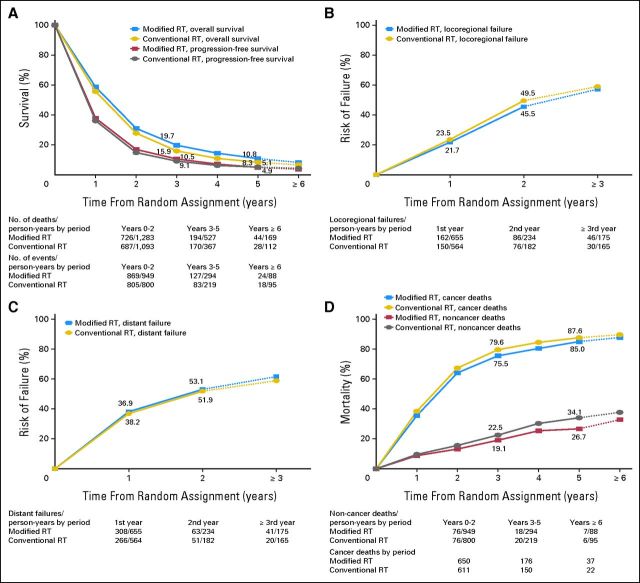
Fig 3.
Survival curves for the non–small-cell lung cancer trials: (A) overall and progression-free survival; (B) locoregional failure; (C) distant failure; (D) lung and non–lung cancer mortality. RT, radiotherapy.
Fig 4.
Effect of modified radiotherapy (RT) versus conventional RT on overall survival by patient characteristics. Conv., conventional; Exp., experimental; O-E, observed-expected.
Other survival outcomes.
Analysis of PFS was based on 2,000 patients and 1,926 events. Patterns of failure as first event were as follows: distant failure (30%), locoregional failure (29%), deaths (26%), locoregional and distant failure (8%), and unspecified (6%). No evidence of a benefit of modified RT was observed on PFS (HR = 0.94; 95% CI, 0.86 to 1.03; P = .19 [Data Supplement]). Absolute benefit was 1.4% at 3 years and −0.2% at 5 years (Fig 3A). There was no evidence of heterogeneity between trials (P = .28, I2 = 18%) and no evidence of interaction between trials subsets (P = .85). No evidence of interaction of treatment effect with subgroup of patients was found (Data Supplement). Similarly, on the basis of nine trials and 1,862 patients, there was no evidence that modified RT had an impact on locoregional failure (550 events, HR = 0.92; 95% CI, 0.77 to 1.09; P = .32) or distant failure (749 events, HR = 1.07; 95% CI, 0.92 to 1.24; P = .37, Figs 3B and 3C). For these two end points, no evidence of heterogeneity was observed. The benefit observed on OS was similar in size when considering lung cancer-related deaths only (2,000 patients and 1,646 events; HR = 0.89; 95% CI, 0.81 to 0.98, P = .02 [Data Supplement]) and non–lung cancer mortality only (1,942 patients and 203 events, HR = 0.87; 95% CI, 0.66 to 1.15; P = .33 [Data Supplement]). Absolute decrease in mortality rates was 4.1% and 3.4% at 3 years and 2.6% and 7.4% at 5 years, respectively, for lung cancer and non–lung cancer deaths (Fig 3D).
Toxicity assessment.
As shown in Table 2, modified RT increased the risk of acute severe esophageal toxicity from 9% to 19% (OR = 2.44; 95% CI, 1.90 to 3.14; P < .001). There was a significant interaction between RT modality and acute severe esophageal toxicity (P = .001), with the very accelerated RT being the most toxic (OR = 3.21; 95% CI, 2.41 to 4.28 [Data Supplement]). Overall, modified RT significantly reduced the risk of platelet toxicity (OR = 0.55; 95% CI, 0.32 to 0.96; P = .03), but no severe platelet toxicity was reported in the trials without CT. Severe hematologic toxicity was mainly reported in trials with CT, which principally influenced overall results. Consequently, no differences between trials with CT and trials without CT could be observed (interaction test, P = .72). Modified RT had no impact on other acute hematologic toxicities as well as long-term toxicity (Table 2).
Table 2.
Effect of Modified Radiotherapy Compared With Conventional Radiotherapy on Toxicity Events
| Severe Toxicity | Availability |
Toxicity Rate in Control Arm (%) | Toxicity Rate in Experimental Arm (%)* | Result |
P Efficacy | I2 (%) | P Heterogeneity | ||
|---|---|---|---|---|---|---|---|---|---|
| No. of Trials | No. of Patients | OR | 95% CI | ||||||
| Non–small-cell lung cancer | |||||||||
| Acute toxicity | |||||||||
| Esophageal | 10 | 1,968 | 9 | 19 | 2.44 | 1.90 to 3.14 | < .001 | 57 | .01† |
| Pulmonary | 9 | 1,390 | 7 | 5 | 0.67 | 0.42 to 1.05 | .08 | 0 | .65 |
| Cardiac | 6 | 940 | 1 | 1 | 1.33 | 0.46 to 3.83 | .59 | 0 | .92 |
| Hematologic‡ | 5 | 607 | 34 | 29 | 0.79 | 0.48 to 1.32 | .38 | 0 | .54 |
| Neutrophils | 5 | 600 | 33 | 28 | 0.80 | 0.46 to 1.40 | .44 | 3 | .39 |
| Platelets | 5 | 595 | 13 | 8 | 0.55 | 0.32 to 0.96 | .03 | 0 | .98 |
| Hemoglobin | 6 | 677 | 1 | 1 | 1.36 | 0.46 to 4.08 | .58 | 0 | .86 |
| Late toxicity | |||||||||
| Pulmonary | 7 | 866 | 15 | 16 | 1.07 | 0.73 to 1.56 | .73 | 0 | .56 |
| Esophageal | 7 | 861 | 3 | 4 | 1.24 | 0.61 to 2.56 | .55 | 0 | .89 |
| Cardiac | 4 | 515 | 1 | 1 | 1.49 | 0.40 to 5.60 | .55 | 0 | .96 |
| Any of above | 4 | 533 | 13 | 16 | 1.27 | 0.79 to 2.06 | .33 | 0 | .97 |
| Small-cell lung cancer | |||||||||
| Acute esophageal | 2 | 667 | 12 | 25 | 2.41 | 1.62 to 3.59 | < .001 | 0 | .99 |
| Acute pulmonary | 2 | 675 | 5 | 6 | 1.32 | 0.69 to 2.51 | .40 | 0 | .33 |
| Acute cardiac | 2 | 670 | 1 | 3 | 2.96 | 1.13 to 7.73 | .03 | 0 | .76 |
| Hematologic§ | 2 | 674 | 83 | 86 | 1.22 | 0.81 to 1.86 | .34 | 0 | .36 |
| Neutrophils | 2 | 643 | 84 | 87 | 1.31 | 0.84 to 2.04 | .23 | 0 | .70 |
| Platelets | 2 | 666 | 38 | 30 | 0.70 | 0.50 to 0.98 | .04 | 36 | .21 |
| Hemoglobin | 2 | 673 | 18 | 19 | 1.06 | 0.71 to 1.59 | .76 | 0 | .35 |
Abbreviation: OR, odds ratio.
See Statistical Considerations section in the text for the methods used to compute the rate in the experimental arm.
Significant interaction: very accelerated radiotherapy is more toxic than other radiotherapy types (interaction test, P = .001, Data Supplement).
Including three trials (456 patients) with chemotherapy.
Chemotherapy treatment was administered in the two small-cell lung cancer trials.
Compliance and exploratory analyses.
Some individual patient data on RT parameters were available for seven trials. As shown (Data Supplement), compliance to RT was good, with 90% or more patients receiving the treatment as planned in terms of total dose and fractionation, and superior to 80% for the overall treatment time. Compliance in the experimental arm was similar to the one in the control arm. The delivered BED could be calculated for 1,471 patients in six trials (Data Supplement). The BED ranged from 3.7 Gy to 74.9 Gy. The first quartile was 54.7 Gy (53.9 Gy in the conventional arm and 57.2 Gy in the experimental arm). The BED corresponding to the radiotherapy actually delivered to patients was significantly associated with OS: the 1,076 patients receiving a BED ≥ 55 Gy had a decreased risk of death as compared with the 395 patients with BED less than 55 Gy (HR = 0.75; 95% CI, 0.65 to 0.85; P < .001). This resulted in an absolute benefit of 5.1% at 3 years and 3.4% at 5 years (Data Supplement). However, this was at the price of a higher risk of acute esophageal toxicity (OR = 2.9; 95% CI, 1.9 to 4.4; P < .001). Similar results were found when studying the EQD2t (data not shown). Because incomplete treatment could be related to early progression and death, a sensitivity analysis was done to test this effect after exclusion of 306 patients with follow-up less than 6 months. Results were robust and supported the outcome (HR = 0.86; 95% CI, 0.74 to 1.00; P = .04) for OS.
SCLC Trials
OS.
Analyses were based on 685 patients with a median follow-up of 12.1 years and 622 deaths. Effect of modified RT on OS was similar to that of NSCLC, but nonsignificant (HR = 0.87; 95% CI, 0.74 to 1.02; P = .08 [Data Supplement]). No heterogeneity was seen between the two trials (P = .49, I2 = 0%). The absolute benefit was 1.7% at 3 years (from 29.6% to 31.3%) and 5.1% at 5 years (from 18.7% to 23.8% [Data Supplement]). An interaction was seen and confirmed between modified RT effect and PS: patients with poor PS seemed to benefit less from modified RT than patients with good PS (HR = 2.22 in PS 2 v 0.81 and 0.86 in PS = 0 and PS = 1 respectively; P = .03). No interaction was observed between effect of modified RT and age or sex (Data Supplement).
Other outcomes.
There was no evidence that the use of modified RT had an impact on PFS (HR = 0.88; 95% CI, 0.75 to 1.03; P = .11 [Data Supplement]). There was no evidence of heterogeneity between the two trials (P = .45, I2 = 0%). The absolute benefit at 3 years and 5 years was 5.7% and 4.5%, respectively (Data Supplement). As for OS, patients with poor PS seemed to benefit less from modified RT than others (P = .03). Locoregional and distant failures, as well as lung cancer and non–lung cancer mortality, were not studied because of the lack of available data in these trials.
Toxicity assessment.
In SCLC trials, only acute toxicities were assessed (Table 2). As in NSCLC, there was an excess of acute esophageal toxicity in the modified RT arm (OR = 2.41; 95% CI, 1.62 to 3.59; P < .001), without heterogeneity between the two trials, and a reduction of risk of platelet toxicity (OR = 0.70; 95% CI, 0.50 to 0.98; P = .04). Risk of cardiac toxicity was higher with modified RT (OR = 2.96; 95% CI, 1.13 to 7.73; P = .03), but the toxicity rate in the control arm was only 1%. No evidence of impact of modified RT on acute hemoglobin, neutrophils, or pulmonary toxicity was found.
Exploratory Analysis
Individual patient data on RT parameters were only available for one trial, and no exploratory analysis was done.
DISCUSSION
On the basis of this meta-analysis, the use of modified RT has led to a significant 12% to 13% relative reduction of mortality in patients with lung cancer, resulting in a 5-year survival absolute benefit of 2.5% in NSCLC and 5.1% in SCLC. Because there were fewer patients with SCLC, this difference was nonsignificant except for in patients with good PS. Results are based on a minimum follow-up of 5 years in each trial. In exploratory analyses, we could not identify any subgroup (age, sex, performance status, stage, histology) for whom modified RT was any more or less effective, except a detrimental effect was observed in patients with SCLC and poor PS. However, detailed data in histology were not available in several trials, and the effect of the modified RT in adenocarcinoma could not be studied.
Because uncontrolled locoregional disease continues to be a major challenge in lung cancer, there is a conceptual rationale in support of more aggressive RT, as explored in the investigational arm of the various trials included in the meta-analysis. The survival results support this hypothesis. In a previous meta-analysis comparing concomitant and sequential radiochemotherapy in patients with locally advanced NSCLC, the absolute benefit of 4.5% in 5-year overall survival was mainly due to a decrease of locoregional failures,34 but not in the present meta-analysis. There may be several explanations: the relatively low statistical power (only 550 events), the extreme difficulty of local control evaluation in trials mostly performed in the early 1990s, the difficulties to define the patterns of failure, the burden of distant failures that may have outweighed local recurrences, and lastly, an improvement in non–lung cancer mortality. It is possible that better control of distant disease obtained with improved integration of newer systemic therapy agents could uncover improved local control in future trials. Furthermore, the biologic advantage may have been undermined by the use of suboptimal RT techniques (use of split course radiotherapy, no computed tomography–based planning) contributing to an insufficient local outcome.
Even if some toxicity results should be interpreted with caution, data are robust in terms of acute esophageal toxicity, which was increased by a factor of 2.4. It was, however, reversible for most patients. Aggressive types of RT fractionation are not only associated with severity of esophagitis, but also its duration.27,28,35 Interestingly, compliance with modified RT was good, especially in terms of overall treatment duration in very accelerated regimens, as esophageal maximal toxicity occurred after the end of the RT schedule.27,28,31 More modern RT techniques may contribute to diminish esophageal toxicity, which can be severe and disabling. However, reversible toxicity in fit patients may be considered subsidiary to improving survival.
Preclinical and clinical research studies suggest that most cancer cells have a doubling time of less than 1 week.36,37 Prolonging total duration of treatment may be detrimental because it results in accelerated tumor repopulation.38,39 Thus in SCLC, the results, even if not significant, support such accelerated regimens with concomitant CT. In NSCLC, where there is less biologic background to support hyperfractionated regimens, we could also observe a similar HR favoring modified RT regimens, with 64% of individual data issued from very accelerated trials and with good compliance regarding radiation dose and duration. This seemed related to a decrease in non–cancer-related deaths.
To better compare the different regimens, we calculated the BED for each RT schedule. We observed an absolute benefit in terms of 3-year survival of 5.1% in patients who had a BED ≥ 55 Gy (corresponding to 60 Gy over 6 weeks). Similar results were found when studying the EQD2t. Repopulation is hypothesized to be one of the major factors that limit the success of conventional dose-escalation approaches, so that accelerated regimen should be reconsidered in the light of these results, and hyperfractionated regimens may better protect normal tissues and enable concomitant approaches.
Concomitant chemoradiotherapy is at present considered the standard regimen for locally advanced lung cancer. The integration of optimized conformal RT to improve local control as well as the combination with systemic agents to reduce systemic failures using modified RT regimens should be reconsidered. The search for biologic predictive factors that could enable us to better individualize the optimal treatment for patients with lung cancer is also warranted, as this meta-analysis seems to show that there are different possibilities to improve curability of lung cancer. Further research is needed to identify the optimal schedule of modified fraction RT, including new techniques in target volume definition, treatment techniques, and delivery, such as positron emission tomography scans, intensity-modulated RT, and dose-guided RT.40
Supplementary Material
Acknowledgment
We thank the trialists and the following research groups who agreed to share their data: Arbeitsgemeinschaft Radioonkologie der Deutschen Krebsgesellschaft, Eastern Cooperative Oncology Group, Medical Research Council, North Central Cancer Treatment Group, and Radiation Therapy Oncology Group. We also thank Matthieu Faron and Béranger Lueza for statistical help, Denise Avenell for secretarial assistance, and Francine Courtial for electronic literature search.
Appendix
MAR-LC Collaborative Group members are as follows: Secretariat (Institut Gustave-Roussy), R. Arriagada, C. Le Pechoux, A. Mauguen, J.P. Pignon; advisory board, H. Choy (The University of Texas Southwestern), D. De Ruysscher (University Hospital Maastricht), P. Fournel (Institut de Cancérologie de la Loire), S.J. Mandrekar, F. Mornex (Centre Hospitalier Lyon Sud), M.K. Parmar; Investigators, K. Bae (Radiation Therapy Oncology Group), D. Ball (Peter MacCallum Cancer Centre and the University of Melbourne), M. Bauman (University of Dresden), K. Behrendt (Maria Sklodowska—Curie Memorial Cancer Center and Institute of Oncology), C.P. Belani (Penn State Cancer Institute), J. Beresford (Peter MacCallum Cancer Centre), J. Bishop (Victorian Comprehensive Cancer Centre), J.A. Bonner (University of Alabama-Birmingham), S.E. Dahlberg (Dana Farber Cancer Institute), S. Dische (Mount Vernon Hospital), R. Koch (University of Dresden), S.J. Mandrekar (Mayo Clinic), M. Nankivell (MRC Clinical Trials Unit), G. Nelson (Mayo Clinic), M.K. Parmar (MRC Clinical Trials Unit), M.I. Saunders (Mount Vernon Hospital), W. Sause (Intermountain Medical Center), S.E. Schild (Mayo Clinic), A.T. Turrisi (Sinai Grace Hospital), A. Zajusz (Maria Sklodowska–Curie Memorial Cancer Center and Institute of Oncology).
Footnotes
Written on behalf of the Meta-Analysis of Radiotherapy in Lung Cancer Collaborative Group.
Supported by unrestricted grants from French Programme Hospitalier de Recherche Clinique, Ligue Nationale Contre le Cancer, and sanofi-aventis (J.-P.P.). The funding sources had no role in study design, data collection, data analysis, data interpretation, or manuscript writing.
Presented in part at the 2nd European Lung Cancer Conference, April 28-May 1, 2010, Geneva, Switzerland; the 29th Annual Meeting of the European Society of Therapeutic Radiation and Oncology, September 12-16, 2010, Barcelona, Spain; and fully at the 14th World Conference on Lung Cancer,July 3-7, 2011, Amsterdam,the Netherlands.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Jean-Pierre Pignon, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Audrey Mauguen, Cécile Le Péchoux, Michele I. Saunders, Steven E. Schild, Andrew T. Turrisi, Rodrigo Arriagada, Dirk De Ruysscher, Jean-Pierre Pignon
Financial support: Jean-Pierre Pignon
Administrative support: Jean-Pierre Pignon
Provision of study materials or patients: Michele I. Saunders, Steven E. Schild, Andrew T. Turrisi, Michael Baumann, William T. Sause, David Ball, Chandra P. Belani, James A. Bonner, Aleksander Zajusz, Suzanne E. Dahlberg, Matthew Nankivell, Sumithra J. Mandrekar, Rebecca Paulus, Katarzyna Behrendt, Rainer Koch, James F. Bishop, Stanley Dische
Collection and assembly of data: Audrey Mauguen, Michele I. Saunders, Steven E. Schild, Andrew T. Turrisi, Michael Baumann, William T. Sause, David Ball, Chandra P. Belani, James A. Bonner, Aleksander Zajusz, Suzanne E. Dahlberg, Matthew Nankivell, Sumithra J. Mandrekar, Rebecca Paulus, Katarzyna Behrendt, Rainer Koch, James F. Bishop, Stanley Dische, Jean-Pierre Pignon
Data analysis and interpretation: Audrey Mauguen, Cécile Le Péchoux, Michele I. Saunders, Steven E. Schild, Andrew T. Turrisi, Dirk De Ruysscher, Jean-Pierre Pignon
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: A 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 3.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the Surveillance, Epidemiologic, and End Results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 4.Stuschke M, Pöttgen C. Localized small-cell lung cancer: Which type of thoracic radiotherapy and which time schedule. Lung Cancer. 2004;45(suppl 2):S133–S137. doi: 10.1016/j.lungcan.2004.07.981. [DOI] [PubMed] [Google Scholar]
- 5.Arriagada R, Komaki R, Cox JD. Radiation dose escalation in non-small cell carcinoma of the lung. Semin Radiat Oncol. 2004;14:287–291. doi: 10.1016/j.semradonc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Peters L, Ang K. The role of altered fractionation in head and neck cancer. Semin Radiat Oncol. 1992;2:180–194. doi: 10.1053/SRAO00200180. [DOI] [PubMed] [Google Scholar]
- 7.Horiot JC, Bontemps P, van den Bogaert W, et al. Accelerated fractionation (AF) compared to conventional fractionation (CF) improves loco-regional control in the radiotherapy of advanced head and neck cancers: Results of the EORTC 22851 randomized trial. Radiother Oncol. 1997;44:111–121. doi: 10.1016/s0167-8140(97)00079-0. [DOI] [PubMed] [Google Scholar]
- 8.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists Collaborative Group: Effect of systemic treatment of early breast cancer by hormonal, cytotoxic, or immunotherapy therapy: 133 randomized trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Lancet. 1992;339:1–15. [PubMed] [Google Scholar]
- 10.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 11.Chemotherapy in non-small cell lung cancer: A meta-analysis using updated data on individual patient from 52 randomized clinical trials—Non-Small Cell Lung Cancer Collaborative Group. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 12.Aupérin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission: Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476–484. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 13.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung Adjuvant Cisplatin Evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 14.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: An overview of randomised clinical trials. Prog Cardiovasc Dis. 1985;27:335–371. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17.Stewart LA, Parmar MK. Meta-analysis of the literature or meta-analysis of individual patient data: Is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 18.Fisher DJ, Copas AJ, Tierney JF, et al. A critical review of methods for the assessment of patient-level interactions in individual participant data meta-analysis of randomized trials, and guidance for practitioners. J Clin Epidemiol. 2011;64:949–967. doi: 10.1016/j.jclinepi.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 20.Schild SE, Bonner JA, Hillman S, et al. Results of a phase II study of high-dose thoracic radiation therapy with concurrent cisplatin and etoposide in limited-stage small-cell lung cancer (NCCTG 95-20-53) J Clin Oncol. 2007;25:3124–3129. doi: 10.1200/JCO.2006.09.9606. [DOI] [PubMed] [Google Scholar]
- 21.Bentzen SM, Saunders MI, Dische S. From CHART to CHARTWEL in non-small cell lung cancer: Clinical radiobiological modelling of the expected change in outcome. Clin Oncol (R Coll Radiol) 2002;14:372–381. doi: 10.1053/clon.2002.0117. [DOI] [PubMed] [Google Scholar]
- 22.Fu S, Jiang GL, Wang LJ. Hyperfractionated irradiation for non-small cell lung cancer (NSCLC): A phase III clinical trial [in Chinese] Zhonghua Zhong Liu Za Zhi. 1994;16:306–309. [PubMed] [Google Scholar]
- 23.Sun LM, Leung SW, Wang CJ, et al. Concomitant boost radiation therapy for inoperable non-small-cell lung cancer: Preliminary report of a prospective randomized study. Int J Radiat Oncol Biol Phys. 2000;47:413–418. doi: 10.1016/s0360-3016(00)00429-6. [DOI] [PubMed] [Google Scholar]
- 24.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 25.Schild SE, Bonner JA, Shanahan TG, et al. Long-term results of a phase III trial comparing once-daily radiotherapy with twice-daily radiotherapy in limited-stage small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;59:943–951. doi: 10.1016/j.ijrobp.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Sause WT, Kolesar P, Taylor S, 4th, et al. Final results of phase III trial in regionally advanced unresectable non-small cell lung cancer: Radiation Therapy Oncology Group, Eastern Cooperative Oncology Group, and Southwest Oncology Group. Chest. 2000;117:358–364. doi: 10.1378/chest.117.2.358. [DOI] [PubMed] [Google Scholar]
- 27.Ball D, Bishop J, Smith J, et al. A randomised phase III study of accelerated or standard fraction radiotherapy with or without concurrent carboplatin in inoperable non-small cell lung cancer: Final report of an Australian multi-centre trial. Radiother Oncol. 1999;52:129–136. doi: 10.1016/s0167-8140(99)00093-6. [DOI] [PubMed] [Google Scholar]
- 28.Saunders M, Dische S, Barrett A, et al. Continuous, hyperfractionated, accelerated radiotherapy (CHART) versus conventional radiotherapy in non-small cell lung cancer: Mature data from the randomised multicentre trial—CHART Steering committee. Radiother Oncol. 1999;52:137–148. doi: 10.1016/s0167-8140(99)00087-0. [DOI] [PubMed] [Google Scholar]
- 29.Bonner JA, McGinnis WL, Stella PJ, et al. The possible advantage of hyperfractionated thoracic radiotherapy in the treatment of locally advanced non-small cell lung cancer: Results of a North Central Cancer Treatment Group phase III study. Cancer. 1998;82:1037–1048. [PubMed] [Google Scholar]
- 30.Schild SE, Stella PJ, Geyer SM, et al. Phase III trial comparing chemotherapy plus once-daily or twice-daily radiotherapy in stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:370–378. doi: 10.1016/s0360-3016(02)02930-9. [DOI] [PubMed] [Google Scholar]
- 31.Baumann M, Herrmann T, Koch R, et al. On behalf of the CHARTWEL-bronchus group: Final results of the randomized phase III CHARTWEL-trial (ARO 97-1) comparing hyperfractionated-accelerated vs conventionally fractionated radiotherapy in non-small cell lung cancer (NSCLC) Radiother Oncol. 2011;100:76–85. doi: 10.1016/j.radonc.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 32.Belani CP, Wang W, Johnson DH, et al. Phase III study of the Eastern Cooperative Oncology Group (ECOG 2597): Induction chemotherapy followed by either standard thoracic radiotherapy or hyperfractionated accelerated radiotherapy for patients with unresectable stage IIIA and B non-small-cell lung cancer. J Clin Oncol. 2005;23:3760–3767. doi: 10.1200/JCO.2005.09.108. [DOI] [PubMed] [Google Scholar]
- 33.Zajusz A, Behrendt K, Nowicka E, et al. Early results of continuous accelerated radiotherapy (CAIR) for LA-NSCLC patients. Radiother Oncol. 2006;81(suppl 1):S386. [Google Scholar]
- 34.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 35.Werner-Wasik M. Treatment-related esophagitis. Semin Oncol. 2005;32(suppl 3):S60–S66. doi: 10.1053/j.seminoncol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Kerr KM, Lamb D. Actual growth rate and tumour cell proliferation in human pulmonary neoplasms. Br J Cancer. 1984;50:343–349. doi: 10.1038/bjc.1984.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trott KR. Cell repopulation and overall treatment time. Int J Radiat Oncol Biol Phys. 1990;19:1071–1075. doi: 10.1016/0360-3016(90)90036-j. [DOI] [PubMed] [Google Scholar]
- 38.Mehta M, Scrimger R, Mackie R. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2001;49:23–33. doi: 10.1016/s0360-3016(00)01374-2. [DOI] [PubMed] [Google Scholar]
- 39.De Ruysscher D, Pijls-Johannesma M, Bentzen SM, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057–1063. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 40.De Ruysscher D, Faivre-Finn C, Nestle U, et al. European Organisation for Research and Treatment of Cancer recommendations for planning and delivery of high-dose, high-precision radiotherapy for lung cancer. J Clin Oncol. 2010;28:5301–5310. doi: 10.1200/JCO.2010.30.3271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.