CT imaging features of lung adenocarcinomas can be an image biomarker for epidermal growth factor receptor mutation status, and combining imaging-based features with clinical variables can be used to discriminate epidermal growth factor receptor mutation status better than use of clinical variables alone.
Abstract
Purpose
To retrospectively identify the relationship between epidermal growth factor receptor (EGFR) mutation status, predominant histologic subtype, and computed tomographic (CT) characteristics in surgically resected lung adenocarcinomas in a cohort of Asian patients.
materials and Methods
This study was approved by the institutional review board, with waiver of informed consent. Preoperative chest CT findings were retrospectively evaluated in 385 surgically resected lung adenocarcinomas. A total of 30 CT descriptors were assessed. EGFR mutations at exons 18–21 were determined by using the amplification refractory mutation system. Multiple logistic regression analyses were performed to identify independent factors of harboring EGFR mutation status. The final model was selected by using the backward elimination method, and two areas under the receiver operating characteristic curve (ROC) were compared with the nonparametric approach of DeLong, DeLong, and Clarke-Pearson.
Results
EGFR mutations were found in 168 (43.6%) of 385 patients. Mutations were found more frequently in (a) female patients (P < .001); (b)those who had never smoked (P < .001); (c)those with lepidic predominant adenocarcinomas (P = .001) or intermediate pathologic grade (P < .001); (e) smaller tumors (P < .001); (f)tumors with spiculation (P = .019), ground-glass opacity (GGO) or mixed GGO (P < .001), air bronchogram (P = .006), bubblelike lucency (P < .001), vascular convergence (P = .024), thickened adjacent bronchovascular bundles (P = .027), or pleural retraction (P < .001); and (g) tumors without pleural attachment (P = .004), a well-defined margin (P = .010), marked heterogeneous enhancement (P = .001), severe peripheral emphysema (P = .002), severe peripheral fibrosis (P = .013), or lymphadenopathy (P = .028). The most important and significantly independent prognostic factors of harboring EGFR-activating mutation for the model with both clinical variables and CT features were those who had never smoked and those with smaller tumors, bubblelike lucency, homogeneous enhancement, or pleural retraction when adjusting for histologic subtype, pathologic grade, or thickened adjacent bronchovascular bundles. ROC curve analysis showed that use of clinical variables combined with CT features (area under the ROC curve = 0.778) was superior to use of clinical variables alone (area under the ROC curve = 0.690).
Conclusion
CT imaging features of lung adenocarcinomas in combination with clinical variables can be used to prognosticate EGFR mutation status better than use of clinical variables alone.
© RSNA, 2016
Introduction
During the past few decades, great advances have been taken place in pathologic analysis and molecular biology of lung adenocarcinoma. An international multidisciplinary classification sponsored by the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) was proposed in 2011 (1), and it has been reported that this classification has substantial correlations with molecular changes (2–5). In particular, epidermal growth factor receptor (EGFR) mutations had a high frequency among some histologic subtypes of adenocarcinoma; however, the relationships between EGFR mutations and the new lung adenocarcinoma classification schema are unclear.
The discovery of activating mutations in EGFR of lung adenocarcinomas has led to a paradigm shift in the care of patients with lung cancer. Several studies have revealed that mutation in patients with EGFR is a strong factor with which to predict outcomes in patients who received receptor tyrosine kinase inhibitors (TKIs), and approximately 70%–80% of patients with EGFR-mutant lung cancer initially respond to EGFR TKI therapy (6). In patients with mutated EGFR lung cancer, TKIs may significantly contribute to improve patients’ disease control, symptoms, and quality of life when compared with those in patients who underwent standard platinum-based chemotherapy (7,8). Thus, it is important to determine EGFR mutation status prior to the use of TKIs in patients with non–small cell lung cancer. However, sometimes it is not feasible to acquire adequate tissue for EGFR mutational analysis before the initiation of treatment because of inoperability, small biopsy specimens, or sampling artifact. In this situation, nonsmoking status, female sex, Asian ethnicity, and adenocarcinoma histologic type have been initially identified as potential prerequisites to achieve response in patients who received erlotinib and gefitinib (9,10). These predictors have been subsequently shown to be only representative of a clinically selected population with a high likelihood of harboring the sensitizing mutation. Furthermore, tumor cells harvested for molecular analysis with needle biopsy represent only a very small fraction of tumor burden, and this fraction might be even smaller in patients who underwent fine-needle aspiration. Taniguchi et al (11) analyzed 50–60 areas of tumor tissue in 21 patients with a known EGFR mutation and documented intratumoral heterogeneity in six (28.6%) of the 21 tumors, which contained both EGFR-mutated and wild-type cells. Hence, more detailed factors are needed to enrich the treated population via EGFR mutation status, and we contend that it is prudent to profoundly analyze pretreatment computed tomographic (CT) images, as these data are commonly available. For instance, they can provide complimentary data for genomics and can potentially be used to identify patients at risk for EGFR mutations. In case of negative mutation results from biopsy, which is prone to sampling artifacts, or from less sensitive techniques, such as direct sequencing, resampling of the tumor in nonsurgical cases should be considered if CT findings suggest a high probability of EGFR mutation; otherwise, these mutations would be missed, and these patients would not receive potentially beneficial TKI therapy. In this study, we detected EGFR mutations in lung cancer samples with the Amplification Refractory Mutation System (ARMS) technique (12,13), which is a more sensitive and robust assay used to detect somatic mutations of EGFR in patients with lung cancer, compared with direct sequencing.
To date, relatively few studies have investigated the relationships between CT imaging features with EGFR mutations (14–20). Most of these studies have focused on ground-glass opacity (GGO) ratio, and some of the findings are conflicting. Thus, the aim of the present study was to retrospectively identify the relationship between EGFR mutation status, predominant histologic subtype, and CT characteristics in surgically resected lung adenocarcinomas in a cohort of Asian patients.
Materials and Methods
Patients
This retrospective study included patients with histologically confirmed adenocarcinoma of the lung for primary lung cancer from December 2012 to March 2014. The institutional review board approved this study, with waiver of informed consent. Subjects were collected consecutively. A total of 756 patients initially were included in the present study according to the following inclusion criteria: (a) preoperative thin-section CT images were acquired with our picture archiving and communication system, (b) available pathology reports with diagnosis of lung adenocarcinoma, (c) available test results for EGFR mutation status, and (d) available clinical data, including age, sex, smoking history (patients were classified as nonsmokers [ie, those who had never smoked] or smokers [former and current smokers]), and TNM stage (21). Among them, 371 patients were excluded due to the following exclusion criteria: (a) patients did not undergo surgery (n = 280); (b) patients received preoperative treatment, such as radiation therapy or chemotherapy (n = 73); and (c) interval between CT and subsequent surgery exceeded 1 month (n = 18). Finally, 385 patients were included. All patients were ethnically Asian. Female patients (213 of 385 [55.3%]) and nonsmokers (210 of 385 [54.5%]) were predominant in this study. The most common histologic subtype among patients with invasive adenocarcinoma was acinar predominant subtype (166 of 382 [43.5%]). Most patients had early stage disease, which included stage I or II disease (245 of 385 [63.6%]) and intermediate pathologic grade (270 of 384 [70.3%]). EGFR mutations were identified in 168 of 385 tumors (43.6%), 75 had an exon 19 mutation, 84 had an exon 21 mutation, four had an exon 20 mutation, four had an exon 18 mutation, and one patient had two mutations (exons 20 and 21).
CT Scanning Protocol
Chest CT examinations were performed by using one of three multidetector CT systems (Somatom Sensation 64, Siemens, Erlangen, Germany; Lightspeed 16, GE Healthcare, Milwaukee, Wis; Discovery CT750 HD, GE Healthcare). Scanning parameters were as follows: (a) 120 kVp with tube current adjusted automatically and 1.5-mm reconstruction thickness with a 1.5-mm reconstruction interval for the 64-detector scanner and (b) 120 kVp, 150–200 mA, and 1.25-mm reconstruction thickness with a 1.25-mm reconstruction interval for the other two scanners. A total of 346 patients (346 of 385 [89.9%]) underwent contrast material–enhanced CT. Nonionic iodinated contrast material (300 mg of iodine per milliliter, Ultravist; Bayer Pharma, Berlin, Germany) was injected into the antecubital vein at a dose of 1.3–1.5 mL per kilogram of body weight at a rate of 2.5 mL/sec by using an automated injector. CT scanning was performed with a 70-second delay.
CT Image Interpretation
A clinical radiologist with 6 years of experience (Y.L.) in CT imaging of thoracic malignancies and two other radiologists with 3 years of experience (F.Q., S.L.) retrospectively interpreted the CT images independently (Table 1; Figs E1–E2 [online]; Table E1 [online]). All of these radiologists were blinded to clinical and histologic findings. The majority class was used as the final CT feature value in case of disagreement. Mean values were used for continuous variables. CT images were read with both mediastinal (width, 350 HU; level, 40 HU) and lung (width, 1500 HU; level, -600 HU) window settings.
Table 1.
CT Characteristics for Lung Adenocarcinoma

Note.—NA = not applicable.
*If the lesion crosses a fissure, the lobe location is defined as the lobe in which the lesion is predominantly located.
In accordance with Zwirewich et al (22), coarse spiculation was defined as the presence of linear strands at least 2 mm thick that extended from the nodule margin into the lung parenchyma, and fine spiculation was defined as the presence of linear strands thinner than 2 mm. Tumor enhancement was described both quantitatively and qualitatively. Homogeneity was defined as present when more than 90% of the area was occupied by the same attenuation value, as ascertained by visual inspection. The artery on the same section of the tumor was used as a reference site, and relative enhancement (Erel) was calculated with the following formula:
where Apost is contrast-enhanced CT attenuation of tumor, Apre is unenhanced CT attenuation of the tumor, and Eart is enhancement attenuation of the artery.
Histologic Evaluation and EGFR Mutation Analysis
Tumors were diagnosed as adenocarcinoma and were categorized according to the 2011 IASLC/ATS/ERS classification system. Molecular analysis of mutation status of EGFR exons 18, 19, 20, and 21 was examined with a polymerase chain reaction–based ARMS by using the Human EGFR Gene Mutations Detection Kit (Beijing ACCB Biotech, Beijing, China).
Statistical Analysis
All statistical analyses were performed by using statistical software (version 9.4; SAS Institute, Cary, NC). Agreement between three readers was measured with the κ index and Kendall coefficient of concordance. For continuous variable, intraclass correlation of coefficient was calculated. The Wilcoxon rank sum test and the χ2 test were used to compare medians and proportions between two groups, respectively. Comparisons between two areas under the receiver operating characteristic curve were made with the nonparametric approach of DeLong, DeLong, and Clarke-Pearson (23). Two-sided P <.05 indicated a significant difference. Multiple logistic regression analyses were performed to identify independent factors that can be used to predict EGFR mutation status. The final model was selected with the backward elimination method, with a cutoff P value of .15.
Results
Reader Reproducibility
Agreement among three readers was good (Table E2 [online]). The intraclass correlation coefficient for long-axis diameter, short-axis diameter, degree of enhancement, and relative enhancement was 0.99 (range, 0.99–0.99), 0.98 (range, 0.98–0.98), 0.80 (range, 0.76–0.83), and 0.67 (range, 0.63–0.72), respectively.
Correlation of EGFR Mutation Status with Clinical Features
A total of 385 patients (mean age, 59.2 years; age range, 30–80 years) were included in this study. The mean age of male patients (59.8 years; age range, 37–79 years) was not significantly different from that of female patients (58.7 years; age range, 30–80 years) as determined with Wilcoxon rank sum statistics (P = .42). Associations between clinical factors and EGFR mutation status are presented in Table 2. The median age of patients did not differ between the wild-type and EGFR mutant groups (P = .34). EGFR mutation rates were significantly higher (a) in women (113 of 213 [53.1%]) than in men (55 of 172 [32.0%]) (P < .001) and (b) in nonsmokers (116 of 210 [55.2%]) than in smokers (52 of 175 [29.7%]) (P < .001). No significant association was observed between cancer staging and the presence of EGFR mutation (P = .985).
Table 2.
Association between Clinical Characteristics with EGFR Mutation Status

Note.—Data in parentheses are the range. OR = odds ratio.
*P value was based on comparison between EGFR mutation group with wild-type group.
†Data in parentheses are 95% confidence intervals (CIs).
‡Histologic subtype was categorized according to the 2011 IASLC/ATS/ERS classification system.
§Histologic subtype was categorized as lepidic predominant adenocarcinomas (adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic predominant invasive adenocarcinoma) and other subtypes of dominant histologic findings (acinar, papillary, micropapillary, and solid predominant, as well as variants of invasive adenocarcinoma).
||Pathologic grade of the new classification defined adenocarcinoma in situ and minimally invasive adenocarcinoma as low; lepidic, acinar, and papillary adenocarcinoma as intermediate; and micropapillary, solid, and invasive mucinous adenocarcinoma as high. Three cases of minimally invasive adenocarcinoma and one case of enteric adenocarcinoma (variants of invasive adenocarcinoma) were removed from this part of the comparison.
In regard to tumor histology, EGFR mutations were associated with a high frequency of lepidic (49 of 80 [61.3%]) predominant subtypes. EGFR mutations were less frequent in the solid predominant subtype (17 of 82 [20.7%]). There was a significant association between EGFR mutation status and histologic subtype (P < .001). When tumors were divided into lepidic predominant adenocarcinomas (adenocarcinoma in situ, minimally invasive adenocarcinoma, and lepidic predominant invasive adenocarcinoma) and other subtypes of dominant histologic findings (acinar, papillary, micropapillary, and solid predominant adenocarcinoma, as well as variants of invasive adenocarcinoma), EGFR mutations were more likely to be lepidic predominant adenocarcinomas (50 of 83 [60.2%]) than other subtype (118 of 302 [39.1%]) (OR, 2.53; 95% CI: 1.43, 3.86; P = .001). Significantly lower mutation rates were observed in high-pathologic-grade cancers with an OR of 0.36 (95% CI: 0.22, 0.58; P < .001).
Correlation of EGFR Mutation Status with Radiologic Features
Univariate analysis (Table 3) revealed that 16 CT features were associated with EGFR mutation, including long-axis diameter (P < .001), short-axis diameter (P = .003), size category (P < .001), pleural attachment (P = .004), border definition (P = .010), spiculation (P = .019), texture (P < .001), air bronchogram (P = .006), bubblelike lucency (P < .001), enhancement heterogeneity (P = .001), vascular convergence (P = .024), thickened adjacent bronchovascular bundles (P = .027), pleural retraction (P < .001), peripheral emphysema (P = .002), peripheral fibrosis (P = .013), and lymphadenopathy (P = .028). Both long- and short-axis tumor diameter on chest CT images in patients with EGFR mutations were significantly smaller than those in patients with a wild-type mutation, with ORs of 0.78 (95% CI: 0.68, 0.89) and 0.75 (95% CI: 0.63, 0.90), respectively. Tumors with an ill-defined margin (OR of level 2, 5.22; 95% CI: 1.49, 18.26; OR of level 3, 3.75; 95% CI: 1.04, 13.48), spiculation (OR of level 2, 2.01; 95% CI: 1.19, 3.39; OR of level 3, 1.75; 95% CI: 1.07, 2.86), air bronchogram (OR, 1.77; 95% CI: 1.17, 2.66), bubblelike lucency (OR, 3.37; 95% CI: 2.19, 5.18), vascular convergence (OR, 1.67; 95% CI: 1.07, 2.59), thickened adjacent bronchovascular bundles (OR, 1.59; 95% CI: 1.05, 2.39), and pleural retraction (OR, 2.84; 95% CI: 1.86, 4.32) were more frequently found among tumors with EGFR mutation than among wild-type tumors. Tumors with pleural attachment (OR, 0.54; 95% CI: 0.35, 0.83), those without a GGO component (OR, 0.41; 95% CI: 0.26, 0.65), those with marked heterogeneous enhancement (OR, 0.23; 95% CI: 0.10, 0.53), and those with lymphadenopathy (OR, 0.62; 95% CI: 0.40, 0.95) were more frequently found among tumors with wild-type EGFR. No significant associations were observed between EGFR mutation status and other CT features (Table E3 [online]).
Table 3.
Association between CT Features with EGFR Mutation Status
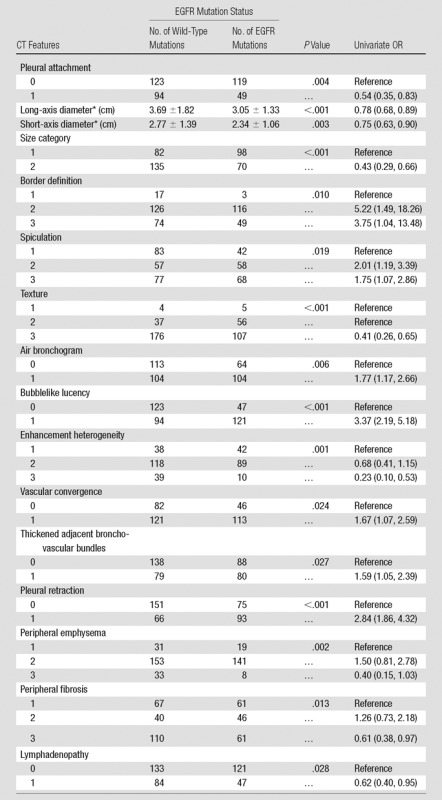
Note.—Data in parentheses are 95% CIs.
*Data for long- and short-axis diameter are mean ± standard deviation.
We then further explored the correlation of EGFR mutation status with peripheral emphysema and fibrosis in nonsmokers and smokers separately (Table E4 [online]). No significant associations were found between EGFR mutation status and peripheral emphysema (P = .08) or fibrosis (P = .20) in nonsmokers. However, in smokers, EGFR mutations were detected more frequently without severe emphysema (P = .025; OR of level 2 vs level 1, 0.75; 95% CI: 0.28, 1.98; OR of level 3 vs level 2, 0.26; 95% CI: 0.09, 0.78; OR of level 3 vs level 1, 0.19; 95% CI: 0.05, 0.76) or severe fibrosis (P = .014; OR of level 2 vs level 1, 0.98; 95% CI: 0.42, 2.30; OR of level 3 vs level 2, 0.37; 95% CI: 0.17, 0.83; OR of level 3 vs level 1, 0.36; 95% CI: 0.16, 0.81).
Multivariable Analyses of Prognostic Factors for EGFR Mutations and ROC Curve Analysis
For the model with clinical variables alone, pathologic grade and smoking history were proved to be independent prognostic factors of harboring EGFR mutation when adjusting for histologic subtype via multivariable logistic regression analysis (Table E5 [online]). For the model with both clinical variables and CT features, these three clinical features were kept in the model, regardless of statistical significance. The most important and significantly independent prognostic factors of harboring EGFR mutation were never having smoked, smaller tumor size, bubblelike lucency, homogeneous enhancement, and pleural retraction when adjusting for histologic subtype, pathologic grade, and thickened adjacent bronchovascular bundles (Table 4). The area under the receiver operating characteristic curve significantly increased from 0.690 to 0.778 when imaging features were added (P < .001) (Fig 1 ; Table E6 [online]).
Table 4.
Multivariable Logistic Regression Analysis of CT Features Combined with Clinical Variables Predicting the Presence of EGFR Mutation in Lung Adenocarcinoma

Note.—NA = not applicable.
Receiver operating characteristic curves used to predict EGFR mutation status with clinical variables alone and combined with CT features.
Discussion
It is now common practice to obtain tissue samples of all lung lesions to test for EGFR. In this study, we detected EGFR mutations in lung cancer samples by using the ARMS technique. The incidence of EGFR mutations in the current cohort of Asian patients was 43.6%, most EGFR-mutated tumors harbored exon 19 or 21 mutations, accounting for 44.6% and 50% of all mutated tumors respectively. As expected, we found that EGFR mutations were more common among female patients and nonsmokers; this finding was consistent with previous results (24–28).
Recently, a few studies have reported correlations between predominant subtype in lung adenocarcinomas and EGFR mutations. Song et al (24) examined 161 surgically resected lung adenocarcinomas in Chinese patients and found that EGFR mutations were significantly more frequent in micropapillary and lepidic predominant subtypes and less common in the solid predominant subtype. Zhang et al (26) found the frequency of EGFR mutations was positively correlated with acinar predominant tumors and negatively correlated with invasive mucinous adenocarcinoma and solid predominant tumors. Sun et al (27) found that papillary predominant adenocarcinomas showed more frequent EGFR mutations. The results of the present work showed that EGFR mutations were associated with a high frequency of lepidic predominant subtypes and were uncommon in the solid predominant subtype, all of which were similar to the findings published by Song et al (24). The different outcomes from literature between EGFR mutations and histologic subtypes may be related to study sample size and a difference in ethnicity. Furthermore, we found that EGFR mutations were more frequent in patients with lepidic predominant adenocarcinoma than in patients with other types of adenocarcinoma. This can be supported by the results of a prior study which found that EGFR exon 21 mutations were significantly more common in patients with lepidic predominant adenocarcinomas (15).
There are limited numbers of reports regarding the association between EGFR mutations and CT imaging features. Yano et al (18) found that tumors with a GGO ratio of 50% or more and a diameter of 3 cm or less often have EGFR mutation in peripheral pulmonary adenocarcinoma. Sugano et al (16) found no significant association between GGO, spiculation, and EGFR mutation status. A recent study by Zhou et al (20) compared the clinicoradiologic characteristics of lung adenocarcinomas with anaplastic lymphoma kinase rearrangements or EGFR mutations. They found that spiculated margin, pleural retraction, and air bronchogram were more frequent in the EGFR mutation group than in the wild-type group, but there was no significant difference between these groups. In another study (19), there were significantly higher percentages of air bronchogram, pleural retraction, or absence of emphysema among patients with an EGFR mutation. However, CT features related to contrast material enhancement of the lesion and histologic features were not included in their analysis. In the current study, we quantified 30 detailed CT features and found that tumors of smaller size, as well as those with spiculation, a GGO component, air bronchogram, bubblelike lucency, vascular convergence, thickened adjacent bronchovascular bundles, or pleural retraction were more likely to be tumors with EGFR mutations, while tumors with pleural attachment, well-defined margins, marked heterogeneous enhancement, severe peripheral emphysema, severe peripheral fibrosis, or lymphadenopathy were more likely to be wild-type tumors. Some of these findings are consistent with findings of previous studies (18,19) but are contrary to the findings of Sugano et al (16). These conflicting results among studies may be due to differences in study design and grouping method. More importantly, some CT characteristics, especially those related to contrast enhancement of the lesion, which have not been previously evaluated for the purpose of finding prognostic factors for EGFR mutations, were found to have associations with EGFR mutation status. It is reported that the frequency of EGFR was low in patients with emphysema and fibrosis compared with the frequency of EGFR in patients without emphysema and fibrosis (25); however, the correlation between underlying lung diseases and EGFR mutation status in smokers with lung cancer has not been thoroughly investigated. We found that in smokers, those without severe emphysema or fibrosis have a higher possibility of developing EGFR-mutated tumors; this finding was in agreement with findings of a prior study (28). This suggests that it may be possible to prognosticate the presence of EGFR mutations from CT findings of peripheral emphysema or fibrosis in smokers. In clinical practice, EGFR TKIs may be effective for lung cancer, even in smokers who develop EGFR-mutant lung cancer.
One important aim of promoting the application of CT features in patients with lung adenocarcinoma is to more accurately enrich patient populations for EGFR TKI treatment. In a clinical study conducted by Zhou et al (29), it was confirmed that median progression-free survival of patients with a low abundance of EGFR mutations (positive for mutation with ARMS but negative with direct DNA sequencing) was significantly longer than for those with wild-type tumors (negative for mutation with both direct DNA sequencing and ARMS), and the difference between patients with high (positive for mutation with both direct DNA sequencing and ARMS) and low abundance of EGFR mutations was not significant regarding overall response rate and overall survival. Thus, we believe that identifying this genetically unique subset of patients is of great importance; even patients with a small portion of EGFR mutant cells will receive benefits from TKI therapy. We report a significant relationship between CT imaging characteristics and EGFR mutation status in patients with resected lung adenocarcinoma. Multivariable regression analyses indicated that nonsmokers and smaller tumors, as well as tumors with bubblelike lucency, homogeneous enhancement, or pleural retraction, were independent prognostic factors for harboring EGFR mutation and that the combination of clinical variables with CT features improved prognostic ability from 0.690 to 0.778, as measured with the area under the receiver operating characteristic curve.
There were several limitations in this study. First, its retrospective nature may have caused a selection bias. Second, we limited our analyses to adenocarcinomas and did not address other histologic subtypes. This was done because the majority of EGFR mutations are found in adenocarcinomas. Third, in this study, no survival data were provided because cases were collected from 2013 to 2014 and because follow-up time was insufficient. Finally, there might have been some differences in CT features between low and high proportions of EGFR mutant cell groups, but we did not have the data to differentiate these groups in the current study. However, it is expected that future research will reveal relationships between EGFR mutation status, treatment protocol, CT features, and survival.
In conclusion, CT features of lung adenocarcinomas can be an image biomarker for EGFR mutation status, and combining imaging-based features with clinical variables can enable discrimination of EGFR mutation status better than use of clinical variables alone.
Advances in Knowledge
■ Sixteen CT features were significantly associated with epidermal growth factor receptor (EGFR) mutation status, including long-axis diameter (P < .001), short-axis diameter (P = .003), size category (P < .001), spiculation (P = .019), texture (P < .001), air bronchogram (P = .006), bubblelike lucency (P < .001), vascular convergence (P = .024), thickened adjacent bronchovascular bundles (P = .027), pleural retraction (P < .001), pleural attachment (P = .004), border definition (P = .010), enhancement heterogeneity (P = .001), peripheral emphysema (P = .002), peripheral fibrosis (P = .013), and lymphadenopathy (P = .028).
■ A model with clinical variables and CT features enabled better discrimination than did the model with clinical variables alone.
■ The area under the receiver operating characteristic curve significantly increased from 0.690 to 0.778 by adding imaging features (P < .001) (95% confidence interval: 0.043, 0.134).
Implication for Patient Care
■ Our study enabled us to confirm that pretreatment CT images could provide complimentary data to genomics and potentially could be used to identify patients at risk for EGFR mutations.
SUPPLEMENTAL TABLES
SUPPLEMENTAL FIGURES
Acknowledgments
Acknowledgments
We thank Steven A. Eschrich, PhD, Department of Biostatistics and Bioinformatics, H. Lee Moffitt Cancer Center and Research Institute, and John J. Heine, PhD, Department of Cancer Imaging and Metabolism, H. Lee Moffitt Cancer Center and Research Institute, for providing helpful discussions and Zhongli Zhan for performing histologic evaluation and EGFR mutation analysis.
Received July 5, 2015; revision requested September 15; revision received September 24; accepted October 23; final version accepted December 11.
Supported by the Tianjin Science and Technology Major Project (grant 12ZCDZSY15500) and Public Science and Technology Research Funds Projects of NHFPC of the P.R. China (grant 201402013).
Funding: This research was supported by the National Institutes of Health (grants U01 CA143062 and 5P30CA076292-16).
Disclosures of Conflicts of Interest: Y.L. disclosed no relevant relationships. J.K. disclosed no relevant relationships. F.Q. disclosed no relevant relationships. S.L. disclosed no relevant relationships. H.W. disclosed no relevant relationships. Y.B. disclosed no relevant relationships. Z.Y. disclosed no relevant relationships. R.J.G. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received nonfinancial support from Health Myne. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ARMS
- amplification refractory mutation system
- CI
- confidence interval
- EGFR
- epidermal growth factor receptor
- GGO
- ground glass opacity
- IASLC/ATS/ERS
- International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society
- OR
- odds ratio
- TKI
- tyrosine kinase inhibitor
References
- 1.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc 2011;8(5):381–385. [DOI] [PubMed] [Google Scholar]
- 2.Villa C, Cagle PT, Johnson M, et al. Correlation of EGFR mutation status with predominant histologic subtype of adenocarcinoma according to the new lung adenocarcinoma classification of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society. Arch Pathol Lab Med 2014;138(10):1353–1357. [DOI] [PubMed] [Google Scholar]
- 3.Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8(1):52–61. [DOI] [PubMed] [Google Scholar]
- 4.Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8(4):461–468. [DOI] [PubMed] [Google Scholar]
- 5.Shim HS, Lee H, Park EJ, Kim SH. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011;135(10):1329–1334. [DOI] [PubMed] [Google Scholar]
- 6.Choi CM, Kim MY, Lee JC, Kim HJ. Advanced lung adenocarcinoma harboring a mutation of the epidermal growth factor receptor: CT findings after tyrosine kinase inhibitor therapy. Radiology 2014;270(2):574–582. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 8.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12(8):735–742. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353(2):123–132. [DOI] [PubMed] [Google Scholar]
- 10.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290(16):2149–2158. [DOI] [PubMed] [Google Scholar]
- 11.Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci 2008;99(5):929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu H, Zhong C, Xue G, et al. Direct sequencing and amplification refractory mutation system for epidermal growth factor receptor mutations in patients with non-small cell lung cancer. Oncol Rep 2013;30(5):2311–2315. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Zhao R, Zhang J, Zhang J. ARMS for EGFR mutation analysis of cytologic and corresponding lung adenocarcinoma histologic specimens. J Cancer Res Clin Oncol 2015;141(2):221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Lee HJ, Kim YT, et al. Imaging characteristics of stage I non-small cell lung cancer on CT and FDG-PET: relationship with epidermal growth factor receptor protein expression status and survival. Korean J Radiol 2013;14(2):375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HJ, Kim YT, Kang CH, et al. Epidermal growth factor receptor mutation in lung adenocarcinomas: relationship with CT characteristics and histologic subtypes. Radiology 2013;268(1):254–264. [DOI] [PubMed] [Google Scholar]
- 16.Sugano M, Shimizu K, Nakano T, et al. Correlation between computed tomography findings and epidermal growth factor receptor and KRAS gene mutations in patients with pulmonary adenocarcinoma. Oncol Rep 2011;26(5):1205–1211. [DOI] [PubMed] [Google Scholar]
- 17.Hsu KH, Chen KC, Yang TY, et al. Epidermal growth factor receptor mutation status in stage I lung adenocarcinoma with different image patterns. J Thorac Oncol 2011;6(6):1066–1072. [DOI] [PubMed] [Google Scholar]
- 18.Yano M, Sasaki H, Kobayashi Y, et al. Epidermal growth factor receptor gene mutation and computed tomographic findings in peripheral pulmonary adenocarcinoma. J Thorac Oncol 2006;1(5):413–416. [PubMed] [Google Scholar]
- 19.Rizzo S, Petrella F, Buscarino V, et al. CT radiogenomic characterization of EGFR, K-RAS, and ALK mutations in non-small cell lung cancer. Eur Radiol 2016;26(1):32–42. [DOI] [PubMed] [Google Scholar]
- 20.Zhou JY, Zheng J, Yu ZF, et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol 2015;25(5):1257–1266. [DOI] [PubMed] [Google Scholar]
- 21.Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E, Jr. The 7th lung cancer TNM classification and staging system: review of the changes and implications. World J Radiol 2012;4(4):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology 1991;179(2):469–476. [DOI] [PubMed] [Google Scholar]
- 23.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44(3):837–845. [PubMed] [Google Scholar]
- 24.Song Z, Zhu H, Guo Z, Wu W, Sun W, Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30(3):645. [DOI] [PubMed] [Google Scholar]
- 25.Usui K, Ushijima T, Tanaka Y, et al. The frequency of epidermal growth factor receptor mutation of nonsmall cell lung cancer according to the underlying pulmonary diseases. Pulm Med 2011;2011:290132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Sun Y, Pan Y, et al. Frequency of driver mutations in lung adenocarcinoma from female never-smokers varies with histologic subtypes and age at diagnosis. Clin Cancer Res 2012;18(7):1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun PL, Seol H, Lee HJ, et al. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol 2012;7(2):323–330. [DOI] [PubMed] [Google Scholar]
- 28.Sekine A, Tamura K, Satoh H, et al. Prevalence of underlying lung disease in smokers with epidermal growth factor receptor-mutant lung cancer. Oncol Rep 2013;29(5):2005–2010. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Zhang XC, Chen ZH, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29(24):3316–3321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



