Abstract
Published atrial fibrillation (AF) guidelines and decision tools offer oral anticoagulant (OAC) recommendations; however, they consider stroke and bleeding risk differently. The aims of our study are: (i) to compare the variation in OAC recommendations by the 2012 American College of Chest Physicians guidelines, the 2012 European Society of Cardiology (ESC) guidelines, the 2014 American Heart Association (AHA) guidelines and two published decision tools by Casciano and LaHaye; (ii) to compare the concordance with actual OAC use in the overall study population and the population stratified by stroke/bleed risk. A cross-sectional study using the 2001–2013 Lifelink claims data was used to contrast the treatment recommendations by these decision aids. CHA2DS2-VASc and HAS-BLED algorithms were used to stratify 15,129 AF patients into nine stroke/bleed risk groups to study the variation in treatment recommendations and concordance with actual OAC use/non-use. The AHA guidelines which were set to recommend OAC when CHA2DS2-VASc = 1 recommended OAC most often (86.30%) and the LaHaye tool recommended OAC the least often (14.91%). OAC treatment recommendations varied considerably when stroke risk was moderate or high (CHA2DS2-VASc > 0). Actual OAC use/non-use was highly discordant (>40%) with all of the guidelines or decision tools reflecting substantial opportunities to improve AF OAC decisions.
Keywords: atrial fibrillation, guidelines, decision tools, overuse, underuse, oral anticoagulants, warfarin, recommendations
1. Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia which increases the risk of ischemic stroke 4 to 5 fold [1]. Oral anticoagulants (OACs) are more effective than aspirin in reducing the stroke risk, but are also associated with an increased bleeding risk. Therefore, anticoagulant recommendations depend upon balancing the expected benefit of stroke risk reduction against the increased harm from bleeding in patients with different factors that are prognostic for strokes and bleeding [2].
Several studies have shown that anticoagulant prescribing is often poorly related to patient stroke risk with underuse of anticoagulants in AF being more common, especially among elevated stroke risk patients [3,4,5]. One of the reasons for the under use of anticoagulants is the variation in physicians’ perceptions of the relative risk of stroke compared to bleeding by their specialty [6]. Physicians with a good personal experience with warfarin are more likely to prescribe warfarin; however, even these physicians prescribe warfarin in only half of their warfarin eligible AF patients [7]. Oral anticoagulants are not only underused but are also overused in the patients with low stroke risk [8].
Given the evidence of inappropriate OAC prescribing, various AF guidelines and decision support tools have been developed to aid clinicians considering oral anticoagulants for their AF patients. These decision aids include various clinical guidelines, model based decision tools and electronic medical record based apps and tools. However, these guidelines and decision support tools do not use the same stroke and bleed risk algorithms for treatment recommendations (Table S1) [9,10,11,12,13,14,15,16]. All the contemporary decision aids rely on the CHADS2 or the CHA2DS2-VASc scores to estimate stroke risk [17,18]. Some tools include formal bleed risk assessments including HAS-BLED and ATRIA, which are validated tools to estimate bleeding risk in AF patients [19,20]. The differences in the preferences for the stroke/bleed risk algorithm by these decision aids are due to the differences in the expert opinion and/or a lack of sufficient evidence supporting use of one approach over the other. The 2012 American College of Chest Physicians (CHEST) guideline recommendations are solely based on the stroke risk (CHADS2) scores as they have been extensively validated and easy for clinicians to remember, whereas the 2012 European Society of Cardiology (ESC) guidelines and the 2014 American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society (AHA/ACC/HRS hereafter “AHA”) guideline recommendations are based on CHA2DS2-VASc [10,12,13]. Existing evidence suggest that the CHA2DS2-VASc has a better ability to identify patients who are at “truly low stroke risk” with a comparable overall discriminative ability in predicting ischemic stroke when compared to CHADS2 score [13]. Two published decision tools by Casciano et al. (hereafter “Casciano tool”) and LaHaye et al. (hereafter “Lahaye tool”) also include bleed risk scores (Casciano based on ATRIA, LaHaye based on HAS BLED) along with the stroke risk scores (Casciano based on CHADS2, LaHaye based on CHA2DS2-VASc) to derive their treatment recommendations. The tool developers did not provide a justification for choice of one bleed risk score over the other but this could be due to the lack of studies comparing the two bleed risk algorithms when the decision tools were developed [9,11].
Currently, there are no population-based studies that have compared the treatment recommendations by the AF guidelines and decision support tools. To address this, we empirically compared the treatment recommendations by the 2012 CHEST guidelines, the 2012 ESC guidelines, the 2014 AHA guidelines, the Casciano tool and the LaHaye tool [9,10,11,12,13]. To compare the acceptance of the guideline and decision tool recommendations in real world practice, we compared the concordance of actual OAC use/non-use between the decision aid recommendations. To understand the differences in the treatment recommendations by the decision aids and their concordance with actual OAC use/non-use at a more granular level, we also stratified our study population by their baseline stroke and bleed risk.
2. Methods
2.1. Study Design
A cross sectional study design using incident cases of AF from a 10% sample of the Lifelink claims database from 2001 to 2013 was used to contrast the treatment recommendations by these decision aids. The Lifelink claims data is nationally representative of the commercially insured population with respect to age, gender, geographic location and the type of insurance coverage. Separate files for inpatient claims, outpatient claims, prescription claims and eligibility data are available in the database. The inpatient and outpatient claim files include diagnosis codes in the International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) format as well as procedure codes in the Healthcare Common Procedure Coding System (HCPCS) format. The prescription claim files include data on national drug code (NDC) level and represents prescriptions covered by the insurance plan. The eligibility file was used to determine monthly enrolment/disenrollment in health plans. This study was approved by the University of Arkansas for Medical Sciences Institutional Review Board.
2.2. Study Subjects
The first primary diagnosis of AF was defined as the index diagnosis. Patients without any AF related claim within 12 months before the index diagnosis were defined as incident cases of AF. To rule out transient AF cases, we required patients to have at least two AF related claims separated by 30 days and have at least one outpatient claim with primary AF diagnosis in the post index period. Patients were also required to have continuous medical and pharmacy benefit eligibility for at least 12 months prior and three months after the index diagnosis. Table S2 shows a flowchart depicting the details of inclusion-exclusion criteria applied for the selection of final study sample.
2.3. Exposure Definition
The Lifelink claims database does not completely capture over-the-counter (OTC) aspirin use. Also, the newer oral anticoagulants were not available during most of the study time period. Therefore, the exposure definition was limited to oral anticoagulant (OAC) exposed and unexposed. A patient with at least one prescription claim for an oral anticoagulant (warfarin, dabigatran, apixaban or rivaroxaban) within 90 days of index diagnosis or at least one International Normalized Ratio/Prothrombin Time (INR/PT) claim within 90 days of the index diagnosis followed by a second INR/PT claim within 90 days of the first INR/PT claim date was considered as an OAC exposed patient. INR/PT test is often used as a proxy for warfarin exposure [21,22]. Patients who did not meet the definition of OAC exposure (including aspirin users) were considered as OAC unexposed patients.
2.4. Stroke Risk and Bleeding Risk
We calculated the stroke risk and bleeding risk using two stroke risk (CHADS2 and CHA2DS2-VASc) and two bleeding risk (ATRIA and HAS-BLED) algorithms. The operational definition to calculate stroke and bleeding risk scores were obtained from previous studies using administrative claims data [9,23,24,25].
2.5. Decision Aid Recommendations
The Casciano recommendations are based on the CHADS2 score, ATRIA score, patient characteristics (age and gender) to estimate the quality-adjusted life expectancy with warfarin/aspirin treatment [11]. The LaHaye tool offers treatment recommendations for aspirin, warfarin and newer oral anticoagulants based on the CHA2DS2-VASc score, HAS-BLED score, a treatment threshold (minimum stroke risk reduction after initiating antithrombotic therapy), a cost threshold (maximum cost that a patient is willing to pay per day in order to realize the specified treatment threshold) and bleed ratio (maximum number of major bleed events that a patient can endure to prevent one stroke event). Our analysis was restricted to warfarin and aspirin (ASA) recommendations of the LaHaye tool that were obtained by using fixed values of the treatment threshold (0.3%), the cost threshold ($0.50) and the bleed ratio (2:1) [9]. The CHEST guideline recommendations are based on the CHADS2 score while the ESC and the AHA guideline recommendations are based on the CHA2DS2-VASc algorithm [10,12,13]. The ESC guideline recommends caution for prescribing OAC when HAS BLED ≥ 3. To operationalize the ESC OAC recommendation, we set the ESC guideline to recommend withholding OAC in patients with CHA2DS2-VASc score = 1 and HAS BLED ≥ 3, and OAC in patients whose HAS-BLED score is less than 3 and CHA2DS2-VASc score = 1, and OAC in all patients when CHA2DS2-VASc score > 1 [13]. The AHA guideline does not offer a clear recommendation for OAC in patients when CHA2DS2-VASc score = 1. Therefore, we compared two scenarios: (i) conservative OAC AHA recommendations which assume that OAC is not recommended (AHA Conservative) and (ii) aggressive OAC AHA recommendations which assume that OAC is recommended (AHA Aggressive) when CHA2DS2-VASc score = 1 [10].
2.6. Main Independent Variable
Concordance Status
When a patient’s OAC exposure status was consistent with the decision aid’s recommendation (OAC recommended—OAC exposed, OAC not recommended—OAC unexposed), that patient was considered to be concordant with the treatment recommendation of the respective decision aid. We also looked at the underuse and overuse of OAC (Underuse: OAC recommended-OAC unexposed; Overuse: OAC not recommended-OAC exposed) separately.
2.7. Analyses
We compared the demographic characteristics of OAC exposed and unexposed. Based on the CHA2DS2-VASc and HAS-BLED scores, AF patients were classified into nine categories to study the variation in treatment recommendations by the decision aids and their concordance with actual OAC use/non-use. These nine categories reflected the nine possible combinations of low, moderate and high CHA2DS2-VASc (low—CHA2DS2-VASc = 0; moderate CHA2DS2-VASc = 1; high—CHA2DS2-VASc > 1) scores and HAS-BLED (low—HAS-BLED = 0; moderate HAS-BLED = 1 or 2; high—HAS-BLED ≥ 3) scores. The number of patients in each subgroup and their treatment recommendations were determined. In addition, the number and percent of patients discordant with the treatment recommendations by each decision aid were also determined. The decision aid recommendations and percent discordance with these recommendations were reported for the entire population to provide an overall acceptance of these decision aids. To assess the factors responsible for the difference in treatment recommendations and actual OAC use, we compared the characteristics of patients who were over-anticoagulated and under-anticoagulated based on the recommendations of each decision aid using a chi square test. Characteristics for comparison included age, gender, geographic region, prior stroke, hypertension, diabetes, heart failure, anemia, major bleed, renal failure, liver failure, vascular disease, alcohol use, medication use (anti-platelets, NASIDs, GI agents, antineoplastic agents, systemic corticosteroids), CHA2DS2-VASc score, CHADS2 score, ATRIA score and HAS-BLED score. All analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
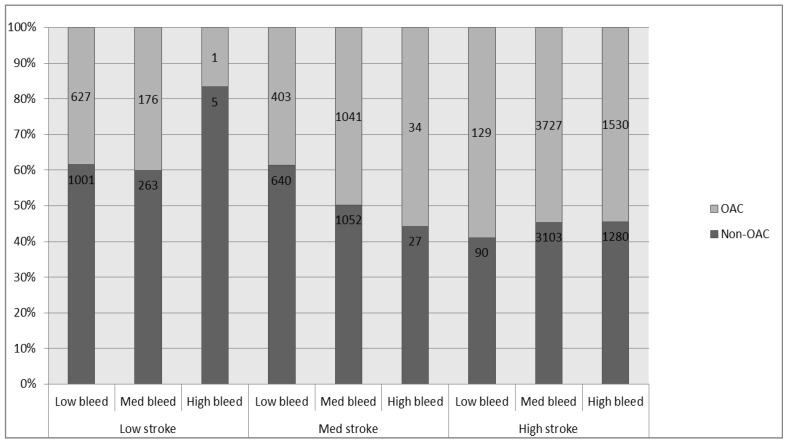
A total of 15,129 incident AF patients met our inclusion-exclusion criteria (Table S2). Of these, 7668 (50.68%) were OAC exposed within first 90 days after the index diagnosis (Table 1). Compared to patients unexposed to OAC, a higher percentage of OAC exposed patients had CHA2DS2-VASc scores ≥2 (70.24% vs. 59.95%, p value < 001; Table 1 and Table S3). Also, the proportion of patients with moderate to high HAS-BLED score was higher among the OAC exposed group compared to the OAC unexposed group (84.89% vs. 76.79%, p value < 001). Actual OAC exposure was not always influenced by the baseline stroke and bleeding risks (Figure 1).
Table 1.
Demographics characteristics of the study population.
| Characteristics | OAC Exposed | OAC Unexposed | Total | p Value |
|---|---|---|---|---|
| Number of patients (n %) | 7668 (50.68%) | 7461 (49.32%) | 15129 | |
| Mean age, y (SD) | 65.04 (11.82) | 62.54 (14.84) | 63.81 (13.45) | <0.001 |
| N (%) | N (%) | N (%) | ||
| Age Category, y | <0.001 | |||
| 18–30 | 29 (0.38%) | 146 (1.96%) | 175 (1.16%) | |
| 31–49 | 584 (7.62%) | 1156 (15.49%) | 1740 (11.50%) | |
| 50–64 | 3453 (45.03%) | 3092 (41.44%) | 6545 (43.26%) | |
| 65–74 | 1745 (22.76%) | 1240 (16.62%) | 2985 (19.73%) | |
| 75–84 | 1570 (20.47%) | 1429 (19.15%) | 2999 (19.82%) | |
| ≥85 | 287 (3.74%) | 398 (5.33%) | 685 (4.53%) | |
| Gender | <0.001 | |||
| Male | 4961 (53.16%) | 4372 (46.84%) | 9333 (61.69%) | |
| Female | 2707 (35.30%) | 3089 (41.40%) | 5796 (38.31%) | |
| Geographic Region | <0.001 | |||
| East | 1591 (20.75%) | 1474 (19.76%) | 3065 (20.26%) | |
| Midwest | 2656 (34.64%) | 2454 (32.89%) | 5110 (33.78%) | |
| South | 2301 (30.01%) | 2271 (30.44%) | 4572 (30.22%) | |
| West | 1120 (14.61%) | 1262 (16.91%) | 2382 (15.74%) | |
| Comorbidities | ||||
| Prior stroke or TIA | 551 (7.19%) | 476 (6.38%) | 1027 (6.79%) | 0.048 |
| Hypertension | 5177 (67.51%) | 4263 (57.14%) | 9440 (62.4%) | <0.001 |
| Diabetes | 1857 (24.22%) | 1265 (16.95%) | 3122 (20.64%) | <0.001 |
| Heart failure | 1506 (19.64%) | 884 (11.85%) | 2390 (15.8%) | <0.001 |
| Anemia | 731(9.53%) | 864 (11.58%) | 1595 (10.54%) | <0.001 |
| History of any bleed | 675 (8.80%) | 732 (9.81%) | 1407 (9.30%) | 0.032 |
| Renal impairment | 493 (6.43%) | 441 (5.91%) | 934 (6.17%) | 0.185 |
| Liver failure | 255 (3.33%) | 258 (3.46%) | 513 (3.39%) | 0.652 |
| Vascular disease | 2377 (31.00%) | 2202 (29.51%) | 4579 (30.27%) | 0.046 |
| Antiplatelet agents | 488 (6.36%) | 403 (5.40%) | 891 (5.89%) | 0.011 |
| NASIDs | 1443 (18.82%) | 1208 (16.19%) | 2651 (17.52%) | <0.001 |
| GI agents | 1532 (19.98%) | 1398 (18.74%) | 2930 (19.37%) | 0.053 |
| Antineoplastic agents | 178 (2.32%) | 135 (1.81%) | 313 (2.07%) | 0.027 |
| Systemic corticosteroids | 1136 (14.81%) | 939 (12.59%) | 2075 (13.72%) | <0.001 |
| Alcohol use | 124 (1.62%) | 119 (1.59%) | 243 (1.61%) | 0.913 |
| Stroke risk scores | ||||
| CHADS2 score | <0.001 | |||
| Low (score = 0) | 1524 (19.87%) | 2426 (32.52%) | 3950 (26.11%) | |
| Medium (score = 1) | 2676 (34.90%) | 2510 (33.64%) | 5186 (34.28%) | |
| High (score ≥ 2) | 3468 (45.23%) | 2525 (33.84%) | 5993 (39.61%) | |
| CHA2DS2-VASc score | <0.001 | |||
| Low (score = 0) | 804 (10.49%) | 1269 (17.01%) | 2073 (13.70%) | |
| Medium (score = 1) | 1478 (19.27%) | 1719 (23.04%) | 3197 (21.13%) | |
| High (score ≥ 2) | 5386 (70.24%) | 4473 (59.95%) | 9859 (65.17%) | |
| Bleeding risk scores | ||||
| ATRIA score | 0.002 | |||
| Low (score ≤ 3) | 6590 (85.94%) | 6391 (85.66%) | 12981 (85.80%) | |
| Medium (score = 4) | 596 (6.29%) | 401 (5.37%) | 883 (5.84%) | |
| High (score ≥ 5) | 596 (7.77%) | 669 (8.97%) | 1265 (8.36%) | |
| HAS BLED score | <0.001 | |||
| Low (score = 0) | 1159 (15.11%) | 1731 (23.20%) | 2890 (19.10%) | |
| Medium (score = 1 or 2) | 4944 (64.48%) | 4418 (59.21%) | 9362 (61.88%) | |
| High (score ≥ 3) | 1564 (20.41%) | 1312 (17.58%) | 2877 (19.02%) | |
| Anticoagulant exposure after index date | ||||
| Warfarin | 5886 (76.76%) | - | 5886 (38.91%) | |
| Dabigatran | 756 (9.86%) | - | 756 (5.00%) | |
| Rivaroxaban | 178 (2.32%) | - | 178 (1.18%) | |
| Apixaban | 5 (0.07%) | - | 5 (0.03%) | |
| Antiplatelet agents | 365 (4.76%) | 434 (5.82%) | 799 (5.28%) | |
SD, standard deviation, TIA: transient ischemic attack, NASIDs: Non-steroidal anti-inflammatory drugs, GI agents: Antacids, proton pump inhibitors, H2 receptor antagonist and other GI protectants, OAC/INR: use of oral anticoagulant or INR test with 90 days after index diagnosis.
Figure 1.
Actual OAC use: Number of patients that received and did not receive OAC stratified by stroke and bleeding risk scores. * Data shown are the number of patients who were exposed to OAC/non-OAC within 90 days after index diagnosis. Low stroke: CHA2DS2-VASc score = 0, Med stroke: CHA2DS2-VASc score = 1, high stroke: CHA2DS2-VASc score ≥ 2, Low bleed: HAS BLED score = 0, Med bleed: HAS BLED score = 1 or 2, high bleed: HAS BLED score ≥ 3).
The AHA Aggressive guideline recommended OAC most often (86.30%) and the LaHaye tool recommended OAC the least often (14.91%; Table 2). None of the decision aids recommended OAC for patients when CHA2DS2-VASc = 0. However, OAC treatment recommendations varied considerably when the stroke risk was moderate or high (CHA2DS2-VASc > 0). For example, the LaHaye tool never recommended OAC for patients with CHA2DS2-VASc = 1 irrespective of bleeding risk, however, the ESC guidelines as we defined them universally recommended OAC for patients with CHA2DS2-VASc = 1 and a HAS-BLED < 3. For those with the highest stroke risk, the ESC and AHA guidelines recommended OAC in all patients with CHA2DS2-VASc score ≥ 2 irrespective of the HAS-BLED score, whereas the CHEST guidelines, Casciano and LaHaye tools recommended OAC between 22.88% and 95.57% of high stroke risk patients.
Table 2.
Oral anticoagulation (OAC) recommendations by the decision aid.
| Stroke Risk | Bleeding risk | AHA Aggressive | AHA Conservative | Casciano | CHEST | ESC | LaHaye |
|---|---|---|---|---|---|---|---|
| Low (CHA2DS2-VASc score = 0) | Low (HAS-BLED score = 0) | 0 | 0 | 0 | 0 | 0 | 0 |
| n (%) = 1628 (10.76%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | |
| Medium (HAS-BLED score = 1 or 2) | 0 | 0 | 0 | 0 | 0 | 0 | |
| n (%) = 439(2.90%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | |
| High (HAS-BLED score ≥ 3) | 0 | 0 | 0 | 0 | 0 | 0 | |
| n (%) = 6 (0.04%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | |
| Sub-total | 0 | 0 | 0 | 0 | 0 | 0 | |
| (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | (0.00%) | ||
| Medium (CHA2DS2-VASc score = 1) | Low (HAS-BLED score = 0) | 1043 | 0 | 193 | 196 | 1043 | 0 |
| n (%) = 1043(6.89%) | (100%) | (0.00%) | (18.50%) | (18.79%) | (100%) | (0.00%) | |
| Medium (HAS-BLED score = 1 or2) | 2093 | 0 | 1371 | 1505 | 2093 | 0 | |
| n (%) = 2093(13.83%) | (100%) | (0.00%) | (65.50%) | (71.91%) | (100%) | (0.00%) | |
| High (HAS-BLED score ≥ 3) | 61 | 0 | 19 | 57 | 0 | 0 | |
| n (%) = 61 (0.40%) | (100%) | (0.00%) | (31.15%) | (93.44%) | (0.00%) | (0.00%) | |
| Sub-total | 3197 | 0 | 1583 | 1758 | 3136 | 0 | |
| (100%) | (0.00%) | (49.51%) | (54.98%) | (98.09%) | (0.00%) | ||
| High (CHA2DS2-VASc score ≥ 2) | Low (HAS-BLED score = 0) | 219 | 219 | 151 | 158 | 219 | 219 |
| n (%) = 219 (1.45%) | (100.00%) | (100.00%) | (68.95%) | (72.15%) | (100.00%) | (100.00%) | |
| Medium (HAS-BLED score = 1 or 2) | 6830 | 6830 | 5819 | 6468 | 6830 | 1102 | |
| n (%) = 6830 (45.15%) | (100.00%) | (100.00%) | (85.20%) | (94.70%) | (100.00%) | (16.13%) | |
| High (HAS-BLED score ≥ 3) | 2810 | 2810 | 2210 | 2795 | 2810 | 935 | |
| n (%) = 2810 (18.57%) | (100.00%) | (100.00%) | (78.65%) | (99.47%) | (100.00%) | (33.27%) | |
| Sub-total | 9859 | 9859 | 8180 | 9421 | 9859 | 2256 | |
| (100.00%) | (100.00%) | (82.96%) | (95.57%) | (100.00%) | (22.88%) | ||
| Overall | 15129 (100%) | 13056 | 9859 | 9763 | 11179 | 12995 | 2256 |
| (86.30%) | (65.17%) | (64.53%) | (73.89%) | (85.89%) | (14.91%) |
Data is presented as n (%) for oral anticoagulant recommendations by each decision aid. AHA Aggressive: AHA guidelines (assuming that guideline recommends OAC at CHA2DS2-VASc = 1), AHA Conservative: AHA guidelines (assuming that guideline recommends non-OAC at CHA2DS2-VASc = 1), Casciano: Casciano tool, CHEST: CHEST guidelines, ESC: ESC guidelines, LaHaye: LaHaye tool.
The overall OAC exposure/non-exposure was most consistent with the Casciano tool (56.75%) and was least consistent with the LaHaye tool (49.94%; Table S4). The overall percent discordance with the decision aid recommendations was fairly consistent over the study period (Table S5). When the underuse of OAC was assessed using the nine stroke/bleed risk groups, underuse of OAC was similar between moderate and high stroke risk patients with OAC not being prescribed between 43.98% and 53.95% of patients recommended OAC (Table 3).
Table 3.
Underuse of OAC (Number of patients discordant with the OAC treatment recommendation by decision aids).
| Stroke Risk | Bleeding Risk | AHA Aggressive | AHA Conservative | Casciano | CHEST | ESC | LaHaye |
|---|---|---|---|---|---|---|---|
| Low (CHA2DS2-VASc score = 0)* | Sub-total | ARNO | ARNO | ARNO | ARNO | ARNO | ARNO |
| Medium (CHA2DS2-VASc score = 1) | Low (HAS-BLED score = 0) | 640 (61.36%) | ARNO | 52 (26.94%) | 54 (27.55%) | 640 (61.36%) | ARNO |
| Medium (HAS-BLED score = 1 or 2) | 1052 (50.26%) | ARNO | 661 (48.21%) | 728 (48.37%) | 1052 (50.26%) | ARNO | |
| High (HAS-BLED score ≥ 3) | 27 (44.26%) | ARNO | 8 (42.11%) | 27 (47.37%) | ARNO | ARNO | |
| Sub-total | 1719 (53.77%) | ARNO | 721 (45.55%) | 809 (46.02%) | 1692 (53.95%) | ARNO | |
| High (CHA2DS2-VAS score ≥ 2) | Low (HAS-BLED score = 0) | 90 (41.10%) | 90 (41.10%) | 47 (31.13%) | 48 (30.38%) | 90 (41.10%) | 90 (41.10%) |
| Medium (HAS-BLED score = 1 or 2) | 3103 (45.43%) | 3103 (45.43%) | 2578 (44.30%) | 2904 (44.90%) | 3103 (45.43%) | 542 (49.18%) | |
| High (HAS-BLED score ≥ 3) | 1280 (45.55%) | 1280 (45.55%) | 973 (44.03%) | 1274 (45.58%) | 1280 (45.55%) | 449 (48.02%) | |
| Sub-total | 4473 (45.37%) | 4473 (45.37%) | 3598 (43.98%) | 4226 (44.86%) | 4473 (45.37%) | 1081 (47.92%) | |
| Overall | Total | 6192 (47.42%) | 4473 (48.01%) | 4319 (44.23%) | 5035 (45.04%) | 6165 (47.44%) | 1081 (47.92%) |
Data is presented as n (%) of patients who are not consistent with the OAC treatment recommendation by each decision aid, ARNO: Always Recommends Non-OAC. * Data not shown by bleed risk because no decision aids recommend OAC when CHA2DS2-VASc = 1 irrespective of HAS-BLED score. AHA Aggressive: AHA guidelines (assuming that guideline recommends OAC at CHA2DS2-VASc = 1), AHA Conservative: AHA guidelines (assuming that guideline recommends non-OAC at CHA2DS2-VASc = 1), Casciano: Casciano tool, CHEST: CHEST guidelines, ESC: ESC guidelines, LaHaye: LaHaye tool.
Most of the OAC unexposed patients who were recommended OAC by the decision aids had a high stroke risk profile (72.55%–100%; Table S6). Since none of the decision aids recommended OAC use among patients with a low stroke risk, the percent of patients who were over-anticoagulated (38.78%) was consistent across all the decision aids at CHA2DS2-VASc score = 0 (Table 4).
Table 4.
Overuse of OAC (Number of patients discordant with the non-OAC treatment recommendation by decision aids).
| Stroke Risk | Bleeding Risk | AHA Aggressive | AHA Conservative | Casciano | CHEST | ESC | LaHaye |
|---|---|---|---|---|---|---|---|
| Low (CHA2DS2-VASc score = 0) | Low (HAS-BLED score = 0) | 627 (38.51%) | 627 (38.51%) | 627 (38.51%) | 627 (38.51%) | 627 (38.51%) | 627 (38.51%) |
| Medium (HAS-BLED score = 1 or 2) | 176 (40.09%) | 176 (40.09%) | 176 (40.09%) | 176 (40.09%) | 176 (40.09%) | 176 (40.09%) | |
| High (HAS-BLED score ≥ 3) | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) | 1 (16.67%) | |
| Sub-total | 804 (38.78%) | 804 (38.78%) | 804 (38.78%) | 804 (38.78%) | 804 (38.78%) | 804 (38.78%) | |
| Medium (CHA2DS2-VASc score = 1 | Low (HAS-BLED score = 0) | ARO | 403 (38.64%) | 262 (30.82%) | 261 (30.81%) | ARO | 403 (38.64%) |
| Medium (HAS-BLED score = 1 or 2) | ARO | 1041 (49.74%) | 331 (45.84%) | 264 (44.90%) | ARO | 1041 (49.74%) | |
| High (HAS-BLED score ≥ 3) | ARO | 34 (55.74%) | 23 (54.76%) | 4 (100.00%) | 34 (55.74%) | 34 (55.74%) | |
| Sub-total | ARO | 1478 (46.23%) | 616 (38.17%) | 529 (36.76%) | 34 (55.73%) | 1478 (46.23%) | |
| High (CHA2DS2-VASc score ≥ 2) | Low (HAS-BLED score = 0) | ARO | ARO | 25 (36.76%) | 19 (31.15%) | ARO | ARO |
| Medium (HAS-BLED score = 1 or 2) | ARO | ARO | 486 (48.07%) | 163 (45.03%) | ARO | 3167 (55.29%) | |
| High (HAS-BLED score ≥ 3) | ARO | ARO | 293 (48.83%) | 9 (60.00%) | ARO | 1044 (55.68%) | |
| Sub-total | ARO | ARO | 804 (47.88%) | 191 (43.61%) | ARO | 4211 (55.38%) | |
| Overall | Total | 804 (38.78%) | 2282 (43.30%) | 2224 (41.45%) | 1564 (39.59%) | 838 (39.27%) | 6493 (50.43%) |
Data is presented as n (%) of patients who are not consistent with the non- OAC treatment recommendation by each decision aid. ARO: Always recommends OAC. AHA Aggressive: AHA guidelines (assuming that guideline recommends OAC at CHA2DS2-VASc = 1), AHA Conservative: AHA guidelines (assuming that guideline recommends non-OAC at CHA2DS2-VASc = 1), Casciano: Casciano tool, CHEST: CHEST guidelines, ESC: ESC guidelines, LaHaye: LaHaye tool.
Among the OAC exposed patients (n = 7668), the decision aids recommended withholding the OAC therapy in 10.49%–84.68% of the patients depending on the decision aid. Most of the patients (35.14%–100%) who were exposed to OAC when not recommended had low to moderate stroke risk profiles (Table S7).
4. Discussion
There is a considerable variation in the treatment recommendations by the decision aids. The AHA and CHEST guidelines base their recommendations exclusively on the stroke risk algorithms, and these recommend OAC to more patients (65.17%–86.30%) compared to the LaHaye (14.91%) and Casciano (64.53%) tools which formally incorporate bleeding risk. The decision tools/guidelines which are based on the same stroke risk algorithm have more similarity in the treatment recommendations compared to those which are based on different stroke risk algorithms. For example, the treatment recommendations by the CHEST guidelines are more similar to the Casciano tool recommendations, both of which are based on the CHADS2 algorithm compared to the CHA2DS2VASc based ESC guideline and LaHaye tool. However, the treatment recommendations by the decisions aids based on the same stroke risk algorithm also differ depending on the weight given to the bleeding risk by the respective decision aids. For example, the LaHaye tool incorporates HAS-BLED score (0 to 7) at all levels of CHA2DS2VASc score (0 to 9) which is different than the ESC guidelines in which we only considered bleed risk to be a factor when CHA2DS2VASc = 1, which led to a 70.98% difference in OAC recommendations.
The treatment recommendations by the decision aids varied considerably among patients with moderate to high stroke risk. The stroke risk algorithm used and the weight given to the bleeding risk by respective decision aids mostly influences this variation. As the CHA2DS2-VASc algorithm accounts for additional stroke factors (vascular disease, female gender and age between 65 and 74 years) [18], the decision aids based on CHA2DS2-VASc classifies a higher proportion of patients in moderate and particularly in high stroke risk groups compared to the decision aids which are based on the CHADS2 algorithm. Although the LaHaye and the Casciano decision support tools consider bleeding risk algorithms in rendering OAC recommendations, both tools are based on different stroke risk and bleeding risk algorithms. Furthermore, the LaHaye tool recommendations are also influenced by bleed, treatment and cost thresholds while the Casciano tool recommendations are based on quality-adjusted life survival which incorporates demographic factors in addition to stroke and bleeding risk to render warfarin/aspirin treatment recommendations [9,11].
Despite the recommendation of withholding OAC in patients with CHA2DS2-VASc = 0 by all the decision aids, we found considerable utilization of OAC in these patients. Nearly 40% of CHA2DS2-VASc = 0 patients were OAC exposed which is similar to the 43.1% found by Lip et al. suggesting that overutilization is a widespread and persistent problem [26].
Our study confirms that OACs are underused in patients with elevated stroke risk [3,4,5]. We found that depending on the decision aid, between 45% and 48% were NOT anticoagulated when recommended. Although the use of OACs reduces the risk of stroke in all patients, it also increases the risk of hemorrhagic events [11]. Therefore, both overuse and underuse can be viewed as a concern in terms of AF related adverse events. When compared with the “real world” data, we found that the direction of OAC misuse was in both directions, patients who were OAC treatment eligible (moderate-high stroke risk) were often not anticoagulated while patients who were not eligible for OAC treatment (moderate–high bleeding risk with low stroke risk) were anticoagulated. The reason for this discrepancy between the actual OAC use and guideline recommendations could be attributed to the difference in physicians’ perceptions and empirical algorithms for risk assessment of stroke/bleed events. Hypertension, heart failure, age and diabetes have a smaller influence on physician assigned stroke risk while prior stroke, severe AF symptoms and not living independently have a higher influence on physician assigned stroke risk when compared to empirical stroke risk algorithms. [27] Our data had similar findings; patients who were under-anticoagulated had a high proportion of hypertensive patients (68.71%–84.67%), females (43.88%–72.43%), patients with vascular disease (35.56%–75.39%) and diabetes (20.43%–44.96%).
5. Limitations
The newer oral anticoagulants were not available over most of the study time thereby limiting the study to more general OAC/non-OAC comparisons. We relied on the prescription claims supplemented by INR test to identify OAC exposure and 6.29% of patients were considered OAC exposed by INR tests alone and a portion of these patients may be misclassified. Furthermore, this study could not assess the degree of guideline concordance with aspirin recommendations. The database used for this study does not contain information on lab values for the INR/PT test, which is one of the risk factors to calculate the HAS-BLED score. The lack of information about this risk factor might underestimate the bleeding risk determined by HAS-BLED for those that are anticoagulated with warfarin over time. However, the aim of our study was to assess the appropriateness of initial anticoagulation, and the lack of information on labile INR would not significantly influence our study findings. When administrative claims data are used, coding errors could influence study measures. The accuracy of identifying the comorbid conditions used to calculate stroke/bleed risk scores using the administrative database has been previous validated (crude agreement: 78%–96%) [23,28] however not all study measures have known validity [11,24,29]. Finally, the data is representative of a commercially insured population and the study findings may not be applicable for other populations.
6. Conclusions
Real world OAC prescribing is frequently discordant with all the common contemporary guidelines and decision aids with both overuse and underuse of OAC being detected. There is a considerable variability in the OAC treatment recommendations by the AF guidelines and decision tools when the ischemic stroke risk is moderate or high. Given the variability in guideline recommendations, empirical evidence is needed to compare the outcomes of OAC treatment recommendations for these decision aids.
Acknowledgments
We would like to thank Gary Moore, Research Assistant who often guided our SAS programming and cleaned the claims file for errant records. The Lifelink data used for the study was supported by the University of Arkansas Translational Research Institute (NIH Grant # 1UL1RR029884).
Supplementary Files
Author Contributions
Anand Shewale was responsible for developing the initial concept, study design, data analysis, data interpretation and writing the manuscript under the supervision of Bradley Martin. Martin played an instrumental role in developing the concept, methodology, interpreting the results and drafting the manuscript. Chenghui Li, Jill Johnson and David Nelsen provided valuable feedback on the initial concept, study design, data interpretation and also helped in drafting and editing the final manuscript.
Conflicts of Interest
Martin was a paid consultant to e-Max Health and served on an advisory board for Daiichi-Sankyo. Li is a paid consultant to e-Max Health on unrelated studies. No financial support was obtained from e-Max Health or Daiichi-Sankyo to support this study and neither had any role in this study.
References
- 1.Singer D.E., Albers G.W., Dalen J.E., Fang M.C., Go A.S., Halperin J.L., Lip G.Y., Manning W.J. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:546S–592S. doi: 10.1378/chest.08-0678. [DOI] [PubMed] [Google Scholar]
- 2.Garg N., Kumar A., Flaker G.C. Antiplatelet therapy for stroke prevention in atrial fibrillation. Mo. Med. 2010;107:44–47. [PMC free article] [PubMed] [Google Scholar]
- 3.Ogilvie I.M., Newton N., Welner S.A., Cowell W., Lip G.Y. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am. J. Med. 2010;123:638–645. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Gage B.F., Boechler M., Doggette A.L., Fortune G., Flaker G.C., Rich M.W., Radford M.J. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31:822–827. doi: 10.1161/01.STR.31.4.822. [DOI] [PubMed] [Google Scholar]
- 5.Cohen N., Almoznino-Sarafian D., Alon I., Gorelik O., Koopfer M., Chachashvily S., Shteinshnaider M., Litvinjuk V., Modai D. Warfarin for stroke prevention still underused in atrial fibrillation: Patterns of omission. Stroke. 2000;31:1217–1222. doi: 10.1161/01.STR.31.6.1217. [DOI] [PubMed] [Google Scholar]
- 6.Chang H.J., Bell J.R., Deroo D.B., Kirk J.W., Wasson J.H. Physician variation in anticoagulating patients with atrial fibrillation. Dartmouth Primary Care COOP Project. Arch. Inter. Med. 1990;150:83–86. doi: 10.1001/archinte.1990.00390130089012. [DOI] [PubMed] [Google Scholar]
- 7.Beyth R.J., Antani M.R., Covinsky K.E., Miller D.G., Chren M.M., Quinn L.M., Landefeld C.S. Why isn’t warfarin prescribed to patients with nonrheumatic atrial fibrillation? J. Gen. Inter. Med. 1996;11:721–728. doi: 10.1007/BF02598985. [DOI] [PubMed] [Google Scholar]
- 8.Akao M., Chun Y.H., Esato M., Abe M., Tsuji H., Wada H., Hasegawa K., Fushimi AF Registry Investigators Inappropriate use of oral anticoagulants for patients with atrial fibrillation. Circ. J. 2014;78:2166–2172. doi: 10.1253/circj.CJ-14-0344. [DOI] [PubMed] [Google Scholar]
- 9.Lahaye S.A., Gibbens S.L., Ball D.G., Day A.G., Olesen J.B., Skanes A.C. A clinical decision aid for the selection of antithrombotic therapy for the prevention of stroke due to atrial fibrillation. Eur. Heart J. 2012;33:2163–2171. doi: 10.1093/eurheartj/ehs167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.January C.T., Wann L.S., Alpert J.S., Calkins H., Cleveland J.C., Jr., Cigarroa J.E., Conti J.B., Ellinor P.T., Ezekowitz M.D., Field M.E., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Casciano J.P., Singer D.E., Kwong W.J., Fox E.S., Martin B.C. Anticoagulation therapy for patients with non-valvular atrial fibrillation: comparison of decision analytic model recommendations and real-world warfarin prescription use. Am. J. Cardiovasc. Drugs. 2012;12:313–323. doi: 10.1007/BF03261840. [DOI] [PubMed] [Google Scholar]
- 12.You J.J., Singer D.E., Howard P.A., Lane D.A., Eckman M.H., Fang M.C., Hylek E.M., Schulman S., Go A.S., Hughes M., et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e531S–e575S. doi: 10.1378/chest.11-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camm A.J., Lip G.Y., de Caterina R., Savelieva I., Atar D., Hohnloser S.H., Hindricks G., Kirchhof P., ESC Committee for Practice Guidelines (CPG) Bax J.J., et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012;33:2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 14.Wess M.L., Schauer D.P., Johnston J.A., Moomaw C.J., Brewer D.E., Cook E.F., Eckman M.H. Application of a decision support tool for anticoagulation in patients with non-valvular atrial fibrillation. J. Gen. Inter. Med. 2008;23:411–417. doi: 10.1007/s11606-007-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DAWN AC Anticoagulation Software—4S Dawn Clinical Software RefGrab-It. [(accessed on 7 February 2014)]. Available online: http://www.4s-dawn.com/products/anticoagulation/dawnac/
- 16.Medical Disclaimer—AFib.ca RefGrab-It. [(accessed on 7 February 2014)]. Available online: http://www.afib.ca/Disclaimer.
- 17.Gage B.F., Waterman A.D., Shannon W., Boechler M., Rich M.W., Radford M.J. Validation of clinical classification schemes for predicting stroke: Results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 18.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 19.Fang M.C., Go A.S., Chang Y., Borowsky L.H., Pomernacki N.K., Udaltsova N., Singer D.E. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. J. Am. Coll. Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lip G.Y., Frison L., Halperin J.L., Lane D.A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. J. Am. Coll. Cardiol. 2011;57:173–180. doi: 10.1016/j.jacc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Singer D.E., Chang Y., Fang M.C., Borowsky L.H., Pomernacki N.K., Udaltsova N., Go A.S. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann. Inter. Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Go A.S., Hylek E.M., Chang Y., Phillips K.A., Henault L.E., Capra A.M., Jensvold N.G., Selby J.V., Singer D.E. Anticoagulation therapy for stroke prevention in atrial fibrillation: How well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 23.Go A.S., Hylek E.M., Borowsky L.H., Phillips K.A., Selby J.V., Singer D.E. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann. Inter. Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kate F., Jonah B., Bruce P., Kosuke I., Winghan Jacqueline K. Utilization of anticoagulation therapy in medicare patients with nonvalvular atrial fibrillation. 2012;5:157–168. [PMC free article] [PubMed] [Google Scholar]
- 25.Hylek E.M., Go A.S., Chang Y., Jensvold N.G., Henault L.E., Selby J.V., Singer D.E. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. New Engl. J. Med. 2003;349:1019–1026. doi: 10.1056/NEJMoa022913. [DOI] [PubMed] [Google Scholar]
- 26.Lip G., Bassand J., Fitzmaurice D., Goldhaber S., Goto S., Verheugt F., Turpie A., Mueller I., Rushton-Smith S., Kakkar A. Inappropriate utilization of anticoagulation in patients with atrial fibrillation: The global anticoagulant registry in the field (GARFIELD) registry. J. Am. Coll. Cardiol. 2012;59:E670. doi: 10.1016/S0735-1097(12)60671-8. [DOI] [Google Scholar]
- 27.Steinberg B.A., Kim S., Thomas L., Fonarow G.C., Hylek E., Ansell J., Go A.S., Chang P., Kowey P., Gersh B.J., et al. Lack of concordance between empirical scores and physician assessments of stroke and bleeding risk in atrial fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Circulation. 2014;129:2005–2012. doi: 10.1161/CIRCULATIONAHA.114.008643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer D.E., Chang Y., Borowsky L.H., Fang M.C., Pomernacki N.K., Udaltsova N., Reynolds K., Go A.S. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J. Am. Heart Assoc. 2013;2:e000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercaldi C.J., Siu K., Sander S.D., Walker D.R., Wu Y., Li Q., Wu N. Long-term costs of ischemic stroke and major bleeding events among medicare patients with nonvalvular atrial fibrillation. Cardiol. Res. Pract. :2012. doi: 10.1155/2012/645469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.