Abstract
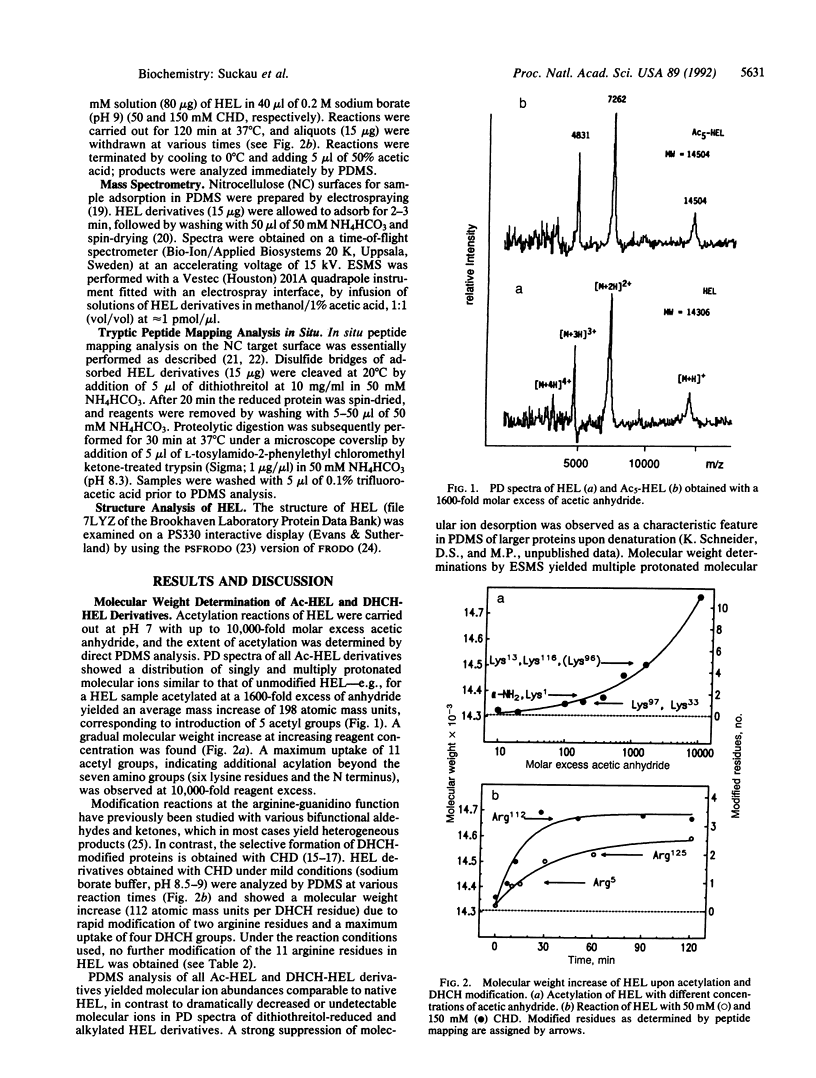
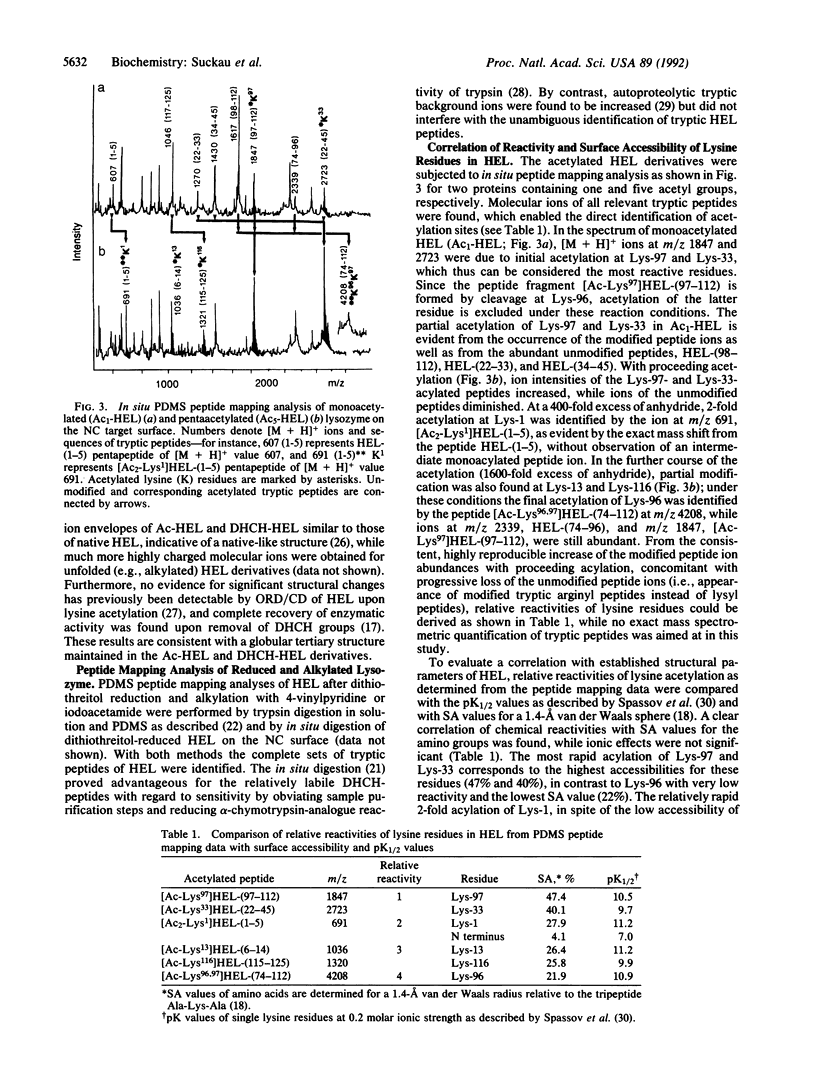
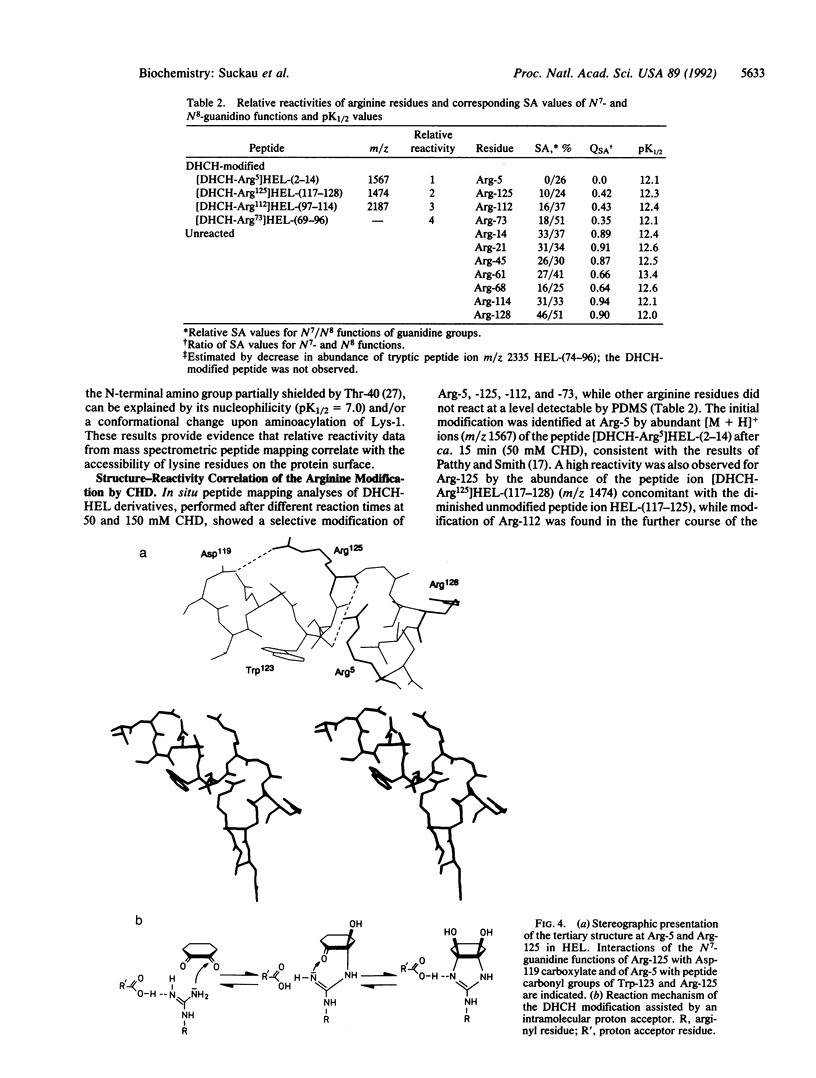
Aminoacetylation of lysine residues and the modification of arginine by 1,2-cyclohexanedione to N7,N8-(dihydroxy-1,2-cyclohexylidene)arginine were used for probing the surface topology of hen-eggwhite lysozyme as a model protein. The molecular identification of lysine and arginine modification sites was provided by molecular weight determinations of modified and unmodified tryptic peptide mixtures (peptide mapping) using 252Cf plasma desorption mass spectrometry. At conditions of limited chemical modification, mass-spectrometric peptide-mapping analyses of lysozyme derivatives enabled the direct assignment of relative reactivities of lysine and arginine residues at different reaction times and reagent concentrations. The relative reactivities of lysine residues showed a direct correlation with their surface accessibilities from x-ray structure data. For the reaction with 1,2-cyclohexanedione, a selective modification at Arg-5, -125, -112, and -73 was identified, and an inverse correlation of relative reactivities with the surface accessibility ratios of the N7- and the N8-guanidino functions was obtained. By examination of the x-ray structural data of lysozyme, this selective modification was attributed to intramolecular catalysis because of the presence of neighboring proton acceptor groups, such as the Asp-119 carboxylate group for Arg-125 and the Trp-123 and Arg-125 carbonyl groups for Arg-5.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artymiuk P. J., Blake C. C., Grace D. E., Oatley S. J., Phillips D. C., Sternberg M. J. Crystallographic studies of the dynamic properties of lysozyme. Nature. 1979 Aug 16;280(5723):563–568. doi: 10.1038/280563a0. [DOI] [PubMed] [Google Scholar]
- Berthou J., Lifchitz A., Artymiuk P., Jollès P. An X-ray study of the physiological-temperature form of hen egg-white lysozyme at 2 A resolution. Proc R Soc Lond B Biol Sci. 1983 Mar 22;217(1209):471–489. doi: 10.1098/rspb.1983.0021. [DOI] [PubMed] [Google Scholar]
- Bhat T. N., Bentley G. A., Fischmann T. O., Boulot G., Poljak R. J. Small rearrangements in structures of Fv and Fab fragments of antibody D1.3 on antigen binding. Nature. 1990 Oct 4;347(6292):483–485. doi: 10.1038/347483a0. [DOI] [PubMed] [Google Scholar]
- Burnens A., Demotz S., Corradin G., Binz H., Bosshard H. R. Epitope mapping by chemical modification of free and antibody-bound protein antigen. Science. 1987 Feb 13;235(4790):780–783. doi: 10.1126/science.2433768. [DOI] [PubMed] [Google Scholar]
- Chait B. T., Field F. H. A rapid, sensitive mass spectrometric method for investigating microscale chemical reactions of surface adsorbed peptides and proteins. Biochem Biophys Res Commun. 1986 Jan 14;134(1):420–426. doi: 10.1016/0006-291x(86)90580-2. [DOI] [PubMed] [Google Scholar]
- Chowdhury S. K., Katta V., Chait B. T. Electrospray ionization mass spectrometric peptide mapping: a rapid, sensitive technique for protein structure analysis. Biochem Biophys Res Commun. 1990 Mar 16;167(2):686–692. doi: 10.1016/0006-291x(90)92080-j. [DOI] [PubMed] [Google Scholar]
- Cotter R. J. Plasma desorption mass spectrometry: coming of age. Anal Chem. 1988 Jul 1;60(13):781A–793A. doi: 10.1021/ac00164a002. [DOI] [PubMed] [Google Scholar]
- Fenn J. B., Mann M., Meng C. K., Wong S. F., Whitehouse C. M. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989 Oct 6;246(4926):64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Stevenson K. J., Hartley B. S. Competitive labelling, a method for determining the reactivity of individual groups in proteins. The amino groups of porcine elastase. Biochem J. 1971 Sep;124(2):289–299. doi: 10.1042/bj1240289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Miranker A., Radford S. E., Karplus M., Dobson C. M. Demonstration by NMR of folding domains in lysozyme. Nature. 1991 Feb 14;349(6310):633–636. doi: 10.1038/349633a0. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Identification of functional arginine residues in ribonuclease A and lysozyme. J Biol Chem. 1975 Jan 25;250(2):565–569. [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- Petros A. M., Mueller L., Kopple K. D. NMR identification of protein surfaces using paramagnetic probes. Biochemistry. 1990 Oct 30;29(43):10041–10048. doi: 10.1021/bi00495a005. [DOI] [PubMed] [Google Scholar]
- Post C. B., Brooks B. R., Karplus M., Dobson C. M., Artymiuk P. J., Cheetham J. C., Phillips D. C. Molecular dynamics simulations of native and substrate-bound lysozyme. A study of the average structures and atomic fluctuations. J Mol Biol. 1986 Aug 5;190(3):455–479. doi: 10.1016/0022-2836(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Silberring J., Nyberg F. Fast atom bombardment mass spectrometric analysis of arginine-containing neuropeptides. Biomed Environ Mass Spectrom. 1990 Dec 20;19(13):819–821. doi: 10.1002/bms.1200191305. [DOI] [PubMed] [Google Scholar]
- Smith L. J., Sutcliffe M. J., Redfield C., Dobson C. M. Analysis of phi and chi 1 torsion angles for hen lysozyme in solution from 1H NMR spin-spin coupling constants. Biochemistry. 1991 Jan 29;30(4):986–996. doi: 10.1021/bi00218a015. [DOI] [PubMed] [Google Scholar]
- Suckau D., Köhl J., Karwath G., Schneider K., Casaretto M., Bitter-Suermann D., Przybylski M. Molecular epitope identification by limited proteolysis of an immobilized antigen-antibody complex and mass spectrometric peptide mapping. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9848–9852. doi: 10.1073/pnas.87.24.9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda M., Przybylski M., Schreurs J., Miyajima A., Hogeland K., Deinzer M. Mass spectrometric determination of glycosylation sites and oligosaccharide composition of insect-expressed mouse interleukin-3. J Chromatogr. 1991 Jan 2;562(1-2):403–419. doi: 10.1016/0378-4347(91)80595-4. [DOI] [PubMed] [Google Scholar]
- Vestling M. M., Murphy C. M., Fenselau C. Recognition of trypsin autolysis products by high-performance liquid chromatography and mass spectrometry. Anal Chem. 1990 Nov 1;62(21):2391–2394. doi: 10.1021/ac00220a025. [DOI] [PubMed] [Google Scholar]



