Abstract
Emerging data is suggesting that the process of dendritic cell (DC) tolerization is an important step in tumorigenesis. Our understanding of the networks within the tumor microenvironment that functionally tolerize DC function is evolving while methods for genetically manipulating DC populations in situ continue to develop. A more intimate understanding of the paracrine signaling pathways which mediate immune evasion by subverting DC function promises to provide novel strategies for improving the clinical efficacy of DC-based cancer vaccines. This will likely require a better understanding of both the antigen expression profile and the immune evasion network of the tumor and its associated stromal tissues.
Introduction
Discovered in 1973 by Ralph Steinman, the dendritic cell (DC) is an antigen-presenting cell (APC) comprised of highly specialized antigen-processing and presentation machinery that allows for the efficient activation of antigen-specific T cells (Steinman and Cohn, 1973). This includes the ability to capture antigen from diverse peripheral tissues and to process this antigen in a manner that allows for effective presentation to effector CD8+ T cells in local lymph node tissues (Steinman et al., 1983). Additional attributes of the DC further enable these APCs to direct the differentiation and ultimate phenotype of the responding T cell, further shaping the adaptive immune response of the host (Banchereau and Steinman, 1998). These abilities explain the notable potency of DC-mediated T cell activation and establish the DC as the central orchestrator of anti-tumor immunity.
Development of DC-based Cancer Vaccines
These remarkable characteristics of the DC led to the concept of DC-based cancer vaccines, an approach that utilizes the myeloid DC as a vector for priming the immune system toward cancer-expressed antigens. While this approach was sought to capitalize on the DCs specialized ability to induce the activation of naïve T cell populations, it offered a seemingly elegant solution for overcoming the self-tolerance of tumor-expressed antigens (Gilboa, 2007). To date, however, this strategy has only resulted in limited benefit in the clinic (Melief, 2008). Since the initial clinical trial examining the efficacy of DC-based vaccination in 1995, steps toward the optimization of multiple parameters involved in the generation and delivery of DC-based cancer vaccines have been taken (Constantino et al., 2015). These have involved the eventual development of phase III clinical trials examining the role of DC-based cancer vaccines in several solid tumors including renal cell carcinoma, melanoma, prostate cancer, and glioblastoma. These studies have led to the first in-class approval of a DC-based vaccine strategy, sipuleucel-T (Provenge®) for metastatic, hormone-refractory prostate cancer after showing an improvement in overall survival relative to placebo. This has been followed by additional DC-based vaccines primarily for the treatment of glioblastoma that have been given orphan drug designations by the Food and Drug Administration (FDA) and the European Medicine Agencies (EMA). Despite this advance in the field, the overall impact of these agents in clinical oncology has remained limited. Part of this limitation is related to the intricacies of DC subset selection and preparation, tumor antigen selection and loading, as well as the route of DC delivery (Palucka et al., 2010). In addition to these technical issues of DC-based vaccine design, there has been a developing literature describing mechanisms by which cancers both evade and actively suppress the generation of anti-tumor immunity (Gajewski et al., 2006). It is has become clear that these mechanisms play an important role in immunotherapy failure and likely contribute to the limited efficacy of DC-based cancer vaccines (Gabrilovich, 2004). Indeed, recent reports have described biochemical mechanisms that cancers have evolved to subvert DC function in order to generate sites of immune privilege and to facilitate disease progression (Da Forno et al., 2008; Hanks et al., 2013a; Holtzhausen et al., 2015). While many of these mechanisms serve to suppress DC-dependent stimulation of T cell activation, some are capable of reprogramming DCs to allow for the differentiation and expansion of regulatory T cell (Treg) populations known to be central mediators of tumor-induce immune tolerance. It follows, therefore, that an improved understanding of these mechanisms will facilitate the engineering of more effective DC-based cancer vaccines. To date, efforts to take advantage of our understanding of these mechanisms to improve DC-based vaccination strategies have been relatively limited, suggesting that opportunities exist to significantly improve this immunotherapy strategy.
Tumor-mediated Immune Evasion and DC-targeted Subversion
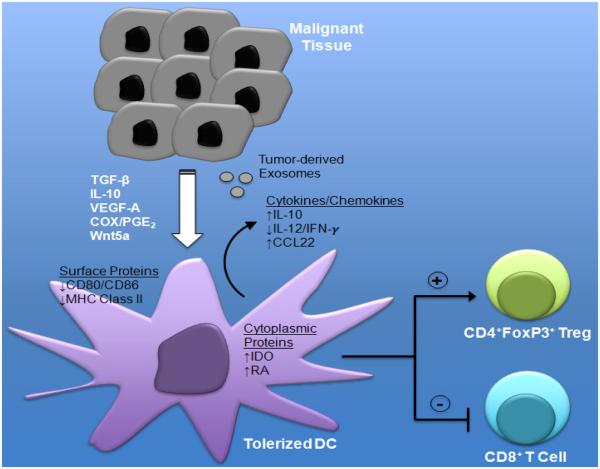
A variety of mechanisms have been described by which developing cancers manipulate host immunity ultimately allowing for their escape and metastatic progression. Many of these mechanisms have been passive, describing ways in which cancers downregulate the expression of more immunogenic antigens and/or class I major histocompatibility complex (MHC) molecules, effectively cloaking them from infiltrating CD8+ T cells (Rabinovich et al., 2007; Swann and Smyth, 2007). Additional studies have described more active mechanisms of immune suppression exemplified by the upregulation of PD-L1 to inhibit effector T cell activity (Kroemer and Galluzzi, 2015; Xu et al., 2014). The importance of these mechanisms is exemplified by the clinical effectiveness of the anti-PD-1 and anti-PD-L1 antibodies to block PD-L1-mediated T cell suppression (Badiee et al., 2007; Topalian et al., 2012; 2014). These immune evasion mechanisms also involve the expression and release of soluble mediators by either the tumor stroma or the tumor cells themselves that drive paracrine signaling networks in the tumor microenvironment (Liao et al., 2009). These networks have been shown to modulate DC function and, in some cases, induce their tolerization via as yet poorly understood biochemical signaling pathways (Figure 1). In light of the central role of the DC in the generation of cellular immunity, these paracrine signaling pathways are likely to be highly potent immune suppression mechanisms and to represent promising targets for augmenting DC-based cancer vaccine efficacy.
Figure 1.
Tumor-mediated mechanisms of dendritic cell (DC) tolerization and immune suppression. Tumor-derived soluble factors and exosomes promote DC-mediated Treg differentiation to generate an immune privileged site conducive to tumor progression. COX, cyclooxgenase; PGE2, prostaglandin E2; IDO, indoleamine 2,3-dioxygenase; RA, retinoic acid.
The transforming growth factor-β (TGF-β) cytokine has been shown by several investigators to suppress the activity of many different components of the immune system including various T cell subsets, natural killer (NK) cells, and DCs either directly or indirectly through the generation of Treg populations (Flavell et al., 2010). Several cancer types exhibit elevated levels of TGF-β expression primarily within their associated stroma and these levels have been correlated with poor clinical prognosis in several studies (Teicher, 2007). In terms of the DC, TGF-β directly suppresses the expression of interferon-γ (IFN-γ), a pro-inflammatory cytokine that plays a critical role in promoting CD8+ cytotoxic T cell activation and the TH1 polarization of differentiating CD4+ helper T cell populations (Travis and Sheppard, 2014). In addition, TGF-β suppresses DC co-stimulatory molecule and MCH class II expression while also promoting the expression of the CCL22 chemokine which is capable of recruiting Tregs to the tumor bed (Flavell et al., 2010; Hanks et al., 2013a). Indeed, transgenic mice harboring DCs engineered to be unresponsive to TGF-β signaling exhibit diminished melanoma growth along with enhanced T cell immunity to melanoma-expressed antigens (Hanks et al., 2013a; Laouar et al., 2008). These findings are consistent with the work of others which have shown myeloid cell TGF-β signaling to be a necessary step in the metastatic progression of pre-clinical murine tumor models (Pang et al., 2013). These immunologic attributes of TGF-β have led to the development of pharmacological treatment strategies to augment the currently available checkpoint inhibitors (Hanks and Morse, 2010; Holtzhausen et al., 2014a).
Although multifunctional and context dependent, the interleukin-10 (IL-10) cytokine has also been found to be expressed at high levels by some developing cancers and to be capable of directly inhibiting myeloid DC expression of several critical antigen presentation and co-stimulatory molecules by interfering with their NF-κB-dependent maturation program (Bhattacharyya et al., 2004; Mocellin et al., 2005). Indeed, IL-10 has been implicated in the inhibition of DC cross-presentation, the critical process of exogenous antigen uptake from the tumor microenvironment with subsequent MHC class I-restricted antigen presentation to cytotoxic T lymphocytes (Moore et al., 2001). Further work has shown tumor-released IL-10 to inhibit DC expression of the pro-inflammatory cytokine IL-12, a potent driver of cytotoxic T cell and NK cell activation, as well as the TH1 polarization of CD4+ helper T cells, all of which is necessary to generate a potent cell-mediated immune response. Consistent with these findings, T cells responding to IL-10 conditioned DCs become functionally anergic. In addition, tolerized DCs themselves have been shown to express the IL-10 cytokine to maintain their anergic state through an autocrine signaling mechanism that involves the anti-inflammatory signaling mediator, signal transducer and activator of transcription 3 (Stat3). This negative feedback loop has been implicated by some investigators to be particularly suppressive to DC-mediated T cell activation (Huang et al., 2011).
Factors that have been traditionally associated with other pro-tumorigenic properties such as angiogenesis as well as tumor cell survival and invasion have also been shown to suppress DC functionality. These include vascular endothelial growth factor A (VEGF-A) which inhibits the DC maturation program by interfering with the RelB, NF-κB subunit (Mimura et al., 2007). Consistent with these findings, anti-VEGF antibody therapy (bevacizumab) has been associated with an increase in peripheral blood DCs and a reduced number of immature myeloid progenitors in patients with advanced cancer (Osada et al., 2008). This impact on DC function may explain the recent data demonstrating improved disease control rates in advanced melanoma patients receiving combination ipilimumab and bevacizumab therapy (Hara et al., 2008).
Another factor that has been demonstrated to negatively influence DC function includes prostaglandin E2 (PGE2). This prostanoid lipid is generated by the cyclooxygenase (COX)-1 and 2 enzymes that are known to be overexpressed by several tumor types including colorectal, breast, and pancreatic cancers (Weeraratna et al., 2002). Previous work has demonstrated that PGE2 suppresses DC-mediated T cell proliferation by signaling through the G protein-coupled receptor, EP2 (van Baren and Van den Eynde, 2015).
Studies have demonstrated that the generation of PGE2 by tumors inhibits DC-induced effector T cell proliferation in vivo and others have found PGE2 to regulate T cell responses by inducing DC expression of the immunoregulatory enzyme, indoleamine 2,3-dioxygenase (IDO) (Jung et al., 2010; Kerner and Hoppel, 2000). Using a transgenic melanoma model, a more recent study has shown tumor-derived PGE2 to suppress the recruitment and activation status of the immunostimulatory CD103+ DC subset (Da Forno et al., 2008). These authors went on to show synergism between pharmacological COX inhibition and anti-PD-1 antibody immunotherapy in a murine melanoma model.
A more recently described mechanism that has been shown to actively tolerize DC populations within the tumor microenvironment includes the tumor-mediated release of soluble Wnt ligands. Although historically characterized as a key signaling pathway in developmental biology, there has been increasing interest in the Wnt-β-catenin signaling axis in the field of immunology (Clevers and Nusse, 2012; Staal et al., 2008). Initial studies implicating a role for β-catenin in regulating DC biology demonstrated the activation of this signaling pathway to suppress inflammatory cytokine expression by myeloid DCs (Jiang et al., 2007). This effect was found to promote the generation of IL-10-expressing CD4+ T cells and to ameliorate the condition of a murine model of multiple sclerosis. Based on this data, the authors conjectured that β-catenin may serve as a mediator of tolerogenic DCs. During a separate route of inquiry, studies identified the Wnt-β-catenin signaling pathway in intestinal DCs to promote the expression of the immunosuppressive cytokines, TGF-β and IL-10, while also suppressing the expression of the proinflammatory cytokines IL-6 and IL-12 (Manicassamy et al., 2010). Indeed, the genetic ablation of β-catenin expression specifically in DCs led to a significant reduction in Tregs within the intestinal epithelium of transgenic mice and promoted the development of inflammatory bowel disease. Further studies have also associated the Wnt3a and Wnt5a ligands with the development of a tolerogenic DC phenotype (Oderup et al., 2013; Valencia et al., 2011). A follow-up report suggested the tolerization effect of the DC β-catenin signaling pathway to be partially dependent upon the induction of retinoic acid synthesis, allowing for downstream induction of Treg differentiation (Hong et al., 2015). More recent work has shown melanomas to hijack this pathway in order to establish a site of immune privilege (Holtzhausen et al., 2014b). By releasing soluble Wnt5a into the tumor microenvironment, melanomas induce expression of the IDO enzyme in local DC populations in a β-catenin-dependent manner (Holtzhausen et al., 2015). IDO enzymatically converts the tryptophan amino acid into kynurenine, a compound capable of directly driving Treg differentiation (Fallarino et al., 2006; Sharma et al., 2007). Indeed, the upregulation of IDO by the Wnt5a-β-catenin signaling axis resulted in potent Treg generation and melanoma progression in an autochthonous BRAFV600EPTEN−/− melanoma mouse model. Interestingly, systemic inhibition of this signaling pathway synergistically enhanced the activity of anti-CTLA-4 antibody immunotherapy in murine melanoma while further work showed melanoma Wnt5a expression to be associated with diminished responses to anti-CTLA-4 antibody immunotherapy in both murine and human melanoma (Zhao et al., 2015).
Exploiting Mechanisms of DC-targeted Immune Evasion to Augment DC-based Cancer Vaccines
Investigators have utilized various approaches to genetically alter these immunosuppressive signaling pathways in DC-based vaccines. Ex vivo manipulation of tumor antigen-pulsed DCs using viral transduction or RNA transfection techniques allows the opportunity to augment DC functionality prior to their delivery to tumor-bearing hosts. While studies have evaluated the impact of ex vivo pharmacological treatment of DC vaccines to reverse these immunosuppressive signaling pathways prior to their administration, significant concerns exist in terms of the duration of the treatment impact on the physiology of the DC in situ. As a result, we focus here on genetic approaches to promoting DC functionality in the immunotolerant tumor microenvironment.
Investigators have utilized lentiviral vectors to overexpress a dominant-negative version of the type II TGF-β receptor (TβRIIDN) in myeloid DC-based vaccines to circumvent the suppressive activity of TGF-β in a prostate cancer mouse model (Lua et al., 2015). This study found tumor antigen-pulsed TβRIIDN DCs to enhance prostate cancer cell killing in vitro while suppressing prostate tumor growth and prolonging the survival of prostate tumor-bearing mice relative to control DC vaccines. An alternative approach has been taken to suppress DC-dependent expression of the IL-10 cytokine via small interfering RNA (siRNA)-targeted transfection (Zhao et al., 2015). This approach demonstrated that DC IL-10 inhibition generated a significant improvement in T cell-mediated leukemic cell killing, a reduction in bone marrow leukemic cells, and prolonged survival in a rat model of acute myeloid leukemia compared with control siRNA-transfected DC vaccines. Cationic microparticles to improve DC vaccine transfection have also been utilized to genetically silence DC IL-10 expression (Carreno et al., 2015). When paired with toll receptor stimulation, the blockade of IL-10 induction resulted in synergistic enhancement of DC pro-inflammatory cytokine expression as well as prolongation of survival in a murine lymphoma model. Silencing the expression of IL-10 in human monocyte-derived DCs similarly enhanced the induction of autologous T cell proliferation and TH1 CD4+ T cell polarization relative to control transfected DCs and has also been shown to enhance the generation of T cell responses targeting the MART1 melanoma antigen (Chhabra et al., 2008; Liu et al., 2004). As an alternative approach to blocking IL-10 signaling in DCs, siRNA-mediated silencing of the DC IL-10 receptor has also been investigated (Kim et al., 2011). Similar to the studies reported above, this maneuver enhanced DC expression of pro-inflammatory cytokines including IL-12 and promoted CD4+ TH1 polarization. In addition, these authors showed this strategy to enhance antigen-specific CD8+ T cell responses while suppressing the progression of a murine lung cancer model.
While studies have not directly altered the Wnt-β-catenin axis in an attempt to augment the T cell stimulatory capacity of DCs, investigators have targeted the IDO enzyme, a critical downstream effector of DCs in this pro-tolerogenic signaling pathway. Using human peripheral blood mononuclear cell-derived DCs, investigators have optimized siRNA transfection protocols to selectively suppress the expression of DC IDO (Flatekval and Sioud, 2009). Further studies showed that IDO-silenced DCs were capable of generating more robust T cell responses in vitro and inducing antigen-specific T cell responses in a small cohort of patients with gynecological malignancies (Sioud et al., 2013). One patient with metastatic ovarian cancer within this cohort was noted to have a prolonged clinical response following treatment with an IDO-silenced DC vaccine (Sioud et al., 2015). Additional work has demonstrated IDO-silenced DCs to enhance the activity of cytotoxic T lymphocytes while also suppressing the development of Treg populations in the tumor-draining lymph node tissues of a murine breast cancer model (Zheng et al., 2013). While the IDO-silenced DC vaccine suppressed growth of the breast cancer model when administered on the same day as implantation, it failed to impact tumor progression if treatment was delayed until primary tumor development was noted.
In order to circumvent the potential pitfalls of ex vivo generation and manipulation of DC vaccines, a contingent of investigators have launched a series of studies to genetically target DC populations in vivo (Tacken et al., 2007). Many of these in vivo DC targeting protocols involve tumor antigen delivery to in situ DCs utilizing a variety of different approaches. These include tumor antigen-conjugated antibodies that are specific to different DC-expressed C-type lectins, including DEC-205, and that target protein antigens to both MHC class I and class II processing pathways in DCs (Bonifaz et al., 2002; Steinman et al., 2003). Fused with the NY-ESO-1 cancer testis antigen, this approach is currently being evaluated in a series of active clinical trials in several different malignancies. This strategy has also been adapted to target siRNA-containing liposomes to in vivo DC populations (Badiee et al., 2007; Fan and Moon, 2015). By incorporating anti-DEC-205 antibodies into a liposomal structure, this approach can be used to deliver siRNA payloads to DCs in vivo (Zheng et al., 2009). Although the authors did not use a DC-specific strategy, a recent manuscript described the use of liposomal nanoparticles to genetically silence TGF-β expression in the microenvironment of a murine melanoma model (Xu et al., 2014). This study found that the addition of TGF-β-targeted silencing to nanoparticle vaccination for the TRP2 antigen was able to suppress growth even in previously established melanomas. Finally, a more recently developed approach using a re-engineered lentiviral vector has been designed to specifically transduce DCs by targeting the C-type lectin, DC-SIGN (Odegard et al., 2015). This technology is currently being utilized to treat patients with a variety of solid tumors by delivering the NY-ESO-1 cancer testis antigen to DCs in a phase I clinical trial (NCT02122861).
While many questions remain including the target cell specificity and transfection efficiencies of these in vivo DC delivery strategies, there are several potential advantages including the transfection of DCs within the natural environment of the tumor-draining lymph node as well as the simpler logistics of vaccine preparation which make this an attractive option for future cancer vaccine development.
Conclusions
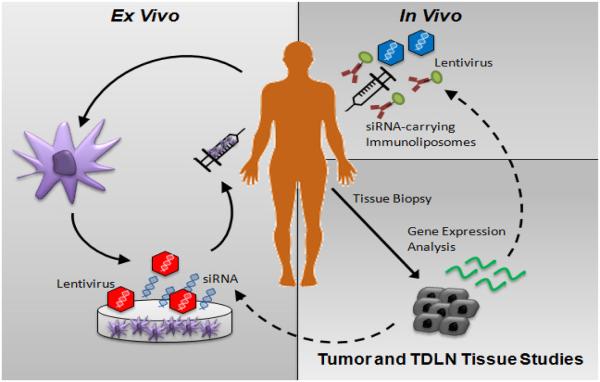
Despite our evolving understanding of the mechanisms utilized by cancers to subvert DC function, few studies to date have attempted to engineer DC-based vaccines to circumvent these suppressive pathways. While checkpoint inhibitor therapies have led the immunotherapy revolution in oncology, these agents do not offer the promise of personalized immunotherapy and, as a result, can be associated with significant autoimmune toxicity particularly when administered in combination (Kroemer and Galluzzi, 2015; Larkin et al., 2015; Postow et al., 2015). The future of cancer immunotherapy is likely to incorporate elements of various approaches including checkpoint blockade to augment effector T cell responses but also personalized cancer vaccines designed based on both selected tumor antigens as well as the incorporation of various modalities to inhibit or reverse active immune evasion and DC subversion mechanisms within the tumor microenvironment. The inclusion of DC-based cancer vaccines into checkpoint inhibitor regimens has been supported by a recent report showing that DC-based vaccination is capable of revealing a larger naïve T cell repertoire that is able to recognize and respond to sub-dominant tumor antigens, effectively enlarging the pool of T cells that can be further activated by checkpoint blockade (Carreno et al., 2015). The selection of which immune evasion pathways to target in these DC-based vaccine protocols will likely be determined based on data derived from various genetic, flow cytometry-based, and immunohistochemical/immunofluorescence-based studies of the tumor and tumor-draining lymph node tissues harvested from the patient prior to undergoing treatment (Figure 2). This rational combinatorial approach offers the optimal chance of generating potent anti-tumor immunity and achieving the therapeutic synergism that will be necessary to effectively treat patients with advanced malignancies.
Figure 2.
Ex vivo and in vivo DC-based vaccine protocol design based on dominant immune evasion mechanisms identified in tumor and tumor-draining lymph node (TDLN) tissues.
Acknowledgments
I would like to thank Dr. Alisha Holtzhausen for reviewing this manuscript and for assistance with figure design.
Footnotes
Disclosure
The author has no known relevant conflicts of interest to disclose.
References
- Badiee A, Davies N, Mcdonald K, Radford K, Michiue H, Hart D, Kato M. Enhanced delivery of immunoliposomes to human dendritic cells by targeting the multilectin receptor DEC-205. Vaccine. 2007;25(25):4757–4766. doi: 10.1016/j.vaccine.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman R. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Sen P, Wallet M, Long B, Baldwin AS, Jr, Tisch R. Immunoregulation of dendritic cells by IL-10 is mediated through suppression of the PI3K/Akt pathway and of IkappaB kinase activity. Blood. 2004;104(4):1100–1109. doi: 10.1182/blood-2003-12-4302. [DOI] [PubMed] [Google Scholar]
- Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, Linette GP. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra A, Chakraborty NG, Mukherji B. Silencing of endogenous IL-10 in human dendritic cells leads to the generation of an improved CTL response against human melanoma associated antigenic epitope, MART-1 27-35. Clin Immunol. 2008;126(3):251–259. doi: 10.1016/j.clim.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Constantino J, Gomes C, Falcao A, Cruz MT, Neves BM. Antitumor dendritic cell-based vaccines: lessons from 20 years of clinical trials and future perspectives. Transl Res. 2015 doi: 10.1016/j.trsl.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14(18):5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, Mcgrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor {zeta}-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines (Basel) 2015;3(3):662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatekval GF, Sioud M. Modulation of dendritic cell maturation and function with mono- and bifunctional small interfering RNAs targeting indoleamine 2,3-dioxygenase. Immunology. 2009;128(1 Suppl):e837–e848. doi: 10.1111/j.1365-2567.2009.03093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nature Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich D. Mechanisms and functional significance of tumor-induced dendritic cell defects. Nature Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- Gajewski T, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213:131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117(5):1195–1203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks BA, Holtzhausen A, Evans KS, Jamieson R, Gimpel P, Campbell OM, Hector-Greene M, Sun L, Tewari A, George A, Starr M, Nixon A, Augustine C, Beasley G, Tyler DS, Osada T, Morse MA, Ling L, Lyerly HK, Blobe GC. Type III TGF-beta receptor downregulation generates an immunotolerant tumor microenvironment. J Clin Invest. 2013a;123(9):3925–3940. doi: 10.1172/JCI65745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks BA, Morse MA. Pharmacological inhibition of TGFbeta as a strategy to augment the antitumor immune response. Curr Opin Investig Drugs. 2010;11(12):1342–1353. [PubMed] [Google Scholar]
- Hara T, Ogasawara N, Akimoto H, Takikawa O, Hiramatsu R, Kawabe T, Isobe K, Nagase F. High-affinity uptake of kynurenine and nitric oxide-mediated inhibition of indoleamine 2,3-dioxygenase in bone marrow-derived myeloid dendritic cells. Immunol Lett. 2008;116(1):95–102. doi: 10.1016/j.imlet.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Holtzhausen A, Evans K, Zhao F, Siska P, Rathmell J, Hanks BA. Combinatorial TGF-beta Signaling Blockade and anti-CTLA-4 Antibody Immunotherapy in a Murine BRAF(V600E)-PTEN−/− Transgenic Model of Melanoma. J Clin Oncol. 2014a;32(5s) [Google Scholar]
- Holtzhausen A, Zhao F, Evans K, Hanks BA. Early carcinogenesis involves the establishment of immune privilege via intrinsic and extrinsic regulation of indoleamine 2,3-dioxygenase-1: translational implications in cancer immunotherapy. Front Immunol. 2014b;5:1–9. doi: 10.3389/fimmu.2014.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzhausen A, Zhao F, Evans K, Tsutsui M, Orabona C, Tyler DS, Hanks BA. Melanoma-derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol Res. 2015;3(9):1082–1095. doi: 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Manoharan I, Suryawanshi A, Majumdar T, Angus-Hill ML, Koni PA, Manicassamy B, Mellor AL, Munn DH, Manicassamy S. beta-Catenin Promotes Regulatory T-cell Responses in Tumors by Inducing Vitamin A Metabolism in Dendritic Cells. Cancer Res. 2015;75(4):656–665. doi: 10.1158/0008-5472.CAN-14-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FP, Chen YX, To CK. Guiding the “misguided” - functional conditioning of dendritic cells for the DC-based immunotherapy against tumours. Eur J Immunol. 2011;41(1):18–25. doi: 10.1002/eji.201040543. [DOI] [PubMed] [Google Scholar]
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27(4):610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ID, Lee JS, Lee CM, Noh KT, Jeong YI, Park WS, Chun SH, Jeong SK, Park JW, Son KH, Heo DR, Lee MG, Shin YK, Kim HW, Yun CH, Park YM. Induction of indoleamine 2,3-dioxygenase expression via heme oxygenase-1-dependant pathway during murine dendritic cell maturation. Biochem Pharmacol. 2010;80(4):491–505. doi: 10.1016/j.bcp.2010.04.025. [DOI] [PubMed] [Google Scholar]
- Kerner J, Hoppel C. Fatty acid import into mitochondria. Biochim Biophys Acta. 2000;1486(1):1–17. doi: 10.1016/s1388-1981(00)00044-5. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kang TH, Noh KH, Bae HC, Ahn YH, Lee YH, Choi EY, Chun KH, Lee SJ, Kim TW. Blocking the immunosuppressive axis with small interfering RNA targeting interleukin (IL)-10 receptor enhances dendritic cell-based vaccine potency. Clin Exp Immunol. 2011;165(2):180–189. doi: 10.1111/j.1365-2249.2011.04410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L. Combinatorial immunotherapy with checkpoint blockers solves the problem of metastatic melanoma-An exclamation sign with a question mark. Oncoimmunology. 2015;4(7):e1058037. doi: 10.1080/2162402X.2015.1058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo V, Flavell R. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2008;105(31):10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, Ferrucci PF, Hill A, Wagstaff J, Carlino MS, Haanen JB, Maio M, Marquez-Rodas I, Mcarthur GA, Ascierto PA, Long GV, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4(11):e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Ng H, Akasaki Y, Yuan X, Ehtesham M, Yin D, Black KL, Yu JS. Small interference RNA modulation of IL-10 in human monocyte-derived dendritic cells enhances the Th1 response. Eur J Immunol. 2004;34(6):1680–1687. doi: 10.1002/eji.200425081. [DOI] [PubMed] [Google Scholar]
- Lua LH, Fan Y, Chang C, Connors NK, Middelberg AP. Synthetic biology design to display an 18kDa rotavirus large antigen on a modular virus-like particle. Vaccine. 2015;33(44):5937–5944. doi: 10.1016/j.vaccine.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329(5993):849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melief CJM. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Mimura K, Kono K, Takahashi A, Kawaguchi Y, Fujii H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol Immunother. 2007;56(6):761–770. doi: 10.1007/s00262-006-0234-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78(5):1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- Moore KW, De Waal Malefyt R, Coffman RL, O’garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Odegard JM, Kelley-Clarke B, Tareen SU, Campbell DJ, Flynn PA, Nicolai CJ, Slough MM, Vin CD, Mcgowan PJ, Nelson LT, Ter Meulen J, Dubensky TW, Jr, Robbins SH. Virological and preclinical characterization of a dendritic cell targeting, integration-deficient lentiviral vector for cancer immunotherapy. J Immunother. 2015;38(2):41–53. doi: 10.1097/CJI.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderup C, Lajevic M, Butcher EC. Canonical and noncanonical Wnt proteins program dendritic cell responses for tolerance. J Immunol. 2013;190(12):6126–6134. doi: 10.4049/jimmunol.1203002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada T, Chong G, Tansik R, Hong T, Spector N, Kumar R, Hurwitz HI, Dev I, Nixon AB, Lyerly HK, Clay T, Morse MA. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol Immunother. 2008;57(8):1115–1124. doi: 10.1007/s00262-007-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity. 2010;33(4):464–478. doi: 10.1016/j.immuni.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Gara SK, Achyut BR, Li Z, Yan HH, Day CP, Weiss JM, Trinchieri G, Morris JC, Yang L. TGF-beta signaling in myeloid cells is required for tumor metastasis. Cancer Discov. 2013;3(8):936–951. doi: 10.1158/2159-8290.CD-12-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, Mcdermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells . Ann Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MD, Baban B, Chandler PR, Hou D-Y, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine-2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M, Mobergslien A, Saeboe-Larssen S. Immunosuppressive factor blockade in dendritic cells via siRNAs results in objective clinical responses. Methods Mol Biol. 2015;1218:269–276. doi: 10.1007/978-1-4939-1538-5_16. [DOI] [PubMed] [Google Scholar]
- Sioud M, Saeboe-Larssen S, Hetland TE, Kaern J, Mobergslien A, Kvalheim G. Silencing of indoleamine 2,3-dioxygenase enhances dendritic cell immunogenicity and antitumour immunity in cancer patients. Int J Oncol. 2013;43(1):280–288. doi: 10.3892/ijo.2013.1922. [DOI] [PubMed] [Google Scholar]
- Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8(8):581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137(5):1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Swann JB, Smyth M. Immune Surveillance of Tumors. J Clin Invest. 2007;117(5):1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacken PJ, De Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7(10):790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- Teicher BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13(21):6247–6251. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, Mcdermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Sznol M, Mcdermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia J, Hernandez-Lopez C, Martinez VG, Hidalgo L, Zapata AG, Vicente A, Varas A, Sacedon R. Wnt5a skews dendritic cell differentiation to an unconventional phenotype with tolerogenic features. J Immunol. 2011;187(8):4129–4139. doi: 10.4049/jimmunol.1101243. [DOI] [PubMed] [Google Scholar]
- Van Baren N, Van Den Eynde BJ. Tumoral Immune Resistance Mediated by Enzymes That Degrade Tryptophan. Cancer Immunol Res. 2015;3(9):978–985. doi: 10.1158/2326-6066.CIR-15-0095. [DOI] [PubMed] [Google Scholar]
- Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1(3):279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8(4):3636–3645. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Evans K, Holtzhausen A, Tsutsui M, Tyler DS, Hanks BA. Targeting the Wnt5a-beta-catenin pathway in the melanoma microenvironment to augment checkpoint inhibitor immunotherapy. J Clin Oncol. 2015;33(suppl) abstr 3054. [Google Scholar]
- Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, Urquhart B, Min W. Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model. Int J Cancer. 2013;132(4):967–977. doi: 10.1002/ijc.27710. [DOI] [PubMed] [Google Scholar]
- Zheng X, Vladau C, Zhang X, Suzuki M, Ichim TE, Zhang ZX, Li M, Carrier E, Garcia B, Jevnikar AM, Min WP. A novel in vivo siRNA delivery system specifically targeting dendritic cells and silencing CD40 genes for immunomodulation. Blood. 2009;113(12):2646–2654. doi: 10.1182/blood-2008-04-151191. [DOI] [PubMed] [Google Scholar]