Abstract
Importance
The likelihood of achieving a live-birth with repeat in-vitro fertilisation (IVF) is unclear, yet treatment is commonly limited to three or four embryo transfers.
Objective
To determine the live-birth rate per initiated IVF cycle and with repeated cycles.
Design, Setting and Participants
Prospective study of 156,947 UK women who received 257,398 IVF ovarian stimulation cycles between 2003 and 2010 and were followed until June 2012.
Main exposure
IVF, with a cycle defined as an episode of ovarian stimulation and all subsequent separate fresh and frozen embryo transfers.
Main Outcome(s)
Live-birth rate per IVF cycle and the cumulative live-birth rates across all cycles in all women and by age and treatment type. Optimal, prognosis-adjusted and conservative cumulative live-birth rates were estimated, reflecting 0%, 30% and 100% of women discontinuing due to poor prognosis and having a live-birth rate of zero had they continued.
Results
In all women the live-birth rate for the first cycle was 29.5% (95%CI: 29.3, 29.7). This remained above 20% up to and including the fourth cycle. The cumulative prognosis-adjusted live-birth rate across all cycles continued to increase up to the ninth, with 65.3% (64.8, 65.8) of women achieving a live-birth by the sixth cycle. In women younger than 40 using their own oocytes, the live-birth rate for the first cycle was 32.3% (32.0, 32.5), and remained above 20% up to and including the fourth cycle. Six cycles achieved a cumulative prognosis-adjusted live-birth rate of 68.4% (67.8, 68.9). For women aged 40-42, the live-birth rate for the first cycle was 12.3% (95%CI: 11.8, 12.8), with six cycles achieving a cumulative prognosis-adjusted live-birth rate of 31.5% (29.7, 33.3). For women older than 42 years all rates within each cycle were less than 4%. No age differential was observed among women using donor oocytes. Rates were lower in those with untreated male factor infertility compared to those with any other cause, but treatment with either intra-cytoplasmic sperm injection or sperm donation removed this difference.
Conclusions and relevance
Among women in the UK undergoing IVF, the cumulative prognosis-adjusted live-birth rate after six cycles was 65.3%, with variations by age and treatment type. These findings support the efficacy of extending the number of IVF cycles beyond three or four.
Introduction
In-vitro fertilization (IVF) is commonly stopped after three or four unsuccessful embryo transfers,1,2 with three unsuccessful transfers labelled ‘repeat implantation failure’.3 This practice has been influenced by a study of 1,328 embryo transfers undertaken twenty-years ago, without use of intra-cytoplasmic sperm injection (ICSI), which reported a decline in live-birth rates after the fourth cycle.4 With one exception,5 previous studies of cumulative pregnancy or live-birth rates have been relatively small, with limited ability to precisely estimate cumulative success beyond four transfers.4,6–9 Previous studies have defined a cycle of IVF as an embryo transfer.5–9 Thus, each initiation of IVF with ovarian stimulation has been treated as several separate cycles whenever there has been a series of repeated embryo transfers. Given the promotion of single embryo transfer and the effective freezing of embryos have increased markedly over the last 10-15 years,10–15 it has been suggested that IVF success should be calculated as the live-birth rate per initiated ovarian stimulation, including all subsequent separate fresh and frozen embryo transfers.5,10–13
The aim of this study was to determine the extent to which repeat IVF cycles continue to increase the likelihood of a live-birth, defining an IVF cycle as the initiation of treatment with ovarian stimulation and all resulting separate fresh or frozen embryo transfers; hereafter we use the term “cycle” for this. Specific objectives were to determine: (i) the live-birth rate within each cycle, and the cumulative rate across all cycles; (ii) how these varied by age and treatment types (use of donor oocyte, ICSI or sperm donation); and (iii) the association between oocyte yield in one cycle and live-birth rate in subsequent cycles.
Methods
Ethical approval for this study was provided by the UK Human Fertilisation and Embryology Authority (HFEA) who have statutory obligations to prospectively collect information on all assisted reproductive treatment (ART) in the UK. Women provided written consent for this information to be used in analyses, audit and publications. The HFEA provided us with data on all ART events occurring in the UK between 1st January 2003 and 30th June 2012, with linkage of cycles to individual women and data on birth outcomes. Because all UK clinics, whether private or public, must provide information on any patients treated with ART, together with the outcomes of that treatment, to the HFEA, they are able to link cycles to individual women for all UK ART. We chose the 2003 start date in order to obtain a large cohort representative of contemporary treatment, and June 2012 was the latest date for which the HFEA could provide validated data. Because the live-birth outcome data were incomplete for cycles commencing between January 2011 and June 2012 (as many of these cycles were still continuing and births from them could occur after June 2012) we limited our potentially eligible cohort to ovarian stimulation cycles initiated between 1st January 2003 and 31st December 2010, with live-birth outcome data collected up to June 2012.
We excluded ART that was not IVF or was undertaken for the purpose of storage, donation or surrogacy. We excluded women who had started IVF before 2003. As in other studies,5–9 once a live-birth occurred women were censored from further analysis. To reflect clinical practice and allow comparisons with other studies,4,5,7,9 we included all embryo transfers, whether the individual transfer was of one or more embryos.
Live-birth was defined as an infant born alive after 24 weeks gestation surviving more than one month. The World Health Organisation (WHO) define live-birth as a birth showing any sign of life irrespective of gestational age. As in other studies,5, 15,16 we modified this to capture births that were likely to be viable. We defined an IVF cycle as the initiation of ovarian stimulation and all resulting separate fresh or frozen embryo transfers. The live-birth rate within a cycle was defined as the probability of a live-birth from an ovarian stimulation encompassing all subsequent fresh and frozen embryo transfers from that stimulation. Thus, for those embarking on IVF the live-birth rate within one cycle answers the question ‘What is my chance of a live-birth with one stimulation and retrieval of oocytes followed by as many subsequent separate embryo transfers as possible from that retrieval?’ The cumulative live-birth rate at a given cycle was defined as the probability of a live-birth from all cycles up to and including that cycle. This answers the question ‘What is my total chance of a live-birth with repeat ovarian stimulation and oocyte retrievals, together with the subsequent embryo transfers from each cycle, up to a given cycle number?’.
Information on age, types of treatment (oocyte donation, sperm donation and ICSI), oocyte yield and other couple characteristics were obtained from the HFEA dataset.
Statistical methods
We calculated the live-birth rates within the first and subsequent cycles up to the ninth, as the proportion of cycles resulting in a live-birth, using a normal approximation to construct confidence intervals. We calculated estimates of cumulative live-birth rates using different assumptions of women who discontinue IVF without a live birth (see below), up to the ninth cycle, using the Kaplan-Meier method with Greenwood’s approximation to calculate confidence intervals (see online supplementary material for full details).17,18 We used a log-rank test19 to compare the live-birth rate within each cycle and cumulatively across all cycles. The first set of comparisons was between woman’s age and oocyte source category and the second was between no male cause of infertility and male cause of infertility with and without treatment by ICSI or sperm donation. We assessed the relationship of oocyte yield in one cycle to live-birth rates in subsequent cycles in women younger than 40 years using their own oocytes, by calculating the within live-birth rate in the first, second, and third cycles by oocytes retrieved in the first cycle, and also calculating the within live-birth rate up to the fifth cycle by oocytes retrieved in the immediately preceding cycle.
Dealing with discontinuation of IVF
Infertile couples discontinue IVF for a number of reasons, with a systematic review of patient perceptions concluding that the commonest reasons were the physical and/or psychological burden of treatment, relationship or personal problems.20 In any study estimating cumulative live-birth rates assumptions have to be made about what the rate in those who discontinue would have been had they continued. To account for this we calculated ‘optimal’ and ‘conservative’ estimates, which are the have been assessed in previous studies. In addition we calculated a prognostic-adjusted estimate. The optimal estimate, is based on the observed data, and whilst not always explicit in previous publications, this assumes that the cumulative live-birth rate in women who discontinue IVF without a live-birth, if they had continued would be equal to the rate in those who continue to have further cycles.5 The conservative estimate assumes those who discontinue IVF would have had a subsequent live-birth rate of zero.5 The true rate is thought to lie between these two.7 The prognostic-adjusted estimate aims to obtain this more realistic value. It assumes a fixed proportion of those who discontinue do so because of poor prognosis and that the live-birth rate in that proportion would have been zero, whereas for those who discontinue for other reasons, such as inability to pay, emotional distress or (in our dataset) emigration from the UK, it would have been similar to those who continue with treatment.
For the prognosis-adjusted estimate we considered the woman’s age at her first cycle and oocyte yield in the previous cycle to be the strongest prognostic factors, because these have been shown to be strongly related to live-birth success.5,7,9,21,22 We checked that these were indicators of live-birth and of discontinuation of treatment in our own data, as well as comparing other available characteristics between those who discontinued and continued treatment after one unsuccessful cycle. To obtain age-adjusted and oocyte yield-adjusted estimates we calculated results for each age strata (18-34, 35-37, 38-39, 40-42, 43-44, 45-50, 50+ years) and for each possible oocyte-yield in the previous cycle and then obtained an average, weighted by the numbers within each category in the first cycle. It was not possible to calculate an age-adjusted estimates for the age stratified analyses as there is too little age variation within the age strata. For any analyses that include women using donor oocytes it is not possible to calculate rates adjusted for oocyte yield in the previous cycle as women using donor oocytes will not have an oocyte yield.
The age and previous oocyte yield adjusted results suggested that 3% of those who discontinued IVF did so because of poor prognosis. However, to calculate a prognostic-adjusted cumulative live-birth rate we assumed 30% of those who discontinued did so because of poor prognosis. We chose a value of ten-times that suggested by our data to obtain a conservative prognostic-adjusted estimate. Full details of how these estimates were calculated are provided in online supplementary material.
As the average population live-birth success rate for a single embryo transfer is between 20-30% in high income countries,10–13 we considered 20% to be a benchmark for a good live-birth rate within a cycle. All analyses were undertaken in Stata version 13 MP2. Two-sided p-values < 0.05 were considered to provide evidence against the null hypothesis.
Comparison with live-birth rates in those not receiving ART
We used data on pregnancy and pregnancy loss rates from published literature to estimate live-birth rates in women who conceive naturally.23-254 Two prospective cohort studies of couples actively trying to conceive provided age specific pregnancy rates attained within twelve menstrual cycles.23,24 Live birth rates were calculated assuming 20% of natural conceptions result in a pregnancy loss.25
Results
Following planned exclusions the eligible cohort included 257,665 cycles in 157,475 women. For all analyses we excluded women with missing linkage information or implausible linkage (i.e. first IVF transfer being a frozen embryo transfer without preceding ovarian stimulation). This resulted in an analysis cohort of 257,398 cycles by 156,947 women (more than 99% of the eligible cohort; Figure 1). Table 1 shows the characteristics of the cohort. eTable 1 shows characteristics by year of treatment. Because of the large sample size there was statistical evidence of differences in all characteristics, but for most these were small and unlikely to be clinically important. For example, median age of the women differed by one-year and median oocyte retrieval differed by one across the study period. Use of ICSI increased by 11%, and transfer of single embryos by 17%, though the live-birth rate increased by just two-percent across the study period.
Figure 1. Definition of eligible and analysis cohort.
Table 1. Characteristics of the analysis cohort of 156,947 women commencing IVF treatment for infertility in the UK in 2003-2010 (with outcomes assessed up to June 2012).
| Characteristic | For all cycles combineda | For first cycleb |
|---|---|---|
| Number of women | 156,947 | 156,947 |
| Total number of cycles 1 2 3 More than 3 |
93,494 (59.6%) 39,707 (25.3%) 15,507 (9.9%) 8,239 (5.2%) |
|
| Number of cycles | 257,398 | 156,947 |
| Live-births (% per cycle) | 70,093 (27.2%) | 46,333 (29.5%) |
| Woman’s age (years) Median (1st quartile, 3rd quartile) |
35 (32, 38) |
35 (32, 38) |
| Duration of infertility (years) Median (1st quartile, 3rd quartile) Missing |
4 (2, 6) 11,165 (4.3%) |
3 (2, 5) 6,586 (4.0%) |
| Causes of infertility (non-exclusive) Tubal Ovulatory Endometriosis Male cause |
46,535 (18.1%) 34,473 (13.4%) 15,889 (6.2%) 105,014 (40.8%) |
28,181 (18.0%) 21,582 (13.8%) 9,654 (6.1%) 63,023 (40.2%) |
| Treated with ICSI | 123,009 (47.8%) | 68,608 (43.7%) |
| Treated with sperm donation | 8,067 (3.1%) | 4,781 (3.05%) |
| Treated with oocyte donation | 7,223 (2.8%) | 3,587 (2.3%) |
| Oocytes retrieved (own) Median (1st quartile, 3rd quartile) |
9 (5, 13) | 9 (5, 13) |
| Embryo transfer events per cycle No embryos transferred Fresh embryo transfer only Fresh and frozen embryo transfer |
31,738 (12.3%) 199,713 (77.6%) 25,947 (10.1%) |
20,794 (13.3%) 119,462 (76.1%) 16,691 (10.6%) |
| Number of embryo transfer events | 257,581 | 157,043 |
| Number of embryos transferred per embryo transfer eventc 1 2 3-4 |
44,330 (17.2%) 201,888 (78.4%) 11,363 (4.4%) |
29,942 (19.1%) 122,483 (78.0%) 4,618 (3.0%) |
The unit of analysis here is cycle (with results the average across all cycles per woman)
As this is just one cycle the unit of analysis is the women at their first treatment cycle
As there are a variable number of transfer events per treatment cycle (which includes all subsequent fresh and frozen transfer events) the % is per the number of transfer events (not per cycle)
Table 2 shows the live-birth rate within each cycle for the whole cohort. In all women the live-birth rate for the first cycle was 29.5% (95%CI: 29.3, 29.7). The live-birth rate within cycles remained above 20% for each cycle up to and including the fourth. After their first cycle there were 110,614 women (70.5% of the analysis cohort) who did not have a live-birth. Of these, 37,704 (34.1%) discontinued treatment and 72,910 (65.9%) had at least one more cycle. eTable 2 compares characteristics between these two groups. Although there was statistical evidence of differences for all characteristics the actual differences were small.
Table 2. Within initiated treatment cycle live-birth rates and cumulative live-birth rate across all cycles in 156,947 women undergoing 257,398 cycles of IVF.
| Cycle number | N Cycles | N live-births | Live-birth rate within each cycle % (95%CI) | Cumulative live-birth across all cycles using different estimates % (95%CI) | |||
|---|---|---|---|---|---|---|---|
| Optimal estimatea | Age adjusted estimateb | Prognostic-adjusted estimatec | Conservative estimated | ||||
| 1st | 156,947 | 46,333 | 29.5 (29.3, 29.7) | 29.5 (29.3, 29.7) | 29.5 (29.3, 29.7) | 29.5 (29.3, 29.7) | 29.5 (29.3, 29.7) |
| 2nd | 63,453 | 15,825 | 24.9 (24.6, 25.3) | 47.1 (46.8, 47.4) | 46.7 (46.4, 47.0) | 45.1 (44.9, 45.4) | 40.5 (40.3, 40.8) |
| 3rd | 23,746 | 5,358 | 22.6 (22.0, 23.1) | 59.0 (58.7, 59.4) | 58.3 (57.9, 58.6) | 54.3 (54.0, 54.6) | 44.6 (44.4, 44.9) |
| 4th | 8,239 | 1,690 | 20.5 (19.6, 21.4) | 67.4 (67.0, 67.9) | 66.4 (66.0, 66.9) | 59.8 (59.4, 60.1) | 46.1 (45.8, 46.3) |
| 5th | 3,012 | 553 | 18.4 (17.0, 19.7) | 73.4 (72.8, 74.0) | 72.2 (71.6, 72.7) | 63.1 (62.6, 63.5) | 46.6 (46.3, 46.8) |
| 6th | 1,162 | 202 | 17.4 (15.2, 19.6) | 78.0 (77.3, 78.8) | 76.7 (76.0, 77.5) | 65.3 (64.8, 65.8) | 46.8 (46.5, 47.0) |
| 7th | 458 | 79 | 17.2 (13.8, 20.7) | 81.8 (80.8, 82.8) | 80.5 (79.5, 81.5) | 66.8 (66.2, 67.4) | 46.9 (46.7, 47.2) |
| 8th | 199 | 37 | 18.6 (13.2, 24.0) | 85.2 (83.9, 86.5) | 83.7 (82.4, 85.0) | 68.0 (67.3, 68.7) | 46.9 (46.7, 47.2) |
| 9th | 83 | 13 | 15.7 (7.8, 23.5) | 87.5 (85.9, 89.1) | 86.3 (84.7, 87.9) | 68.7 (68.0, 69.5) | 46.9 (46.7, 47.2) |
The optimal estimate assumes that the cumulative live-birth rate in women who discontinue IVF without a live-birth, if they had continued, would have been equal to the rate in women who continued to have further IVF. That is it assumes that 0% of women who discontinued IVF did so because of poor prognosis that would have affected their live-birth success had they continued.
The age-adjusted estimate assumes that the cumulative live-birth rate in women who discontinued IVF, if they had continued, would have been equal to the rate in women who were the same age at the start of treatment, and who continued to have further IVF. These results suggested approximately 3% of women who discontinued did so because of poor prognosis and would have had a live-birth rate of zero, had they continued.
The prognostic-adjusted estimate assumes that 30% of women who discontinued IVF did so because of poor prognosis and would have had a live-birth rate of zero, had they continued.
The conservative estimate assumes that the cumulative live-birth rate in all women who discontinued IVF would have been zero, had they continued. That is it assumes that 100% of women who discontinued did so because of poor prognosis and would have had a live-birth rate of zero, had they continued.
Note it is not possible to calculate an oocyte-adjusted estimate for the whole cohort due to the presence of women using donor oocytes.
The cumulative live-birth rate continued to increase up to the ninth cycle, with a cumulative prognosis-adjusted live-birth rate of 65.3% (64.8, 65.8) by the sixth cycle (Table 2). The equivalent optimal (78.0% (77.3, 78.8)) and age-adjusted (76.7% (76.0, 77.5)) estimates for six cycles were similar, while the conservative estimate was 46.8% (46.5, 47.0) (Table 2 and eFigure 1).
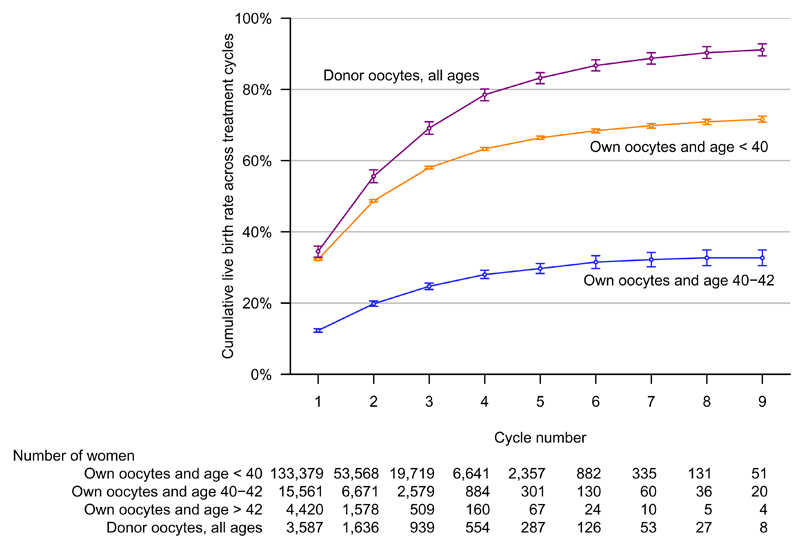
Results varied by age and oocyte source (Figure 2, Table 3, eTables 3 and 4). In women who were younger than 40 years and using their own oocytes (133,379 women, 85% of the cohort), the live-birth rate for the first cycle was 32.3% (32.0, 32.5). This remained above 20% up to and including the fourth cycle. The previous cycle oocyte-yield adjusted and optimal estimates were similar. Six cycles achieved cumulative live-birth rates of 68.4%, (67.8, 68.9), 80.3% (79.5 to 81.0) and 50.7% (50.5, 51.0), for the prognostic-adjusted, optimal and conservative estimates, respectively. For women aged 40-42, the live-birth rate for the first cycle was 12.3% (11.8, 12.8), with six cycles achieving a cumulative live-birth rates of 31.5% (29.7, 33.3), 41.5% (38.0, 44.9), and 19.2% (18.5, 19.8) for prognostic-adjusted, optimal and conservative estimates, respectively. For women older than 42 years all rates within each cycle were less than 4% or based on too few live-births to calculate confidence intervals.
Figure 2. Cumulative live-birth rate across all initiated IVF cycles by age and oocyte source.
The figure shows the prognosis-adjusted estimates of cumulative live-birth rates (i.e. the rate (shown on the y-axis) is the likelihood of a live-birth across all initiated cycles up to and including the numbers on the x-axis), with 95% confidence intervals. These are presented for women in two different age categories at the start of their first IVF treatment cycle (< 40 years and 40-42 years; women in both of these categories used their own oocytes) and also in women who used donor oocytes (these women cover the full age range). Data for women aged over 42 at their first treatment cycle are not shown because rates were so low it would have been difficult to represent them on this same graph (full results for these women are shown in Table 3). The prognostic-adjusted estimate assumes that 30% of those who discontinued IVF did so because of poor prognosis and that the live-birth rate in that 30% would have been zero had they continued. Analyses were completed in 156,947 women undergoing 257,398 cycles. Log-rank tests indicated a difference between the cumulative live-births rates for all groups (p < 0.001 for all comparisons).
Table 3. Within initiated treatment cycle live-birth rates and cumulative live-birth rate across all cycles in 153,360 women, undergoing 250,175 cycles of IVF using their own oocytes, stratified by age at first ovarian stimulation cycle.
| Cycle number | N Cycles | N live-births | Live-birth rate within each cycle % (95%CI) | Cumulative live-birth across all cycles using different estimates % (95%CI) | |||
|---|---|---|---|---|---|---|---|
| Optimal estimatea | Previous oocyte yield-adjusted estimateb | Prognostic-adjusted estimatec | Conservative estimated | ||||
| Aged less than 40 years | |||||||
| 1st | 133,379 | 43,019 | 32.3 (32.0, 32.5) | 32.3 (32.0, 32.5) | 32.3 (32.0, 32.5) | 32.3 (32.0, 32.5) | 32.3 (32.0, 32.5) |
| 2nd | 53,568 | 14,532 | 27.1 (26.8, 27.5) | 50.6 (50.3, 50.9) | 50.7 (50.4, 51.1) | 48.7 (48.4, 49.0) | 44.3 (44.0, 44.5) |
| 3rd | 19,719 | 4,793 | 24.3 (23.7, 24.9) | 62.6 (62.3, 63.0) | 62.7 (62.3, 63.1) | 58.0 (57.7, 58.4) | 48.6 (48.4, 48.9) |
| 4th | 6,641 | 1,419 | 21.4 (20.4, 22.4) | 70.6 (70.1, 71.1) | 70.5 (70.1, 71.0) | 63.3 (62.9, 63.7) | 50.1 (49.8, 50.3) |
| 5th | 2,357 | 449 | 19.0 (17.5, 20.6) | 76.2 (75.6, 76.8) | 76.0 (75.4, 76.6) | 66.4 (66.0, 66.9) | 50.6 (50.3, 50.8) |
| 6th | 882 | 150 | 17.0 (14.5, 19.5) | 80.3 (79.5, 81.0) | 80.1 (79.3, 80.8) | 68.4 (67.8, 68.9) | 50.7 (50.5, 51.0) |
| 7th | 335 | 58 | 17.3 (13.3, 21.4) | 83.7 (82.7, 84.7) | 83.4 (82.4, 84.4) | 69.8 (69.1, 70.4) | 50.8 (50.5, 51.1) |
| 8th | 131 | 25 | 19.1 (12.4, 25.8) | 86.8 (85.4, 88.2) | 86.5 (85.1, 87.9) | 70.9 (70.1, 71.6) | 50.9 (50.6, 51.1) |
| 9th | 51 | 10 | 19.6 (8.7, 30.5) | 89.4 (87.6, 91.2) | 88.8 (87.2, 90.3) | 71.6 (70.8, 72.5) | 50.9 (50.6, 51.2) |
| Aged 40 to 42 years | |||||||
| 1st | 15,561 | 1,914 | 12.3 (11.8, 12.8) | 12.3 (11.8, 12.8) | 12.3 (11.8, 12.8) | 12.3 (11.8, 12.8) | 12.3 (11.8, 12.8) |
| 2nd | 6,671 | 671 | 10.1 (9.3, 10.8) | 21.1 (20.3, 21.9) | 20.8 (20.0, 21.6) | 19.8 (19.1, 20.6) | 16.8 (16.3, 17.4) |
| 3rd | 2,579 | 223 | 8.6 (7.6, 9.7) | 27.9 (26.8, 29.1) | 27.6 (26.5, 28.7) | 24.7 (23.8, 25.6) | 18.5 (17.8, 19.1) |
| 4th | 884 | 69 | 7.8 (6.0, 9.6) | 33.6 (31.9, 35.2) | 33.0 (31.4, 34.7) | 28.0 (26.9, 29.2) | 19.0 (18.4, 19.6) |
| 5th | 301 | 16 | 5.3 (2.8, 7.9) | 37.4 (34.8, 39.4) | 36.5 (34.3, 38.8) | 29.7 (28.3, 31.1) | 19.1 (18.5, 19.8) |
| 6th | 130 | 9 | 6.9 (2.6, 11.3) | 41.5 (38.0, 44.9) | 40.5 (37.3, 43.8) | 31.5 (29.7, 33.3) | 19.2 (18.6, 19.8) |
| 7th | 60 | 2 | 3.3† | 43.4 (39.1, 47.7) | 42.4 (38.4, 46.3) | 32.2 (30.2, 34.2) | 19.2 (18.6, 19.9) |
| 8th | 36 | 1 | 2.8† | 45.0 (39.8, 50.1) | 43.4 (39.1, 47.6) | 32.7 (30.5, 34.9) | 19.2 (18.6, 19.9) |
| 9th | 20 | 0 | 0.0† | 45.0 (39.8, 50.1) | 43.4 (39.1, 47.6) | 32.7 (30.5, 34.9) | 19.2 (18.6, 19.9) |
| Aged more than 42 years | |||||||
| 1st | 4,420 | 164 | 3.7 (3.2, 4.3) | 3.7 (3.2, 4.3) | 3.7 (3.2, 4.3) | 3.7 (3.2, 4.3) | 3.7(3.2, 4.3) |
| 2nd | 1,578 | 52 | 3.3 (2.4, 4.2) | 6.9 (5.9, 7.9) | 6.9 (5.9, 7.9) | 6.3 (5.4, 7.2) | 4.9 (4.3, 5.6) |
| 3rd | 509 | 17 | 3.3 (1.8, 4.9) | 10.0 (8.2, 11.7) | 9.8 (8.1, 11.5) | 8.3 (7.1, 9.6) | 5.4 (4.7, 6.0) |
| 4th | 160 | 2 | 1.3† | 11.1 (8.8, 13.4) | 10.1 (8.5, 11.8) | 8.9 (7.4, 10.5) | 5.5 (4.8, 6.2) |
| 5th | 67 | 3 | 4.5† | 15.1 (10.2, 20.0) | 14.2 (10.7, 17.7) | 10.7 (8.2, 13.2) | 5.5 (4.8, 6.2) |
| 6th | 24 | 0 | 0.0† | 15.1 (10.2, 20.0) | 14.2 (10.7, 17.7) | 10.7 (8.2, 13.2) | 5.6 (4.9, 6.3) |
| 7th | 10 | 2 | 20.0† | 32.1 (10.7, 53.5) | 22.3 (14.0, 30.5) | 15.9 (8.5, 23.2) | 5.6 (4.9, 6.3) |
| 8th | 5 | 0 | 0.0† | 32.1 (10.7, 53.5) | 22.3 (14.0, 30.5) | 15.9 (8.5, 23.2) | 5.6 (4.9, 6.3) |
| 9th | 4 | 0 | 0.0† | 32.1 (10.7, 53.5) | 22.3 (14.0, 30.5) | 15.9 (8.5, 23.2) | 5.6 (4.9, 6.3) |
The optimal estimate assumes that the cumulative live-birth rate in women who discontinue IVF without a live-birth, if they had continued, would have been equal to the rate in women who continued to have further IVF. That is it assumes that 0% of women who discontinued IVF did so because of poor prognosis that would have affected their live-birth success had they continued.
The previous oocyte yeild-adjusted estimate assumes that the cumulative live-birth rate in women who discontinued IVF, if they had continued, would have been equal to the rate in women who had the same oocyte yield in the immediately previous ovarian stimulation treatment, and who continued to have further IVF. These results suggested approximately 3% of women who discontinued did so because of poor prognosis and would have had a live-birth rate of zero, had they continued.
The prognostic-adjusted estimate assumes that 30% of women who discontinued IVF did so because of poor prognosis and would have had a live-birth rate of zero, had they continued.
The conservative estimate assumes that the cumulative live-birth rate in all women who discontinued IVF would have been zero, had they continued. That is it assumes that 100% of women who discontinued did so because of poor prognosis and would have had a live-birth rate of zero, had they continued.
Note it is not possible to calculate an age-adjusted estimate these age stratified analyses and there is too little age variation within the ages stratified groups to further adjust for age.
These are cycles for which there was fewer than six live births and for these standard errors and hence confidence intervals could not be calculated
Use of donor oocytes removed this age differential, as the log-rank test showed no evidence for different cumulative live-birth rates between age categories (eTable 3). Irrespective of age, women using donor oocytes achieved live-birth rates within each cycle of 29.6% or greater for all cycles up to and including the ninth and a cumulative live-birth rate after six cycles of 86.7% (85.2, 88.3), 91.7% (90.3, 93.1) and 75.5% (74.0, 77.1) for the prognostic-adjusted, optimal and conservative estimates, respectively (eTable 4).
Live-birth rates varied by male cause infertility and its treatment (Figure 3 and eTables 5 to 7). Women whose infertility was due to a male related cause and who were not treated with either ICSI or donor sperm had lower live-birth rates than those with a non-male cause of infertility (eTables 3 and 5). Those with a male cause of infertility who were treated with ICSI had cumulative live-birth rates, after six cycles, of 71.3% (70.5, 72.1), 82.2% (81.1,83.4) and 54.7% (54.3, 55.2) using the prognostic-adjusted, optimal and conservative, estimates, respectively (eTable 6). Equivalent results for those with male infertility treated with donor sperm were 81.2% (78.6, 83.9), 90.2% (87.2, 93.1) and 65.9% (63.9, 67.9) respectively (eTable 7). Live-birth rates in both of these groups were greater than in those with a non-male cause of infertility (eTables 3 and 8).
Figure 3. Cumulative live-birth rate across all initiated IVF cycles by ICSI and sperm donation.
The figure shows the prognosis-adjusted estimates of cumulative live-birth rates (i.e. the rate (shown on the y-axis) is the likelihood of a live-birth across all initiated cycles up to and including the numbers on the x-axis), with 95% confidence intervals. These are shown for couples without a male cause of infertility, couples with a male cause who were not treated with ICSI or sperm donation, those with a male cause who were treated with ICSI and those with a male cause who used sperm donation. The prognostic-adjusted estimate assumes that 30% of those who discontinued IVF did so because of poor prognosis and that the live-birth rate in that 30% would have been zero had they continued. Analyses were completed in 156,947 women undergoing 257,398 cycles. Log-rank tests indicated a difference between the cumulative live-births rates for all groups (p < 0.001 for all comparisons).
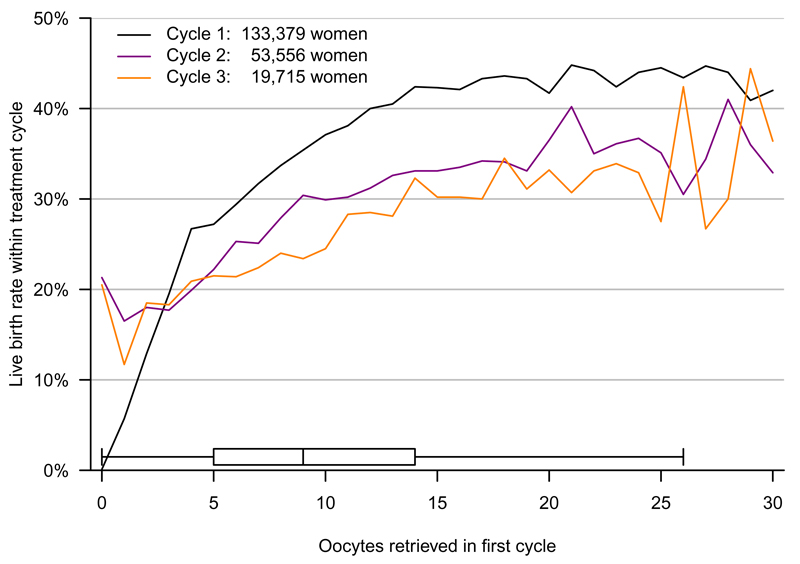
Figure 4 shows the live-birth rate within the first, second and third cycles plotted against the number of oocytes retrieved in the first cycle in women under 40 years of age using their own oocytes. For those in whom no oocytes were retrieved in the first cycle the live-birth rates in the second and third cycles were greater than 20%. The live-birth rates in the first, second and third cycles continued to increase with increasing oocytes retrieved in the first cycle up to around 15 oocytes; thereafter the curves flatten. Plotting the live-birth rate within any cycle against the number of oocytes retrieved in the previous cycle gave a similar pattern (eFigure 2).
Figure 4. Live-birth rate within each single IVF treatment cycle by oocyte retrieval in first cycle.
The figure shows the live-birth rate within each individual first, second and third treatment cycle (i.e. for each line the rate on the y-axis is the rate for just that one treatment cycle), against the number of oocytes retrieved in the first treatment cycle (shown on the x-axis). Analyses are in 134,903 women aged less than 40 years and using their own oocytes. Box and whiskers show the central 95% of the distribution of oocytes retrieved in the first cycle, as well as the median and lower and upper quartiles.
Using published data23–25 we estimated that the live-birth rate for women conceiving naturally, who had been trying for 12 menstrual cycles, varied between 58% and 74% depending on the woman’s age and frequency of intercourse (eTable 9). These estimates are based on studies that only included women younger than 40. Similar cumulative live-birth rates were achieved by the fifth or sixth cycle of IVF treatment in women of this age (Table 3), though, in these women, five cycles took a median of 2 years (1st, 3rd quartile: 2, 3).
Discussion
To our knowledge this is the first study to have linked fresh and frozen embryo transfers to obtain estimates of live-birth rate within each IVF ovarian stimulation cycle and cumulative live-birth rates across repeated stimulation cycles. Despite a decline in the success rate within each cycle as the number of these increased, the cumulative rate across cycles increased up to the ninth in the whole cohort, those younger than 40 (using their own oocytes) and those using donor oocytes (irrespective of age). They also increased up to the eighth or ninth in women aged 40-42, though for women older than 42 (using their own oocytes) the likelihood of success was low and the cumulative live-birth rate did not appear to clearly increase beyond the fourth or fifth cycle. For those women able to use donor oocytes, age was unrelated to success. In those for whom the cause of infertility was related to a male partner problem, treatment with ICSI or donor sperm made a marked difference in the likelihood of success, with cumulative rates increasing up to the eighth or ninth cycle, whereas without treatment rates were lower than in those with other causes of infertility. In women under 40 years with a low oocyte yield in a previous cycle there was benefit in continuing with further cycles. We also found women under 40 years could achieve cumulative live-birth rates after five or six cycles that were similar to published live-birth rates achieved naturally within 12 menstrual cycles.23–25 It should be noted, however, that, in these women, five cycles took a median of 2 years.
Widespread adoption of single embryo transfer has reduced multiple pregnancies and adverse perinatal outcomes, but has meant that the chance of a live-birth from a single ovarian stimulation cycle is spread across multiple embryo transfers, which we have assessed here. Since this method of assessing IVF success combines all embryo transfer events following an ovulation stimulation into one analysis unit, we were unable to examine the effect of the number of embryos transferred per event. However, this method of assessing IVF success is increasingly recommended.5,10–13 Our results show how success rates per embryo transfer event are misleadingly lower, compared with the rate within each ovarian stimulation cycle. Furthermore, we have previously shown, using unlinked data from the same population, that the number of embryos transferred in one event has a relatively modest effect on live-birth rate, with a difference of 9% in women younger than 40 years and 16% in those aged 40 years or older, comparing double to single embryo transfer.15
Despite the differences in the definition of cumulative success between our study and the previous largest study (from the US), in which cumulative live-birth rates were estimated on the basis of each embryo transfer,5 and differences in health systems between the US and UK, both studies found age differences in rates and that these were removed with the use of donor oocytes. In the US study, those with a male cause of infertility had one of the highest cumulative live-birth rates per embryo transfer, but that study did not examine the effect of different treatments (ICSI or sperm donation) and it may be that all of those with male cause infertility in the US receive one of these treatments.
The key limitation of all studies looking at cumulative outcomes with repeat IVF is how one treats those who discontinue treatment. As seen in our data, and in previous studies,5,7 the extremes of the optimal and conservative estimates often vary markedly, for example in our data the optimal and conservative estimates were 78.0% and 46.8%, respectively, for the whole cohort. This is because of the differences between these two, in what they assume would have been the live-birth rate in those who discontinued IVF, had they continued; for the optimal estimate this is assumed to be the same as those who did continue, whereas the conservative estimate it is assumed to be zero. We examined the likelihood that such discontinuation was due to poor prognosis based on age and previous cycle oocyte retrieval. These analyses suggested approximately 3% of those who discontinued did so because of poor prognosis. This small proportion was because although these two were important predictors of live-birth, few women receiving IVF are older than 40 years (only 15% in our national population cohort) and most women have a high oocyte yield (median 9 per cycle in our cohort). However, to account for other factors, for example pre-treatment reproductive hormone levels, smoking and body mass index (BMI), which have been linked to live-birth success, 7,22 but that were not available in this study, we assumed a 30% discontinuation due to poor prognosis. Because of the legal requirement for all UK clinicians to provide data on all ART patients, the HFEA were able to link cycles to individual women even if they moved between clinics within the UK. However, treatment abroad would be absent from our data. A European study, conducted 6 years ago, found very few UK couples travelled for ART to 49 clinics in six (non-UK) European countries with high rates of cross-border patients.26 We were only able to assess live-birth as an outcome: future studies should also consider potential adverse effects of continued treatment, including ovarian hyper-stimulation syndrome and possible increased risk of preterm birth, low birth weight or congenital anomalies.16,27,28
We acknowledge that for some couples the emotional stress of repeat treatments may be undesirable and the cost of a prolonged treatment course, with several repeat oocyte stimulation cycles, may be unsustainable for health services, insurers or couples. However, we think the potential for success with further cycles should be discussed with couples. A cost-effectiveness analysis is beyond the scope of this study, and the difficulties of undertaking such analyses for IVF, in which decisions related to how one values a new life and whether ‘benefits’ and ‘costs’ for both parents and the child should be included, are well-documented.29 The costs of IVF treatment vary between countries, whether publicly or privately funded, and the treatment type used, but are in the range of $14,000 (£9,000, €12,000) to $17,000 (£11,000, €15,000) per cycle.1,29,30 These costs exclude assessment prior to starting IVF and are based on transfer of one fresh embryo. Assuming each addition frozen embryo transfer costs $4000 to $5000,30 the cost per couple of continuing to six, rather than having just three cycles, could be as much as $132,000 compared to $66,000 (assuming one fresh and one frozen transfer per cycle).
Conclusions
Among women in the UK undergoing IVF, the cumulative prognosis-adjusted live-birth rate after six cycles was 65.3%, with variations by age and treatment type. These findings support the efficacy of extending the number of IVF cycles beyond three or four.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff of the HFEA and the contributing UK clinics for these data. All views are those of the authors and not necessarily those of the HFEA.
Funding
ADACS, KT and DAL work in a Unit that receives funding from the UK Medical Research Council (www.mrc.ac.uk) (MC_UU_12013/5 and MC_UU_12013/9) and DAL receives support from a NIHR Senior Investigator awards (NF-SI-0611-10196). This work is also supported by a grant from the Wellcome Trust (www.wellcome.ac.uk) which pays ADACS’ salary (WT094311MA). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author contributions: DAL and SMN designed the study, developed the aims and obtained data. All authors contributed to developing the statistical analysis plan. ADACS completed all statistical analyses. DAL and ADACS wrote the first draft of the paper and all authors contributed to interpreting results and making critical comments on subsequent paper drafts. DAL and ADACS had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.National Institute for Health and Care Excellence (NICE) Fertility: assessment and treatment for people with fertility problems. NICE clinical guideline CG156. 2013 Feb; http://guidance.nice.org.uk/CG156
- 2.Berg Brigham K, Cadier B, Chevreul K. The diversity of regulation and public financing of IVF in Europe and its impact on utilization. Hum Reprod. 2013;28:666–75. doi: 10.1093/humrep/des418. [DOI] [PubMed] [Google Scholar]
- 3.Margolioth EJ, Ben-Chetrit A, Gal M, Eldar-Geva T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. 2006;21:3036–43. doi: 10.1093/humrep/del305. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda J, Kumagai J, Kodama H, Murata M, Kawamura K, Tanaka T. Upper limit of the number of IVF-ET treatment cycles in different age groups, predicted by cumulative take-home baby rate. Acta Obstet Gynecol Scand. 2001;80:71–3. doi: 10.1034/j.1600-0412.2001.800114.x. [DOI] [PubMed] [Google Scholar]
- 5.Luke B, Brown MB, Wantman E, et al. Cumulative birth rates with linked assisted reproductive technology cycles. N Engl J Med. 2012;366:2483–91. doi: 10.1056/NEJMoa1110238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern JE, Brown MB, Luke B, et al. Calculating cumulative live-birth rates from linked cycles of assisted reproductive technology (ART): data from the Massachusetts SART CORS. Fertil Steril. 2010;94:1334–40. doi: 10.1016/j.fertnstert.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 7.Malizia BA, Hacker MR, Pernzias AS. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236–43. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 8.Elizur SE, Lerner-Geva L, Levron J, Shulman A, Bider D, Dor J. Cumulative live birth rate following in vitro fertilization: study of 5310 cycles. Gynecol Endocrinol. 2006;22:25–30. doi: 10.1080/09513590500453916. [DOI] [PubMed] [Google Scholar]
- 9.Sharma V, Allgar V, Rajkhowa M. Factors influencing the cumulative conception rate and discontinuation of in vitro fertilization treatment for infertility. Fertil Steril. 2002;78:40–6. doi: 10.1016/s0015-0282(02)03160-6. [DOI] [PubMed] [Google Scholar]
- 10.Kupka MS, Ferraretti AP, de Mouzon J, et al. Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE. Human Reproduction. 2014;29:2099–113. doi: 10.1093/humrep/deu175. [DOI] [PubMed] [Google Scholar]
- 11.CDC, Centres for Disease Control and Prevention. Reproductive health. Assisted reproductive technology. National Summary and Fertility Clinic Reports 2013. http://www.cdc.gov/art/pdf/2013-report/art-2013-fertility-clinic-report.pdf
- 12.Human Fertilisation and Embryology Authority. London: 2015. Fertility Treatment in 2013: Trends and Figures.http://www.hfea.gov.uk/docs/HFEA_Fertility_Trends_and_Figures_2013.pdf [Google Scholar]
- 13.Macaldowie A, Wang YA, Chambers GM, Sullivan EA. National Perinatal Statistical Unit and Fertility Society of Australia; 2012. Australian Institute of Health and Welfare, Assisted Reproduction Technology in Australia and New Zealand (AIHW) 2012.http://www.aihw.gov.au/publication-detail/?id=10737423259 Assisted Reproduction Technology Series. [Google Scholar]
- 14.Maheshwari A, Griffiths S, Bhattacharya S. Global variations in the uptake of single embryo transfer. Hum Reprod Update. 2011;17:107–20. doi: 10.1093/humupd/dmq028. [DOI] [PubMed] [Google Scholar]
- 15.Lawlor DA, Nelson SM. Effect of age on decisions about the numbers of embryos to transfer in assisted conception: a prospective study. Lancet. 2012;379:521–7. doi: 10.1016/S0140-6736(11)61267-1. [DOI] [PubMed] [Google Scholar]
- 16.Nelson SM, Lawlor DA. Predicting live birth, preterm delivery, and low birth weight in infants born from in vitro fertilisation: a prospective study of 144,018 treatment cycles. PLoS Medicine. 2011;8:e1000386. doi: 10.1371/journal.pmed.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–81. [Google Scholar]
- 18.Greenwood MA. Report on the natural duration of cancer. Rep Public Health Med Subj (Lond) 1926;33:1–26. [Google Scholar]
- 19.Bland JM, Altman DG. The logrank test. BMJ. 2004;328:1073. doi: 10.1136/bmj.328.7447.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gameiro S, Boivin J, Peronace L, Verhaak CM. Why do patients discontinue fertility treatment? A systematic review of reasons and predictors of discontinuation in fertility treatment. Hum Reprod Update. 2012;18:652–659. doi: 10.1093/humupd/dms031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sunkara SK, Khalaf Y, Maheshwari A, Seep P, Coomarasamy A. Association between response to ovarian stimulation and miscarriage following IVF: an analysis of 124 351 IVF pregnancies. Hum Reprod. 2010;29:1218–24. doi: 10.1093/humrep/deu053. [DOI] [PubMed] [Google Scholar]
- 22.van Loendersloot LL, van Wely M, Limpens J, Bossuyte PM, Repping S, van der Veen F. Predictive factors in in vitro fertilisation (IVF): a systematic review and meta-analysis. Hum Reprod Update. 2010;16:577–89. doi: 10.1093/humupd/dmq015. [DOI] [PubMed] [Google Scholar]
- 23.Dunson DB, Baird DD, Colombo B. Increased infertility with age in men and women. Obstet Gynecol. 2004;103:51–56. doi: 10.1097/01.AOG.0000100153.24061.45. [DOI] [PubMed] [Google Scholar]
- 24.Rothman KJ, Wise LA, Sorensen HT, Riis AH, Mikkelsen EM, Hatch EE. Volitional determinants and age-related decline in fecundability: a general population prospective cohort study in Denmark. Fertil Steril. 2013;99:1958–64. doi: 10.1016/j.fertnstert.2013.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savitz DA, Hertz-Picciotto I, Poole C, Olshan AF. Epidemiologic measures of the course and outcome of pregnancy. Epidemiol Rev. 2002;24:91–101. doi: 10.1093/epirev/mxf006. [DOI] [PubMed] [Google Scholar]
- 26.Shenfield F, de Mouzon J, Pennings G, et al. Cross border reproductive care in six European countries. Hum Reprod. 2010;25:1361–68. doi: 10.1093/humrep/deq057. [DOI] [PubMed] [Google Scholar]
- 27.Delvinge A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8:559–77. doi: 10.1093/humupd/8.6.559. [DOI] [PubMed] [Google Scholar]
- 28.Davies MJ, Moore VM, Willson KJ, et al. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–13. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- 29.ESHRE Capri Working Group. Economic aspects of infertility care: a challenge for researchers and clinicians. Hum Reprod. 2015;30:2243–48. doi: 10.1093/humrep/dev163. [DOI] [PubMed] [Google Scholar]
- 30.The cost of IVF: 4 things I learned while battling infertility. By Jennifer Gerson Uffalussy; [Accessed 12th October 2015]. http://www.forbes.com/sites/learnvest/2014/02/06/the-cost-of-ivf-4-things-i-learned-while-battling-infertility/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.