Abstract
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm defined by erythrocytosis and often accompanied by leukocytosis and thrombocytosis. Current treatment options, including IFN-α and hydroxyurea, effectively manage PV in many patients. However, some high-risk patients, particularly those who become hydroxyurea-intolerant/resistant, may benefit from IFN-α or new treatment options. A better understanding of PV pathophysiology, including the role of the JAK/STAT pathway, has inspired the development of new therapies. Several JAK inhibitors directly target JAK/STAT pathway activation and have been evaluated in Phase II/III trials with promising results. Pegylated variants of IFN-α, which reduce dosing frequency and toxicity associated with recombinant IFN-α, have yielded favorable efficacy results in Phase II trials. Finally, histone deacetylase inhibitors have been developed to manage PV at the level of chromatin-regulated gene expression. The earliest Phase III results from these next-generation therapies are expected in 2014.
Keywords: IFN-α, interferon-α, JAK2, MPN, myeloproliferative neoplasms, polycythemia vera, ruxolitinib
Introduction
Polycythemia vera (PV) is a chronic myeloproliferative neoplasm (MPN) that results from the clonal expansion of a hematopoietic progenitor, leading to erythrocytosis and potentially leukocytosis and/or thrombocytosis [1]. Although the life expectancy of patients with PV is relatively long (median, 13.5 years [2]) compared with many other types of cancer, PV is associated with an increased risk of mortality [3,4]. This is particularly true in patients who are resistant to hydroxyurea (HU) treatment, the usual first-line therapy for PV [5]. The leading causes of death in patients with PV include cardiovascular events such as stroke, heart attack, and venous thrombosis. Hemorrhage and transformation to myelofibrosis (MF) or, rarely, acute myeloid leukemia are additional complications of PV [4,6]. Patients often develop splenomegaly, which can result in pain and discomfort [7], and a broad range of other symptoms that often include headache, pruritus, and fatigue [7–13]. Together, these conditions negatively affect overall health-related quality of life [7–13]. In the United States, the age-adjusted prevalence of PV in 2010 was estimated to be 48–57 cases per 100,000 residents, with a mean age of approximately 55 years and a slight male predominance (~65%) [14]. Prevalence in Europe has been estimated at 5–30 cases per 100,000 residents; the discrepancy with the reported US prevalence rate may reflect challenges associated with evaluating a rare disease rather than a true significant difference [15].
Polycythemia vera is associated with dysregulated activation of the JAK/STAT pathway, which in about 98% of patients results from one of two types of constitutively active somatic mutations [16–18]: mutations in JAK2 exon 12 or, in most cases, the recurrent JAK2V617F mutation [18–21]. In normal hematopoiesis, JAK2 is specifically activated by the growth factor erythropoietin (EPO) binding to the EPO receptor and the growth factor thrombopoietin (TPO) binding to its receptor (MPL) [22]. JAK2 can also be activated in response to the growth factors granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) to promote proliferation or prevent apoptotic cell death [23–26]. Activated JAK2 then phosphorylates and activates STAT family transcription factors, leading to hematopoietic stem cell proliferation and differentiation [22,27]. JAK2V617F and JAK2 exon 12 mutations are associated with constitutive activation of JAK2 and the JAK/STAT signaling pathway, leading to exaggerated hematopoietic proliferation in the absence of EPO, TPO, G-CSF, or GM-CSF [18,20,21,27]. JAK/STAT signaling may also contribute to PV-related inflammation and resulting symptoms. Serum inflammatory cytokine levels are increased in patients with PV [28,29], and inflammation, as measured by serum C-reactive protein (CRP), is significantly correlated with JAK2V617F allele burden [30]. In patients with MF, altered cytokine levels are associated with several symptoms, including itching, night sweats, loss of weight and/or appetite, and poor sleep quality; a similar association may exist in patients with PV [31]. In addition to JAK2, JAK1 may also participate in the signaling pathways that underlie PV-related inflammation; selective inhibition of JAK1 has been shown to have anti-inflammatory activity in preclinical models of inflammatory diseases [32]. Importantly, some clinical data indicate that erythrocytosis, leukocytosis, mutant JAK2 allele burden [33], and serum CRP levels [30] are associated with an increased risk of thrombosis in patients with PV.
Diagnostic and therapeutic guidelines for PV have been established by the World Health Organization (WHO) [34] and individual clinicians [16,35]. However, these guidelines were primarily derived from expert opinion and may warrant revisions based on currently available and emerging clinical evidence. For example, WHO major diagnostic criteria for PV include consideration of hematocrit, hemoglobin, or nuclear red cell mass and the presence of JAK2V617F or JAK2 exon 12 mutations (TABLE 1). However, the validity of measuring hematocrit or hemoglobin rather than nuclear red blood cell mass is under debate [36–40]. Current treatment strategies stratify patients with PV based on risk of thrombosis [16,35] and aim to achieve a hematocrit goal of <45% to reduce the risk of cardiovascular and thrombotic events [41,42]. For low-risk patients (<60 years of age with no history of thrombotic events [16,35]), phlebotomy and antiplatelet therapy with low-dose aspirin (100 mg/d) are recommended [16,35]. However, a recent Cochrane meta-analysis indicated that aspirin conferred nonsignificant benefits in terms of all-cause mortality and mortality from thrombotic events in patients with PV [43], and further evaluation may be required to determine if aspirin is safe and effective in all patients with PV [44]. High-risk patients are defined as those aged ≥60 years or with a history of thrombotic events [16,35]; future treatment guidelines may be revised to include leukocytosis and/or thrombocytosis as indicators of high-risk patients based on their associations with patient mortality risk [45]. The current treatment recommendations for high-risk patients suggest phlebotomy, low-dose aspirin, and cytoreductive therapy with HU or recombinant IFN-α as first-line therapy, with HU being the preferred option in many countries [16,35,46]. It has also been suggested that patients may benefit from early treatment with IFN-α–based treatment [47,48]. In the acute setting of cardiovascular events, cytoreductive therapy is recommended in addition to phlebotomy. Allogeneic hematopoietic transplantation is not usually considered for patients with chronic-Phase PV; a recent systematic review and decision analysis reported superior survival in this setting with phlebotomy/aspirin (plus a cytoreductive agent as needed) compared with allogeneic hematopoietic stem cell transplantation [49]. Despite treatment guideline endorsement of HU [16,35], clinical evidence of HU efficacy in patients with PV is limited. An older study (initial findings published in 1986) compared patients with PV treated with HU (n = 51) to historical controls treated with phlebotomy (n = 134); the overall survival difference was not statistically significant between groups [50]. A more recent study (results published in 2011) demonstrated a statistically significant survival advantage for patients with PV (n = 285) who received HU compared with those who received pipobroman; however, a noncytoreductive treatment group was not included [4]. It is also important to note that HU is not adequately effective for a subgroup of patients with PV. Up to one quarter of patients may become intolerant of or resistant to HU (BOX 1) [5,51]. Moreover, some patients and clinicians may avoid HU treatment because of increasing concerns about risks of skin cancer [52] and leukemic transformation [5,50,53]. Indeed, some centers have replaced HU with IFN-α–based treatments as first-line therapy in patients <60 years of age because of potential leukemogenicity [48,54]. Finally, HU may not provide antithrombotic benefit [53,55] or mitigate symptom burden [9,56], which should be the primary goals of treatment for patients with PV [16,35]. Second-line treatment options include recombinant IFN-α, pipobroman, 32P, and busulfan [16,35,46]. Recombinant IFN-α may effectively reduce dependence on phlebotomy [57,58], normalize platelet [57,58] and leukocyte counts [57], and manage splenomegaly [58], with clinical benefits observed in patient populations that include those who are intolerant of or resistant to HU [58]. While the toxicity profile of recombinant IFN-α, particularly at higher doses, has led to high discontinuation rates in some clinical trials and may therefore be impractical for long-term therapy for some individuals [54], lower doses are well tolerated by many patients [58,59]. Pipobroman, 32P, and busulfan may effectively improve the patient’s condition but are associated with significantly increased risk for leukemic transformation [4,60,61]. New therapeutic options are needed to better address the burden of PV, most notably in high-risk patients who are intolerant of or resistant to HU, which is the subject of this review.
Table 1.
2008 WHO diagnostic criteria for polycythemia vera†
| Criteria | |
|---|---|
| Major criteria |
|
| Minor criteria |
|
Diagnosis requires a patient to meet either both major criteria and 1 minor criterion or the first major criterion and 2 minor criteria.
Hb: Hemoglobin; Hct: Hematocrit; WHO: World Health Organization.
Adapted with permission from [34].
Box 1. Current European LeukemiaNet criteria for resistance to or intolerance of hydroxyurea.
| 1. Phlebotomy requirement to maintain hematocrit <45% after receiving ‡2 g/d hydroxyurea for ‡3 months |
| OR |
| 2. Uncontrolled myeloproliferation after receiving ‡2 g/d hydroxyurea for ‡3 months (platelet count >400 × 109/l AND white blood cell count >10 × 109/l) |
| OR |
| 3. Massive† splenomegaly reduced by £50% as measured by palpation, OR persistent splenomegaly-related symptoms, after receiving ‡2 g/d hydroxyurea for ‡3 months |
| OR |
| 4. Absolute neutrophil count <1.0 × 109/l, OR platelet count <100 × 109/l, OR hemoglobin <100 g/l, with the lowest dose of hydroxyurea required to achieve complete or partial clinicohematologic response‡ |
| OR |
| 5. Presence of hydroxyurea-related nonhematologic toxicities, including leg ulcers, mucocutaneous manifestations, gastro-intestinal symptoms, pneumonitis, or fever |
Emerging treatment options
A major hindrance to the development of targeted therapy options for PV before 2005 was the absence of a molecular mechanism as an underlying cause of the disease. The discovery of JAK2V617F, and subsequently a more complete understanding of the pathophysiology underlying PV, including the identification of JAK/STAT pathway activation as a unifying, underlying, biological abnormality, has opened the door to more specific treatment options.
JAK inhibitors
In recent years, JAK inhibitors have received preclinical and clinical attention owing to their ability to target the central molecular feature of PV, JAK/STAT pathway activation. Several JAK1/JAK2 and JAK2 inhibitors have been investigated in patients with PV, with results supportive of this drug class as a promising treatment option.
Ruxolitinib
Ruxolitinib (Novartis International AG; Basel, Switzerland) is an oral JAK1/JAK2 inhibitor currently indicated for intermediate or high-risk MF [62]. Worsening severity of anemia has been a limitation of JAK inhibitor treatment for some patients with MF; however, an anemic tendency could be potentially salutary in a disease with erythrocytosis, such as PV.
The efficacy and safety of ruxolitinib in patients with PV who are resistant to or intolerant of HU have been explored in a Phase II clinical trial. Overall, clinicohematologic response per modified 2009 European LeukemiaNet (ELN) criteria was achieved in most patients with ruxolitinib (TABLES 2 & 3) [29]. Of 34 patients receiving ruxolitinib, 97% achieved a clinicohematologic response, with a complete response achieved by 59%. In particular, treatment with ruxolitinib was associated with a reduction in white blood cell (WBC) and platelet counts for most patients with elevated cell counts at baseline, and a ≥50% reduction in palpable spleen by week 24 in a majority of patients with palpable spleen at baseline. Response was durable; after 48 weeks, 85% of patients maintained a hematocrit <45% without phlebotomy, and only 4 patients required phlebotomy during the 144-week study. Patients receiving ruxolitinib also experienced a reduction in PV-related symptom burden. Within the first 4 weeks of treatment, most patients experienced a ≥50% reduction in the severity of baseline pruritus, night sweats, and bone pain. Some data suggest that the JAK2V617F allele burden positively correlates with several measures of disease severity, including thrombosis, splenomegaly, and fibrotic transformation [17,33]. After 3 years of treatment with ruxolitinib, nearly one quarter of patients achieved a ≥ 50% reduction in JAK2V617F allele burden [29]. Adverse events (AEs) were primarily of grades 1 or 2. Grade ≥3 AEs included anemia and thrombocytopenia, which were observed in 3 patients each, and neutropenia, pyrexia, vomiting, and asthenia, which were reported in 1 patient each. These results are consistent with the tolerability profile reported for ruxolitinib in patients with MF, with the exception of cytopenia, which is a common AE in patients with MF but rare in patients with PV [63,64].
Table 2.
Response criteria in Phase II trials
| Study (year) | Drug | Partial response criteria | Complete response criteria | Ref. |
|---|---|---|---|---|
| Verstovsek et al. (2014) | ruxolitinib |
|
|
[29] |
| Kiladjian et al. (2008) | PEG–IFN-α2a |
|
|
[94] |
| Quintás-Cardama et al. (2009) Quintás-Cardama et al. (2013) |
PEG–IFN-α2a PEG–IFN-α2a |
|
|
[93,95] |
| Gisslinger et al. (2013) | PEG–IFN-α2b |
|
|
[100] |
| Gowin et al. (2012)† Andersen et al. (2013)† Rambaldi et al. (2010)† Finazzi et al. (2013)† |
PEG–IFN-α2a vorinostat givinostat givinostat |
|
Hematocrit <45% without phlebotomy Platelet count ≤400 × 109/l WBC count ≤10 × 109/l Normal spleen size on imaging No disease-related symptoms |
[96,108–110] |
European LeukemiaNet 2009 criteria [41].
PEG-IFN: Pegylated interferon; WBC: White blood cell.
Table 3.
Phase II efficacy results in patients with polycythemia vera
| Drug | Study (year) | Dosing method | Patients with PV | Response rate | Patients without phlebotomy requirement | Planned or ongoing clinical trials | Ref. |
|---|---|---|---|---|---|---|---|
| JAK Inhibitors | |||||||
| Ruxolitinib | Verstovsek et al. (2014) | Oral BID | 34 | PR, 38% CR, 59%† |
33 (97.1%) at 15 d 30 (88.2%) at 144 wk |
Ruxolitinib Phase III RESPONSE (NCT01243944) RELIEF (NCT01632904) |
[29,65,70] |
| Lestaurtinib | Hexner et al. (2014) | Oral BID | 27 (18 evaluable) | No significant change in Hb, neutrophil count, or platelet count | 6 (33.3%) at 18 wk | No trials registered | [74] |
| PEG–IFN-α | |||||||
| PEG–IFN-α2a | Kiladjian et al. (2008) | SC QW | 40 (37 evaluable) | PR, 5% CR, 95%‡ |
37 (100%) at 6 mo 36 (97.3%) at 30 mo |
PEG–IFN-α2a Phase III (NCT01259856) |
[94,98] |
| Quintás-Cardama et al. (2009) | SC QW | 40 | PR, 10% CR, 70%§ |
Not reported | [93] | ||
| Gowin et al. (2012) | SC QW | 55 | PR, 87% CR, 54%¶ |
Not reported | [96] | ||
| Quintás-Cardama et al. (2013) | SC QW | 43 | PR, 3% CR, 76%§ |
Not reported | [95] | ||
| PEG–IFN-α2b | Gisslinger et al. (2013) | SC Q2W | 51 (35 evaluable patients after 6 mo; 19 evaluable patients after 18 mo) | PR, 42% CR, 47%# |
29 (83%) at 6 mo 18 (95%) at 18 mo |
PEG–IFN-α2b Phase III PROUD-PV (NCT01949805) |
[100,102] |
| Histone deacetylase inhibitors | |||||||
| Vorinostat | Andersen et al. (2013) | Oral QD | 44 plus 19 with ET (30 evaluable patients after 24 wk) | PR, 63.3% CR, 10%¶ |
Not reported | No trials registered | [108] |
| Givinostat | Rambaldi et al. (2010) | Oral BID or TID | 12 (10 evaluable) | PR, 60% CR, 10%¶ |
3 (30%) at 12 wk 4 (40%) at 24 wk |
Givinostat Phase II (NCT01901432) |
[109,111] |
| Givinostat plus HU | Finazzi et al. (2013) | Oral QD or BID | 44 | PR, 47.7% CR, 4.5%¶ |
13 (29.5%) at 12 wk | [110] | |
PR=hematocrit <45% after week 4 without phlebotomy. CR=hematocrit <45% without phlebotomy, WBC count ≤10 × 109/l, platelet count ≤400 × 109/l, normal spleen per palpation, pruritus-free for ≥1 week.
PR=hematocrit <45% in men and <42% in women with splenomegaly or platelet count <400 × 109/l, or ≥50% reduced phlebotomy requirement. CR=hematocrit <45/<42% in men/women without phlebotomy, absence of splenomegaly, WBC count <10 × 109/l, platelet count <400 × 109/l.
PR=≥50% reduction in phlebotomy requirement or spleen size. CR=hematocrit <45% in men and <42% in women; absence of thromboembolic events; and WBC count ≤10 × 109/l, platelet count ≤400 × 109/l, and normalized spleen size without phlebotomy, HU, or anagrelide.
Per European LeukemiaNet 2009 criteria [41].
PR=hematocrit <45% without phlebotomy or phlebotomy requirement reduced by ≥50%. CR=hematocrit <45% without phlebotomy, platelet count ≤400 × 109/l, WBC count ≤10 × 109/l, normal spleen size, absence of thrombotic events.
BID: Twice daily; CR: Complete response; ET: Essential thrombocythemia; Hb:Hemoglobin; HU: Hydroxyurea; JAK: Janus kinase; PEG-IFN: Pegylated interferon; PR: Partial response; PV: Polycythemia vera; QD: Once daily; Q2W: Once every 2 weeks; QW: Once weekly; SC: Subcutaneous; TID: 3 times daily; WBC: White blood cell.
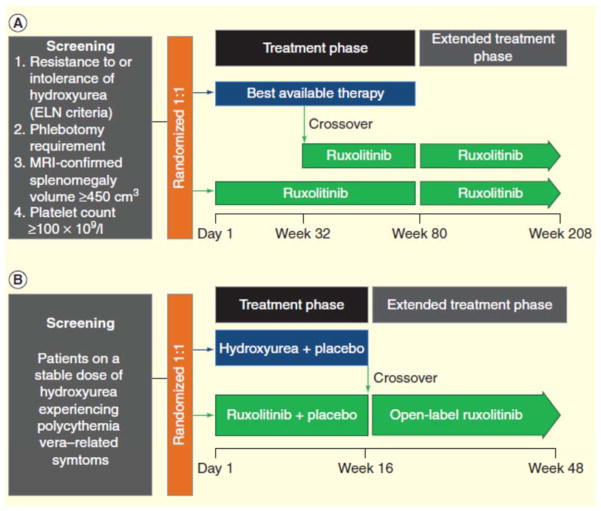
Ruxolitinib is currently being evaluated for the treatment of PV in a Phase III clinical trial, with results expected in mid to late 2014. RESPONSE [65] is a randomized, open-label, Phase III clinical trial that aims to evaluate efficacy and safety of ruxolitinib in patients with PV who have splenomegaly and are intolerant of or resistant to first-line HU (FIGURE 1). Overall, 222 patients with PV have been enrolled in RESPONSE [66], and preliminary results indicate that treatment with ruxolitinib is superior to best available therapy (BAT) for managing hematocrit without phlebotomy, reducing splenomegaly, and improving symptom burden [67]. The primary end (hematocrit control without phlebotomy eligibility and a ≥35% reduction in spleen volume) was achieved by 21 versus 1% (p < 0.0001) of patients randomized to ruxolitinib versus BAT, respectively, and 91% of patients maintained their response at week 48. At least 1 component of the primary end point was achieved by 77% of patients receiving ruxolitinib. Treatment with ruxolitinib was associated with greater benefit than BAT for both components of the primary end point: hematocrit control without phlebotomy eligibility (ruxolitinib, 60%; BAT, 20%) and a ≥35% reduction in spleen size (ruxolitinib, 38%; BAT, 1%). Complete hematologic response was achieved by 24 versus 9% of patients randomized to ruxolitinib versus BAT. Symptom burden, as assessed by the Myeloproliferative Neoplasm Symptom Assessment Form, was reduced by at least half in 49 versus 5% of patients randomized to ruxolitinib versus BAT. Among patients receiving ruxolitinib, grade ≥3 AEs included anemia (1.8%) and thrombocytopenia (5.5%); thromboembolic events were reported in 1 patient receiving ruxolitinib and 6 patients receiving BAT. RESPONSE 2 [68] is a randomized double-blind study that compares ruxolitinib and BAT for efficacy and safety in patients who are intolerant of or resistant to first-line HU and do not have enlarged spleens [69]. RELIEF [70] is a randomized double-blind study that compares ruxolitinib and HU for improvement of PV-related symptoms (FIGURE 1); patients who are currently on HU but still have uncontrolled signs and symptoms of PV are eligible to participate. At the time of this writing, no clinical data from RESPONSE 2 or RELIEF have been published.
Figure 1. Ruxolitinib Phase III clinical trial designs.
(A) RESPONSE is a randomized, open-label, Phase III clinical trial that aims to compare ruxolitinib and best available therapy for efficacy and safety in patients with polycythemia vera who are resistant to or intolerant of hydroxyurea [65]. (B) RELIEF is a randomized, double-blind clinical trial in patients experiencing polycythemia vera–related symptoms while receiving a stable dose of hydroxyurea that aims to evaluate ruxolitinib versus hydroxyurea for the relief of symptoms [70].
ELN: European LeukemiaNet; MRI: Magnetic resonance imaging.
Other JAK inhibitors
Lestaurtinib (CEP-701; Cephalon, Frazer, PA) is an oral multikinase inhibitor that blocks the activity of JAK2, as well as the tyrosine kinases Fms-like tyrosine kinase 3 (FLT3) and neurotrophic tyrosine kinase receptor type 1 (TrkA), at nanomolar concentrations [71–73]. Lestaurtinib has been evaluated for the treatment of MPNs, including a Phase II study that enrolled 27 patients with PV [74,75]. Evidence to date suggests that lestaurtinib has limited efficacy in this setting. Of 18 evaluable patients, 2 (11%) met the primary end point of a ≥15% reduction in JAK2V617F allele burden within 18 weeks (TABLE 3) [74]. Phlebotomy requirement persisted on treatment for 75% of patients who required phlebotomy at baseline. Ten of 18 (56%) evaluable patients experienced any reduction in spleen size. Toxicity may be a concern with lestaurtinib; 11 (41%) patients discontinued within the 18-week treatment period because of AEs, including diarrhea, nausea, increased appetite, or muscle spasms [74]. These results emphasize the importance of a well-tolerated agent when long-term administration is anticipated, as is the case with PV. Additional clinical trials of lestaurtinib in patients with PV are not currently being planned.
LY2784544 (Eli Lilly, Indianapolis, IN) is an oral JAK2 inhibitor in early development for the treatment of patients with MPNs. Preclinical work has demonstrated selective inhibition of wild-type JAK2 and JAK2V617F signaling in vitro and in vivo and reduced spleen volume and JAK2V617F tumor burden in a murine MPN model [76]. A Phase I clinical study of patients with MF, essential thrombocythemia (ET), or PV identified a daily dose of 120 mg LY2784544 for use in Phase II studies [77]. Serious AEs associated with LY2784544 include increased creatinine/acute renal insufficiency, hyperuricemia, hyperkalemia, and anemia [77]. LY2784544 is currently being evaluated in a Phase II clinical trial in patients with PV, ET, or MF who are intolerant of or resistant to ruxolitinib [78].
Momelotinib (CYT387; Gilead, Foster City, CA) is an oral JAK1/JAK2 inhibitor that is currently being evaluated in a Phase II clinical trial in patients with PV or ET with no previous exposure to a JAK inhibitor [79]. Preclinical studies have demonstrated selective inhibition of JAK1 and JAK2 at nanomolar concentrations and antiproliferative activity in cell lines with constitutively active JAK2 mutations, including JAK2V617F colonies from patients with PV [80,81]. In a murine MPN model, treatment with momelotinib was associated with improved WBC counts, lowered hematocrit, reduced spleen size, and a reduced JAK2V617F allele burden [81]. A Phase I/II clinical study of momelotinib in patients with MF (n = 60) reported spleen and anemia responses in 48 and 59% of patients, respectively, based on 2006 International Working Group-Myeloproliferative Neoplasms Research and Treatment criteria [82]. Grade ≥3 treatment-emergent AEs included thrombocytopenia (n = 19), increased lipase (n = 3), increased aspartate aminotransferase (n = 2), increased alanine aminotransferase (n = 2), and headache (n = 2) [82].
Pacritinib (SB1518; Cell Therapeutics, Seattle, WA) is another oral JAK2 inhibitor. Preclinical work has demonstrated that pacritinib selectively inhibits JAK2, JAK2V617F, and FLT3 and reduces spleen size in a JAK2V617F murine model. In a cell culture system with human erythroid progenitor cells, treatment with pacritinib was shown to inhibit colony formation and reduce cell viability, with similar activity in cells derived from healthy volunteers and patients with PV [83]. Clinical trials of pacritinib in patients with PV are not currently being planned.
The development of fedratinib (SAR302503; Sanofi, Bridgewater, NJ), an oral JAK2 inhibitor, was recently terminated during a Phase II trial [84] because of safety concerns related to Wernicke-like encephalopathy. The failure of fedratinib development emphasizes that JAK inhibitors have structural differences that may impact clinical outcomes; off-target effects may result in meaningful differences in clinical outcomes.
Pegylated IFN-α
The mechanism by which IFN-α-based therapies function to treat PV remains unclear and may result from a combination of antiproliferative, proapoptotic, antiangiogenic, and immunomodulatory activities [85]. IFN-α signaling in melanoma tumor tissue and lymphocytes has been associated with the activation of the tumor suppressor STAT1 and inhibition of a promoter of immunosuppression, STAT3 [85,86]. In hematopoietic cells, IFN-α signals through a pathway that requires phosphorylation and activation of p38 mitogen-activated protein kinase to inhibit growth and induce apoptosis [87]. Furthermore, the expression of hematopoietic cytokines, including GM-CSF and interleukin-1β, is negatively regulated by IFN-α [88].
Clinical trials with recombinant IFN-α have reported encouraging response rates in patients with PV; however, long-term patient tolerability of recombinant IFN-α can be challenging because of toxicity concerns and inconvenient treatment schedules, limiting the duration of treatment and patient benefit (as reviewed by Quintas-Cardama and colleagues [89], Hasselbalch [54], and Silver and colleagues [48]). Interferon-based therapy may be considered treatment of choice for younger women seeking pregnancy or during pregnancy [35].
The conjugation of polyethylene glycol (PEG) to a protein can be used to lengthen exposure time and reduce toxicity; these effects primarily result from PEG interference with proteolytic enzymes and PEG disruption of immune cell interactions, which can lead to reduced immunogenicity and antigenicity [90]. PEG–IFN-α variants have plasma half-lives of 40.0 to 72.4 h, 10 times longer than that of recombinant IFN-α, and reduced renal excretion, allowing for weekly rather than daily dosing [89,91].
Pegylated IFN-α2a
Pegylated interferon-α2a (Roche, Nutley, NJ) is approved by the US Food and Drug Administration for the treatment of patients with chronic hepatitis C with compensated liver disease and chronic hepatitis B with compensated liver disease, viral replication, and liver inflammation [92]. Phase II trials have reported high response rates in patients with PV, with clinicohematologic response observed in 79–100% of patients and 54–95% of patients experiencing a complete response (defined as hematocrit maintenance <45% in men and either <42 or <45% in women [study dependent] without phlebotomy, absence of thromboembolic events, WBC count ≤10 × 109/l, platelet count ≤400 × 109/l, and absence of splenomegaly; TABLES 2 and 3) [93–96]. The JAK2V617F allele burden was reduced in most patients (54–90%) after median follow-up times of 21 months (range, 2–45 months) and 31.4 months (Q1–Q3, 26.4–35.1 months) [93,94]; after 12 months of therapy, the median allele burden was reduced by 42–50% [93,94]. In a Danish long-term analysis (median follow-up, 42 months) of treatment with IFN-α-based therapies (primarily PEG–IFN-α2a) in patients with MPNs, complete hematologic remission was reported in 68% of patients with PV, with a median decrease in JAK2V617F allele burden of 59–35% (95% CI: 17–33%) [97]. A subset of patients with ET or PV (11%) had an allele burden reduction to ≤2%. Furthermore, normalized bone marrow morphology was observed in 3 of 6 patients with PV who paused IFN-α treatment to evaluate the sustainability of remission; all 6 patients had normalized blood cell counts that persisted for a median of 45 months after discontinuation. Treatment of early disease (i.e., within 2 years of diagnosis) may portend a better response, given the high response rates and absence of thrombosis that have been observed after treatment for a median of 30 months [94]. Importantly, patients with mutations in TET2 may be resistant to PEG–IFN-α; these mutations are associated with significantly higher JAK2V617F allele burden at baseline compared with patients without a TET2 mutation [93].
Adverse events were reported by 89–96% of patients, with most being of grade 1 or 2 severity [93–96]. No grade 4 toxicities were observed; grade 3 AEs reported for >1 patient included neutropenia (8% of patients) and liver toxicity (5% of patients) [93–95]. Toxicity resulted in discontinuation of 10–24% of patients during the 24–92-month study periods. Toxicities that resulted in the discontinuation of >1 patient included depression, fatigue, dyspnea, muscular pain, and neutropenia [94,95].
A Phase III clinical trial [98] is currently ongoing to compare the efficacy and safety of PEG–IFN- α2a versus HU in patients with high-risk PV (defined as JAK2V617F-positive and hemoglobin >18.5 g/dl [men] or >16.5 g/dl [women], hematocrit >99th percentile, red cell mass >25% above average, or hemoglobin >17.0 g/dl [men] or >15.0 g/dl [women] if it is sustained above baseline with no known cause) or ET. The study is estimated to be completed in late 2014.
Pegylated IFN-α2b
Pegylated interferon-α2b (Merck, Whitehouse Station, NJ) is currently being investigated in a Phase II clinical study, with the final data collection estimated for mid-2014 [99]. Preliminary results indicate high response rates; of 19 evaluable patients, 17 (89%) had a hematologic response after 18 months of therapy, and 9 (47%) had a complete response (defined as hematocrit maintenance <45% without phlebotomy, absence of thromboembolic events, WBC count ≤10 × 109/l, platelet count ≤400 × 109/l, and normalized spleen size; TABLES 2 and 3) [100]. After 12 months of therapy, 1 (5%) patient still required phlebotomy for hematocrit maintenance. AEs were reported by 48 (94%) patients, with 26 of 645 (4%) considered serious [100]. The most commonly reported AEs were pruritus, headache, fatigue, arthralgia, diarrhea, and vertigo. Within the 18-month study period, AEs led to the discontinuation of 10 (20%) patients. Cases of long-term reductions in JAK2V617F allele burden that persist for months following discontinuation of PEG–IFN-α2b have been reported [47,101], but prospective clinical trials will be required to confirm this finding. Evaluation of pegylated interferon-α2b has also begun in a Phase III clinical trial [102]. PROUD-PV aims to compare the efficacy and safety of PEG–IFN-α2b and HU in patients with JAK2V617F-positive PV, with an estimated completion date of late 2015.
Histone deacetylase inhibitors
Histone deacetylases (HDACs) regulate gene expression through chromatin modifications [103] and may play a critical role in facilitating the proliferative and cytokine-mediated aspects of the PV disease state. In patients with PV, HDAC expression has been observed at elevated levels [104]. Inhibition of HDACs has been shown to have antiproliferative and proapoptotic activity in leukemia and melanoma model cell lines [105,106]. Furthermore, HDAC inhibition has been associated with decreased cytokine production by mesenchymal stromal cells in vitro [106].
Vorinostat
Vorinostat (Merck, Whitehouse Station, NJ) is indicated as third-line therapy for patients with cutaneous manifestations of cutaneous T-cell lymphoma [107]. A Phase II clinical study evaluating efficacy and safety of vorinostat in patients with MPNs reported moderate response rates but also raised concern regarding toxicity-related discontinuation. Clinicohematologic response was observed in 35% of 63 patients with MPNs (including 44 patients with PV) at the end of the 24-week intervention period (TABLES 2 and 3) [108]. Forty-six percent of patients with palpable splenomegaly at baseline were splenomegaly-free after 24 weeks of treatment. Among the 48% of patients who completed the study, the most commonly reported AEs were fatigue and gastrointestinal toxicity [108]. Grade 3/4 AEs reported by >1 patient included fatigue (13% of patients), gastrointestinal toxicity (11%), diarrhea (5%), dry mouth (5%), nausea (5%), and anorexia (5%). After 24 weeks of treatment with vorinostat, 28 (44%) patients had discontinued because of toxicity, with the most common causes of discontinuation reported as diarrhea, fatigue, renal impairment, hair loss, and nausea. No additional trials with vorinostat in patients with PV have been registered with ClinicalTrials.gov at the time of this writing.
Givinostat
Oral givinostat (Italfarmaco, Milan, Italy) has been evaluated in 2 Phase II clinical studies in patients with PV, with promising efficacy results. In 1 open-label multicenter study of 10 patients with PV who completed the 24-week schedule, 7 (70%) experienced a hematologic response and 1 (10%) a complete response [109] per ELN criteria [41] (TABLES 2 and 3). Baseline splenomegaly per palpation was resolved in 5 of 8 patients. A second open-label, multicenter, Phase II clinical study randomized patients to HU plus either 50 or 100 mg/d givinostat [110]. Overall, of 44 patients with PV, 52.3% had a hematologic response after 12 weeks, with 4.5% reporting a complete response [110] per ELN criteria [41]. Treatment with givinostat was associated with resolution of pruritus in 65.2% of patients with grade ≥2 pruritus at baseline. In contrast to the first Phase II clinical study, treatment with givinostat was ineffective for the resolution of baseline splenomegaly in 91.9% of patients. AEs (grade 2 thrombocytopenia, hyperkalemia, and arrhythmia; grade 3 psychiatric symptoms [disorientation], pulmonary embolism, and anemia) resulted in discontinuation of 16.7 and 18.2% of patients in the 2 studies, respectively. Givinostat is currently being evaluated in a Phase II clinical trial [111] for safety and efficacy in patients with PV.
Expert commentary
Patients with PV have an increased risk of thrombotic events and splenomegaly, and they may experience a broad range of symptoms that negatively affect overall quality of life [3,4,6–13]. Patients with PV have increased risk of morbidity due to disease complications and may have shorter life expectancy than normal. Although treatment consistent with current guidelines can effectively manage PV in many patients, these guidelines are based on expert opinion rather than clinical evidence. For example, no prospective randomized clinical trials to date have evaluated HU efficacy in reducing thrombotic risk and managing PV-related symptoms, which are primary treatment goals in this setting. Furthermore, suggested therapeutic strategies are not effective in all patient populations; a subpopulation that includes high-risk patients who are intolerant of or resistant to HU does not receive adequate benefit from current therapy options [5,51]. New therapies will be required to address unmet needs in this patient population, and it is critical that randomized clinical studies are designed to evaluate thrombotic risk and PV-symptom burden to ensure that future treatment guidelines are supported by adequate clinical evidence.
Several clinical trials have highlighted the potential of new therapy options currently in development in this setting. Clinical results to date suggest that JAK inhibitors are a promising therapeutic option. Although it is challenging to make cross-study comparisons, there do appear to be important efficacy and toxicity differences among JAK inhibitors currently in development. These differences may result from JAK inhibition specificity (JAK1/JAK2 vs JAK2 only), activity against other kinases, or other nonspecific activities. IFN-α–based treatments also have a place in therapy for patients with PV, and clinical results with PEG–IFN-α are supportive of an improvement over recombinant IFN-α; however, Phase III clinical trial results will be required to substantiate reduced toxicity and long-term tolerability of PEG–IFN-α in patients with PV [93–96,100]. Physicians will have to balance the efficacy of this drug class against a patient’s ability to tolerate the medicine and, therefore, derive benefit. An intriguing treatment option for patients who are intolerant of or resistant to IFN-α–based therapies is PEG–IFN-α plus ruxolitinib combination therapy [112,113], although clinical trial data will be required to adequately evaluate this novel combination. Based on clinical results to date, HDAC inhibitors are associated with high toxicity levels, and their role, if any, needs to be evaluated further in clinical studies.
Five-year view
New treatment options currently in Phase III development, including ruxolitinib and therapies based on PEG–IFN-α, are positioned to become standard second-line therapy options in coming years. Initial Phase III results for ruxolitinib have been reported, suggesting that ruxolitinib may receive approval as a second-line therapy in the near future. Beyond ruxolitinib, the number of clinical trials focused on new treatment options in this setting emphasizes the potential for approval of several new therapies in coming years. Determination of how new agents will be incorporated into updated treatment algorithms will be an important challenge for physicians to overcome during the next 5 years. Given the chronic nature of PV, preference should be given to treatments that are not only efficacious, but also well tolerated over the course of long-term treatment.
Key Issues.
PV is a chronic MPN that is associated with excessive proliferation of erythrocytes, leukocytes, and platelets, as well as splenomegaly.
Patients with PV have increased risk of thrombosis, as well as a broad symptom burden that includes fatigue and pruritus. Taken together, these can meaningfully detract from a patient’s quality of life.
Current treatment guidelines are based on expert opinion and need to be validated with randomized clinical trials in patients with PV.
Currently available treatment options provide clinical benefit for some patients; however, for other patients, PV is not adequately managed by current treatment options, including up to one quarter of patients who become resistant to or intolerant of HU.
Several new therapy options are in the clinical trial phase of development, including JAK inhibitors, pegylated variants of IFN-α (as opposed to recombinant IFN-α), and HDAC inhibitors.
The JAK1/JAK2 inhibitor ruxolitinib, PEG–IFN-α2a, and PEG–IFN-α2b have exhibited promising efficacy and safety results in Phase II clinical trials and are currently in Phase III development.
Acknowledgments
We thank Elizabeth Hexner (University of Pennsylvania) for her review of the manuscript.
Footnotes
Financial & competing interests disclosure
S Verstovsek and RS Komrokji have received clinical research funding and served on advisory boards for Incyte Corporation. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing assistance was provided by Cory Pfeiffenberger, PhD (Complete Healthcare Communications, Inc.,) whose work was funded by Incyte Corporation.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Barbui T, Finazzi G, Falanga A. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122(13):2176–84. doi: 10.1182/blood-2013-03-460154. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly-annotated essential thrombocythemia, polycythemia vera and myelofibrosis. Blood. 2014 doi: 10.1182/blood-2014-05-579136. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. J Clin Oncol. 2012;30(24):2995–3001. doi: 10.1200/JCO.2012.42.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiladjian JJ, Chevret S, Dosquet C, et al. Treatment of polycythemia vera with hydroxyurea and pipobroman: final results of a randomized trial initiated in 1980. J Clin Oncol. 2011;29(29):3907–13. doi: 10.1200/JCO.2011.36.0792. [DOI] [PubMed] [Google Scholar]
- 5•.Alvarez-Larran A, Pereira A, Cervantes F, et al. Assessment and prognostic value of the European LeukemiaNet criteria for clinicohematologic response, resistance, and intolerance to hydroxyurea in polycythemia vera. Blood. 2012;119(6):1363–9. doi: 10.1182/blood-2011-10-387787. Synopsis: This retrospective study aimed to evaluate the ELN 2009 criteria for intolerance of and resistance to HU in patients with PV. Resistance to HU was associated with increased risks of mortality and disease transformation to acute myeloid leukemia or MF. [DOI] [PubMed] [Google Scholar]
- 6.Polednak AP. Recent decline in the U.S. death rate from myeloproliferative neoplasms, 1999–2006. Cancer Epidemiol. 2012;36(2):133–6. doi: 10.1016/j.canep.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109(1):68–76. doi: 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- 8.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–103. doi: 10.1200/JCO.2012.42.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson P, Mesa R, Scherber R, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012;53(3):441–4. doi: 10.3109/10428194.2011.619608. [DOI] [PubMed] [Google Scholar]
- 10.Scherber R, Dueck AC, Johansson P, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011;118(2):401–8. doi: 10.1182/blood-2011-01-328955. [DOI] [PubMed] [Google Scholar]
- 11.Siegel FP, Tauscher J, Petrides PE. Aquagenic pruritus in polycythemia vera: characteristics and influence on quality of life in 441 patients. Am J Hematol. 2013;88(8):665–9. doi: 10.1002/ajh.23474. [DOI] [PubMed] [Google Scholar]
- 12.Diehn F, Tefferi A. Pruritus in polycythaemia vera: prevalence, laboratory correlates and management. Br J Haematol. 2001;115(3):619–21. doi: 10.1046/j.1365-2141.2001.03161.x. [DOI] [PubMed] [Google Scholar]
- 13.Finelli C, Gugliotta L, Gamberi B, et al. Relief of intractable pruritus in polycythemia vera with recombinant interferon alfa. Am J Hematol. 1993;43(4):316–18. doi: 10.1002/ajh.2830430419. [DOI] [PubMed] [Google Scholar]
- 14.Mehta J, Wang H, Iqbal SU, Mesa R. Epidemiology of myeloproliferative neoplasms in the United States. Leuk Lymphoma. 2014;55(3):595–600. doi: 10.3109/10428194.2013.813500. [DOI] [PubMed] [Google Scholar]
- 15.Moulard O, Mehta J, Fryzek J, et al. Epidemiology of myelofibrosis (MF), essential thrombocythemia (ET), and polycythemia vera (PV) in the European Union (EU) Eur J Haematol. 2014;92(4):289–97. doi: 10.1111/ejh.12256. [DOI] [PubMed] [Google Scholar]
- 16.Tefferi A. Polycythemia vera and essential thrombocythemia: 2013 update on diagnosis, risk-stratification, and management. Am J Hematol. 2013;88(6):507–16. doi: 10.1002/ajh.23417. [DOI] [PubMed] [Google Scholar]
- 17.Passamonti F, Rumi E, Pietra D, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010;24(9):1574–9. doi: 10.1038/leu.2010.148. [DOI] [PubMed] [Google Scholar]
- 18.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- 19.Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- 21.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 22.Drachman JG, Millett KM, Kaushansky K. Thrombopoietin signal transduction requires functional JAK2, not TYK2. J Biol Chem. 1999;274(19):13480–4. doi: 10.1074/jbc.274.19.13480. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe S, Itoh T, Arai K. JAK2 is essential for activation of c-fos and c-myc promoters and cell proliferation through the human granulocyte-macrophage colony- stimulating factor receptor in BA/F3 cells. J Biol Chem. 1996;271(21):12681–6. doi: 10.1074/jbc.271.21.12681. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Xue J, Zadorozny EV, Robinson LJ. G-CSF stimulates Jak2-dependent Gab2 phosphorylation leading to Erk1/2 activation and cell proliferation. Cell Signal. 2008;20(10):1890–9. doi: 10.1016/j.cellsig.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shimoda K, Iwasaki H, Okamura S, et al. G-CSF induces tyrosine phosphorylation of the JAK2 protein in the human myeloid G-CSF responsive and proliferative cells, but not in mature neutrophils. Biochem Biophys Res Commun. 1994;203(2):922–8. doi: 10.1006/bbrc.1994.2270. [DOI] [PubMed] [Google Scholar]
- 26.Simon HU, Yousefi S, Dibbert B, et al. Anti-apoptotic signals of granulocyte-macrophage colony-stimulating factor are transduced via Jak2 tyrosine kinase in eosinophils. Eur J Immunol. 1997;27(12):3536–9. doi: 10.1002/eji.1830271256. [DOI] [PubMed] [Google Scholar]
- 27.Quintás-Cardama A, Kantarjian H, Cortes J, Verstovsek S. Janus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyond. Nat Rev Drug Discov. 2011;10(2):127–40. doi: 10.1038/nrd3264. [DOI] [PubMed] [Google Scholar]
- 28.Vaidya R, Gangat N, Jimma T, et al. Plasma cytokines in polycythemia vera: phenotypic correlates, prognostic relevance, and comparison with myelofibrosis. Am J Hematol. 2012;87(11):1003–5. doi: 10.1002/ajh.23295. [DOI] [PubMed] [Google Scholar]
- 29••.Verstovsek S, Passamonti F, Rambaldi A, et al. A phase 2 study of ruxolitinib, an oral JAK1 and JAK2 inhibitor, in patients with advanced polycythemia vera who are refractory or intolerant to hydroxyurea. Cancer. 2014;120(4):513–20. doi: 10.1002/cncr.28441. Synopsis: This Phase II study evaluated ruxolitinib for safety and efficacy in patients with PV who were intolerant of or resistant to HU. Ruxolitinib was associated with clinicohematologic response in 97% of patients, and a $50% reduction in the severity of pruritus, night sweats, and bone pain in most patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barbui T, Carobbio A, Finazzi G, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96(2):315–18. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Squires M, Harrison CN, Barosi G, et al. The relationship between cytokine levels and symptoms in patients (pts) with myelofibrosis (MF) from COMFORT-II, a phase 3 study of ruxolitinib (RUX) vs best available therapy (BAT) [abstract] Blood. 2013;122(21):4070. [Google Scholar]
- 32.Van Rompaey L, Galien R, van der Aar EM, et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013;191(7):3568–77. doi: 10.4049/jimmunol.1201348. [DOI] [PubMed] [Google Scholar]
- 33.Vannucchi AM, Antonioli E, Guglielmelli P, et al. Prospective identification of high-risk polycythemia vera patients based on JAK2 (V617F) allele burden. Leukemia. 2007;21(9):1952–9. doi: 10.1038/sj.leu.2404854. [DOI] [PubMed] [Google Scholar]
- 34.Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. doi: 10.1038/sj.leu.2404955. [DOI] [PubMed] [Google Scholar]
- 35.Passamonti F. How I treat polycythemia vera. Blood. 2012;120(2):275–84. doi: 10.1182/blood-2012-02-366054. [DOI] [PubMed] [Google Scholar]
- 36.Barbui T, Thiele J, Vannucchi AM, Tefferi A. Rethinking the diagnostic criteria of polycythemia vera. Leukemia. 2014;28:1191–5. doi: 10.1038/leu.2013.380. [DOI] [PubMed] [Google Scholar]
- 37.Cassinat B, Laguillier C, Gardin C, et al. Classification of myeloproliferative disorders in the JAK2 era: is there a role for red cell mass? Leukemia. 2008;22(2):452–3. doi: 10.1038/sj.leu.2404908. [DOI] [PubMed] [Google Scholar]
- 38.Johansson PL, Safai-Kutti S, Kutti J. An elevated venous haemoglobin concentration cannot be used as a surrogate marker for absolute erythrocytosis: a study of patients with polycythaemia vera and apparent polycythaemia. Br J Haematol. 2005;129(5):701–5. doi: 10.1111/j.1365-2141.2005.05517.x. [DOI] [PubMed] [Google Scholar]
- 39.Alvarez-Larran A, Ancochea A, Angona A, et al. Red cell mass measurement in patients with clinically suspected diagnosis of polycythemia vera or essential thrombocythemia. Haematologica. 2012;97(11):1704–7. doi: 10.3324/haematol.2012.067348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silver RT, Chow W, Orazi A, et al. Evaluation of WHO criteria for diagnosis of polycythemia vera: a prospective analysis. Blood. 2013;122(11):1881–6. doi: 10.1182/blood-2013-06-508416. [DOI] [PubMed] [Google Scholar]
- 41.Barosi G, Birgegard G, Finazzi G, et al. Response criteria for essential thrombocythemia and polycythemia vera: result of a European LeukemiaNet consensus conference. Blood. 2009;113(20):4829–33. doi: 10.1182/blood-2008-09-176818. [DOI] [PubMed] [Google Scholar]
- 42.Marchioli R, Finazzi G, Specchia G, et al. Cardiovascular events and intensity of treatment in polycythemia vera. N Engl J Med. 2013;368(1):22–33. doi: 10.1056/NEJMoa1208500. [DOI] [PubMed] [Google Scholar]
- 43.Squizzato A, Romualdi E, Passamonti F, Middeldorp S. Antiplatelet drugs for polycythaemia vera and essential thrombocythaemia. Cochrane Database Syst Rev. 2013;4:CD006503. doi: 10.1002/14651858.CD006503.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrono C, Rocca B, De Stefano V. Platelet activation and inhibition in polycythemia vera and essential thrombocythemia. Blood. 2013;121:1701–11. doi: 10.1182/blood-2012-10-429134. [DOI] [PubMed] [Google Scholar]
- 45.Tefferi A, Rumi E, Finazzi G, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013;27(9):1874–81. doi: 10.1038/leu.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barbui T, Barosi G, Birgegard G, et al. Philadelphia-negative classical myeloproliferative neoplasms: critical concepts and management recommendations from European LeukemiaNet. J Clin Oncol. 2011;29(6):761–70. doi: 10.1200/JCO.2010.31.8436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen TS, Moller MB, de Stricker K, et al. Minimal residual disease and normalization of the bone marrow after long-term treatment with alpha-interferon2b in polycythemia vera. A report on molecular response patterns in seven patients in sustained complete hematological remission. Hematology (Am Soc Hematol Educ Program) 2009;14(6):331–4. doi: 10.1179/102453309X12473408860587. [DOI] [PubMed] [Google Scholar]
- 48.Silver RT, Kiladjian JJ, Hasselbalch HC. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev Hematol. 2013;6(1):49–58. doi: 10.1586/ehm.12.69. [DOI] [PubMed] [Google Scholar]
- 49.Lacevic J, Reljic T, El Jurdi N, et al. Conservative management vs. allogeneic hematopoietic cell transplantation for polycythemia vera: a systematic review and decision-analysis. [abstract] Blood. 2013;122(21) abstract 5372. [Google Scholar]
- 50.Fruchtman SM, Mack K, Kaplan ME, et al. From efficacy to safety: a Polycythemia Vera Study Group report on hydroxyurea in patients with polycythemia vera. Semin Hematol. 1997;34(1):17–23. [PubMed] [Google Scholar]
- 51.Barosi G, Birgegard G, Finazzi G, et al. A unified definition of clinical resistance and intolerance to hydroxycarbamide in polycythaemia vera and primary myelofibrosis: results of a European LeukemiaNet (ELN) consensus process. Br J Haematol. 2010;148(6):961–3. doi: 10.1111/j.1365-2141.2009.08019.x. [DOI] [PubMed] [Google Scholar]
- 52.Best PJ, Petitt RM. Multiple skin cancers associated with hydroxyurea therapy. Mayo Clin Proc. 1998;73(10):961–3. doi: 10.4065/73.10.961. [DOI] [PubMed] [Google Scholar]
- 53.Najean Y, Rain JD. Treatment of polycythemia vera: the use of hydroxyurea and pipobroman in 292 patients under the age of 65 years. Blood. 1997;90(9):3370–7. [PubMed] [Google Scholar]
- 54.Hasselbalch HC. A new era for IFN-alpha in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Expert Rev Hematol. 2011;4(6):637–55. doi: 10.1586/ehm.11.63. [DOI] [PubMed] [Google Scholar]
- 55.Najean Y, Rain JD. Treatment of polycythemia vera: use of 32P alone or in combination with maintenance therapy using hydroxyurea in 461 patients greater than 65 years of age. The French Polycythemia Study Group. Blood. 1997;89(7):2319–27. [PubMed] [Google Scholar]
- 56.Scherber R, Dueck AC, Kiladjian JJ, et al. Conventional therapeutic options have limited impact on MPN symptoms: insights from a prospective analysis of the MPN-SAF. Presented at: European Hematology Association; 14–17 June 2012; Amsterdam, Netherlands. [Google Scholar]
- 57.Sacchi S, Leoni P, Liberati M, et al. A prospective comparison between treatment with phlebotomy alone and with interferon-alpha in patients with polycythemia vera. Ann Hematol. 1994;68(5):247–50. doi: 10.1007/BF01737425. [DOI] [PubMed] [Google Scholar]
- 58.Silver RT. Long-term effects of the treatment of polycythemia vera with recombinant interferon-alpha. Cancer. 2006;107(3):451–8. doi: 10.1002/cncr.22026. [DOI] [PubMed] [Google Scholar]
- 59.Gilbert HS. Long term treatment of myeloproliferative disease with interferon- alpha-2b: feasibility and efficacy. Cancer. 1998;83(6):1205–13. doi: 10.1002/(sici)1097-0142(19980915)83:6<1205::aid-cncr21>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 60.Berk PD, Goldberg JD, Silverstein MN, et al. Increased incidence of acute leukemia in polycythemia vera associated with chlorambucil therapy. N Engl J Med. 1981;304(8):441–7. doi: 10.1056/NEJM198102193040801. [DOI] [PubMed] [Google Scholar]
- 61.Treatment of polycythaemia vera by radiophosphorus or busulphan: a randomized trial. “Leukemia and Hematosarcoma” Cooperative Group, European Organization for Research on Treatment of Cancer (E.O.R.T.C) Br J Cancer. 1981;44(1):75–80. doi: 10.1038/bjc.1981.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Full Prescribing Information. Incyte Corporation; Wilmington, DE: 2011. Jakafi® (ruxolitinib) [Google Scholar]
- 63.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–98. doi: 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 65.Study of efficacy and safety in polycythemia vera subjects who are resistant to or intolerant of hydroxyurea: JAK inhibitor INC424 (INCB018424) tablets versus best available care: (the RESPONSE trial) ((RESPONSE)) Available from: http://clinicaltrials.gov/show/NCT01243944.
- 66.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Baseline characteristics and symptom burden in RESPONSE: a randomized, open-label, phase 3 study of ruxolitinib in polycythemia vera patients resistant to or intolerant of hydroxyurea [abstract] Blood. 2013;122(21):4071. [Google Scholar]
- 67.Verstovsek S, Kiladjian JJ, Griesshammer M, et al. Results of a prospective, randomized, open-label phase 3 study of ruxolitinib (RUX) in polycythemia vera (PV) patients resistant to or intolerant of hydroxyurea (HU): the RESPONSE trial. J Clin Oncol. 2014;32(5s) abstract 7026. [Google Scholar]
- 68.Ruxolitinib efficacy and safety in patients with HU resistant or intolerant polycythemia vera vs best available therapy. (RESPONSE 2). Available from: http://clinicaltrials.gov/show/NCT02038036.
- 69.Passamonti F, Saydam G, Lim L, et al. RESPONSE 2: A phase 3b study evaluating the efficacy and safety of ruxolitinib in patients with hydroxyurea (HU)-resistant/intolerant polycythemia vera (PV) versus best available therapy (BAT) J Clin Oncol (ASCO Annual Meeting Abstracts) 2014;32(5s) abstract TPS7128. [Google Scholar]
- 70.Switch study from hydroxyurea to ruxolitinib for RELIEF of polycythemia vera symptoms: the RELIEF study. Available from: http://clinicaltrials.gov/show/NCT01632904.
- 71.Hexner EO, Serdikoff C, Jan M, et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111(12):5663–71. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.George DJ, Dionne CA, Jani J, et al. Sustained in vivo regression of Dunning H rat prostate cancers treated with combinations of androgen ablation and Trk tyrosine kinase inhibitors, CEP-751 (KT- 6587) or CEP-701 (KT-5555) Cancer Res. 1999;59(10):2395–401. [PubMed] [Google Scholar]
- 73.Levis M, Allebach J, Tse KF, et al. A FLT3-targeted tyrosine kinase inhibitor is cytotoxic to leukemia cells in vitro and in vivo. Blood. 2002;99(11):3885–91. doi: 10.1182/blood.v99.11.3885. [DOI] [PubMed] [Google Scholar]
- 74.Hexner E, Roboz G, Hoffman R, et al. Open-label study of oral CEP-701 (lestaurtinib) in patients with polycythaemia vera or essential thrombocythaemia with JAK2-V617F mutation. Br J Haematol. 2014;164(1):83–93. doi: 10.1111/bjh.12607. [DOI] [PubMed] [Google Scholar]
- 75.Santos FP, Kantarjian HM, Jain N, et al. Phase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or post-polycythemia vera/essential thrombocythemia myelofibrosis. Blood. 2010;115(6):1131–6. doi: 10.1182/blood-2009-10-246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma L, Clayton JR, Walgren RA, et al. Discovery and characterization of LY2784544, a small-molecule tyrosine kinase inhibitor of JAK2V617F. Blood Cancer J. 2013;3:e109. doi: 10.1038/bcj.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verstovsek S, Mesa RA, Salama ME, et al. Phase I study of LY2784544, a JAK2 selective inhibitor, in patients with myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET) Blood. 2013;122(21):665. [Google Scholar]
- 78.A study of LY2784544 in participants with myeloproliferative neoplasms. Available from: http://clinicaltrials.gov/show/NCT01594723.
- 79.Safety and efficacy of momelotinib in subjects with polycythemia vera or essential thrombocythemia. Available from: http://clinicaltrials.gov/show/NCT01998828.
- 80.Pardanani A, Lasho T, Smith G, et al. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia. 2009;23(8):1441–5. doi: 10.1038/leu.2009.50. [DOI] [PubMed] [Google Scholar]
- 81.Tyner JW, Bumm TG, Deininger J, et al. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115(25):5232–40. doi: 10.1182/blood-2009-05-223727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pardanani A, Laborde RR, Lasho TL, et al. Safety and efficacy of CYT387, a JAK1 and JAK2 inhibitor, in myelofibrosis. Leukemia. 2013;27(6):1322–7. doi: 10.1038/leu.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hart S, Goh KC, Novotny-Diermayr V, et al. SB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignancies. Leukemia. 2011;25(11):1751–9. doi: 10.1038/leu.2011.148. [DOI] [PubMed] [Google Scholar]
- 84.Study with SAR302503 in patients with polycythemia vera or essential thrombocythemia. Available from: http://clinicaltrials.gov/show/NCT01420783.
- 85.Hasselbalch HC, Larsen TS, Riley CH, et al. Interferon-alpha in the treatment of Philadelphia-negative chronic myeloproliferative neoplasms. Status and perspectives. Curr Drug Targets. 2011;12(3):392–419. doi: 10.2174/138945011794815275. [DOI] [PubMed] [Google Scholar]
- 86.Wang W, Edington HD, Rao UN, et al. Modulation of signal transducers and activators of transcription 1 and 3 signaling in melanoma by high-dose IFNalpha2b. Clin Cancer Res. 2007;13(5):1523–31. doi: 10.1158/1078-0432.CCR-06-1387. [DOI] [PubMed] [Google Scholar]
- 87.Lu M, Zhang W, Li Y, et al. Interferon-alpha targets JAK2V617F-positive hematopoietic progenitor cells and acts through the p38 MAPK pathway. Exp Hematol. 2010;38(6):472–80. doi: 10.1016/j.exphem.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aman MJ, Keller U, Derigs G, et al. Regulation of cytokine expression by interferon-alpha in human bone marrow stromal cells: inhibition of hematopoietic growth factors and induction of interleukin-1 receptor antagonist. Blood. 1994;84(12):4142–50. [PubMed] [Google Scholar]
- 89.Quintás-Cardama A, Kantarjian HM, Giles F, Verstovsek S. Pegylated interferon therapy for patients with Philadelphia chromosome-negative myeloproliferative disorders. Semin Thromb Hemost. 2006;32:409–16. doi: 10.1055/s-2006-942761. 4 Pt 2. [DOI] [PubMed] [Google Scholar]
- 90.Nucci ML, Shorr R, Abuchowski A. The therapeutic value of poly(ethylene glycol)- modified proteins. Adv Drug Deliv Rev. 1991;6:133–51. [Google Scholar]
- 91.Garcia-Garcia I, Gonzalez-Delgado CA, Valenzuela-Silva CM, et al. Pharmacokinetic and pharmacodynamic comparison of two “pegylated” interferon alpha-2 formulations in healthy male volunteers: a randomized, crossover, double-blind study. BMC Pharmacol. 2010;10:15. doi: 10.1186/1471-2210-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Full Prescribing Information. Hoffman-La Roche Inc; Nutley, NJ: 2002. Pegasys (pegylated interferon alpha-2a) [Google Scholar]
- 93••.Quintás-Cardama A, Kantarjian H, Manshouri T, et al. Pegylated interferon alfa-2a yields high rates of hematologic and molecular response in patients with advanced essential thrombocythemia and polycythemia vera. J Clin Oncol. 2009;27(32):5418–24. doi: 10.1200/JCO.2009.23.6075. Synopsis: This Phase II study evaluated PEG–IFN-α2a for efficacy and safety in patients with advanced ET and PV. Among patients with PV, the hematologic response rate was 80%, and more than half had a $20% reduction in JAK2V617F allele burden. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94••.Kiladjian JJ, Cassinat B, Chevret S, et al. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112(8):3065–72. doi: 10.1182/blood-2008-03-143537. Synopsis: This Phase II study investigated PEG–IFN-α2a for efficacy and toxicity in patients with PV. After 12 months of treatment with PEG–IFN-α2a, hematologic response was achieved by all patients, and the JAK2V617F allele burden was reduced from 45% at baseline to 22.5%. [DOI] [PubMed] [Google Scholar]
- 95.Quintás-Cardama A, Abdel-Wahab O, Manshouri T, et al. Molecular analysis of patients with polycythemia vera or essential thrombocythemia receiving pegylated interferon alpha-2a. Blood. 2013;122(6):893–901. doi: 10.1182/blood-2012-07-442012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gowin K, Thapaliya P, Samuelson J, et al. Experience with pegylated interferon alpha-2a in advanced myeloproliferative neoplasms in an international cohort of 118 patients. Haematologica. 2012;97(10):1570–3. doi: 10.3324/haematol.2011.061390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Larsen TS, Iversen KF, Hansen E, et al. Long term molecular responses in a cohort of Danish patients with essential thrombocythemia, polycythemia vera and myelofibrosis treated with recombinant interferon alpha. Leuk Res. 2013;37:1041–5. doi: 10.1016/j.leukres.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 98.Randomized trial of pegylated interferon alfa-2a versus hydroxyurea in polycythemia vera (PV) and essential thrombocythemia (ET) Available from: http://clinicaltrials.gov/show/NCT01259856.
- 99.Safety study of pegylated interferon alpha 2b to treat polycythemia vera (PEGINVERA) Available from: http://clinicaltrials.gov/show/NCT01193699.
- 100.Gisslinger H, Buxhofer-Ausch V, Greil R, et al. Analysis of molecular responses and chromosomal aberrations in patients with polycythemia vera treated with peg-proline-interferon alpha-2b (AOP2014/P1101) in the Peginvera Study [abstract] Blood. 2013;122(21):1589. doi: 10.1002/ajh.23928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Larsen TS, Bjerrum OW, Pallisgaard N, et al. Sustained major molecular response on interferon alpha-2b in two patients with polycythemia vera. Ann Hematol. 2008;87(10):847–50. doi: 10.1007/s00277-008-0498-4. [DOI] [PubMed] [Google Scholar]
- 102.Pegylated interferon alpha-2b versus hydroxyurea in polycythemia vera (PROUD-PV) Available from: http://clinicaltrials.gov/show/NCT01949805.
- 103.Shi B, Xu W. The development and potential clinical utility of biomarkers for HDAC inhibitors. Drug Discov Ther. 2013;7(4):129–36. [PubMed] [Google Scholar]
- 104.Skov V, Larsen TS, Thomassen M, et al. Increased gene expression of histone deacetylases in patients with Philadelphia-negative chronic myeloproliferative neoplasms. Leuk Lymphoma. 2012;53(1):123–9. doi: 10.3109/10428194.2011.597905. [DOI] [PubMed] [Google Scholar]
- 105.Barbetti V, Gozzini A, Rovida E, et al. Selective anti-leukaemic activity of low-dose histone deacetylase inhibitor ITF2357 on AML1/ETO-positive cells. Oncogene. 2008;27(12):1767–78. doi: 10.1038/sj.onc.1210820. [DOI] [PubMed] [Google Scholar]
- 106.Golay J, Cuppini L, Leoni F, et al. The histone deacetylase inhibitor ITF2357 has anti-leukemic activity in vitro and in vivo and inhibits IL-6 and VEGF production by stromal cells. Leukemia. 2007;21(9):1892–900. doi: 10.1038/sj.leu.2404860. [DOI] [PubMed] [Google Scholar]
- 107.Full Prescribing Information. Merck & Co. Inc; Whitehouse Station, NJ: 2006. Zolinza (vorinostat) [Google Scholar]
- 108•.Andersen CL, McMullin MF, Ejerblad E, et al. A phase II study of vorinostat (MK- 0683) in patients with polycythaemia vera and essential thrombocythaemia. Br J Haematol. 2013;162(4):498–508. doi: 10.1111/bjh.12416. Synopsis: This Phase II study aimed to evaluate the HDAC inhibitor vorinostat for safety and efficacy in patients with PV and ET. Clinicohematologic response was achieved by 35% of patients after 24 weeks of treatment; however, 44% of patients discontinued owing to toxicity in this timeframe. [DOI] [PubMed] [Google Scholar]
- 109.Rambaldi A, Dellacasa CM, Finazzi G, et al. A pilot study of the histone-deacetylase inhibitor givinostat in patients with JAK2V617F positive chronic myeloproliferative neoplasms. Br J Haematol. 2010;150(4):446–55. doi: 10.1111/j.1365-2141.2010.08266.x. [DOI] [PubMed] [Google Scholar]
- 110•.Finazzi G, Vannucchi AM, Martinelli V, et al. A phase II study of givinostat in combination with hydroxycarbamide in patients with polycythaemia veraunresponsive to hydroxycarbamide monotherapy. Br J Haematol. 2013;161(5):688–94. doi: 10.1111/bjh.12332. Synopsis: This Phase II study investigated givinostat plus HU combination therapy for efficacy and safety in patients with PV who were intolerant of or resistant to HU. Addition of givinostat was associated with a 52.3% response rate after 12 weeks and resolution of grade $2 baseline pruritus in 65.2% of patients. [DOI] [PubMed] [Google Scholar]
- 111.A two-part study to assess the safety and preliminary efficacy of givinostat in patients with polycythemia vera. Available from: http://clinicaltrials.gov/show/NCT01901432.
- 112.Bjørn M, de Stricker K, Kjaer L, et al. Combination therapy with interferon and JAK1-2 inhibitor is feasible: proof of concept with rapid reduction in JAK2V617F-allele burden in polycythemia vera. Leuk Res Rep. 2014;3(2):73–5. doi: 10.1016/j.lrr.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasselbalch HC. Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert Rev Hematol. 2014;7(2):203–16. doi: 10.1586/17474086.2013.876356. [DOI] [PubMed] [Google Scholar]