Abstract
Pancreatic cancer, the 4th leading cause of cancer death in the US, is highly resistant to all current chemotherapies, and its growth is facilitated by chronic inflammation. An important mediator of inflammation is the nuclear factor kappa B (NFκB), a transcription factor that regulates over 500 genes including the regulation of anti-apoptotic proteins, cell cycle progression and cytokine production. NFκB is constitutively activated in pancreatic cancer cells contributing to their resistance to apoptosis and high metastatic potential. Although many small molecules that inhibit NFκB have been identified, none are currently used in the clinic, perhaps due to their lack of specificity. To identify novel inhibitors of NFκB, the HBOI library of enriched fractions from marine organisms was screened using a reporter cell line that produces luciferin under the transcriptional control of NFκB. Fractions from the sponge Amphibleptula were active in this screen and contained the antifungal cyclic peptide microsclerodermin A. Microsclerodermin A is shown here to inhibit NFκB transcriptional activity in a reporter cell line, to reduce levels of phosphorylated (active) NFκB in the AsPC-1 cell line, to have an IC50 for cytotoxicity in the low micromolar range against the AsPC-1, BxPC-3, MIA PaCa-2 and PANC-1 pancreatic cancer cell lines, and to induce significant apoptosis in the AsPC-1, BxPC-3 and the PANC-1 cell lines. Treatment of AsPC-1 cells with microsclerodermin A also resulted in an increase in IL-8 production without apparent induction of angiogenic factors and there is the possibility that inhibition of NFκB by microsclerodermin A is mediated by the glycogen synthase kinase 3β pathway.
Pancreatic cancer is an extremely aggressive disease that ranks 4th in the US for cancer-induced deaths[1]. Only 6% of patients survive 5 years after diagnosis. Current treatments do little to prolong life or ameliorate symptoms and there is an urgent need for new treatments [1]. In at least a sub-set of the population, chronic or hereditary pancreatitis (inflammation of the pancreas) leads to a significantly higher risk of progression to pancreatic cancer [2]. In these patients, treatment with agents that target key inflammatory signaling pathways associated with tumorigenesis may provide the best hope of treatment through preventing tumor formation, proliferation and metastasis.
While the exact mechanism by which inflammation leads to cancer development is not completely understood, the microenvironment elicited by the inflammatory response appears to facilitate cancer growth [3,4]. Chronic inflammation creates a microenvironment that includes reactive oxygen species, cytokines, growth factors, angiogenic factors and the activation of signaling pathways that result in increased cell turnover, cell proliferation, angiogenesis, resistance to apoptosis, invasion and metastasis [5]. Many important inflammatory signaling molecules have been validated as chemopreventative drug targets for treatment of pancreatic cancer[5–8], and one of these is the nuclear factor kappa B (NFκB). NFκB regulates over 500 genes [9], including the regulation of anti-apoptotic proteins, cell cycle progression and cytokine production. Constitutive activation of NFκB is frequently found in inflammatory diseases and in cancer. In pancreatic cancer and pancreatic cancer cell lines, NFκB is constitutively activated [10] and its activation correlates with metastatic potential [11] and resistance to apoptosis [12]. Therefore, any drugs that can interfere with the activation of NFκB have the potential to be effective therapies against inflammatory diseases and cancer. Although many small molecules that inhibit NFκB have been identified, none are currently used in the clinic, perhaps because their mode of action is not specific to this pathway [9].
The oceans are a rich source of bioactive natural products [13–15]. The uniqueness, chemical diversity and structural complexity of marine natural products represent an unexploited source of lead structures for use as biological probes or in drug discovery and development. The Marine Biomedical and Biotechnology Research Program at Harbor Branch Oceanographic Institute (HBOI) has a unique library of both pure natural products and chromatographically enriched fractions derived from marine organisms. In an effort to discover small molecule inhibitors of NFκB from our library of marine natural products, the HBOI enriched fraction library was screened using a luciferase reporter cell line that contains luciferin under transcriptional control of NFκB. Fractions from the sponge Amphibleptula were found to be active in the assays and contained the cyclic peptide microsclerodermin A [16], known to have antifungal [16] and anti-proliferative activity in select cancer cells [17]. The effects of microsclerodermin A on pancreatic cancer cells were unknown. We show here that microsclerodermin A has the previously unreported activity of inhibiting NFκB in a reporter cell-based assay, as well as in pancreatic cancer cells. In addition, microsclerodermin A significantly induced apoptosis in the AsPC-1, BxPC-3 and PANC-1 cell lines. Initial studies towards understanding its mode of action proved unsuccessful. Microsclerodermin A does not appear to affect the levels of expression of the inhibitor of NF-κB kinases a (Iκκα), the Iκκα regulator TNFAIP3, or the toll-like receptor pathway. Changes were seen in the levels of expression of proteins in the glycogen synthase kinase 3 pathway, but these failed to be statistically significant. Further studies are necessary to understand how microsclerodermin A exerts its effects on pancreatic cancer cells.
Materials and Methods
Reagents
Microsclerodermin A was obtained from the Harbor Branch Oceanographic Institute pure compound library. The material was isolated from a sponge of the genus Amphibleptula collected off the coast of Florida. The microsclerodermin A stock solution was at a concentration of 1 mg/ml in methanol. Methanol and isopropanol used in the experiments were purchased from Fisher Scientific, Fair Lawn, NJ. The 3-[4,5-Dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide (MTT) used for cell viability assays was purchased from Sigma Chemical Co., St. Louis, MO.
Purification of Microsclerodermin A
A sample of a sponge of the genus Amphibleptula cf. madrepora (HBOI Sample Identifier: 31-III-89-2-003; Museum Number 003:00944; 257 g) was extracted exhaustively with aliquots of ethyl acetate/ethanol (9:1 v/v). The filtered extracts were combined and concentrated by distillation under reduced pressure to yield 4.16 g of crude extract. This extract was partitioned between ethyl acetate and water and the aqueous phase further partitioned with n-butanol. The n- butanol partition (650 mg) was chromatographed using medium performance liquid chromatography (MPLC) on an Isco Combiflash™ Companion as follows: Redisep C-18 column (12 g); flow rate = 30 ml/min; Solvent A: H2O, Solvent B: CH3CN; t=0 min, 100% A, t=20 100% B, t= 26 min 100% B. The fraction containing microsclerodermin A eluted as a broad peak between 9 and 14 minutes. This fraction was purified further by high performance liquid chromatography (HPLC) as follows: Vydac C-18 Protein and Peptide column (10 mm x 250 mm); flow rate = 3 ml/min; Solvent A: H2O:CH3CN: CF3COOH 95:5:0.1 (v/v/v), Solvent B: CH3CN:CF3COOH 100:0.1 (v/v); t=0 min, 100% A, t=10 min, 69:31 v/v A:B, t=30 min, 69:31v/v A:B t= 35 min, 100% B. Microsclerodermin A was detected by UV at 210 nm, Rt = 24.0 min. It was identified by comparison of NMR and mass spectrometry data to the published data [16]. A total of 9.6 mg of microsclerodermin A was recovered (0.0037% of wet weight).
Cell Culture
The human pancreatic cancer cell lines PANC-1 (CRL-1469), AsPC-1 (CRL-1682), BxPC-3 (CRL-1687) and MIA PaCa-2 (CRL-1420) cell lines were obtained from ATCC (Manassas, VA), grown, aliquotted and maintained in liquid nitrogen. Aliquots of AsPC-1, PANC-1, and BxPC-3 were thawed and grown in RPMI-1640 (ATCC, Manassas, VA) supplemented with 10% Fetal Bovine Serum (Hyclone, Logan, UT), 0.11mg/ml Sodium Pyruvate, 4.5g/L D-glucose, 18mM HEPES Buffer, 100U/mL penicillin G sodium, 100μg/ml streptomycin sulfate, 0.25μg/ml amphotericin B, 2mM L-glutamine and 50μg/mL gentamicin (Complete RPMI). Sodium pyruvate and glucose were purchased from Sigma, St Louis, MO. All other supplements were purchased from Gibco, Carlsbad, CA. The MIA PaCa-2 cell line was grown in DMEM media (ATCC, Manassas, VA) supplemented with 5% horse sera (ATCC, Manassas, VA), 10% Fetal Bovine Serum (Hyclone, Logan, UT), 100U/ml penicillin G sodium, 100μg/ml streptomycin sulfate, 0.25μg/ml amphotericin B and 50μg/mL gentamicin. Cells were maintained in a humidified incubator at 37°C and 5% CO2.
NFκB Inhibition (A549/NFκB-luc) Screening Assay
The A549 NFκB-luc cell line was obtained from Panomics (RC0002; Freemont, CA) and is the lung cancer A549 cell line stably transfected with a luciferase reporter under the transcriptional control of NFκB. Cells were received at passage 3 from Panomics and were propagated and maintained in complete media containing Dulbecco’s Modified Eagles Medium (ATCC, Mannassas, VA), 10% Fetal Bovine Serum, 100units/mL penicillin, 100 μg/mL Streptomycin Penicillin, and 100μg/mL hygromycin B. Cells were plated at a concentration of 5 x 104 cells per well on a clear 96-well plate, allowed to adhere and treated with media containing 100ng/ml TNF-α (Alexis Biochemicals,) in addition to 5μg/mL of HBOI compounds or vehicle control and incubated for 6 hours. Media was removed by aspiration and cells were lysed with detergent lysis buffer (Promega, Madison WI). Cell solutions were mixed before transferring 20 μL of each lysate to a white 96-well plate. Luciferase substrate (Promega P/N E1500) was added at a volume of 20μL to each well, mixed, and the light produced immediately read using the luminescence setting on a BMG NOVOstar plate reader. To determine cell viability, 20 μL of each lysate were also plated on a clear-bottom black 96-well plated and equilibrated with ethidium bromide for 30 minutes. The resulting fluorescence was read on the fluorescence setting of the BMG NOVOstar plate reader (excitation 340nm; emission 590 nm). A compound was considered a hit if it showed 50% inhibition of light production with less than 20% cytotoxicity. To determine the concentration of microsclerodermin A necessary to produce 50% inhibition (IC50), serial dilutions of microsclerodermin ranging from 0.00013 to 5 μg/mL were used in the assay described above. The resulting absorbencies were normalized against methanol treated cells using Microsoft Excel, and this data was subjected to a non-linear regression curve fit with GraphPad Prism 5 software.
Cell Viability Assay (MTT)
12,000 cells were plated on a 96-well tissue culture plate. Cells were allowed to adhere for 24 hours. At the end of this incubation, 100 μl of medium were removed from each test well and 100 μl of medium containing treatment were added. Treatment consisted of a range of concentrations from 0.00013 to 5 μg/mL of microsclerodermin A or media with methanol. The cells were then incubated for either 72 hours at 37°C and 5% CO2. After this incubation, 75 μl 5 mg/ml MTT were added to each well. The cells were then incubated for 3 hours at 37°C. The plates were centrifuged for 10 minutes at 800 rpm. The supernatant was removed and 200 μl acidified isopropyl alcohol (1:500 solution of hydrochloric acid to isopropanol) were added to each well. The plates were shaken for 15 minutes. The absorbencies of these solutions were measured at 570 nm with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC). The resulting absorbencies were normalized against methanol treated cells using Microsoft Excel. Determination of the dose at which 50% inhibition is found (IC50) was done using a non-linear regression curve fit with GraphPad Prism 5 software.
Caspase-Glo
To determine induction of apoptosis, a commercially available kit from Promega (G0890; Madison, WI) to measure cleavage of caspase 3/7 was used following manufacturer’s protocol. Briefly, 10,000 cells were plated in a 96-well flat bottom white plate. Cells were allowed to adhere overnight then treated with 2.4 μg/mL microsclerodermin A (2X IC50 for NFκB inhibition) or its vehicle control for 1, 3, 6 or 24 hours. At the end of treatment an equal volume of pro-luminescent caspase 3–7 substrate was added to the wells and incubated at room temperature with mild shaking for 30–60 minutes. In cells with active caspase 3/7, the tetrapeptide sequence DEVD gets cleaved to release aminoluciferin, and the resulting luminescence was read with a plate reader (NOVOstar, BMG Labtech Inc., Durham, NC). Luminescence results are presented as fold induction compared to the vehicle control treated sample. Statistical significance was determined using a student’s T test comparing results to vehicle control.
TUNEL
To determine induction of apoptosis, a commercially available kit from Promega (G3250; Madison, WI) to measure fragmented DNA by incorporating fluorescein 12-dUTP at 3′-OH DNA ends using the Terminal Deoxynucleotidyl Transferase enzyme was used following manufacturer’s protocol. Briefly, 500,000 cells were plated in a 6-well plate. Cells were allowed to adhere overnight then treated with 2.4 μg/mL microsclerodermin A (2X IC50 for NFκB inhibition) or its vehicle control for 24 hours. At the end of treatment, cells were trypsinized, fixed with 4% paraformaldehyde and permeabilized with ice cold ethanol. Cells were transferred to a 96 well U-bottom plate, equilibrated for five minutes and then treated with 25 μl of a reaction mix containing the nucleotide mix and the enzyme to label the fragmented DNA for 60 minutes at 37°C. The reaction was stopped by the addition of 20mM EDTA. The resulting fluorescence was visualized via flow cytometry in a BD FACSCanto equipped with a plate adapter, acquiring 20,000 events. Statistical significance was determined using a student’s T test comparing results to vehicle control.
Intracellular Staining
To determine the levels of particular signal transduction molecules affected by microsclerodermin A, AsPC-1 cells were treated for 6 hours with 2.4 μg/mL microsclerodermin A (2X IC50 for NFκB inhibition) or its vehicle control. At the end of treatment, cells were trypsinized, fixed with 4% paraformaldehyde and permeabilized with ice cold ethanol. Cells were labeled with primary antibodies specific for the active (phosphorylated) form of NFκB using a phycoerythrin conjugated antibody (PE Mouse anti-NFκB p65 (pS529); 558423; BD Pharmingen, San Diego, CA, USA) or a mouse IgG isotype (Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour at room temperature. For differential protein expression studies cells were labeled with unconjugated antibodies against Toll-like receptor (TLR) 1 (2209), TLR3 (6961), IRAK (4504), phosphorylated Elk Ser 383 (9181), TRAIL (3219S), cyclin D1(2978), phosphorylated cyclin D1 Thr 286 (2921S), VEGF Receptor 2 (2479S), phosphorylated VEGF R2 Tyr 1059 (3817S), IκBα (2682P), TNFAIP3 (5630S), GSK3β (9315), phosphorylated GSK3β Ser9 (9323), phosphorylated Fos Ser 32 (5348P), phosphorylated Jun Ser73 (3270P), Akt (9272), phosphorylated Akt Ser 473 (9271S) (Cell Signaling Technologies, Danvers, MA, USA) or a rabbit IgG isotype (Jackson ImmunoResearch, West Grove, PA, USA) for 1 hour at room temperature. Cells were labeled with a secondary phycoerythrin conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA) for all antibodies except NFκB for 30 min at room temperature. Cells were washed and resuspended in 2% FBS in PBS and analyzed via flow cytometry in a BD FACSCanto equipped with a plate adapter, acquiring 20,000 events. Statistical significance was determined using a student’s T test comparing the average of 3 experiments for the results to the vehicle control.
IL-8 ELISA
A commercially available ELISA antibody pair from R& D Systems (DY208E; Minneapolis, MN) was used to detect IL-8 secretion by AsPC-1 cells following manufacturer’s instructions. Briefly, 25,000 AsPC-1 pancreatic cancer cells per well were plated on a flat bottom clear 96 well plate and allowed to adhere overnight. Next day, media was removed and substituted with 100 μL media containing 2.4μg/mL microsclerodermin A or vehicle control. After 6 hours, media was removed and transferred to a 96-well EIA/RIA Plate (Costar 3590) coated with 400ng capture antibody. After overnight incubation at 4°C, the media was removed, the plate washed repeatedly and 2 ng of detection antibody were added for 1 hour at room temperature. At the end of this incubation and repeated washing, streptavidin conjugated to horseradish was added for 20 minutes, followed by 3,3′,5,5′ tetramethyl-benzidine (Sigma, St. Louis, MO) substrate for 20 minutes at room temperature. Reaction was terminated with the addition of 2N H2SO4, and absorbance was read in a NOVOstar plate reader (BMG Labtech Inc., Durham, NC) at 450nm.
Results
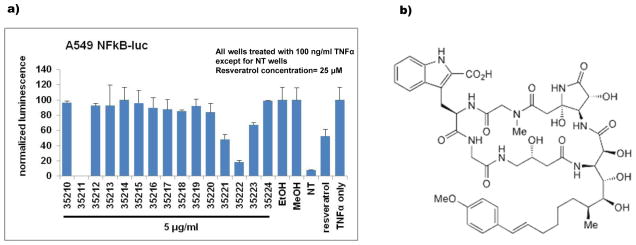
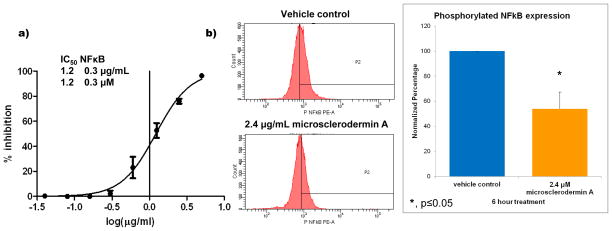
In an effort to identify novel inhibitors of the nuclear factor kappa B (NFκB) among our collection of marine secondary metabolites, a commercially available reporter cell line with luciferin under the transcriptional control of NFκB (A549 NFκB-luc) was used to screen the HBOI enriched fraction library. While this reporter cell line is not a pancreatic cancer cell line, it was chosen as it had been validated by other researchers, was commercially available and it could test for inhibition of transcriptional activity of NFκB. Cells were treated first with either vehicle control or marine compounds, immediately followed by treatment with tumor necrosis factor alpha (TNF-α) to stimulate NFκB activity. Compounds with no effect on NFκB produce luciferin which upon treatment with luciferase produces visible light which can be read as luminescence on a plate reader. Those that inhibit NFκB show reduced light production. Fractions from the sponge Amphibleptula showed significant inhibition (≥ 50% decrease from control with ≤ 20% cytotoxicity; Figure 1a). After confirming inhibition in the screening assay and in pancreatic cancer cells, bioassay-guided fractionation was conducted to identify the active compound. The active compound was identified as the previously known cyclic peptide microsclerodermin A [16] whose structure is shown in Figure 1b. Serial dilutions of the compound were used in the screening assay to calculate the concentration at which 50% inhibition of NFκB activity (IC50) was seen. This concentration was 1.2 ± 0.3 μM (Figure 2a).
Figure 1. Screening of Amphibleptula fractions shows NFκB inhibition.
a) Fractions from the sponge Amphibleptula showed significant inhibition (≥ 50% decrease from control with ≤ 20% cytotoxicity) of NFκB activity in the cell-based reporter assay when tested at 5μg/mL. b) Bioassay guided fractionation, dereplication, and structure elucidation studies revealed that the compound responsible for the activity was microsclerodermin A whose structure is shown here.
Figure 2. Inhibition of NFκB by microsclerodermin A.
a) Serial dilutions of the compound in the screening assay and subsequent non-linear regression analysis showed that the concentration of microsclerodermin A required to obtain 50% inhibition (IC50) of NFκB activity in the reporter assay was 1.2 μM with a standard deviation of 0.3 μM. b) Significant inhibition of NFκB was also observed in AsPC-1 pancreatic cancer cells treated with 2X the IC50 for 6 hours as analyzed by flow cytometry for the phosphorylated form of NFκB (Ser 529). One representative histogram shown; gates were set up against isotype control. Graphical representation of the flow cytometry data normalized to vehicle control shows the average of 3 experiments ± standard deviation. Statistical significance was determined through a student’s T test.
Because a major focus in our laboratory is to find compounds with the potential to be useful in the treatment of pancreatic cancer, we studied the effects of microsclerodermin A in pancreatic cancer cells known to have constitutively active NFκB [10]. In the reporter cell line, NFκB activity was induced through stimulation with TNFα while in the pancreatic cancer cells no further stimulation was required due to the constitutive activation of NFκB in these cells. To maximize the opportunity of observing the effects in the pancreatic cancer cell lines, microsclerodermin A was tested at two times the IC50 found for the reporter cell line (2.4 μM or 2.4 μg/mL). Inhibition of NFκB and effects on downstream signaling was measured at 6 hours to replicate the timing used in the screening assay. Cytotoxicity was measured in the pancreatic cancer cell lines at 72 hours using a standard MTT-based protocol.
The pancreatic adenocarcinoma cell line AsPC-1 was used to investigate whether microsclerodermin A could reduce levels of constitutively activated NFκB in a highly metastatic cell line. As shown in Figure 2b, flow cytometry indicated that treatment with 2.4 μg/mL microsclerodermin A for 6 hours significantly reduced the levels of phosphorylated (active) NFκB p65 (pS529) in AsPC-1 cells with respect to vehicle control.
While microsclerodermin A exhibited little or no cytotoxicity in the reporter cell line during the 6 hours treatment time (which was of importance to eliminate false positives), we expected that it would have a different effect in cells that express constitutively active NFκB. NFκB is a known regulator of anti-apoptotic molecules [9] and the inhibition of constitutively activated NFκB has been shown to sensitize cells to apoptosis in pancreatic cancer cells [12]. The cytotoxicity of microsclerodermin A in four pancreatic cancer cell lines was determined using a standard MTT-based protocol. The average of three experiments for cytotoxicity resulted in the following IC50 values for microsclerodermin A ± standard deviation: AsPC-1, 2.3 ± 0.5 μM; BxPC-3, 0.8 ± 0.2 μM; MIA PaCa-2, 4.3 ± 0.4 μM; PANC-1, 4.0 ± 0.4 μM. The IC50 was in the low micromolar range for all cell lines. The second most sensitive line was the AsPC-1 cell line, which is known to have the highest metastatic potential and resistance to apoptosis [18,19].
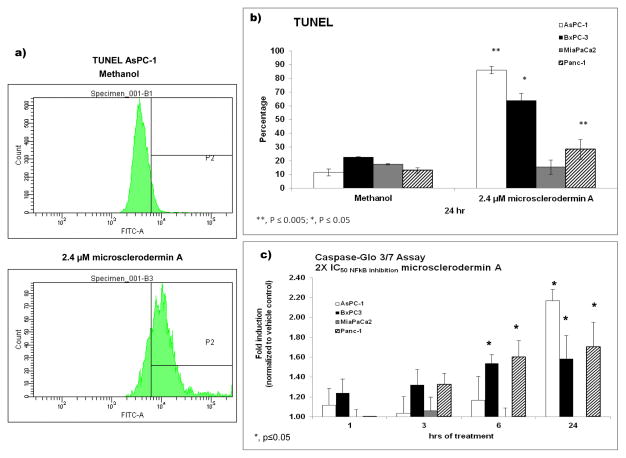
To ascertain if the observed cytotoxicity was caused by apoptosis, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) was used to detect DNA fragmentation via flow cytometry. In addition, cleavage of caspase 3/7 was also monitored using the commercially available Caspase-Glo assay. As shown in Figure 3, microsclerodermin A induced statistically significant apoptosis in the AsPC-1, BxPC-3 and PANC-1 cell lines. Caspase 3/7 cleavage was seen in the AsPC-1, BxPC-3 and PANC-1 cells as well, with increases in the amount of cleavage as the time of treatment was increased. A movie showing induction of apoptosis by microsclerodermin A in the AsPC-1 cell line obtained through high content imaging has been submitted as supplementary data.
Figure 3. Induction of apoptosis by microsclerodermin A in pancreatic cancer cells.
a) Flow cytometry histograms from one representative experiment for TUNEL in AsPC-1 cells treated for 6 hours with vehicle control or 2.4 μM microsclerodermin A. b) Graphical representation of the flow cytometry data shows the average of 3 experiments ± standard deviation. Microsclerodermin A induced statistically significant apoptosis in the AsPC-1, BxPC-3 and PANC-1 cell lines. DNA fragmentation was not detected in the MIA PaCa2 cell line. c) Graphical representation of the caspase cleavage data showing the average of 3 experiments ± standard error of the mean. Caspase 3/7 cleavage was seen in the AsPC-1, BxPC-3 and PANC-1 cells as well, with increases in the amount of cleavage as the time of treatment was increased.
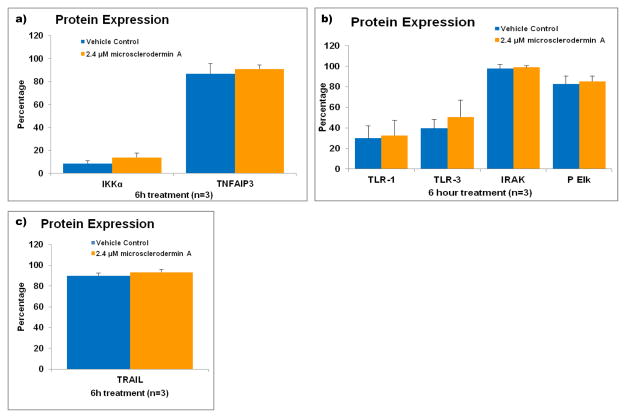
NFκB regulates over 200 different genes and is part of many signal transduction cascades [9]. To obtain insight on the mechanism by which microsclerodermin A inhibited NFκB, protein expression of a few key signal transduction pathways was followed by flow cytometry in AsPC-1 cells treated with microsclerodermin A or its vehicle control for 6 hours. IKKα and TNFAIP3 are known to maintain NFκB sequestered in the cytoplasm. However, no changes at the protein level were seen for these proteins (Figure 4a). The toll-like receptor pathway can regulate expression of NFκB and its inhibition could provide a plausible explanation for the observed NFκB inhibition. No changes at the protein level were seen in key members of this pathway with microsclerodermin A treatment (Figure 4b). No changes were seen in the levels of expression of the TNF-related apoptosis–inducing ligand (TRAIL) (Figure 4c).
Figure 4. Flow cytometric analysis of pathways involved in the regulation of NFκB in AsPC-1 cells treated with microsclerodermin A.
AsPC-1 cells were treated with either 2.4 μM microsclerodermin A or methanol (vehicle control) for 6 hours and analyzed via flow cytometry to determine changes in expression of a) Iκκα and TNFAIP3, which keep NFκB in its inactive form in the cytoplasm; b) the members of the Toll-like Receptor signaling pathway TLR-1, TLR-3, IRAK and phosphorylated Elk; c) the TNF-related apoptosis-inducing ligand (TRAIL). No significant changes were observed in these pathways in cells treated with microsclerodermin A compared to cells treated with vehicle control. Graphical representation of the flow cytometry data shows the average of 3 experiments. Error bars represent standard deviation.
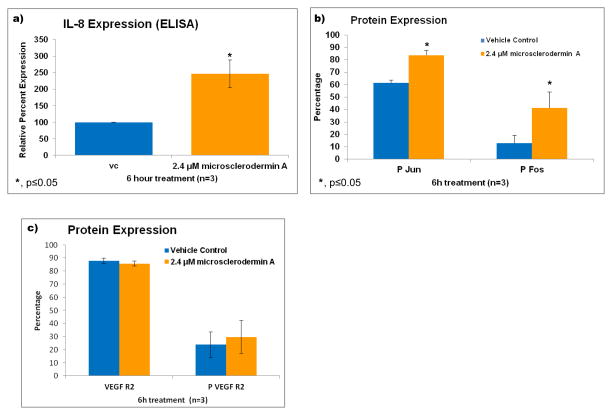
The levels of IL-8 were measured using an ELISA assay and a significant increase (about 2.5 fold) in IL-8 production was observed in AsPC-1 cells treated with microsclerodermin A for 6 hours compared to controls (Figure 5a). This was a puzzling result as we expected either no change or a decrease in the levels of IL-8 as a result of the inhibition of NFκB. Fos and Jun are known to control the transcription factor AP-1, which can also regulate production of IL-8 [20]. Flow cytometry showed that both the phosphorylated forms of Fos and Jun were statistically significantly upregulated in microsclerodermin A treated AsPC-1 cells compared to controls (Figure 5b). No significant changes in the levels of the vascular endothelial growth factor receptor 2 (VEGF R2) or its phosphorylation were seen in microsclerodermin A treated cells compared to controls (Figure 5c).
Figure 5. Treatment of AsPC-1 cells with microsclerodermin A increases IL-8 secretion possibly through the upregulation of the transcription factor AP-1, but without affecting VEGF Receptor 2 expression.
a) An increase in IL-8 production by AsPC-1 cells treated with microsclerodermin A for 6 hours compared to control was observed using an ELISA assay. Data shown is the average of 3 experiments ± standard deviation. b) Graphical representation of the flow cytometry data for intracellular staining of AsPC-1 cells treated for 6 hours with 2.4μM microsclerodermin A or vehicle control. Data shown is the average of 3 experiments ± standard deviation. The phosphorylated forms of Fos and Jun were statistically significantly increased in microsclerodermin A treated AsPC-1 cells compared to controls. c) Graphical representation of the flow cytometry data for intracellular staining of AsPC-1 cells treated for 6 hours with 2.4μM microsclerodermin A or vehicle control. Data shown is the average of 3 experiments, error bars represent standard error of the mean. No significant differences in expression of the VEGF receptor 2 in its native and phosphorylated form were observed between control and microsclerodermin A treated AsPC-1 cells.
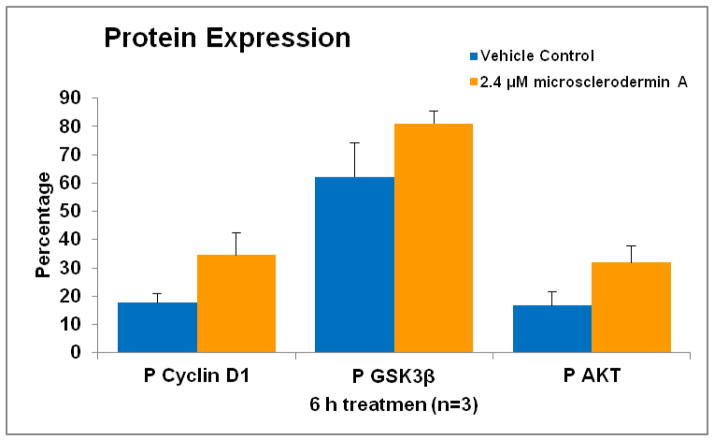
Since inhibition of glycogen synthase kinase 3 beta (GSK3β) is known to lead to inactivation of NFκB and induction of apoptosis in pancreatic cancer cells [21,22], the levels of key components of this signaling pathway were also studied in AsPC-1 cells. As shown in Figure 6, levels of phosphorylated (inactive) GSK3β are increased by treatment with microsclerodermin A. Levels of phosphorylated AKT, which phosphorylates GSK3β, were also increased upon treatment with microsclerodermin A. Levels of phosphorylated cyclin D1 which is downstream of GSK3β were also increased. This data suggests that microsclerodermin A may inhibit NFκB through inactivation of the GSK3β signaling pathway. However, none of these increases was statistically significant using a student’s T test and further work needs to be done to confirm this.
Figure 6. Possible involvement of the Glycogen Synthase Kinase 3 beta pathway in NFκB inhibition by microsclerodermin A.
Graphical representation of the flow cytometry data for intracellular staining of AsPC-1 cells treated for 6 hours with 2.4μM microsclerodermin A or vehicle control. Data shown is the average of 3 experiments, error bars represent standard error of the mean. The levels of phosphorylated (inactive) GSK3β, phosphorylated AKT and phosphorylated cyclin D1 are increased by treatment of AsPC-1 cells with microsclerodermin A. These increases suggest that microsclerodermin A may inhibit NFκB through inactivation of the GSK3β signaling pathway. However, none of these increases was statistically significant using a student’s T test.
Discussion
Microsclerodermin A is a marine natural product known to have antifungal activity and anti-proliferative effects against the A549 lung cancer cell line and the p388 leukemia cell line. The effects of microsclerodermin A on pancreatic cancer cells were unknown. We show here that microsclerodermin A can significantly reduce NFκB activity in a luciferase reporter cell line and significantly reduce the levels of phosphorylated NFκB present in the AsPC-1 pancreatic cancer cell line. In addition, microsclerodermin A significantly induced apoptosis in the AsPC-1, BxPC-3 and PANC-1 cell lines. These cell lines are known to have constitutively activated NFκB [10]. While The MIA PaCa-2 cell line also has constitutively activated NFκB and microsclerodermin A did induce cytotoxicity in this cell line, no markers of apoptosis were seen in this cell line with the assays used; further testing is needed to determine how the cytotoxicity caused by microsclerodermin A in this cell line occurs. The mechanism of action of microsclerodermin A remains to be elucidated, although the data shown suggests the possible involvement of the GSK3β pathway and more work is needed to determine if this is the case.
One of the most puzzling results presented in this paper is the increase in IL-8 levels in the AsPC-1 cells as a result of microsclerodermin A treatment, although other NFκB inhibitors are known to have similar effects. Further experiments are needed to determine what the effects of this increase are in functional assays. A single endothelial tube formation assay experiment performed in the presence of microsclerodermin A showed decreased tube formation by HUVEC cells in the presence of the compound (Data not shown). While preliminary, this result along with the lack of changes in the VEGF receptor suggests that microsclerodermin A does not promote angiogenesis.
One of the major obstacles to studies with marine organisms is obtaining sufficient quantities of the compound. However, numerous studies have been undertaken to synthetically produce microsclerodermin A and related compounds [23–28]. Related microsclerodermin class metabolites have been recently isolated from cultured terrestrial myxobacteria which may provide a renewable supply for further preclinical evaluation [29]. In conclusion we have demonstrated that microsclerodermin A inhibits NFκB, induces apoptosis and has many effects against pancreatic cancer cells.
Supplementary Material
AsPC-1 cells were plated and allowed to adhere overnight in a 96-well clear bottom black plate. Prior to imaging, media was replaced with complete RPMI media with no phenol red that contained 2.4μM microsclerodermin A (2X IC50 of NFκB inhibition) or its vehicle control methanol, as well as 1 drop NucBlue Live Cell Stain Hoechst 33342 (Molecular Probes R37605) per 1000mL complete RPMI, 5 μL/well 7-Aminoactinomycin D (7AAD; BD Pharmingen, San Jose California) and 0.75 μL/well NucView 488 Caspase 3 substrate (Biotum Inc., Hayward, CA). Images were captured every 20 minutes for about 16 hours at 20X magnification with an ImageXpress Micro XLS Widefield High-Content Analysis System (Molecular Devices, Sunnyvale, CA). Images were captured in bright field as well to determine cell morphology. The nuclei of all cells were stained with Hoechst. Cells that cleaved caspase 3 show a green color. Those cells that lost membrane integrity allowed the introduction of the 7AAD dye shown in red. Cells undergoing apoptosis should stain green first, followed by red.
Acknowledgments
Grant Support
Funding for this project was provided by the State of Florida Biomedical Research Program Bankhead Coley New Investigator Award 09BN-08-23088 (Guzmán) and by NIH R01 CA 093455 and U19 CA 52955 awarded to Dr. Amy Wright. Funding for the student’s stipend (K. Maers) was provided by the Gertrude E. Skelly Charitable Foundation.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
This is HBOI contribution number XXXX.
References
- 1.American Cancer Society. Cancer Facts and Figures. 2014 http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- 2.McKay CJ, Glen P, McMillan DC. Chronic inflammation and pancreatic cancer. Best Pract Res Clin Gastroenterol. 2008;22 (1):65–73. doi: 10.1016/j.bpg.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7 (3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420 (6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi M, Mutoh M, Ishigamori R, Fujii G, Imai T. Involvement of inflammatory factors in pancreatic carcinogenesis and preventive effects of anti-inflammatory agents. Seminars in immunopathology. 2013;35 (2):203–227. doi: 10.1007/s00281-012-0340-x. [DOI] [PubMed] [Google Scholar]
- 6.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8 (1):33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran RB, Contino G, Deshpande V, Tzatsos A, Conrad C, Benes CH, Levy DE, Settleman J, Engelman JA, Bardeesy N. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 2011;71 (14):5020–5029. doi: 10.1158/0008-5472.CAN-11-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karin M. Inflammation and cancer: the long reach of Ras. Nat Med. 2005;11 (1):20–21. doi: 10.1038/nm0105-20. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799 (10–12):775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5 (1):119–127. [PubMed] [Google Scholar]
- 11.Fujioka S, Sclabas GM, Schmidt C, Frederick WA, Dong QG, Abbruzzese JL, Evans DB, Baker C, Chiao PJ. Function of nuclear factor kappaB in pancreatic cancer metastasis. Clin Cancer Res. 2003;9 (1):346–354. [PubMed] [Google Scholar]
- 12.Braeuer SJ, Buneker C, Mohr A, Zwacka RM. Constitutively activated nuclear factor-kappaB, but not induced NF-kappaB, leads to TRAIL resistance by up-regulation of X-linked inhibitor of apoptosis protein in human cancer cells. Molecular cancer research : MCR. 2006;4 (10):715–728. doi: 10.1158/1541-7786.MCR-05-0231. [DOI] [PubMed] [Google Scholar]
- 13.Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR. Marine natural products. Natural product reports. 2012;29 (2):144–222. doi: 10.1039/c2np00090c. [DOI] [PubMed] [Google Scholar]
- 14.Mayer AMS. Marine Pharmacology and the Late 2011 Marine Pharmaceuticals Pipeline. Toxicon. 2012;60:104. [Google Scholar]
- 15.Newman DJ, Cragg GM. Natural products from marine invertebrates and microbes as modulators of antitumor targets. Current drug targets. 2006;7 (3):279–304. doi: 10.2174/138945006776054960. [DOI] [PubMed] [Google Scholar]
- 16.Bewley CA, Debitus C, Faulkner DJ. Microsclerodermins A and B. Antifungal Cyclic Peptides from the Lithistid Sponge Microscleroderma sp. J Am Chem Soc. 1994;116:7631–7636. [Google Scholar]
- 17.Wright AE, Pomponi SA, Longley RE, Isbrucker RA. Antiproliferative activity of microsclerodermins. US6384187B1. United States Patent. 2002 May 7;
- 18.Takamori H, Hiraoka T, Yamamoto T. Expression of tumor-associated carbohydrate antigens correlates with hepatic metastasis of pancreatic cancer: clinical and experimental studies. Hepato-gastroenterology. 1996;43 (9):748–755. [PubMed] [Google Scholar]
- 19.Tan MH, Chu TM. Characterization of the tumorigenic and metastatic properties of a human pancreatic tumor cell line (AsPC-1) implanted orthotopically into nude mice. Tumour biology. 1985;6 (1):89–98. [PubMed] [Google Scholar]
- 20.Hipp MS, Urbich C, Mayer P, Wischhusen J, Weller M, Kracht M, Spyridopoulos I. Proteasome inhibition leads to NF-kappaB-independent IL-8 transactivation in human endothelial cells through induction of AP-1. European journal of immunology. 2002;32 (8):2208–2217. doi: 10.1002/1521-4141(200208)32:8<2208::AID-IMMU2208>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clinical cancer research. 2006;12 (17):5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65 (6):2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekhar S, Sultana SS. Stereoselective synthesis of the C1–C20 segment of the Microsclerodermins A and B. Tetrahedron Lett. 2006;47:7255–7258. [Google Scholar]
- 24.Sasaki S, Hamada Y, Shioiri T. Synthetic studies of microsclerodermins. A stereoselective synthesis of a core building block for (2S, 3R, 4S, 5S, 6S, 11E)-3-amino-6-methyl-12-(4-methoxyphenyl)-2,4,5-trihydroxydodec-11-enoic acid (AMMTD) Tetrahedron Lett. 1997;38:3013–3016. [Google Scholar]
- 25.Sasaki S, Hamada Y, Shioiri T. The efficient stereoselective synthesis of (2S,3R,4S,5S,6S,11E)-3-amino-6-methyl-12-(4-methoxyphenyl)-2,4,5-trihydroxydodec-11-enoic acid (AMMTD), a component of microsclerodermins of marine sponge origin, as its protected form. Tetrahedron Lett. 1999;40:3187–3190. [Google Scholar]
- 26.Sasaki S, Hamada Y, Shioiri T. Construction of three building blocks for the total synthesis of microsclerodermins. Synlett. 1999:453–455. [Google Scholar]
- 27.Shioiri T, Sasaki S, Hamada Y. Synthetic approach to microsclerodermins: construction of three building blocks. ARKIVOC (Gainesville, FL, U S) 2003:103–122. [Google Scholar]
- 28.Shuter EC, Duong H, Hutton CA, McLeod MD. The enantioselective synthesis of APTO and AETD: Polyhydroxylated β-amino acid constituents of the microsclerodermin cyclic peptides. Org Biomol Chem. 2007;5:3183–3189. doi: 10.1039/b707891a. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann T, Muller S, Nadmid S, Garcia R, Muller R. Microsclerodermins from terrestrial myxobacteria: an intriguing biosynthesis likely connected to a sponge symbiont. J Am Chem Soc. 2013;135 (45):16904–16911. doi: 10.1021/ja4054509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AsPC-1 cells were plated and allowed to adhere overnight in a 96-well clear bottom black plate. Prior to imaging, media was replaced with complete RPMI media with no phenol red that contained 2.4μM microsclerodermin A (2X IC50 of NFκB inhibition) or its vehicle control methanol, as well as 1 drop NucBlue Live Cell Stain Hoechst 33342 (Molecular Probes R37605) per 1000mL complete RPMI, 5 μL/well 7-Aminoactinomycin D (7AAD; BD Pharmingen, San Jose California) and 0.75 μL/well NucView 488 Caspase 3 substrate (Biotum Inc., Hayward, CA). Images were captured every 20 minutes for about 16 hours at 20X magnification with an ImageXpress Micro XLS Widefield High-Content Analysis System (Molecular Devices, Sunnyvale, CA). Images were captured in bright field as well to determine cell morphology. The nuclei of all cells were stained with Hoechst. Cells that cleaved caspase 3 show a green color. Those cells that lost membrane integrity allowed the introduction of the 7AAD dye shown in red. Cells undergoing apoptosis should stain green first, followed by red.