Summary
Sniffing and whisking are two rhythmic orofacial motor activities that enable rodents to localize and track objects in their environment. They have related temporal dynamics, possibly as a result of both shared musculature and shared sensory tasks. Sniffing and whisking also constitute the overt expression of an animal's anticipation of a reward. Yet, the neuronal mechanisms that underlie the control of these behaviors have not been established. Here, we review the similarities between sniffing and whisking and suggest that such similarities indicate a mechanistic link between these two rhythmic exploratory behaviors.
Keywords: Central pattern generator, Exploratory behavior, Olfaction, Vibrissae, Reward
Introduction
When one observes rodents introduced in a new environment, one immediately notices that they are extremely curious [1]. They run about, stand up on their hind legs, crane their necks forward, and repeatedly explore the whole environment by sniffing and whisking vigorously. Psychologists have described curiosity as a drive like hunger and thirst, which implies that gathering information is rewarding. In experimental studies, sniffing and whisking are commonly considered as motor strategies for rapidly gathering detailed information about the location, texture, and scent of objects. Yet, it is intriguing that decorticated rats, or rats deprived of olfactory and vibrissa afferents, continue to sniff and whisk in a relatively normal manner. Therefore, beyond the obvious intuition that vibrissae are ‘for touch’ and noses ‘for smell’, it is not clear why rhythmic activity is associated with the use of these sensors. Here, we examine the dynamics of rhythmic sniffing and whisking, and how they shape olfactory and tactile sensations in rodents. We place special emphasis on the behavioral significance of the apparent coordination of these patterned activities.
A link between sniffing and whisking
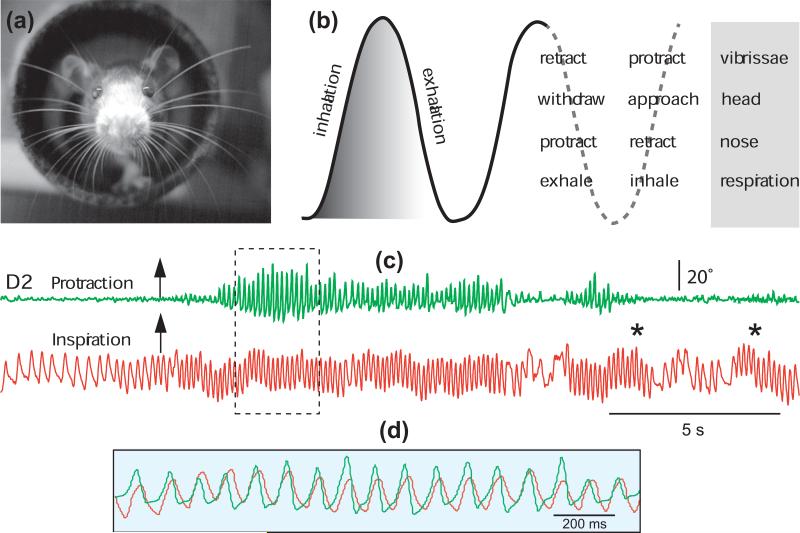
In a now classic paper published in 1964, Welker [2] provided the first descriptive account of the sniffing behavior in rats. Using cinematographic technique, he reported that sniffing consists of an integrated movement sequence involving: (1) bursts of polypnea, (2) recurrent protraction and retraction of mystacial vibrissae, (3) repetitive retraction and protraction of the tip of the snout, and (4) a rapid series of head movements and fixations. These four components occur at rates between 5 and 11 Hz, in bursts of various durations, and “exhibit a fixed temporal relationship to one another” (Fig. 1b). Welker further made the little noticed remark that ”Polypnea and nose movements together may occur independently of vibrissae and head movements, but rapid vibrissae or head movements were never observed to occur independently of polypnea and nose movements”. In short, Welker noted that whisking was always coupled to sniffing. This temporal relationship is illustrated in Figure 1c,d, where whisking and sniffing were recorded as a rat explores the exit of a tunnel (Fig. 1a).
Figure 1. Co-occurrence of sniffing and whisking in rodents.
(a) Photograph of a rat whisking at the exit of a tunnel. Image was obtained in the dark under near infrared illumination. (b) Sniff and whisk cycles are coordinated with nose and head movements. Adapted from Welker [2]. (c) Whisking occurs in phase with sniffing, but sniffing can occur without whisking; asterisks denote sniffing bouts without whisking. Overlapping traces in (d) show the framed area in (c). The motion of vibrissa D2 was monitored by high-speed videography (250 frames per second), and sniffing was recorded by means of a thermocouple implanted in the nasal cavity (unpublished data).
Welker's seminal observations did not raise immediate interest in the sensorimotor community as the rodent vibrissa (barrel) cortex had yet to be discovered [3], and most sensorimotor work at that time was focused on primates. Later studies in the field of respiratory physiology alluded to Welker's findings in reporting instances of facial nerve, muscle, or motoneuron activity that were time-locked to different phases of the respiratory cycle [4 - 6]. Interestingly, the nasolabial muscle, which contributes to flare of the naris during sniffing, also acts as a retractor muscle during whisking [7, 8]. This dual role suggests an obligate neuromuscular coupling between whisking and sniffing that requires coordinated control to ensure that these behaviors do not interfere with each other.
Rhythmogenesis of sniffing and whisking
Sniffing in rats is usually defined as rapid, rhythmic respiration [2, 9]. Rats, unlike humans, always breathe nasally, so lower frequencies (1-3 Hz) correspond to basal respiration while higher frequencies (4-12 Hz) correspond to “sniffing” per se. Although rats and mice are commonly used in studies of respiratory rhythmogenesis, the neural mechanisms underlying the generation of sniffing remain unknown. One possibility is that sniffing relies on an increased rate of bursts in the central pattern generator (CPG) that controls breathing, e.g., the Bötzinger/pre-Bötzinger complex and the parafacial group [10,11]. Yet, a mechanism for generating rhythmic sniffing remains to be demonstrated.
As with respiration, it has long been postulated that whisking is driven by a CPG located in the brainstem. This hypothesis is derived from the observation that whisking persists after decortication [2, 12], and that bilateral section of the infraorbital branch of the trigeminal nerve, i.e., sensory denervation, has only little effect on the generation, kinematics, and bilateral coordination of the normal whisking pattern [2, 7, 13]. Attempts to identify neurons that form the core of the whisking CPG have so far yielded unconvincing results. Beyond the obvious participation of facial motoneurons as downstream command neurons, the premotor whisking circuitry remains elusive. It has been proposed that facial motoneurons themselves generate whisking in response to modulatory input from serotonergic pre-motoneurons [14]. Yet, this hypothesis does not explain the synchrony of motoneuron discharges, nor how different pools of facial motoneurons are activated both bilaterally, and in a multiphasic manner, to generate the protraction/retraction cycle [7, 8]. Alternatively, and on the basis of Welker's description of the close phase relationship between sniffing and whisking [2], one might speculate that both rhythmic activities share a common neural circuitry.
The function of the vibrissae in sensation
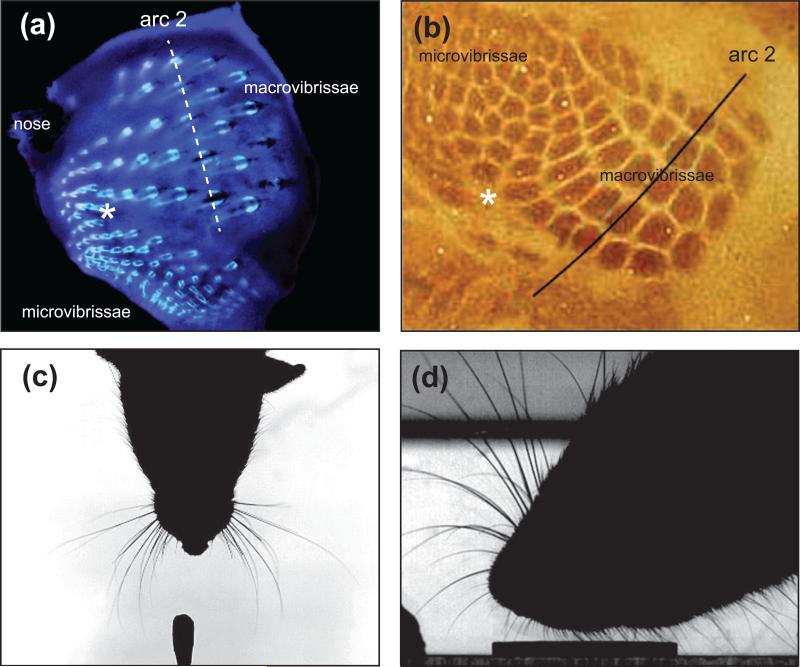
It is necessary to first consider the anatomical organization and function of the vibrissa sensorimotor plant itself to understand the behavioral significance of whisking. Rats and mice are nocturnal animals with poor visual acuity. They have laterally-placed eyes, which allows very little overlap of the visual fields. Panoramic vision gives them a wide field of view, which likely helps them detect predators but provides poor depth discrimination. At close range they rely on sniffing and whisking to sense objects and move about, search for food and shelter, and interact with conspecifics [15]. They are equipped with two sets of vibrissae (Fig. 2); one set is the large macrovibrissae that form a matrix of approximately 25 motile sensors on each side of the face, while the other is a dense array of shorter vibrissae, or microvibrissae, around the mouth, chin and nose. Each of the macrovibrissae has a corresponding “intrinsic” papillary muscle responsible for the protraction phase of the whisk cycle [16]. Microvibrissae do not have intrinsic muscles and do not protract, but they are distinguished from the surrounding pelagic fur by a thick shaft that is housed within a follicle-blood sinus complex. The entire mystacial pad, including the microvibrissae, is under active control of three major “extrinsic” muscles that are responsible for pad translation [8, 16].
Figure 2. Rodents have two sets of vibrissae.
(a) Vibrissa follicles on the mystacial pad and perioral region. (b) The folliciles are mapped in a one-to-one manner in layer 4 of the primary somatosensory cortex. Note the presence of small-sized follicles, i.e., microvibrissae, that are represented by smaller sized barrels in cortex. Asterisks indicate the transition point between macro- and microvibrissae and their representation in cortex. (c) Photograph of a rat whisking upon presentation of a new odor on the cotton swab in the lower part of the image (unpublished data). (d) Photograph of a rat sampling a coin with its microvibrissae. Adapted from Fox et al. [50].
Though both macro and microvibrissae are capable of detecting tactile features such as texture and shape, there appears to be specialization and complementarity of function. Macrovibrissae serve principally to locate objects in space (Fig. 2c), whereas the microvibrissa array is primarily used for close examination of shape and surface properties [17] (Fig. 2d). The functional importance of both sets of vibrissae is reflected by their extensive representation in the primary somatosensory cortical area (Fig. 2b). However, very little is known about the psychophysics and physiology of tactile sensation mediated by the microvibrissae nor about the anatomical organization of pathways that convey microvibrissa information from the trigeminus to cortical stations.
Sniffing and whisking shape sensory inputs
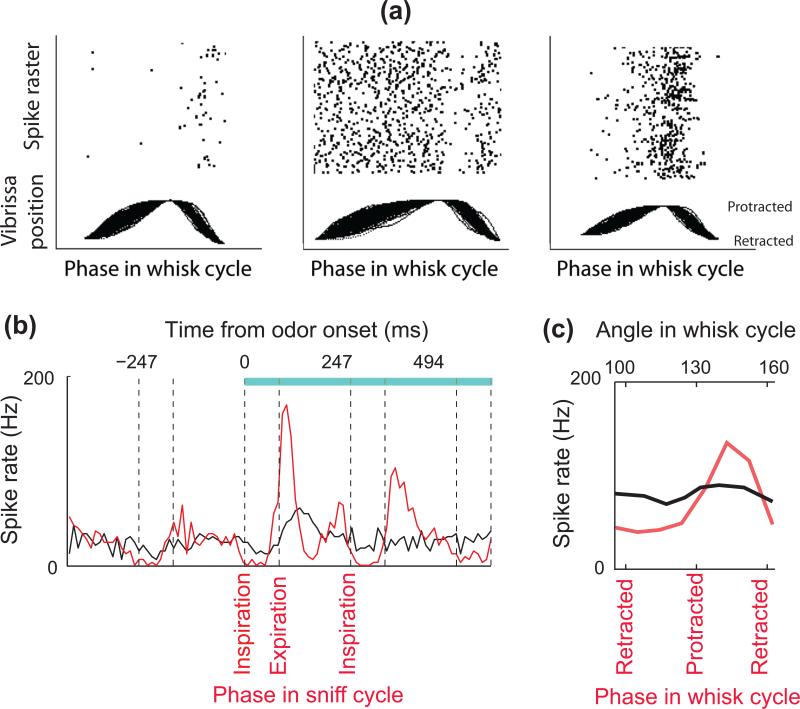
Sniffing and whisking affect both the encoding and central processing of sensory inputs. Sniffing and whisking per se, in the absence of odor or touch, modulate the activity of mitral cells and first-order vibrissa afferents respectively [18 - 21] (Fig. 3a). Though air flow varies in amplitude, velocity and frequency, individual mitral cells preferentially discharge at a specific phase of the sniffing cycle [21], such that odor evoked responses are normalized to the duration of the sniff cycle (Fig. 3b). Similarly, there is analogous coding of touch information in terms of normalized parameters of whisking in the vibrissa system, where neuronal activity locks to the phase in the whisk cycle in primary somatosensory cortex [22 - 25] (Fig. 3c). This is thought to provide a phase reference signal for decoding object location independently of the amplitude, midpoint, and frequency of whisking [24, 26]. The encoding of sensory inputs in a manner that is pegged to the rhythmic motor control of the sensory organs demonstrates the fundamental link of motor control of behavior to sensation for both somatosensation [24] and olfaction [27].
Figure 3. Encoding of odorants and vibrissa contact during the sniffing and whisking cycles.
(a) Whisking solely in air modulates the activity of trigeminal ganglion cells. Unit recordings were obtained from a head-fixed rat together with simultaneous optical tracking of vibrissa position. Vibrissa movements are aligned to protraction onset, retraction onset, and end. Raster plots the spike times above the movements. Adapted from Khatri et al. [19]. (b) Peristimulus time histograms for a mitral/tufted cell in response to an odor stimulus (blue bar, stimulus duration): synchronized by odor onset (black), and temporally warped to the phase in the sniff cycle (red). Note that mitral cell activity is modulated by sniffing before the odor presentation, and that odor-induced responses are more tightly time-locked to the sniff phase than to the time after odor onset. Vertical dashed lines indicate the beginning and end of inhalation intervals. Adapted from Shusterman et al. [21]. (c) Touch response in vibrissa sensory cortex is strongly modulated by the phase in the whisk cycle. The red trace shows the spike rate of a neuron in response to object contact parsed according to the phase in the whisk cycle at which the contact occurred. The black line is the same data parsed according to the angular position of the vibrissa where the contact occurred. Unlike the case for phase, there is no significant tuning for angle. Adapted from Curtis and Kleinfeld [24].
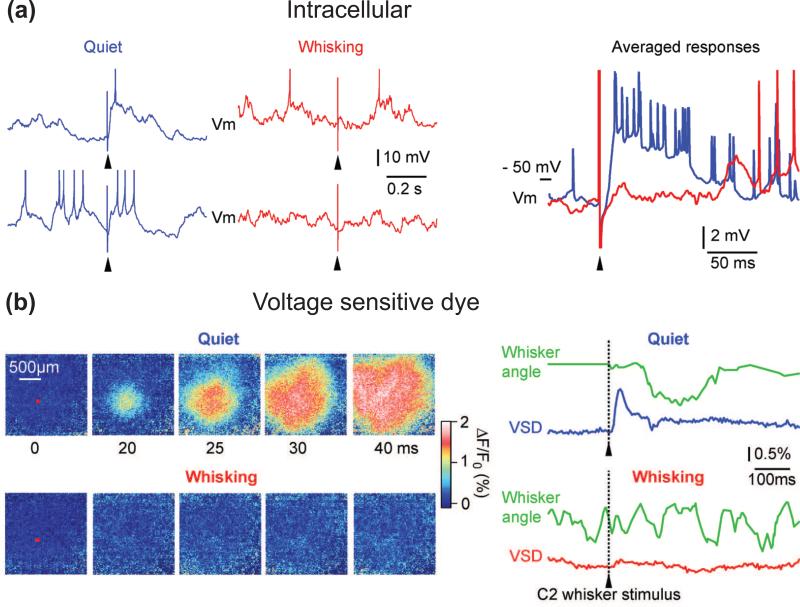
The modulation of touch sensation by motor activity has precedents from other studies as well. Whisking gates touch along afferent pathways of the vibrissa system [28-30]. The study of Ferezou et al. [28] is particularly interesting in this regard (Fig. 4). Using voltage-sensitive dyes in freely moving mice, the authors find that in awake but otherwise sessile mice, an unexpected vibrissa deflection produces a large response in barrel cortex. When the same stimulus is delivered unexpectedly during a whisking bout, the evoked response is markedly attenuated. Finally, ‘expected’ contact of the vibrissae with an object during exploratory whisking leads to a pronounced cortical response. These results are clear indication that both the state of arousal and the behavioral context impact on the transfer of sensory messages along the vibrissa-to-barrel pathway. The results of anatomical and physiological studies indicate that gating occurs at the first relay station in brainstem, via inhibitory projections from the spinal trigeminal complex to the principal trigeminal nucleus [30, 31]. In the olfactory system, however, there is no clear evidence that a central signal related to sniffing gates sensory inputs [32].
Figure 4. State-dependent gating of vibrissa responses in barrel cortex.
(a) Vibrissa stimuli (arrowheads) were delivered randomly during recording from a layer 4 barrel neuron. Intracellular recordings show that the depolarizing sensory response is strongly reduced during active whisking (red) compared to during quiescent wakefulness (blue). Adapted from Crochet and Petersen [23]. (b) The above state-dependent reduction in sensory processing can be imaged with voltage-sensitive dye. Passively evoked sensory responses during quiescent wakefulness have large amplitude and spread across large cortical areas, whereas the response is almost suppressed during whisking. The red square on the images at 0 ms indicates the region of interest centered on the C2 cortical column from which voltage-sensitive dye fluorescence changes are quantified in the adjacent traces (lower right). Adapted from Ferezou et al. [28].
Context- and state-dependent sniffing and whisking modes
There is evidence to suggest that the expression of both whisking and sniffing is dependent on the motivational state and behavioral goals of the animal. Analysis of sniffing in rats performing a go-nogo odor discrimination task reveal two modes of sniffing: a 7 to 8 Hz sniffing mode associated with odor sampling, and a 10 to 12 Hz sniffing mode in anticipation of reward delivery [33]. Correspondingly, several other studies report that sniffing is associated with reward anticipation. For example, sniffing becomes part of the conditioned response as rats learn to predict the occurence of a reward based on a sensory cue [34, 35]. Electrical stimulation of brain regions whose activation is intrinsically rewarding, such as the lateral hypothalamus, elicits exploratory behaviors as well as high-frequency sniffing [36]. Although whisking was not quantified in these latter studies, the authors incidentally report that whisking was associated with sniffing. Further, in a situation where rewarding brain stimulation is repeatedly delivered at fixed intervals, regardless of response, rats eventually exhibit vigorous sniffing just prior to the delivery of the stimulation [37].
The context of exploratory behavior can shape how sensory input is processed in the nervous system. In a study in which rats whisked in air either spontaneously or for a reward, oscillations in the local field potential in primary sensory cortex showed drastically increased coherence with the whisking rhythm when the rats were motivated by the reward [38]. This occurred without an increase in field potential amplitude and thus implies a decrease in the variability of the cortical response under conditions of a near-term reward, possibly as a result of increased attention to salient features in the environment.
As with sniffing, different whisking frequencies could subserve different behavioral goals. First, a wide range of whisking frequencies, i.e., 2 to 20 Hz, was reported in an early study of texture discrimination [39]. Later work showed that whisking could be divided into two overall frequency ranges, each with a different pattern and associated behavior [7]. Large amplitude whisks at 8 to 9 Hz are associated with exploration, while a second pattern of whisking, termed foveal whisking, occurs when the animal is perched and the vibrissae are thrust forward and stretched toward an object of interest. This latter pattern consists of relatively low-amplitude whisks in the frequency range of 15 to 25 Hz, and are reminiscent of 25 Hz oscillatory muscle activity that occurs during the hold period of a precision grip task in monkey [40]. For the moment it is unresolved whether fast whisks are part of a scanning motor strategy, or whether they are rhythmic events related to a strategy of maintaining vibrissae in a protracted position.
The above observations suggest that sniffing and whisking not only serve odor sampling and touch, but they also constitute activity patterns that are the overt expression of reward expectation, and thus serve to automatically sample and identify objects of potential interest. This motivates the question of whether there are specific behavioral contexts in which whisking and sniffing are related. When whisking and sniffing occur together, they appear to be synchronous [2] (asterisk in Fig. 1c), yet no study has examined the precise conditions under which the two rhythms couple. Casual observations suggest that rats sniff without whisking when the object of interest is near the nose, and can be inspected with the microvibrissae in conjunction with head and forepaw movements. Beyond behavioral contexts, it is of theoretical interest to understand how two CPGs phase lock and decouple in a dynamic manner that depends on their synaptic interactions and the action of modulators that control emotional and motivational states [41]. Such an understanding of these mechanisms could provide insight into the nature of coupling of other rhythmic behaviors to respiration [42].
Why do rodents sniff and whisk?
Although sniffing clearly subserves olfaction and strongly patterns olfactory processing, several studies reported that basal respiratory rhythm is sufficient for delivering odorants to olfactory receptors, and that rats and mice can perform simple odor discrimination after a single sniff [32, 43, 44]. Likewise, rodents can locate objects and gauge aperture widths with a single whisk and, rather dramatically, severing the facial nerve to block whisking does not affect performance [45, 46]. Lastly, rodents can perform many tactile tasks without whisking per se, either by using head and body movements to move the vibrissae or by maintaining their vibrissae still in a region of interest where contact or stimulation is expected [46, 47].
Given the above evidence that smelling can occur without sniffing and vibrissa touch can be effective without whisking, the questions remain as to why rodents sniff and why they whisk. There are likely to be multiple answers. First, since rodents have relatively limited visual capabilities and are the targets of predators, fast sampling of the immediate environment has clear survival value [48]. The few extra hundreds of milliseconds of warning that are gained by scanning the environment may be sufficient for escape. Second, since rodents live in burrows and locomote in a bumpy, cluttered environment, they must rely on tactile inputs to look ahead and avoid obstacles. The speed of locomotion could account for variability in the whisking frequency given the monotonic relation between whisking frequency and the speed of locomotion across species that whisk [49]. Finally, sniffing and whisking not only serve odor sampling and touch, but also represent the overt expression of an animal's interest in its environment. In this regard, casual observation suggests a marked difference in behavior between strains of rats, with Long Evans rats being much more enthusiastic sniffers and whiskers than their Sprague Dawley congeners. The use of transgenic mice, in which the effects of neuromodulators are up or down regulated, might be useful models to understand the neural basis of the drive to sniff and whisk.
Conclusions
Many rhythmic behaviors in rodents have been reported to alternately couple and decouple to breathing. These include whisking and head movements [2], licking, mastication, and swallowing [42]. These rhythmic activities are all generated by pattern generators located in the brainstem. The case of sniffing and whisking is of particular interest as these behaviors often occur spontaneously, are phase locked, and are related to the sensory exploration of the immediate environment. From a practical standpoint, they can be controlled by classical and operant conditioning. The accessibility of the vibrissa system for electrophysiological studies and the use of high-speed videography and indwelling sensors for electromyography, air flow, and related measurements make the rodent an ideal system to understand the neural mechanisms that underly the generation of these rhythms. Such studies may further provide a framework for the nature of the circuitry, the modulation of sensory inputs, and the computations that underly coupling between other brainstem-controlled rhythmic behaviors.
Beyond sensory coding, sniffing and whisking constitute overt expression of the emotional and motivational state of rodents. A detailed knowledge of these behaviors could provide simple metrics for assessing the animal's subjective valuation of objects and experiences. Such metrics would be of particular interest, for instance, to assess motor and motivational deficits in animal models of neuropsychiatric disorders.
Highlights.
Sniffing and whisking in rodents serve not only to gather olfactory and tactile information, but are also the overt expression of reward expectation.
These rhythmic activities affect both the encoding and central processing of sensory inputs.
Because sniffing and whisking exhibit a fixed temporal relationship to one another, it is suggested that both rhythms share a common neural circuitry.
Acknowledgements
We thank Adam Kepecs and Dima Rindberg for discussions that shaped this review, and the Canadian Institutes of Health Research (grant MT-5877), the National Institutes of Health (grants NS058668 and NS066664), and the US-Israeli Binational Foundation (grant 2003222) for their support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benjamini Y, Fonio E, Galili T, Havkin GZ, Golani I. Quantifying the buildup in extent and complexity of free exploration in mice. Proc Natl Acad Sci USA. 2011;108:15580–15587. doi: 10.1073/pnas.1014837108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welker WI. Analysis of sniffing of the albino rat. Behavior. 1964;22:223–244. [Google Scholar]
- 3.Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- 4.Sherrey JH, Megirian D. State dependence of upper airway respiratory motoneurons: functions of the cricothyroid and nasolabial muscles of the unanesthetized rat. EEG Clin Neurophysiol. 1977;43:218–228. doi: 10.1016/0013-4694(77)90129-8. [DOI] [PubMed] [Google Scholar]
- 5.Hwang JC, St John WM. Respiratory-modulated activities of motor units of the facial nerve. Respir Physiol. 1988;73:189–200. doi: 10.1016/0034-5687(88)90066-7. [DOI] [PubMed] [Google Scholar]
- 6.Huangfu D, Koshiya N, Guyenet PG. Central respiratory modulation of facial motoneurons in rats. Neurosci Letts. 1993;151:224–228. doi: 10.1016/0304-3940(93)90025-g. [DOI] [PubMed] [Google Scholar]
- 7.Berg RW, Kleinfeld D. Rhythmic whisking by rat: retraction as well as protraction of the vibrissae is under active muscular control. J Neurophysiol. 2003;89:104–117. doi: 10.1152/jn.00600.2002. [DOI] [PubMed] [Google Scholar]
- 8.Hill DN, Bermejo R, Zeigler HP, Kleinfeld D. Biomechanics of the vibrissa motor plant in rat: rhythmic whisking consists of triphasic neuromuscular activity. J Neurosci. 2008;28:3438–3455. doi: 10.1523/JNEUROSCI.5008-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 10.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semba K, Komisaruk BR. Neural substrates of two different rhythmical vibrissal movements in the rat. Neuroscience. 1984;12:761–774. doi: 10.1016/0306-4522(84)90168-4. [DOI] [PubMed] [Google Scholar]
- 13.Gao P, Bermejo R, Zeigler HP. Whisker deafferentation and rodent whisking patterns: behavioral evidence for a central pattern generator. J Neurosci. 2001;21:5374–5380. doi: 10.1523/JNEUROSCI.21-14-05374.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattox A, Li Y, Keller A. Serotonin regulates rhythmic whisking. Neuron. 2003;39:343–352. doi: 10.1016/s0896-6273(03)00391-x. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe J, Mende C, Brecht M. Social facial touch in rats. Beh Neurosci. 2011 doi: 10.1037/a0026165. in press. [DOI] [PubMed] [Google Scholar]
- 16.Haidarliu S, Simony E, Golomb D, Ahissar E. Muscle architecture in the mystacial pad of the rat. Anat Rec. 2010;293:1192–1206. doi: 10.1002/ar.21156. [DOI] [PubMed] [Google Scholar]
- 17.Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. doi: 10.1016/s0166-4328(97)83328-1. [DOI] [PubMed] [Google Scholar]
- 18.Leiser SC, Moxon KA. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron. 2007;53:117–133. doi: 10.1016/j.neuron.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Khatri V, Bermejo R, Brumberg JC, Keller A, Zeigler HP. Whisking in air: encoding of kinematics by trigeminal ganglion neurons in awake rats. J Neurophysiol. 2009;101:1836–1846. doi: 10.1152/jn.90655.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron. 2010;68:570–585. doi: 10.1016/j.neuron.2010.09.040. [By recording spike trains from the olfactory bulb in awake, behaving rats this study shows that odor coding is invariant to changes in respiration frequency. This supports the notion that olfactory stimuli are encoded according to the phase of sniffing.] [DOI] [PubMed] [Google Scholar]
- 21.Shusterman R, Smear MC, Koulakov AA, Rindberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- 22.Fee MS, Mitra PP, Kleinfeld D. Central versus peripheral determinants of patterned spike activity in rat vibrissa cortex during whisking. J Neurophysiol. 1997;178:1144–1149. doi: 10.1152/jn.1997.78.2.1144. [DOI] [PubMed] [Google Scholar]
- 23.Crochet S, Petersen CC. Correlating whisker behavior with membrane potential in barrel cortex of awake mice. Nat Neurosci. 2006;9:608–610. doi: 10.1038/nn1690. [DOI] [PubMed] [Google Scholar]
- 24••.Curtis JC, Kleinfeld D. Phase-to-rate transformations encode touch in cortical neurons of a scanning sensorimotor system. Nat Neurosci. 2009;12:492–501. doi: 10.1038/nn.2283. [Unit recordings in barrel cortex during whisking show that the touch response is greatest at a particular phase of the whisk cycle, suggesting that the reafferent signal associated with the active movement of the whiskers gates cortical activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Kock CP, Sakmann B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc Natl Acad Sci U S A. 2009;106:16446–16450. doi: 10.1073/pnas.0904143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Kleinfeld D, Deschênes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron. 2011;72:455–68. doi: 10.1016/j.neuron.2011.10.009. [This review examines how rodents locate objects with their whiskers. It is proposed that neurons in barrel cortex receive reafferent input related to the phase of the whisking cycle, while motor cortex provides information about the amplitude and set point of whisking.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27••.Smear M, Shusterman R, O'Connor R, Bozza T, Rindberg D. Perception of sniff phase in mouse olfaction. Nature. 2011;479:397–400. doi: 10.1038/nature10521. [Electrophysiological recordings in the olfactory bulb of awake mice show that mitral cells encode the timing of photoactivation in relation to the sniff in both the timing and the amplitude of their responses. The authors further show that mice can behaviorally report the sniff phase of optogenetically driven activation of olfactory sensory neurons.] [DOI] [PubMed] [Google Scholar]
- 28.Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 29.Hentschke H, Haiss F, Schwarz C. Central signals rapidly switch tactile processing in rat barrel cortex during whisker movements. Cereb Cortex. 2006;16:1142–1156. doi: 10.1093/cercor/bhj056. [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci. 2008;12:1430–1438. doi: 10.1038/nn.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta T, Timofeeva E, Nakamura K, Okamoto-Furuta K, Togo M, Kaneko T, Deschênes M. Inhibitory gating of vibrissal inputs in the brainstem. J Neurosci. 2008;28:1789–1797. doi: 10.1523/JNEUROSCI.4627-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [This review discusses the role of active sensing in shaping olfactory system function at multiple levels and draws parallels with other sensory modalities, particularly whisking.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem. Senses. 2006;31:167–179. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- 34.Bindra D, Campbell JF. Motivational effects of rewarding intracranial stimulation. Nature. 1967;215:375–376. doi: 10.1038/215375a0. [DOI] [PubMed] [Google Scholar]
- 35.Clarke S, Trowill JA. Sniffing and motivated behavior in the rat. Physiol Behav. 1971;6:49–52. doi: 10.1016/0031-9384(71)90013-8. [DOI] [PubMed] [Google Scholar]
- 36.Ikemoto S, Panksepp J. The relationship between self-stimulation and sniffing in rats: does a common brain system mediate these behaviors? Behav Brain Res. 1994;61:143–162. doi: 10.1016/0166-4328(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 37.Waranch HR, Terman M. Control of the rat's sniffing behavior by response-independent and dependent schedules of reinforcing brain stimulation. Physiol Behav. 1975;15:365–370. doi: 10.1016/0031-9384(75)90105-5. [DOI] [PubMed] [Google Scholar]
- 38.Ganguly K, Kleinfeld D. Goal-directed whisking increases phase-locking between vibrissa movement and electrical activity in primary sensory cortex in rat. Proc Natl Acad Sci USA. 2004;101:12348–12353. doi: 10.1073/pnas.0308470101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvell GE, Simons DJ. Biometric analyses of vibrissal tactile discrimination in the rat. J Neurosci. 1990;10:2638–2648. doi: 10.1523/JNEUROSCI.10-08-02638.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J Physiol. 1977;501:225–241. doi: 10.1111/j.1469-7793.1997.225bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grannan E, Kleinfeld D, Sompolinsky H. Stimulus dependent synchronization of neuronal assemblies. Neural Computation. 1993;5:550–569. [Google Scholar]
- 42.Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neurosci Biobehav Rev. 1997;5:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- 43.Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;11:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- 44.Kepecs A, Uchida N, Mainen ZF. Rapid and precise control of sniffing during olfactory discrimination in rats. J Neurophysiol. 2007;98:205–213. doi: 10.1152/jn.00071.2007. [DOI] [PubMed] [Google Scholar]
- 45.Krupa DJ, Matell MS, Brisben AJ, Oliveira LM, Nicolelis MA. Behavioral properties of the trigeminal somatosensory system in rats performing whisker-dependent tactile discriminations. J Neurosci. 2001;21:5752–5763. doi: 10.1523/JNEUROSCI.21-15-05752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Connor DH, Peron SP, Huber D, Svoboda K. Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron. 2010;67:1048–1061. doi: 10.1016/j.neuron.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 47.Hutson KA, Masterton RB. The sensory contribution of a single vibrissa's cortical barrel. J Neurophysiol. 1986;56:1196–1223. doi: 10.1152/jn.1986.56.4.1196. [DOI] [PubMed] [Google Scholar]
- 48.Wesson DW, Verhagen JV, Wachowiak M. Why sniff fast? The relationship between sniff frequency, odor discrimination, and receptor neuron activation in the rat. J Neurophysiol. 2009;101:1089–1102. doi: 10.1152/jn.90981.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchinson B, Grant RA, Arkley KP, Rankov V, Perkn I, Prescott TJ. Active vibrissal sensing in rodents and marsupials. Phil Trans Royal Soc Lond B - Biol Sci. 2011;336:3037–3048. doi: 10.1098/rstb.2011.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [ref. fig. 2d] Fox CW, Mitchinson B, Pearson MJ, Pipe AG, Prescott TJ. Contact type dependency of texture classification in a whiskered mobile robot. Autonomous robots. 2009 Doi :1007/s10514-009-9109-z.