Abstract
Tissue preconditioning, whereby various short term stressors initiate organ resistance to subsequent injury, is well recognized. However, clinical preconditioning of the kidney for protection against acute kidney injury (AKI) has not been established. Here we tested whether a pro-oxidant agent, iron sucrose, combined with a protoporphyrin (Sn protoporphyrin), can induce preconditioning and protect against acute renal failure. Mice were pre-treated with iron sucrose, protoporphyrin, cyanocobalamin, iron sucrose and protoporphyrin, or iron sucrose and cyanocobalamin. Eighteen hours later, ischemic-, maleate-, or glycerol-models of AKI were induced and its severity was assessed the following day (blood urea nitrogen, plasma creatinine concentrations; post-ischemic histology). Agent impact on cytoprotective gene expression (heme oxygenase 1, hepcidin, haptoglobin, hemopexin, α1-antitrypsin, α1-microglobulin, IL-10) was assessed as renal mRNA and protein levels. AKI-associated myocardial injury was gauged by plasma troponin I levels. Combination agent administration up-regulated multiple cytoprotective genes, and unlike single agent administration, conferred marked protection against each tested model of acute renal failure. Heme oxygenase was shown to be a marked contributor to this cytoprotective effect. Preconditioning also blunted AKI-induced cardiac troponin release. Thus, iron sucrose and protoporphyrin administration can up-regulate diverse cytoprotective genes and protect against acute renal failure. Associated cardiac protection implies potential relevance to both AKI and its associated adverse downstream effects.
Keywords: acute kidney injury, nephrotoxicity, ischemia reperfusion
INTRODUCTION
Despite great advancements in our understanding of AKI pathogenesis, no effective prophylaxis for it currently exists. The need for such a therapy is underscored by a plethora of data indicating that AKI increases morbidity, mortality, and can initiate the onset of progressive renal disease (1-7). One potentially promising prophylactic approach could be the administration of agents that up-regulate cytoprotective proteins in kidney (e.g., heme oxygenase 1, haptoglobin, IL-10). Indeed, a plethora of experimental data support the concept that such redox sensitive ‘stress proteins’ can exert dramatic renal protective effects (reviewed in ref. 8, 9). However, the most effective and safest way(s) of inducing these proteins in kidney remains to be defined.
We recently reported that administration of a sub-toxic dose of nitrited myoglobin (NMgb), in combination with Sn protoporphyrin, can safely and synergistically up-regulate potent cytoprotective proteins (e.g., HO-1, haptoglobin, IL-10) in mouse kidney (8). Thus, by 18 hrs post agent administration, striking protection against both ischemia-reperfusion and toxic (maleate, glycerol) ARF was induced. Furthermore, these same cytoprotective genes were activated in extra-renal organs (most notably in liver). As a result, marked protection against ischemic and toxic hepatic injuries was expressed (8). The rationale for the above pharmacologic approach was as follows: 1) heme proteins, such as myoglobin, are potent inducers of redox sensitive cytoprotective genes (8-11); 2) nitrite binding to myoglobin Fe decreases its cytotoxic potential (8,12-15); 3) concomitant Sn protoporphyrin administration enhances nitrite-myoglobin signaling (8); and 4) SnPP can independently up-regulate HO-1 and haptoglobin expression, and synergize nitrited myoglobin-mediated cytoprotective gene induction (8,16).
Despite the observed efficacy and safety of the above approach (8), theoretical reservations might exist vis à vis the use of a potential nephrotoxin (myoglobin) as a renal cytoprotective agent. Furthermore, although SnPP has been safely administered to patients (e.g., to mitigate neonatal jaundice), no clinically available Sn protoporphyrin preparation exists. Thus, the present study was undertaken to address the following two questions: 1) Can a widely used Fe containing macromolecule, iron sucrose (FeS; Venofer), be substituted for nitrited myoglobin in the above prophylactic strategy? and 2) Can a readily available protoporphyrin (the vitamin B12 analogue, cyanocobalamin) be substituted for SnPP and used with FeS in a renal prophylaxis strategy? These two issues formed the basis of this report.
RESULTS
BUN and plasma creatinine levels at 4 and 18 hrs post agent administration
BUN and PCr values for normal mice were 24±2 and 0.31±0.01 mg/dl. None of the test agents, used either alone or in combination, induced any significant changes in these BUN / PCr levels.
Effects of FeS / SnPP on the severity of AKI
Maleate- induced AKI (ref. 17,18)
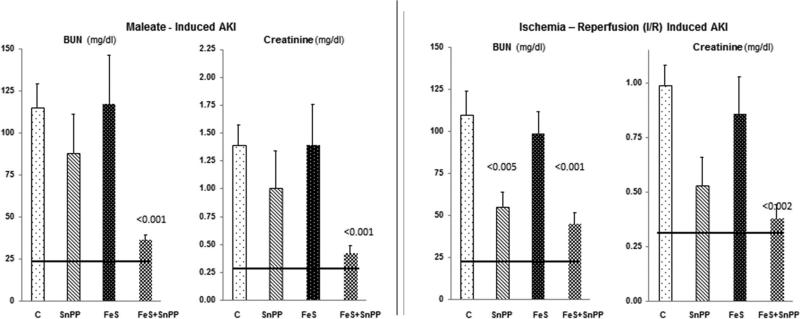
Maleate injection into control (C) mice caused severe AKI as denoted by marked BUN and PCr increases (Fig. 1, left). Neither SnPP alone nor FeS alone significantly reduced injury severity. However, combined FeS+SnPP conferred marked protection, denoted by ~75% decreases in BUN/PCr concentrations.
Figure 1. Severity of AKI without and with single or combined FeS/SnPP pre-treatment.
Left panel: Neither test agent alone induced significant protection against maleate- induced AKI. Conversely, when used together, marked protection against AKI resulted (p values vs. maleate alone). The horizontal lines represent mean BUN and creatinine concentrations for normal mice.
Right panel: Ischemia-reperfusion induced marked BUN and creatinine increases which were significantly reduced by either SnPP or FeS+SnPP pre-treatment. (p values vs. I/R alone). The degree of protection was modestly greater with combined pre-treatment, as judged by lower plasma creatinine concentrations (0.53 ± 013 vs. 038 ±0.06 mg/dl; SnPP vs FeS+SnPP, respectively; <0.05).
Renal ischemia-reperfusion (IRI)- induced AKI
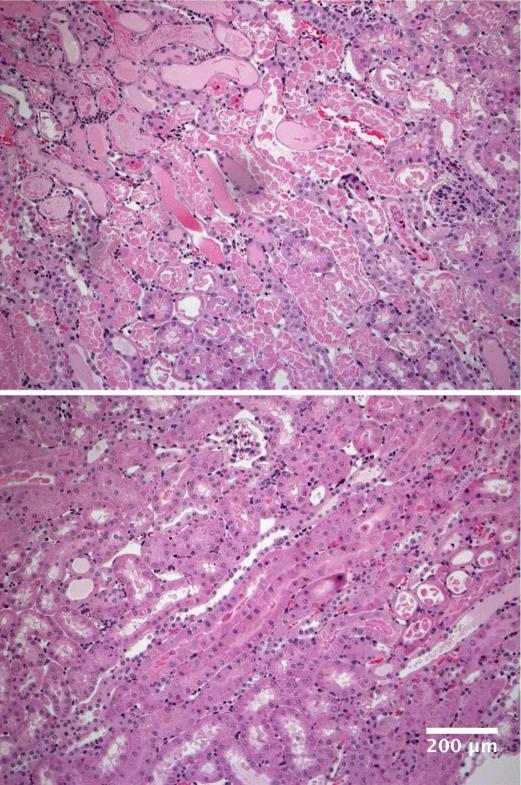
Within 18 hrs of inducing IRI in control mice, ~4 fold elevations in BUN and PCr concentrations resulted (Fig. 1, right panel). Pre-treatment with FeS+SnPP conferred significant protection, lowering the PCr to near normal levels. SnPP alone, but not FeS alone, exerted a modest independent protective effect. Renal protection was also denoted by a marked diminution of histologic injury (tubule necrosis, cast formation; p<0.01), as presented in Fig. 2.
Figure 2. Renal morphologic injury induced by ischemia– reperfusion under control conditions or with FeS+SnPP administration.
By 18 hrs post ischemia-reperfusion, extensive proximal tubule necrosis and cast formation in the inner cortex/outer medullary stripe were observed in unconditioned mice (panel A). The extent of that injury was markedly reduced in FeS+SnPP conditioned mice (panel B). The scale bar = 200 microns. The extent of injury was graded on a scale of 1-4+ (least to most severe injury observed), and was significantly decreased by FeS+SnPP pre-treatment (3.5±0.3; 1.5±0.3; <0.01).
Effects of FeS / CCB on AKI severity
Maleate induced AKI
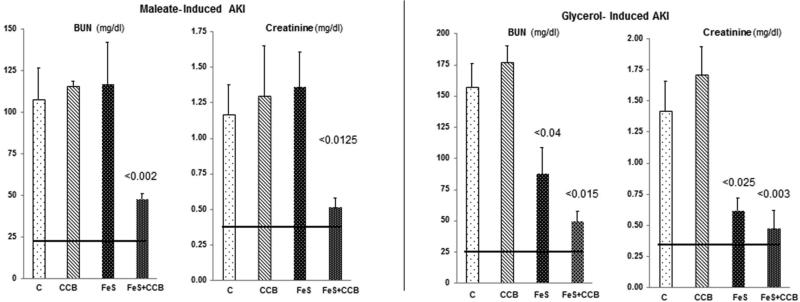
Again, maleate injection induced severe AKI in control mice (Fig. 3, left panel). Pre-treatment with either FeS alone or CCB alone failed to alter AKI severity. Conversely, combination FeS+CCB induced marked renal protection, as denoted by dramatic BUN/ PCr reductions.
Figure 3. Severity of AKI without and with single or combined FeS/CCB pre-treatment.
Left Panel. Neither test agent alone mitigated the severity of maleate- induced AKI. Conversely, marked protection was induced by combination FeS+CCB treatment (p vs. maleate alone).
Right panel. Glycerol injection induced severe AKI. FeS pre-treatment conferred modest protection, lowering the BUN from ~150 to 75 mg/dL. This protection was enhanced when combined with CCB pretreatment. CCB exerted no independent protective effect.
Glycerol model of AKI
Severe renal failure resulted within 18 hrs of glycerol injection into control mice (Fig. 3, right panel). FeS+CCB conferred substantial protection, as denoted by marked reductions in both 18 hr BUN/PCr concentrations. FeS alone also exerted an independent protective effect, albeit not as great as that seen with combined FeS + CCB administration.
Glycerol AKI induction 3 days post preconditioning
Three days following FeS/CCB administration, significant protection against glycerol AKI was still observed (BUNs: 86±32 vs. 154±4 mg/dl; p<0.05; plasma creatinine concentrations, 1.04±0.43 vs. 1.80±0.18 p <0.03). However, it was less pronounced than the protection with the one day post FeS/CCB preconditioning, as shown in Fig. 3.
Renal cortical HO-1 and haptoglobin mRNA and protein levels with FeS / SnPP treatment
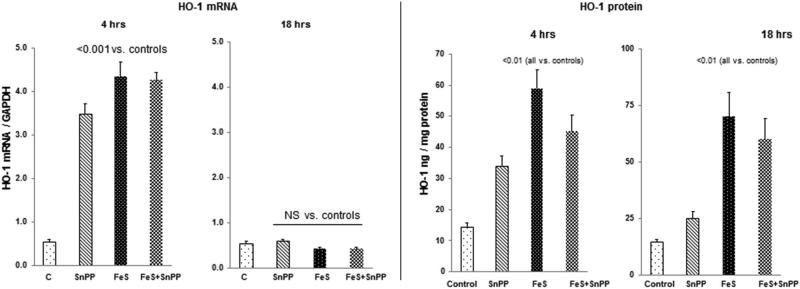
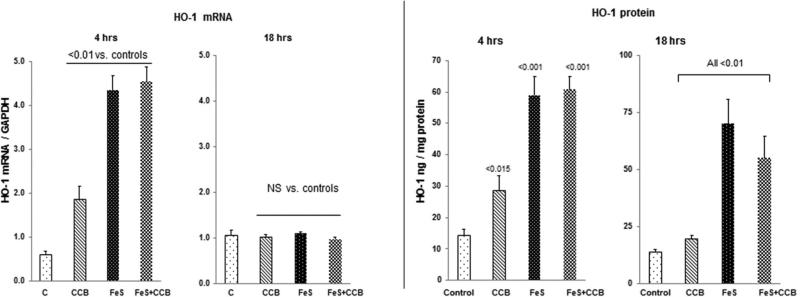
HO-1 mRNA
As shown in Fig. 4, left panel, FeS and SnPP each caused dramatic increases in renal cortical HO-1 mRNA levels by 4 hrs post injection. However, when administered together, no additive HO-1 mRNA increase was observed. By 18 hrs post agent injection, HO1 mRNA levels had returned to normal levels for all treatment groups.
Figure 4. Renal cortical HO-1 mRNA and protein levels following FeS / SnPP administration.
As shown in the left hand panel, both FeS + SnPP induced marked elevations in HO-1 mRNA levels at 4 hrs post injection. However, no additive mRNA elevations were observed. By 18 hrs, the HO-1 mRNA levels had returned to control levels. As shown in the right hand panel, both FeS + SnPP markedly increased HO-1 protein levels, as assessed at both the 4 and 18 hr time points. As with the mRNAs, no additive HO-1 protein increases were observed.
HO-1 Protein
As shown in Fig. 4, right panel, a correlate of the 4 hr mRNA increases were significant increases in HO-1 protein levels. These remained significantly elevated at the 18 hr time point, particularly with FeS alone or the FeS+SnPP combination. No additive FeS/SnPP effects on HO-1 protein levels were observed.
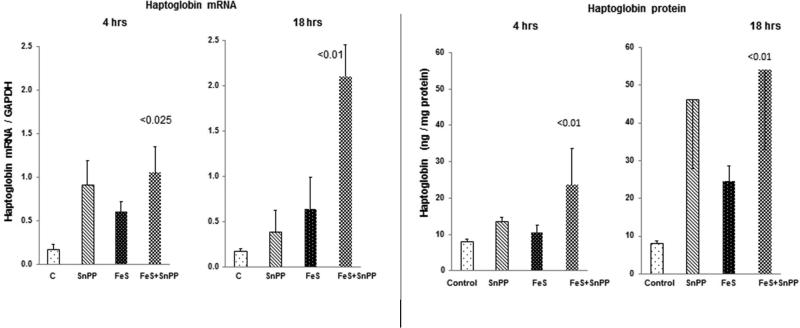
Haptoglobin mRNA
As shown in Fig. 5 left panel, at 4 hrs post administration, SnPP alone, FeS alone, and combined FeS+SnPP, modestly raised renal cortical haptoglobin mRNA levels. At 18 hrs post agent administration, a synergistic FeS/SnPP mediated haptoglobin mRNA increase was observed (greater than with either agent alone; p<0.01).
Figure 5. Renal cortical haptoglobin mRNA and protein levels following FeS / SnPP administration.
Each test agent increased haptoglobin mRNA, and at 18 hrs, a synergistic increase was observed (left hand panel). As shown in the right hand panel, progressive haptoglobin protein elevations developed, reaching 5 fold greater than control levels at the 18 hr time point. Of note, the haptoglobin protein levels varied considerably and followed a non parametric distribution. Hence, they were analyzed vs. control values by Wilcoxon rank sum test. Not shown, renal cortical haptoglobin and plasma haptoglobin levels were tightly correlated (r, 0.92), indicating that plasma haptoglobin levels could potentially serve as a biomarker for renal haptoglobin gene induction.
Haptoglobin protein
At the 4 hr time point, only the combined therapy caused a significant rise in renal cortical haptoglobin levels (Fig. 5, right panel). By 18 hrs, all three treatments led to cortical haptoglobin increases, with the greatest elevations being observed with combined FeS+SnPP treatment.
Renal cortical HO-1 and haptoglobin mRNA and protein levels with FeS / CCB treatment
HO-1 mRNA
As shown in Fig. 6 (left panel), each of the test agents alone and in combination caused significant increases in HO-1 mRNA levels at 4 hrs. However, by 18 hrs, all of the values reverted to normal (recapitulating the findings with FeS+SnPP).
Figure 6. Renal cortical HO-1 mRNA and protein levels following FeS / CCB treatment.
Both FeS and CCB independently raised HO-1 mRNA at 4 hrs, and returned to control values at the 18 hr time point. Conversely, an early (4 hrs) and sustained (18 hrs) increase in HO-1 protein levels was observed. In neither the case of the mRNA or protein levels did additive increases occur with combined treatment.
HO-1 protein
Corresponding with the 4 hr HO-1 mRNA increases were significant increases in HO-1 protein (Fig. 6, right). Unlike the 18 hr HO-1 mRNA levels that reverted to normal at 18 hrs, significant increases in HO-1 protein levels persisted to the 18 hr time point, particularly with FeS or Fe+CCB treatment.
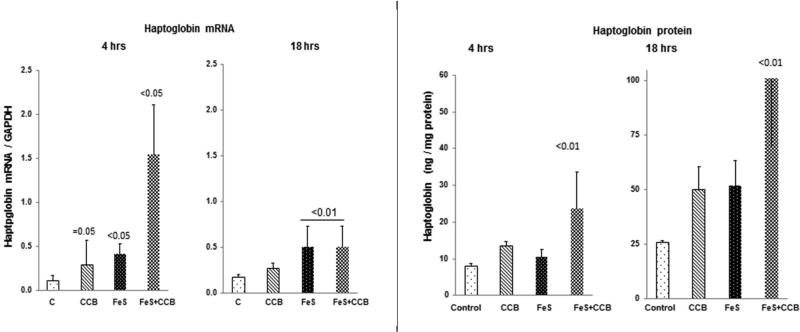
Haptoglobin mRNA
CCB alone and FeS alone each caused small but significant increases in haptoglobin mRNA at the 4 hr but not at the 18 hr time point (Fig. 7 left). Conversely, combined FeS+CCB caused dramatic haptoglobin mRNA increases. By 18 hrs post FeS or FeS+CCB injection, modest haptoglobin mRNA increases were still observed.
Figure 7. Renal cortical haptoglobin mRNA and protein levels following FeS / CCB treatment.
Combined FeS + CCB caused a synergistic increase in haptoglobin mRNA, observed at the 4 hr time point (left panel). By 18 hrs, both FeS and CCB raised haptoglobin proteins levels, effects that were additive with combined therapy.
Haptoglobin protein
FeS+CCB treatment evoked significant haptoglobin increases at both 4 hrs and 18 hrs post injection (Fig. 7, right). These exceeded the values observed with single agent injection.
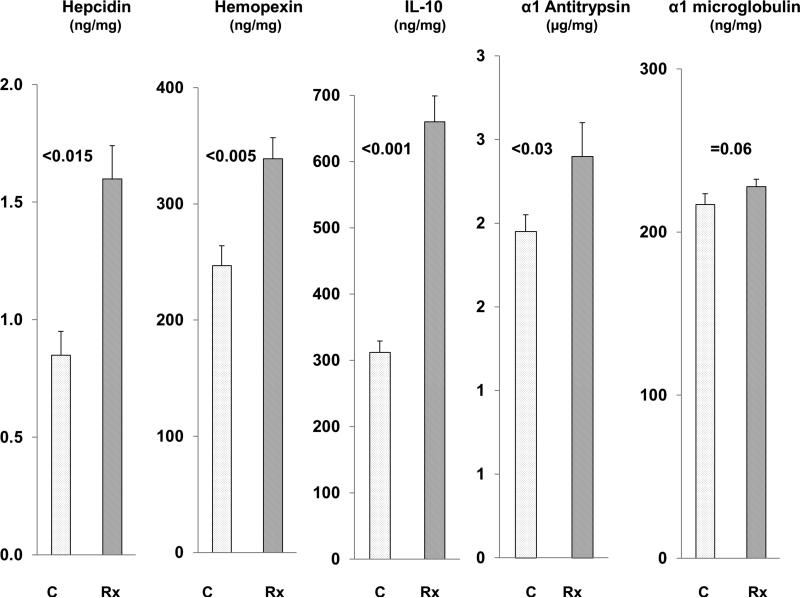
Alpha 1 antitrypsin, hemopexin, α1 microglobulin, hepcidin, and IL-10 expression post FeS+SnPP treatment
To assess whether a potentially more broad based increase in cytoprotective genes might result from Fe/protoporphyrin administration, protein levels for the above noted cytoprotective genes were assessed 18 hrs post FeS+SnPP injection. As shown in Fig. 8, with the exception of α1 microglobin which showed a borderline increase, each of the remaining 4 proteins was significantly increased at the 18 hr time point (i.e., the point in time at which superimposed AKI was induced in the previous experiments). To assess relative individual contributions of FeS or SnPP in this induction, their individual and combined effects of these agents on cognate mRNAs were assessed. As shown in Table 1, combination agent administration raised the mRNAs for hepcidin, hemopexin, α1 antitrypsin and α1 microglobulin at both the 4 and 18 hr time points, with these increases typically being greater than those seen with either FeS or SnPP alone. Surprisingly, IL-10 mRNA increases were not observed, despite a doubling of renal cortical IL-10 protein levels. This suggests that the increased IL-10 levels may have arisen from increased mRNA translation, a widely recognized determinant of IL-10 production (8).
Figure 8. FeS + SnPP up-regulate the expression of diverse cytoprotective proteins.
Hemopexin, hepcidin, IL-10, α1 antitrypsin, and α1 microglobulin protein levels in renal cortex were measured by ELISA 18 hrs following FeS+SnPP administration. With the exception of α1 microglobulin, increased renal cortical protein levels of each were observed.
Table 1.
mRNAs at 4 and 18 hrs of FeS, SnPP, or Fes+SnPP treatment
| Control mRNA | 4 hr FeS | 4 hr SnPP | 4 hr FeS+SnPP | |
|---|---|---|---|---|
| Hepcidin | 0.10 ±0.02 | 0.86±0.54 | 0.23±0.09 | 1.1±0.53** |
| Hemopexin | 0.1±0.02 | 0.41/0.2 | 0.56/0.31 | 0.73±0.32 *** |
| α1 microglobulin | 0.40±0.08 | 0.76/0.13 | 1.03/0.42 | 1.3±0.30* |
| α 1 antitrypsin | 0.95±0.08 | 1.96/0.66 | 2.15/ 0.90 | 2.62±0.93 ** |
| IL-10 | 0.58±0.09 | 0.55±0.29 | 0.29±0.07 | 0.34±0.06 NS |
| Control mRNA | 18 hr FeS | 18 hr SnPP | 18 Hr FeS+SnPP | |
| Hepcidin | 0.10±0.03 | 0.12±0.05 | 0.20±0.09 | 1.47±0.12 *** |
| Hemopexin | 0.11±0.02 | 0.45/0.25 | 0.26/0.16 | 1.01±0.14* |
| α1 microglobulin | 0.41±0.09 | 0.61/0.10 | 0.83/.027 | 1.1±0.21* |
| α1 antitrypsin | 0.91±0.08 | 2.36/1.1 | 2.73/1.5 | 3.86±0.61*** |
| IL-10 | 0.59±0.09 | 0.35±0.09 | 0.65±0.18 | 0.40±0.06 NS |
Either 4 or 18 hrs post administration of FeS, SnPP or FeS+SnPP, the above noted mRNAs in renal cortex were measured (see text). Excepting IL-10 mRNA, all of the other mRNAs were significantly elevated at the 4 and 18 hr time points with combination, but not single agent, treatments. In general, the combination therapy induced greater increases than either agent alone. Values vs. control:
p<0.05
p<0.01
p <0.001.
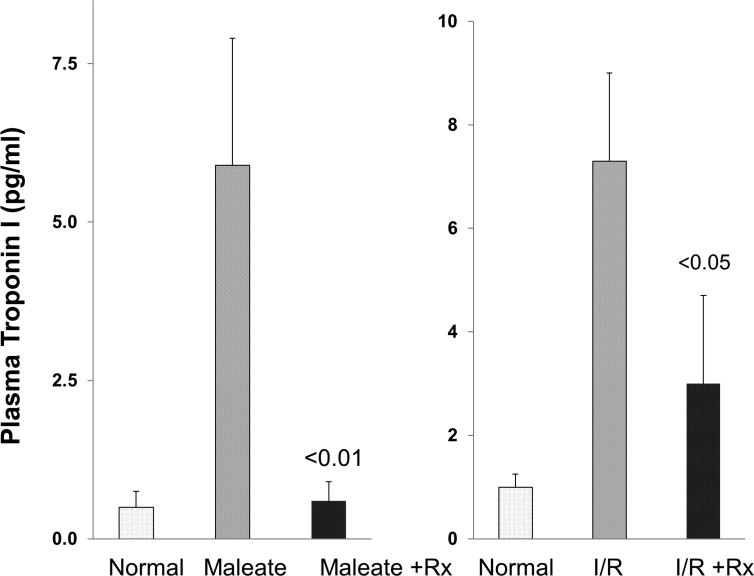
Plasma troponin levels following induction of AKI
By 18 hrs post induction of maleate and I/RI-induced AKI in control mice, ~6-8 fold increases in plasma troponin I levels were observed (Fig. 9). Combined FeS+SnPP pretreatment markedly blunted these troponin increases. This finding was particularly pronounced in the maleate model, where >90% reductions in troponin levels were observed.
Figure 9. AKI induces marked increases in plasma troponin I levels, and these increases are attenuated by FeS+SnPP pretreatment.
Troponin I levels rose ~8 fold by 18 hrs post induction of either maleate or ischemia-reperfusion (I/R) induced AKI. Pre-treatment with FeS+SnPP markedly attenuated these troponin increases, particularly in the case of the maleate AKI model.
Test of whether HO-1 activity is mechanistically involved in FeS+SnPP mediated cytoprotection
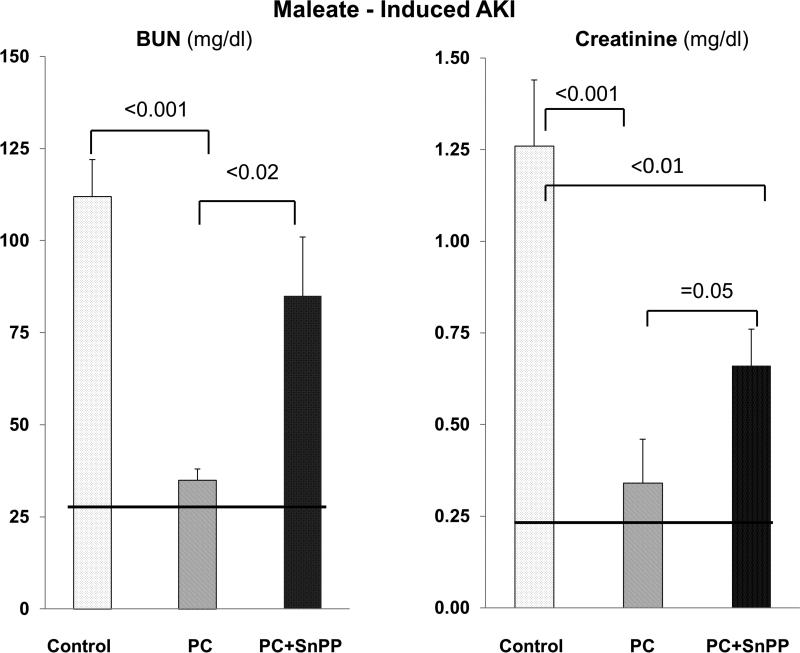
Maleate injection into non conditioned (control) mice caused severe AKI, as depicted in Fig. 10. Preconditioning (PC) with FeS+SnPP conferred marked protection (Fig 10). When a second dose of SnPP was administered into preconditioned mice at the time of maleate injection (to inhibit pre-formed HO-1; see Methods section), a significant diminution of renal protection resulted (Fig. 10). However, some degree of protection persisted, given that both BUN and PCr concentrations remained lower in the preconditioned mice that had received a second SnPP dose vs. untreated maleate controls. Thus, these experiments indicate that increased HO-1 activity is mechanistically important to the induction of the cytoresistant state.
Figure 10. SnPP administered at the time of maleate injection mitigates prior FeS/SnPP mediated preconditioning and resistance to ARF.
Either unconditioned (control) mice or FeS+SnPP preconditioned (PC) mice were challenged with maleate. Immediately following maleate injection, the preconditioned mice were either left alone (n,6), or they received a second SnPP dose (n, 10). Eighteen hrs later, AKI severity was assessed. The preconditioned mice showed marked protection against AKI, but the administration of a second dose of SnPP at the time of maleate injection negated a large part of the preconditioning cytoprotective effect. This supports the concept that increased HO-1 activity is mechanistically involved in the emergence of the cytoresistant state.
Confirming that HO-1 was indeed active at 18 hrs post FeS/SnPP administration, renal cortical and plasma HO-1 enzymatic activity was determined. [Of note, plasma HO-1 levels tightly correlate with intrarenal HO-1 levels; ref. 19)]. Two to three fold increases in HO-1 enzyme activity were observed (plasma: 30±8.9 vs. 8±0.9, p<0.01; tissue: 42±5 vs. 20±1.5, p<0.01 (μg HO-1 activity/ml plasma or μg/mg tissue protein).
DISCUSSION
FeS has become a mainstay in the treatment of anemia of CKD. Although rare episodes of anaphylactoid reactions have occurred following its IV administration, its widespread and repetitive use attests to its overall safety in patients with renal and non renal diseases. As with other Fe containing macromolecules, such as heme proteins, FeS has the capacity to induce transient oxidative stress which can then up-regulate the expression of diverse cytoprotective proteins (e.g., heme oxygenase 1; HO-1, haptoglobin, hemopexin, ferritin) (20-22). Given this property, we previously demonstrated that by 18 hrs post FeS administration in mice, partial renal resistance to the glycerol ARF model emerges (21). However, this protection was relatively weak, and its ability to protect against other forms of AKI was not assessed. We recently demonstrated that administration of another Fe containing macromolecule (nitrited Mgb), in combination with SnPP, induces striking and broad ranging protection against diverse forms of AKI as well as acute liver disease (ischemia/reperfusion, hepatotoxicity) (8). Thus, we have now tested whether IV FeS +/− SnPP, or a clinically available protoporphyrin (the vitamin B12 analogue, CCB), can also up-regulate renal cytoprotective proteins, and thus, induce a broad based renal protected state.
As shown in Fig. 1, when administered alone, FeS had an inconstant effect on AKI: although it decreased the severity of glycerol- induced AKI, it had no discernible impact on I/R-induced ARF. In contrast, when given alone, SnPP conferred partial protection against I/R injury, but it could not mitigate maleate-induced ARF. However, when FeS and SnPP were administered together, dramatic protection against both AKI models resulted. To ascertain whether CCB could be substituted for SnPP, we administered it either alone or in combination with FeS. Eighteen hrs later, protection against both glycerol- and maleate- induced injuries was assessed. Despite the fact that CCB had no independent influence, it increased FeS-mediated protection in both models of ARF (Fig. 2). Finally, that significant protection was also observed when the glycerol challenge was imposed 72 hrs post FeS/CCB administration indicates that the induced preconditioning response was not simply a momentary effect.
HO-1 is widely considered to be a highly potent renal protective protein (8-11). Therefore, to ascertain the impact of our test agents on HO-1 expression, its mRNA and protein levels were determined 4 and 18 hrs post agent administration. As shown in Figs. 4 and 6, FeS, SnPP, and CCB each up-regulated HO-1 mRNA at 4 hrs, and by 18 hrs, the time of cytoresistance, marked increases in HO-1 protein levels were observed. Given that combination therapies were more effective in producing broad based protection against AKI than any single agent, we predicted that combination therapies would produce higher renal cortical HO-1 protein levels than any single agent alone. However, in no case was this observed. This suggests two possibilities: first, that FeS, SnPP, and CCB signal through a shared pathway, thereby obviating additive effects; and second, HO-1 up-regulation is not a complete explanation for the broad based renal protection that followed combination (FeS+SnPP; FeS+CCB) therapy. Had it been the only mediator of protection, then additive HO-1 protein increases should have matched the greater renal protection that followed combination vs. single agent administration.
To further explore the theory that multiple protective pathways are activated by FeS/protoporphyrin therapy, we tested whether an up-regulation of other redox sensitive cytoprotective genes, in addition to HO-1, were induced. To first explore this possibility, haptoglobin gene expression was assessed. Indeed, FeS, SnPP, and CCB each up-regulated haptoglobin mRNA and protein levels, and in general, this effect was greater with combination vs. single agent therapy. We next questioned whether five additional cytoprotective genes, α1 antitrypsin (24), hemopexin (25), α1 microglobulin (26), hepcidin (27), and IL-10 might also have been induced by FeS+SnPP. As shown in Fig. 8, with the exception of α1 microglobulin, increased protein levels for each were observed. Given these findings, it seems quite plausible that combination FeS/protoporphyrin administration can up-regulate diverse cytoprotective pathways which could then act in concert to induce the full expression of the renal cytoresistant state. Clearly, the above test proteins do not represent a complete list of potentially induced cytoprotective pathways. For example, SnPP has been reported to exert anti-apoptotic effects (28), and these have been previously implicated in conferring protection against post ischemic ARF (23).
HO-1 generates cytoprotective molecules, e.g. bilirubin, biliverdin, and carbon monoxide, via its enzymatic cleavage of heme. However, HO-1 may also exert renal protective effects that are independent of its enzymatic activity (23). Thus, we needed to explore whether HO-1 activity was, indeed, involved in the observed cytoprotected state. First, we observed a 2-3 fold increase in HO-1 enzymatic activity at 18 hrs post FeS/SnPP treatment, confirming active HO-1 generation (i.e., vs. production of a ‘dead’ enzyme). Second, we utilized a functional way (29) of determining HO-1's enzymatic participation in the observed cytoresistance. To this end, we preconditioned mice with FeS/SnPP, driving up renal HO-1 protein levels, and then 18 hrs later, we administered SnPP to inhibit the pre-formed HO-1 activity at the time of injury induction. This second SnPP dose produced an approximate 50-75% loss of renal protection, as assessed by BUN and creatinine concentrations (Fig. 10). Thus, this finding is consistent with one of two concepts: first, that an increase in HO-1 activity is, indeed, required for the full expression of FeS/SnPP mediated cytoprotection; and second, given that some protection was still expressed suggests that non HO-1 dependent mediators of protection (e.g., haptoglobin, hemopexin, hepcidin, IL-10) may also have been involved (assuming that the second dose of SnPP completely inhibited HO-1 throughout all of the critical injury phases). Finally, when evaluating the potential mechanistic contributions of individual cytoprotective proteins, it is important to recognize that different injury pathways exist for different inducers / types of ARF. Thus, one cannot assume that a single protectant, e.g., HO-1, is uniquely responsible for inducing protection in divergent forms of ARF.
It has become well recognized that AKI can negatively impact extra-renal organs, such as heart, lung, and brain, via the release of inflammatory cytokines and DAMPs from the injured kidney (30-32). Thus, we questioned whether reducing AKI severity via FeS+SnPP preconditioning could also decrease AKI- associated extra-renal damage. To gain an insight into this issue, we assessed AKI- associated cardiac injury by measuring plasma levels of cardio-specific troponin I in the setting of maleate- and I/R- induced ARF. Each model induced ~8 fold plasma troponin increases, and in each case, preconditioning markedly attenuated this response. This was particularly true in the maleate model where an essentially complete blockade of cardiac troponin increases was observed (Fig. 9). In a previous study, we documented that heme Fe + SnPP up-regulate HO-1 and haptoglobin in heart and liver, as well as in kidney (8). Thus, at least two plausible mechanisms exist by which FeS/SnPP preconditioning could have blunted AKI- associated cardiac troponin release: 1) a diminution of AKI severity, leading to less secondary cardiac injury; and 2) an up-regulation of cytoprotective proteins in heart (e.g., HO-1 and haptoglobin), which then confers a direct cardio-protective effect. Of note, the present findings that FeS/SnPP preconditioning can elicit cardiac protection in the setting of AKI could have substantial clinical relevance, given that cardiovascular dysfunction is a leading cause of death in patients with ARF (32).
In conclusion, the present study suggests that FeS+protoporphyrin pre-treatment could have utility for inducing protection against diverse forms of clinical AKI/ARF. That FeS is a widely accepted therapeutic agent, and that SnPP has already been found to be safe for human use (e.g., 33, 34), underscores the potential for clinical application. The mechanism for the observed protection is dependent in large part on an up-regulation of HO-1. However, a number of other well documented cytoprotective proteins, e.g., hepcidin, haptoglobin, α1 anti-trypsin, and IL-10 may also be involved. Indeed, that multiple protective pathways are induced would seemingly increase the likelihood of success in clinical AKI, where multifactorial precipitating pathways are typically involved (e.g., ischemia, toxins, sepsis). Finally, when considering the current results, it is worth noting that in our previous studies which used N-myoglobin rather than Fe sucrose as the Fe carrier, approximately 2- fold greater HO-1 induction was observed (8). Furthermore, unlike the current studies, we demonstrated that NMgb+SnPP conferred dramatic protection against hepatic ischemic injury (8). Thus, although both pharmacologic approaches induce potent cytoresistance, the preferred set of agents for clinical use remains to be defined. Despite this question, when these two studies are viewed together, it seems abundantly clear that renal Fe delivery systems, in concert with protoporphyrin administration, represents a promising approach for induction of a potent and broad based renal, and potential extra-renal, cytoresistant state.
METHODS
Male CD-1 mice (30-40 grams; Charles River, Wilmington, MA) were used for all experiments. They were housed under standard vivarium conditions with free food and water access. All experiments were approved by the Fred Hutchinson Cancer Research Center IACUC in accordance with NIH guidelines.
Effects of Fe sucrose (FeS) / tin protoporphyrin (SnPP) on AKI severity
Maleate model of AKI
Thirty two mice were subjected to 200 μL tail vein injections of one of the following: 1) vehicle (phosphate buffered saline, PBS; n, 10); 2) 1 mg FeS (American Regent; Shirley, NY; n, 5); 3) 1μmole SnPP (Frontier Scientific, Logan, UT; n, 7), or FeS +SnPP (n, 10). Eighteen hrs later, all mice received an IP injection of Na maleate (800 mg/Kg; in ~500 μl saline, pH 7). Eighteen hrs post maleate injection, the mice were deeply anesthetized with pentobarbital (~50 mg/Kg IP), the abdominal cavities were opened, and blood samples were obtained from the abdominal vena cava. AKI severity was assessed by BUN and plasma creatinine (PCr) concentrations (BioAssay Systems; San Francisco, CA: BUN assay, DIUR-500; Creatinine assay, DICT-500).
Renal ischemic–reperfusion injury (IRI) model of AKI
Mice received 200 μl tail vein injections of either PBS vehicle (n, 13), FeS alone (n, 6), SnPP alone (n, 5), or combined FeS+SnPP (n, 7), as noted above. Eighteen hrs later, the mice were anesthetized with pentobarbital, the abdominal cavities were opened, the renal pedicles were identified, and both were occluded with microvascular clamps. Body temperature was maintained at 36-37°C throughout. Following 22 minutes of bilateral renal ischemia, the clamps were removed, uniform reperfusion was visually confirmed by the loss of tissue cyanosis, and then the abdominal cavities were closed in two layers with silk suture. Eighteen hrs later, the mice were re-anesthetized, the abdominal cavities were re-opened, and terminal blood samples were obtained from the vena cava. AKI severity was determined by BUN and PCr concentrations. Renal histology was also evaluated, as described in Fig. 2 legend.
Effects of FeS and CCB on AKI severity
Glycerol model of AKI
Mice received tail vein injections of either PBS vehicle (n, 6), FeS alone (n, 6); 1 μmole CCB alone (n, 4); or combination FeS + CCB (n, 5; CCB from Alfa Aesar, Ward Hill, MA). Eighteen hrs later, the mice were lightly anesthetized with isoflurane, and then the glycerol model of rhabdomyolysis AKI was induced (9 ml/Kg 50% glycerol, administered in two equally divided IM injections into the hind limbs) (8).Eighteen hrs post glycerol injection, the mice were deeply anesthetized with pentobarbital and terminal vena cava blood samples were obtained. AKI severity was gauged by terminal BUN and PCr concentrations.
The above experiment was repeated in 16 mice (8 preconditioned; 8 non preconditioned), except that a 3 day lapse in time was allowed prior to the glycerol challenge.
Maleate model of AKI
Mice received tail vein injections of PBS alone (n, 9), FeS alone (n, 3), CCB, alone (n, 3), or combination of FeS+ CCB (n, 8). Eighteen hrs later, they received IP maleate injections, as noted above. The severity of AKI was determined 18 hrs post maleate injection by terminal BUN and PCr concentrations.
Effects of FeS, SnPP, and CCB on renal cortical HO-1 and haptoglobin mRNA and protein levels
Mice were injected with FeS alone, SnPP alone, CCB alone, FeS+SnPP, or FeS+CCB (n,10 per group). Ten normal mice provided control kidney samples. Either 4 or 18 hrs later (5 mice per group at each time), they were anesthetized and the kidneys were removed through a midline abdominal incision. The renal cortices were dissected on ice, extracted for protein and mRNA (19,20), and assayed for HO-1 and haptoglobin protein and mRNA levels by ELISA and competitive RT-PCR respectively (19, 35). The mRNA values were factored by simultaneously determined GAPDH levels.
Effects of treatments on renal function in the absence of AKI
BUN and plasma creatinine levels were measured in the 4 hr and 18 hr terminal blood samples obtained at the time of kidney resection for mRNA and protein extraction (see above).
Effect of FeS+SnPP on myocardial troponin I release during AKI
Plasma samples, obtained 18 hrs following maleate- induced AKI and ischemic/reperfusion- induced AKI ± prior FeS+SnPP prophylaxis were assayed for the cardiac specific enzyme, troponin I (Ultra-Sensative mouse cardiac troponin-I; Life Diagnostics, Inc 2010-1-US; Westchester, PA) (n, 7-8 per group).
Effect of FeS +SnPP on additional cytoprotective genes
Alpha 1 anti-trypsin (24), hemopexin (25), α1 microglobulin (26), hepcidin (35), and IL-10 (8) mRNAs were assessed in mouse renal cortical RNA extracts obtained at 4 and 18 hrs post FeS/SnPP administration and compared to values observed in normal kidney samples (n, 5 per group). α1 microglobulin mRNA was assessed by RT-PCR, using the following primer pairs: sense: gcaatctccccatagtccaa; antisense, ctaccctctggcttgcagac. Values were factored by simultaneously measured GAPDH product. To ascertain whether increased protein translation occurred, each of these 5 proteins were measured by ELISA at 18 hr post Fe+SnPP administration (24,25,36).
Test whether HO-1 up-regulation is mechanistically involved in FeS+SnPP - mediated cytoprotection
Ten mice were pre-treated with FeS+SnPP. Eighteen hrs later, IP maleate was administered, along with a second 1 μmole SnPP IV dose (to inhibit pre-formed HO-1 enzyme activity). 18 hrs later, AKI severity was assessed by BUN/ PCr levels. The values were compared to those from control maleate injected mice (n,15), and maleate injected mice preconditioned with FeS/SnPP (n,6).
Measurement of HO-1 enzymatic activity
Plasma and renal cortical extracts were assayed for HO-1 enzymatic activity using a fluorometric assay that determines heme to bilirubin conversion (37). Plasma and tissue values were presented as μg/ml and μg/mg tissue protein, using a standard curve constructed with mouse recombinant HO-1 (a gift from Roland Strong, PhD, Fred Hutchinson Cancer Research Center).
Calculations and Statistics
All values are presented as means ± 1SEM. Statistical comparisons were made by unpaired Student's t test (except plasma troponin levels and renal cortical haptoglobin protein levels, which were compared by Wilcoxon Rank Sum test, given their non parametric distribution). If multiple comparisons were made the Bonferroni correction was applied. Significance was judged at a p value of <0.05.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (DK 38432)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009;20:223–228. doi: 10.1681/ASN.2007080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ. Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol. 2006;17:1135–1142. doi: 10.1681/ASN.2005060668. [DOI] [PubMed] [Google Scholar]
- 3.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 4.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302:1179–85. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg A, Kogan E, Hammerman H, Markiewicz W, Aronson D. The impact of transient and persistent acute kidney injury on long-term outcomes after acute myocardial infarction. Kidney Int. 2009;76:900–906. doi: 10.1038/ki.2009.295. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–999. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zager RA. Marked protection against acute renal and hepatic injury after nitrited myoglobin + tin protoporphyrin administration. Transl Res. 2015 Jun 10;:S1931–5244(15)00209-1. doi: 10.1016/j.trsl.2015.06.004. doi: 10.1016/j.trsl.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill-Kapturczak N, Jarmi T, Agarwal A. Growth factors and heme oxygenase-1: perspectives in physiology and pathophysiology. Antioxid Redox Signal. 2007;9:2197–2207. doi: 10.1089/ars.2007.1798. [DOI] [PubMed] [Google Scholar]
- 10.Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, Rosenberg ME. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992;90:267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A, Bolisetty S. Adaptive responses to tissue injury: role of heme oxygenase-1. Trans Am Clin Climatol Assoc. 2013;124:111–122. [PMC free article] [PubMed] [Google Scholar]
- 12.Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114:1601–1610. doi: 10.1161/CIRCRESAHA.114.303822. [DOI] [PubMed] [Google Scholar]
- 13.Totzeck M, Hendgen-Cotta UB, Luedike P. Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation. Circulation. 2012;126:325–334. doi: 10.1161/CIRCULATIONAHA.111.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal A, Semwal BC, Yadav HN. Abrogated cardioprotective effect of ischemic preconditioning in ovariectomized rat heart. Hum Exp Toxicol. 2015 Aug 11; doi: 10.1177/0960327115597980. pii: 0960327115597980. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sardana MK, Kappas A. Dual control mechanism for heme oxygenase: tin(IV)-protoporphyrin potently inhibits enzyme activity while markedly increasing content of enzyme protein in liver. Proc Natl Acad Sci U S A. 1987;84:2464–2468. doi: 10.1073/pnas.84.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellerman PS. Exogenous adenosine triphosphate (ATP) preserves proximal tubule microfilament structure and function in vivo in a maleic acid model of ATP depletion. J Clin Invest. 1993;92:1940–1949. doi: 10.1172/JCI116787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zager RA, Johnson AC, Naito M, Bomsztyk K. Maleate nephrotoxicity: mechanisms of injury and correlates with ischemic/hypoxic tubular cell death. Am J Physiol. 2008;294:F187–197. doi: 10.1152/ajprenal.00434.2007. [DOI] [PubMed] [Google Scholar]
- 19.Zager RA, Johnson AC, Becker K. Plasma and urinary heme oxygenase-1 in AKI. J Am Soc Nephrol. 2012;23:1048–1057. doi: 10.1681/ASN.2011121147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zager RA, Johnson AC, Hanson SY, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40:90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 21.Johnson AC, Becker K, Zager RA. Parenteral iron formulations differentially affect MCP-1, HO-1, and NGAL gene expression and renal responses to injury. Am J Physiol Renal. 2010;299:F426–35. doi: 10.1152/ajprenal.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zager RA. Parenteral iron compounds: potent oxidants but mainstays of anemia management in chronic renal disease. Clin J Am Soc Nephrol Suppl. 2006;1:S24–31. doi: 10.2215/CJN.01410406. [DOI] [PubMed] [Google Scholar]
- 23.Kaizu T, Tamaki T, Tanaka M, Uchida Y, Tsuchihashi S, Kawamura A, Kakita A. Preconditioning with tin-protoporphyrin IX attenuates ischemia/reperfusion injury in the rat kidney. Kidney Int. 2003;63:1393–403. doi: 10.1046/j.1523-1755.2003.00882.x. [DOI] [PubMed] [Google Scholar]
- 24.Zager RA, Johnson AC, Frostad KB. Rapid renal alpha-1 antitrypsin gene induction in experimental and clinical acute kidney injury. PLoS One. May 21. 2014;9(5):e9838. doi: 10.1371/journal.pone.0098380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zager RA, Johnson AC, Becker K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am J Physiol. 2012;303:F1460–1472. doi: 10.1152/ajprenal.00426.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Åkerström B, Gram M. A1M, an extravascular tissue cleaning and housekeeping protein. Free Radic Biol Med. 2014;74:274–282. doi: 10.1016/j.freeradbiomed.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 27.Scindia Y, Dey P, Thirunagari A, Liping H, Rosin DL, Floris M, Okusa MD, Swaminathan S. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J Am Soc Nephrol. 2015;26:2800–2814. doi: 10.1681/ASN.2014101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumenthal SB, Kiemer AK, Tiegs G, Seyfried S, Höltje M, Brandt B, Höltje HD, Zahler S, Vollmar AM. Metalloporphyrins inactivate caspase-3 and -8. FASEB J. 2005;19:1272–1279. doi: 10.1096/fj.04-3259com. [DOI] [PubMed] [Google Scholar]
- 29.Issan Y, Kornowski R, Aravot D, Shainberg A, Laniado-Schwartzman M, Sodhi K, Abraham NG, Hochhauser E. Heme oxygenase-1 induction improves cardiac function following myocardial ischemia by reducing oxidative stress. PLoS One. Mar 21. 2014;9(3):e92246. doi: 10.1371/journal.pone.0092246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virzì GM, Day S, de Cal M, Vescovo G, Ronco C. Heart-kidney crosstalk and role of humoral signaling in critical illness. Crit Care. 2014;18:201. doi: 10.1186/cc13177. doi: 10.1186/cc13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Curr Opin Crit Care. 2009;5:481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 32.Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81:942–948. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- 33.Kappas A, Drummond GS, Manola T, Petmezaki S, Valaes T. Sn-protoporphyrin use in the management of hyperbilirubinemia in term newborns with direct Coombs-positive ABO incompatibility. Pediatrics. 1988;81:485–497. [PubMed] [Google Scholar]
- 34.Reddy P, Najundaswamy S, Mehta R, Petrova A, Hegyi T. Tin-mesoporphyrin in the treatment of severe hyperbilirubinemia in a very-low-birth-weight infant. J Perinatol. 2003;23:507–512. doi: 10.1038/sj.jp.7210943. [DOI] [PubMed] [Google Scholar]
- 35.Zager RA, Vijayan A, Johnson AC. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury “stress response”. Am J Physiol. 2012;303:F139–148. doi: 10.1152/ajprenal.00168.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zager RA, Johnson AC, Frostad KB. Acute hepatic ischemic-reperfusion injury induces a renal cortical “stress response,” renal “cytoresistance,” and an endotoxin hyperresponsive state. Am J Physiol Renal Physiol. 2014;307:F856–868. doi: 10.1152/ajprenal.00378.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klemz R, Mashreghi MF, Spies C, Vold HD, Kotsch K. Amplifying the fluorescence of bilirubin enables the real-time detection of heme oxygenase activity. Fre Rad Biol Med. 2009;42:305–311. doi: 10.1016/j.freeradbiomed.2008.10.044. [DOI] [PubMed] [Google Scholar]