ABSTRACT
The homeostatic turnover of adult organs and their regenerative capacity following injury depend on a careful balance between stem cell self-renewal (to maintain or enlarge the stem cell pool) and differentiation (to replace lost tissue). We have recently characterized the role of the Drosophila Snail family transcription factor escargot (esg) in testis cyst stem cells (CySCs)1,2 and intestinal stem cells (ISCs). 3,4 CySCs mutant for esg are not maintained as stem cells, but they remain capable of differentiating normally along the cyst cell lineage. In contrast, esg mutant CySCs that give rise to a closely related lineage, the apical hub cells, cannot maintain hub cell identity. Similarly, Esg maintains stemness of ISCs while regulating the terminal differentiation of progenitor cells into absorptive enterocytes or secretory enteroendocrine cells. Therefore, our findings suggest that Esg may play a conserved and pivotal regulatory role in adult stem cells, controlling both their maintenance and terminal differentiation. Here we propose that this dual regulatory role is due to simultaneous control by Esg of overlapping genetic programs and discuss the exciting challenges and opportunities that lie ahead to explore the underlying mechanisms experimentally.
KEYWORDS: DE-cadherin, Escargot, intestine, stem cells, testis
Introduction
Stem cell self-renewal and the production of progenitor cells are tightly controlled by both intrinsic and extrinsic mechanisms. While most adult or tissue stem cells divide asymmetrically to self-renew and give rise to differentiating progeny, stem cell divisions are not invariantly asymmetric, and the relative abundance of cell types that derive from them in a tissue will often be consistent with a “neutral drift” model of stochastic symmetric or asymmetric divisions.5,6 Moreover, in response to diverse forms of stress (wounding, aging, metabolic stress), stem cells can undergo dynamic waves of symmetric self-renewing or differentiating divisions to quickly repair damaged tissue.7-9 Furthermore, differentiating progeny are often multipotent and their cell fate decisions must be tightly regulated for proper homeostasis and regeneration.
While significant progress has been made in the identification, characterization and manipulation of tissue stem cells in several organisms, a complete understanding of the genetic networks that coordinate self-renewal and differentiation decisions is still largely lacking. A fuller picture of how decisions between alternative fates is achieved will advance our ability to manipulate stem cells and unleash their full potential for regenerative medicine.
Research focused on Drosophila stem cells has been instrumental in characterizing basic mechanisms of stem cell regulation, including the interactions between stem cells and their niche10-12 and the role of asymmetric divisions in controlling stem cell behavior (reviewed in13,14). In addition, more recent work has underscored the use of Drosophila as an excellent model system to explore the response of stem cells to various forms of physiological, metabolic and genotoxic stress, from infections to starvation and aging.7,15-18
In our laboratory, we have used 2 well-established stem cell model systems in flies, the posterior midgut epithelium and the testis, to explore how mechanisms regulating stem cell behavior are altered in response to aging and acute or chronic changes in metabolism.16,19-22
The adult Drosophila midgut is a simple epithelium composed of 2 terminally differentiated cell types: secretory enteroendocrine cells (EEs) and absorptive enterocytes (ECs), both of which originate from intestinal stem cells, or ISCs (Fig. 1a).23,24 The majority of ISCs undergo an asymmetric self-renewing division, generating a new ISC and a transient enteroblast (EB) that differentiates into an EC through activation of the Notch pathway. On the other hand, a smaller subset of Prospero-expressing ISCs gives rise to EE cells through asymmetric mitosis (ISC+EE) or direct differentiation.25-28
Figure 1.
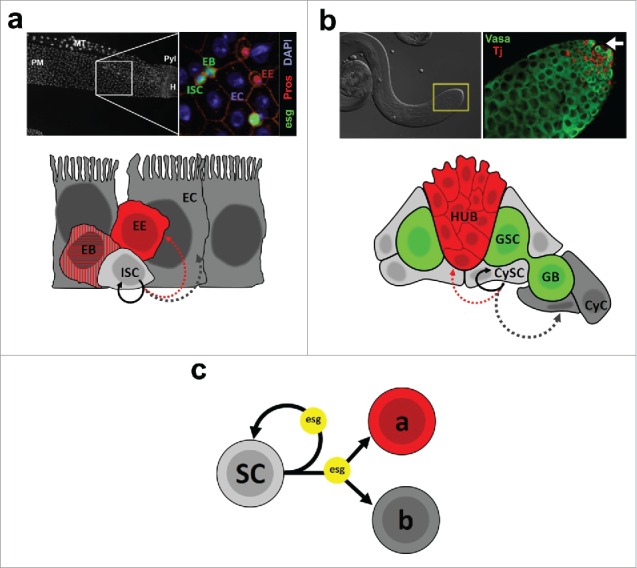
(a) The Drosophila midgut epithelium. Top left: DAPI nuclear staining of the posterior midgut (PM). MT: Malpighian tubule; Pyl: Pyloric ring; H: Hindgut. Top right: Immunostaining of a region that corresponds approximately to the area depicted in the left image. ISC/EBs are labeled by expression of an esg-GFP reporter (esg), EEs are identified by Prospero (Pros) nuclear staining, and ECs are identified based on their polyploid nuclei (DAPI). Images by Christopher Koehler, L. Jones lab. Bottom: Cartoon representing the 4 cell types that make up the midgut epithelium and their lineage relationships. ISCs can self-renew (solid arrow) or differentiate into EEs or ECs (dashed arrows - see main text for details). (b) The Drosophila testis. Top left: DIC image of a testis. The inset marks the apical tip of the gonad, where the stem cell niche resides. Top right: Immunostaining of a testis tip. Germ cells are identified based on expression of Vasa, whereas somatic cells are labeled by expression of Traffic jam (Tj). The arrow points to the approximate location of the apical hub (not shown). Bottom: Schematic representation of the testis apical tip, showing somatic hub cells, germline and somatic stem cells (GSCs and CySCs, respectively) and their progeny (GB and CyC, respectively). Lineage relationships are shown only for CySCs, which can self-renew or differentiate into CyCs or, more rarely, give rise to hub cells. (c) Abstract synthesis of our preliminary work on the role of escargot (esg), which is simultaneously required for the maintenance of stem cells (SC - testis CySCs 2 and midgut ISC/EBs 4), while also controlling the differentiation of their progeny into alternative fates (“a” and “b”). 1,4
Drosophila testes produce sperm throughout life due to asymmetric self-renewing divisions of germline stem cells (GSCs), which reside at the tip of the gonad within a well-characterized niche (Fig. 1b).12 During spermatogenesis, GSCs divide to produce a new GSC and a differentiating daughter that will undergo a series of mitotic divisions before committing to terminal differentiation into sperm. Every GSC daughter that progresses through spermatogenesis is encapsulated by a pair of somatic cyst cells, which are in turn generated by the asymmetric division of cyst stem cells (CySCs) that also reside at the testis tip in contact with GSCs. Both GSCs and CySCs depend upon a cluster of post-mitotic somatic cells known as the hub for their maintenance. Hub cells not only anchor GSCs and CySCs within the niche, but they also produce and secrete factors that are essential for maintaining the self-renewing capacity of both stem cell populations. Hub cells are specified during development.29-31 However, using several lineage-tracing strategies, our data suggest that under circumstances that remain to be better understood CySCs can either become and/or generate new hub cells in adult males.1
Regulation of Drosophila stem cells by escargot
Escargot (Esg) is a Snail family transcription factor32 that is specifically expressed in stem and progenitor cells in various fly organs, including the testis and posterior midgut. In the testis, Esg expression is largely restricted to GSCs, CySCs and hub cells.2 In the midgut, Esg is specifically expressed in ISCs and EBs and is frequently used as a marker for these cell types.23,33
Such restricted expression in stem cells across tissues is highly unusual; therefore, we sought to characterize and compare the role of Esg in stem cells from both tissues. Clonal analysis to remove Esg function specifically from CySCs resulted in loss of stem cell fate, differentiation into apparently normal cyst cells,2 and the generation of morphologically abnormal hub cells.1 In the posterior midgut, loss of Esg function in ISCs resulted in loss of stem cells and an increased proportion of EE cells.3,4
One interesting observation from these studies is that Esg simultaneously regulates the self-renewal potential of the stem cell and the terminal differentiation of its progeny in both systems (Fig. 1c). Moreover, Snail 1 (Snai1), one of the mammalian homologues of Esg, has recently been shown to play an analogous role in the maintenance of mouse intestinal stem cells and the fate choices made by their differentiating progeny.34 Therefore, we propose that Esg plays a highly conserved role in the coordination between self-renewal and differentiation in stem cells across tissues and animal species.
In order to understand the molecular mechanisms involved in stem cell regulation by Esg, we and others have mapped the genomic binding of Esg by DamID,4 identified putative protein interactors by co-immunoprecipitation followed by mass spectrometry (IP/MS)2 and analyzed changes in gene expression by RNA-sequencing in vivo3 or in cultured S2 cells by microarray (S. Sandall and L. Jones, unpublished data). We surmise, however, that the data obtained from each of these screens includes a mixture of targets that are in charge of maintaining stem cells in an undifferentiated state and/or regulating differentiation decisions. On one hand, “-omics” approaches applied to dynamic processes, such as differentiation, are likely to generate a mix of hits that correspond to early and late steps of the process, due to an inescapable degree of biological heterogeneity, with some cells further along the process than others. In addition, Esg could regulate the expression of stemness and differentiation genes in pre-mitotic stem cells simultaneously. Therefore, it will be difficult to distinguish between these phenomena a priori without additional experimental data at finer phenotypic resolution.
Teasing apart the loss of stemness from terminal differentiation: a case for DE-cadherin
Enrichment of the cell adhesion protein Drosophila E-cadherin (DE-cad) is characteristic of ISC/EB ‘nests’ and can be used to identify these cells in the midgut epithelium in the absence of other specific markers (Fig. 2a and refs.3,24). Furthermore, a cell type-specific transcriptome profiling in the Drosophila midgut showed that DE-cadherin mRNA is more abundant in ISCs than in committed EBs or ECs.35
Figure 2.
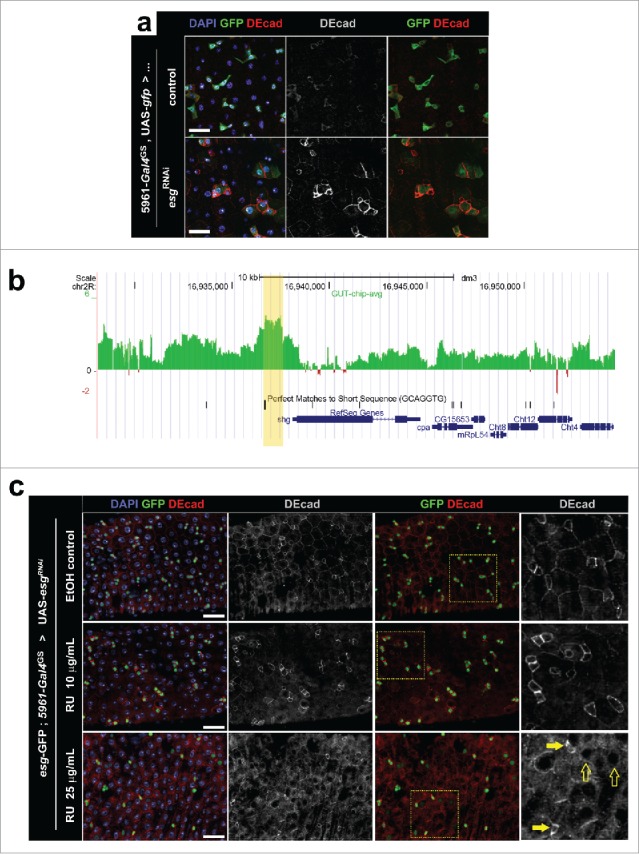
(a) Immunostaining for DE-cad in posterior midguts following knockdown of Esg expression. Flies expressing a UAS-esgRNAi transgene under control of the ISC/EB specific driver 5961-gal4GS (“esgRNAi”) and control flies carrying only the Gal4GS driver (“control”) were kept for 4 d in food containing the inducer RU486 (10 μg/mL). Midguts were probed for GFP expression (ISC/EBs) and DE-cad, and all nuclei were stained with DAPI (as indicated). Scale bars = 20μm. (b) DamID data revealing a peak of Esg binding just upstream of shotgun (shg). DamID was used to map the binding of Esg to the genome; the x-axis corresponds to genomic coordinates in chromosome 2R, while the y-axis corresponds to extent of binding of an Esg:dam fusion construct to DNA in midguts (see ref. 4 for details). The yellow shade marks the Esg-bound region. Notice that the EBR contains a perfect match of the consensus Esg binding sequence. 49 (c) Immunostaining for DE-cad in posterior midguts following different degrees of Esg knockdown. Flies carrying a UAS-esgRNAi transgene under control of the ISC/EB specific driver 5961-gal4GS were kept for 3 d in food that contained only ethanol (EtOH control), or food containing a lower (10μg/mL) or higher (25μg/mL) dose of the inducer RU486 (as indicated). The tissue was stained as in (a) and all images were captured using the same acquisition times. Notice that these flies carried a separate esg-GFP reporter, which allows for ISC/EB identification in flies kept in EtOH control food. Rightmost panels are zoomed regions that correspond to the yellow boxes in the adjacent images. Full and empty arrows point to examples of progenitor cells that express higher and lower levels of esg-GFP respectively. Notice that cells that retain high esg-GFP expression maintain high levels of DE-cad expression. Scale bars = 40μm.
When comparing our observations on the loss of Esg function in ISCs with those by Korzelius and colleagues,3 an apparent inconsistency between our studies arose that prompted us to investigate our findings further. Korzelius et al. observed that RNAi-mediated depletion of Esg resulted in a significant reduction in the expression of DE-cad in ISCs and EBs, which is consistent with the conclusion that the progenitor cells had been induced to differentiate (Fig. S1D-D″ in ref.3). Our experiments, however, revealed different results. We observed a marked increase in the level of DE-cad expression in ISC/EBs following the depletion of Esg by RNAi (Fig. 2a). Of note, our results were consistent with DamID data that had identified a distinct Esg-binding region proximal to shotgun (shg), the locus that codes for DE-cad (Fig. 2b). Esg is thought to act predominantly as a transcriptional repressor.32 Therefore, if shg expression is repressed by Esg as suggested by the DamID data, then the RNAi-mediated downregulation of Esg would be expected to cause an upregulation of DE-cad expression.
To reconcile this apparent inconsistency in the data, we hypothesized that the different strengths of the GAL4 ‘drivers’ used in our studies resulted in different rates of differentiation caused by the loss of Esg. In other words, that the relative higher and lower levels of DE-cad corresponded to different degrees of ISC/EB differentiation. Korzelius et al. used the strong esgGAL4, tubGAL80ts driver line (esgGAL4ts) to express esgRNAi transgenes, which would induce a more abrupt Esg knock-down and a more rapid ISC/EB differentiation. In contrast, we utilized a relatively weaker Geneswitch driver, 5961-gal4GS,36-38 which would knock-down Esg more gradually and allow for a window during which ISC/EBs express less Esg and yet maintain stem cell identity.
We decided to test our hypothesis by modulating GeneSwitch driver activity by using different amounts of the inducer, RU486, in the fly food. In agreement with our hypothesis, we found that a lower dose of RU486 led to a noticeable accumulation of DE-cad in ISC/EBs that have not yet significantly differentiated, as determined by their relatively high levels of esg-GFP expression. In contrast, a higher dose of RU486 caused a significant decrease in DE-cad expression and an overall decrease in esg-GFP expression (Fig. 2c), a pattern similar to that reported by Korzelius et al.3 Furthermore, at higher doses of RU486, we would often observe a small number of ISC/EBs that retained their identity (based on esg-GFP expression) and expressed relatively high levels of DE-cad, which serve as an internal control for the DE-cad staining and confirmed that various degrees of differentiation can be observed upon depletion of Esg (Fig. 2c, arrows).
The role of DE-cad in ISCs is likely complex and context-specific, given its dual function in mediating cell-cell adhesion and controlling cytoplasmic signaling pathways (e.g. the Wnt/Wg pathway39). In flies, E-cadherin expression has been found to be critical for the regulation of stem cells via control of their proper attachment to their niche40-43 (reviewed in ref.44). On the other hand, Snail family proteins seem to repress the expression of E-cadherin in diverse stem cell models.34,45-47 In ISC/EBs, previous work has shown that inhibiting DE-cad expression affects the fate choice made by ISCs42 and affects their rate of proliferation.48 Therefore, it seems plausible that stem cells may require finely tuned levels of E-cadherin expression, permitting proper adhesion to their niche while not interfering with signaling pathways controlling stem cell proliferation and differentiation.
Concluding remarks
At first glance, the positive correlation between Esg and DE-cad expression in ISCs and ECs,35 as well as the decrease in DE-cad expression following a more abrupt induction of ISC differentiation3 would not support a model in which Esg inhibits DE-cad expression. This relationship became apparent only after a gradual induction of ISC/EB differentiation achieved through inducible depletion of Esg using the Geneswitch system.38 These results stress the power of inducible and scalable genetic manipulations as a way to dissect between early, intermediate and later phenotypes in the continuum from commitment to full differentiation. Use of such tools will complement one-dimensional genome-wide screens that set artificial endpoints for dynamic processes such as differentiation. Fortunately, the cost of high throughput screening is steadily dropping, making it possible to combine “-omics” approaches with new and more refined inducible systems for genetic manipulation. These advances will soon make it possible to sample various stages of a dynamic process at a genome wide level providing an even better insight into the mechanisms regulating stem cell behavior across tissues and species.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to acknowledge J. Korzelius and B. Edgar for sharing data prior to publication and T. Southall and A. Brand for their help with DamID and keeping an open line of communication. We apologize to those colleagues whose work could not be referenced directly due to space constraints.
Funding
M.A.L.C is funded by the California State University, Northridge and D.L.J. is funded by the the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California, Los Angeles, the Rose Hills Foundation, the Hillblom Foundation and the NIH: AG028092 and AG040288.
References
- [1].Voog J, D'Alterio C, Jones DL. Multipotent somatic stem cells contribute to the stem cell niche in the Drosophila testis. Nature 2008; 454:1132-6; PMID:18641633; http://dx.doi.org/ 10.1038/nature07173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Voog J, Sandall SL, Hime GR, Resende LP, Loza-Coll M, Aslanian A, Yates JR 3rd, Hunter T, Fuller MT, Jones DL. Escargot restricts niche cell to stem cell conversion in the Drosophila testis. Cell reports 2014; 7:722-34; PMID:24794442; http://dx.doi.org/ 10.1016/j.celrep.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Korzelius J, Naumann SK, Loza-Coll MA, Chan JS, Dutta D, Oberheim J, Glasser C, Southall TD, Brand AH, Jones DL, et al.. Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. EMBO J 2014; 33:2967-82; PMID:25298397; http://dx.doi.org/ 10.15252/embj.201489072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Loza-Coll MA, Southall TD, Sandall SL, Brand AH, Jones DL. Regulation of Drosophila intestinal stem cell maintenance and differentiation by the transcription factor Escargot. EMBO J 2014; 33:2983-96; PMID:25433031; http://dx.doi.org/ 10.15252/embj.201489050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].de Navascues J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martinez-Arias A, Simons BD. Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 2012; 31:2473-85; PMID:22522699; http://dx.doi.org/ 10.1038/emboj.2012.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Amoyel M, Simons BD, Bach EA. Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J 2014; 33:2295-313; PMID:25092766; http://dx.doi.org/ 10.15252/embj.201387500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].O'Brien LE, Soliman SS, Li X, Bilder D. Altered modes of stem cell division drive adaptive intestinal growth. Cell 2011; 147:603-14; PMID:22036568; http://dx.doi.org/ 10.1016/j.cell.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wabik A, Jones PH. Switching roles: the functional plasticity of adult tissue stem cells. EMBO J 2015; 34:1164-79; PMID:25812989; http://dx.doi.org/ 10.15252/embj.201490386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Edgar BA. Intestinal stem cells: no longer immortal but ever so clever. EMBO J 2012; 31:2441-3; PMID:22580826; http://dx.doi.org/ 10.1038/emboj.2012.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell 2011; 21:159-71; PMID:21763616; http://dx.doi.org/ 10.1016/j.devcel.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Palasz A, Kaminski M. Stem cell niche in the Drosophila ovary and testis; a valuable model of the intercellular signalling relationships. Adv Med Sci 2009; 54:143-9; PMID:19808162; http://dx.doi.org/ 10.2478/v10039-009-0032-5 [DOI] [PubMed] [Google Scholar]
- [12].Resende LP, Jones DL. Local signaling within stem cell niches: insights from Drosophila. Curr Opin Cell Biol 2012; 24:225-31; PMID:22296770; http://dx.doi.org/ 10.1016/j.ceb.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Inaba M, Yamashita YM. Asymmetric stem cell division: precision for robustness. Cell Stem Cell 2012; 11:461-9; PMID:23040475; http://dx.doi.org/ 10.1016/j.stem.2012.09.003 [DOI] [PubMed] [Google Scholar]
- [14].Wu PS, Egger B, Brand AH. Asymmetric stem cell division: lessons from Drosophila. Semin Cell Dev Biol 2008; 19:283-93; PMID:18328747; http://dx.doi.org/ 10.1016/j.semcdb.2008.01.007 [DOI] [PubMed] [Google Scholar]
- [15].Biteau B, Hochmuth CE, Jasper HCPCN. Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 2011; 9:402-11; PMID:22056138; http://dx.doi.org/ 10.1016/j.stem.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol 2010; 20:2100-5; PMID:21055942; http://dx.doi.org/ 10.1016/j.cub.2010.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tower J. Stress and stem cells. Wiley Interdiscip Rev Dev Biol 2012; 1:789-802; PMID:23799624; http://dx.doi.org/ 10.1002/wdev.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jasper H, Jones DL. Metabolic regulation of stem cell behavior and implications for aging. Cell Metab 2010; 12:561-5; PMID:21109189; http://dx.doi.org/ 10.1016/j.cmet.2010.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boyle M, Wong C, Rocha M, Jones DL. Decline in self-renewal factors contributes to aging of the stem cell niche in the Drosophila testis. Cell Stem Cell 2007; 1:470-8; PMID:18371382; http://dx.doi.org/ 10.1016/j.stem.2007.08.002 [DOI] [PubMed] [Google Scholar]
- [20].Hur JH, Bahadorani S, Graniel J, Koehler CL, Ulgherait M, Rera M, Jones DL, Walker DW. Increased longevity mediated by yeast NDI1 expression in Drosophila intestinal stem and progenitor cells. Aging (Albany NY) 2013; 5:662-81; PMID:24038661; http://dx.doi.org/ 10.18632/aging.100595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rera M, Bahadorani S, Cho J, Koehler CL, Ulgherait M, Hur JH, Ansari WS, Lo T Jr., Jones DL, Walker DW. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab 2011; 14:623-34; PMID:22055505; http://dx.doi.org/ 10.1016/j.cmet.2011.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang L, McLeod CJ, Jones DL. Regulation of adult stem cell behavior by nutrient signaling. Cell Cycle 2011; 10:2628-34; PMID:21814033; http://dx.doi.org/ 10.4161/cc.10.16.17059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 2006; 439:475-9; PMID:16340959; http://dx.doi.org/ 10.1038/nature04371 [DOI] [PubMed] [Google Scholar]
- [24].Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 2006; 439:470-4; PMID:16340960; http://dx.doi.org/ 10.1038/nature04333 [DOI] [PubMed] [Google Scholar]
- [25].Amcheslavsky A, Song W, Li Q, Nie Y, Bragatto I, Ferrandon D, Perrimon N, Ip YT. Enteroendocrine cells support intestinal stem-cell-mediated homeostasis in Drosophila. Cell Rep 2014; 9:32-9; PMID:25263551; http://dx.doi.org/ 10.1016/j.celrep.2014.08.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Biteau B, Jasper H. Slit/Robo signaling regulates cell fate decisions in the intestinal stem cell lineage of Drosophila. Cell Rep 2014; 7:1867-75; PMID:24931602; http://dx.doi.org/ 10.1016/j.celrep.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zeng X, Hou SX. Enteroendocrine cells are generated from stem cells through a distinct progenitor in the adult Drosophila posterior midgut. Development 2015; 142:644-53; PMID:25670791; http://dx.doi.org/ 10.1242/dev.113357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Guo Z, Ohlstein B. Stem cell regulation. Bidirectional Notch signaling regulates Drosophila intestinal stem cell multipotency. Science 2015; 350 [Epub ahead of print]; http://dx.doi.org/ 10.1126/science.aab0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dinardo S, Okegbe T, Wingert L, Freilich S, Terry N. lines and bowl affect the specification of cyst stem cells and niche cells in the Drosophila testis. Development 2011; 138:1687-96; PMID:21486923; http://dx.doi.org/ 10.1242/dev.057364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Le Bras S, Van Doren M. Development of the male germline stem cell niche in Drosophila. Dev Biol 2006; 294:92-103; PMID:16566915; http://dx.doi.org/ 10.1016/j.ydbio.2006.02.030 [DOI] [PubMed] [Google Scholar]
- [31].Okegbe TC, DiNardo S. The endoderm specifies the mesodermal niche for the germline in Drosophila via Delta-Notch signaling. Development 2011; 138:1259-67; PMID:21350008; http://dx.doi.org/ 10.1242/dev.056994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002; 3:155-66; PMID:11994736; http://dx.doi.org/ 10.1038/nrm757 [DOI] [PubMed] [Google Scholar]
- [33].Toledano H, D'Alterio C, Loza-Coll M, Jones DL. Dual fluorescence detection of protein and RNA in Drosophila tissues. Nat Protoc 2012; 7:1808-17; PMID:22976352; http://dx.doi.org/ 10.1038/nprot.2012.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Horvay K, Jarde T, Casagranda F, Perreau VM, Haigh K, Nefzger CM, Akhtar R, Gridley T, Berx G, Haigh JJ, et al.. Snai1 regulates cell lineage allocation and stem cell maintenance in the mouse intestinal epithelium. EMBO J 2015; 34:1319-35; PMID:25759216; http://dx.doi.org/ 10.15252/embj.201490881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dutta D, Buchon N, Xiang J, Edgar BA. Regional Cell Specific RNA Expression Profiling of FACS Isolated Drosophila Intestinal Cell Populations. Curr Protoc Stem Cell Biol 2015; 34:2F 1-2F 14; PMID:26237570 [DOI] [PubMed] [Google Scholar]
- [36].Biteau B, Karpac J, Supoyo S, Degennaro M, Lehmann R, Jasper H. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS genetics 2010; 6:e1001159; PMID:20976250; http://dx.doi.org/ 10.1371/journal.pgen.1001159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science 2010; 327:210-3; PMID:20056890; http://dx.doi.org/ 10.1126/science.1181958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A 2001; 98:12596-601; PMID:11675495; http://dx.doi.org/ 10.1073/pnas.221303298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol 2005; 15:R64-7; PMID:15668160; http://dx.doi.org/ 10.1016/j.cub.2004.12.058 [DOI] [PubMed] [Google Scholar]
- [40].Gao H, Wu X, Fossett N. Drosophila E-cadherin functions in hematopoietic progenitors to maintain multipotency and block differentiation. PLoS One 2013; 8:e74684; PMID:24040319; http://dx.doi.org/ 10.1371/journal.pone.0074684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Inaba M, Yuan H, Salzmann V, Fuller MT, Yamashita YM. E-cadherin is required for centrosome and spindle orientation in Drosophila male germline stem cells. PLoS One 2010; 5:e12473; PMID:20824213; http://dx.doi.org/ 10.1371/journal.pone.0012473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells 2008; 13:1219-27; PMID:19021776; http://dx.doi.org/ 10.1111/j.1365-2443.2008.01239.x [DOI] [PubMed] [Google Scholar]
- [43].Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci U S A 2002; 99:14813-8; PMID:12393817; http://dx.doi.org/ 10.1073/pnas.232389399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gonzalez-Reyes A. Stem cells, niches and cadherins: a view from Drosophila. J Cell Sci 2003; 116:949-54; PMID:12584239; http://dx.doi.org/ 10.1242/jcs.00310 [DOI] [PubMed] [Google Scholar]
- [45].Dang H, Ding W, Emerson D, Rountree CB. Snail1 induces epithelial-to-mesenchymal transition and tumor initiating stem cell characteristics. BMC Cancer 2011; 11:396; PMID:21929801; http://dx.doi.org/ 10.1186/1471-2407-11-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, Escriva M, Hernandez-Munoz I, Di Croce L, Helin K, et al.. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol 2008; 28:4772-81; PMID:18519590; http://dx.doi.org/ 10.1128/MCB.00323-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhou W, Lv R, Qi W, Wu D, Xu Y, Liu W, Mou Y, Wang L. Snail contributes to the maintenance of stem cell-like phenotype cells in human pancreatic cancer. PLoS One 2014; 9:e87409; PMID:24489910; http://dx.doi.org/ 10.1371/journal.pone.0087409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Choi NH, Lucchetta E, Ohlstein B. Nonautonomous regulation of Drosophila midgut stem cell proliferation by the insulin-signaling pathway. Proc Natl Acad Sci U S A 2011; 108:18702-7; PMID:22049341; http://dx.doi.org/ 10.1073/pnas.1109348108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Fuse N, Hirose S, Hayashi S. Diploidy of Drosophila imaginal cells is maintained by a transcriptional repressor encoded by escargot. Genes & development 1994; 8:2270-81; PMID:7958894; http://dx.doi.org/ 10.1101/gad.8.19.2270 [DOI] [PubMed] [Google Scholar]


