ABSTRACT
A fraction of HIV-1 patients are able to generate broadly neutralizing antibodies (bNAbs) after 2 to 4 years of infection. In rare occasions such antibodies are observed close to the first year of HIV-1 infection but never within the first 6 months. In this study, we analyzed the neutralization breadth of sera from 157 antiretroviral-naive individuals who were infected for less than 1 year. A range of neutralizing activities was observed with a previously described panel of six recombinant viruses from five different subtypes (M. Medina-Ramirez et al., J Virol 85:5804–5813, 2011, http://dx.doi.org/10.1128/JVI.02482-10). Some sera were broadly reactive, predominantly targeting envelope epitopes within the V2 glycan-dependent region. The neutralization breadth was positively associated with time postinfection (P = 0.0001), but contrary to what has been reported for chronic infections, no association with the viral load was observed. Notably, five individuals within the first 6 months of infection (two as early as 77 and 96 days postinfection) showed substantial cross-neutralization. This was confirmed with an extended panel of 20 Env pseudoviruses from four different subtypes (two in tier 3, 14 in tier 2, and four in tier 1). Sera from these individuals were capable of neutralizing viruses from four different subtypes with a geometric mean 50% infective dose (ID50) between 100 and 800. These results indicate that induction of cross-neutralizing responses, albeit rare, is achievable even within 6 months of HIV-1 infection. These observations encourage the search for immunogens able to elicit this kind of response in preventive HIV-1 vaccine approaches.
IMPORTANCE There are very few individuals able to mount broadly neutralizing activity (bNA) close to the first year postinfection. It is not known how early in the infection cross-neutralizing responses can be induced. In the present study, we show that bNAbs, despite being rare, can be induced much earlier than previously thought. The identification of HIV-1-infected patients with these activities within the first months of infection and characterization of these responses will help in defining new immunogen designs and neutralization targets for vaccine-mediated induction of bNAbs.
INTRODUCTION
One of the main challenges for the development of a preventive human immunodeficiency virus type 1 (HIV-1) vaccine is the design of immunogens and immunization strategies that allow the induction of neutralizing antibody responses against multiple HIV-1 isolates. The main target of neutralizing antibodies is the trimeric envelope glycoprotein spike (Env). Unfortunately, when these recombinant proteins are used as immunogens, there is minimal induction of cross-reactive neutralizing antibody responses (1, 2). Despite these hurdles, high titers of broadly neutralizing antibodies (bNAbs) have been found in some chronically infected patients (3, 4). Data from many studies indicate that between 10 and 25% of patient sera displayed broadly neutralizing capacity against diverse HIV-1 strains (2–5), and one large study showed that 50% of sera from chronic infection can neutralize 50% of virus strains (6). Antibody responses have been studied extensively in these chronically infected patients, new bNAbs have been isolated, and the molecular determinants recognized by these antibodies have been characterized. Although these antibodies do not protect infected patients, they exert selective pressure on the virus (7–9). On the other hand, and more importantly, passive transfer of broadly neutralizing antibodies to monkeys and humanized mice effectively protects them against chimeric simian-human immunodeficiency virus (SHIV) infection and HIV infection, respectively (10–18). Additionally, promising results have been obtained in recent years with approaches based on the production of broadly neutralizing antibodies against HIV and SHIV, or molecules resembling these antibodies, by adeno-associated vectors in animal models (19, 20). In humans, passive transfer of bNAbs also has shown some efficacy in controlling viral replication in two recent studies, showing that passive transfer of monoclonal antibody targeting the CD4 binding site can reduce HIV-1 viremia (21, 22).
Taking into account all of the above-described findings, it is reasonable to think that the knowledge of the mechanisms involved in the development of this type of antibody will provide valuable information for the design of an efficient HIV vaccine. Several studies of chronically infected patients have found a positive correlation between viral load and neutralization breadth (5, 23, 24). Broadly cross-reactive neutralizing activity also has been associated with partial B cell restoration (24). In addition, it has been proposed that long periods of viral replication were required to induce the high levels of somatic mutation found in the vast majority of bNAbs (25–27). These data suggest that the design of immunogens and immunization strategies that produce bNAbs will be complicated. To help guide such strategies, the identification and characterization of broadly neutralizing responses in recent infection would provide valuable information.
Upon infection with HIV-1, essentially all individuals develop a strong antibody response against the viral Env. Within 1 week of detectable viremia (10 to 12 days after infection), antigen-antibody complexes are detected. Following this phase, antibodies directed against gp41 and gp120 and to conserved regions (CD4-binding site [CD4bs] and membrane-proximal external region [MPER]) are generated after 23, 38, and 40 to 70 days postinfection, respectively. Unfortunately, despite these antibodies exerting selective pressure on the virus, there is no effect on viremia levels (28).
There is some evidence that the induction of broadly neutralizing antibodies during the first year of infection is possible, although it is less frequent than that in chronic infection. Two recent reports described three patients that developed broadly neutralizing activity (bNA), defined as the ability of serum to neutralize viruses from different clades with a geometric mean 50% infective concentration (IC50) between 100 and 500, at 9.8 and 12 months postseroconversion (29, 30).
In order to better understand bNA induction during recent infection, in the present study we have studied neutralization breadth in patients within the first year of HIV-1 infection. In addition, we have explored the possibility of finding these responses as early in HIV-1 infection as possible. In order to do so, we have studied the neutralization breadth in a cohort of 157 antiretroviral-naive recently infected individuals (<1 year postinfection) and compared the results obtained with a similar analysis done in a cohort of 170 untreated chronically infected individuals (>1 year postinfection).
MATERIALS AND METHODS
Study participants.
Samples from 327 HIV-1-infected individuals treated at the Hospital Clinic of Barcelona (Spain) were collected, 157 within less than 365 days postinfection (dpi) and 170 after more than 365 dpi. A cross-sectional study with samples of these patients was carried out. All individuals gave informed written consent, and the study was reviewed and approved by the Institutional Review Board (IRB) of the Hospital Clinic of Barcelona (Spain).
Recent infection testing algorithm.
In order to detect the presence of HIV-specific antibodies, a conventional sensitive enzyme immunoassay (AxSYM system based; Abbott Laboratories, North Chicago, IL, USA) or Western blotting (Inno-LIA HIV I/II score; Innogenetics, Ghent, Belgium) was used. For the detection of plasma HIV-1, an RNA Cobas Amplicor monitor was used (Roche Molecular Systems, Branchburg, NJ, USA). For the identification of recent infections, the test for recent infection (t.r.i.) used was BED-CEIA (modified version of the Vironostika HIV-1-1 enzyme immunoassay; bioMérieux Durham, NC, USA) (31).
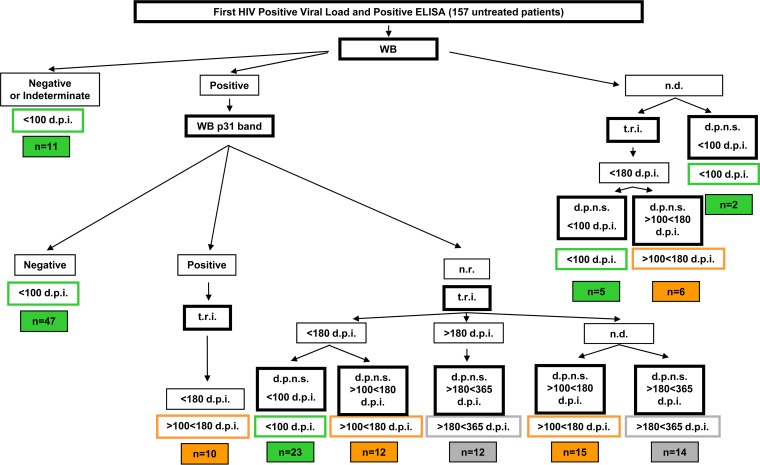
For the <365-dpi group of patients, we designated a specific recent infection testing algorithm to identify cases of recent infection and to divide them into three subgroups depending on the days postinfection: <100 (early recent), 100 to 180 (late recent), and 180 to 365 (early chronic) dpi. The detailed flow chart used for dating HIV infection based on Western blot results, the test for recent infection, and days since previous negative serology is shown in Fig. 1. The characteristics of the cohort of patients with early recent and late recent HIV infection, belonging to the acute/recent HIV infection cohort at the hospital clinic, are described elsewhere (32).
FIG 1.
Algorithm for dating of HIV-1 infection in patient sera (<100 dpi, >100 but <180 dpi, and >180 but <365 dpi). d.p.n.s., days postnegative serology; t.r.i., test for recent infection; n.d., not done; n.r., not reported. The following categories were used for samples from patients infected for <100 dpi: (i) negative or indeterminate Western blot (WB); (ii) positive Western blot with a negative p31 band; (iii) positive Western blot with no data for the p31 band and t.r.i. indicating fewer than 180 days of infection, and d.p.n.s. indicating fewer than 100 days of infection; (iv) no Western blot information, with t.r.i. indicating less than 180 days of infection and d.p.n.s. indicating less than 100 days of infection; and (v) d.p.n.s. within 100 days prior to sampling. The following categories were used for samples from patients infected for 100 to 180 dpi: (i) positive Western blot with a positive p31 band and a t.r.i. indicating less than 180 days of infection; (ii) positive Western blot with no data for the p31 band, a t.r.i. indicating less than 180 days of infection, and a d.p.n.s. indicating 100 to 180 days prior to the sampling; (iii) positive Western blot with no p31 band data reported, no t.r.i. data, and a d.p.n.s. of within 100 to 180 days prior to the sampling; and (iv) no Western blot information, t.r.i. indicating less than 180 days of infection, and a d.p.n.s. within 100 to 180 days prior to the sampling. The following categories were used for samples of patients infected for 180 to 365 dpi: (i) positive Western blot with no data for the p31 band, t.r.i. indicating more than 180 days of infection, and a documented previous negative serology within 180 to 365 days prior to sampling; (ii) a positive Western blot with no data for the p31 band, no t.r.i. data, and a d.p.n.s. of within 180 to 365 days prior to sampling.
For the group of chronically infected patients we selected 170 serum samples from different individuals known to have tested positive by ELISA and viral load at least 1 year prior to the sample.
Neutralization assays.
Serum samples were tested in triplicate at a 1/200 dilution with the six recombinant viruses representing 5 different genetic subtypes using TZM-bl target cells as previously described (33). The minipanel of replication-competent recombinant viruses was previously generated by replacing the env sequence of HIV NL4-3 with env sequences from isolates of 5 different subtypes: VI 191, 92BR025, 92UG024, CM244, and AC10.0.29 (33). An amphotropic vesicular stomatitis virus (VSV) Env pseudotyped on an HIV-1 core was added to the panel as a specificity control virus in neutralization assays. Pseudotyped viruses for neutralization assays were generated by manual or automated large-scale production of HIV by cotransfection of an HIV-1 env expression plasmid and an env-deficient HIV-1 backbone (pSG3ΔEnv), as previously described (34, 35). Clades, tropisms, and tier categorizations are indicated in Fig. 4. Viruses have been classified into tiers according to their sensitivity to neutralization: very high (tier 1A), above average (tier 1B), moderate (tier 2), and low (tier 3) sensitivity (36). The Fiebig classification (I to VI) in different stages of primary infection is based on the emergence of viral markers (37).
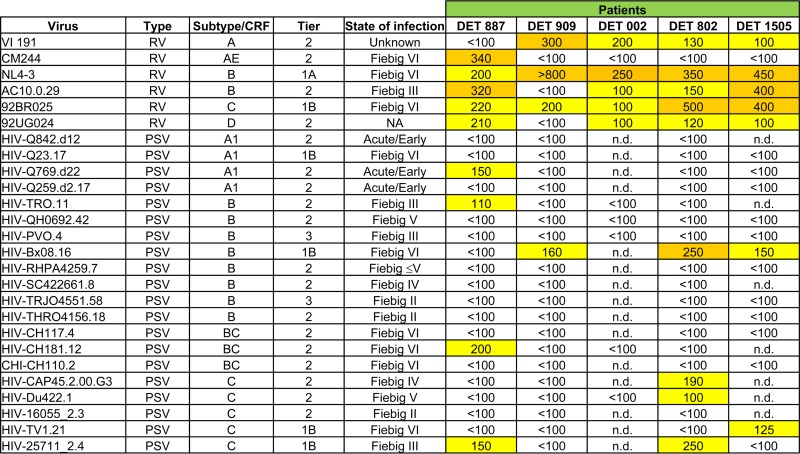
FIG 4.
Serum neutralization (ID50s) data from 5 patients with a broad neutralization profile within the first 6 months of infection. Numbers indicate ID50s. An orange box indicates an ID50 of ≥250, a yellow box indicates an ID50 of ≥100 but <250, and a white box indicates an ID50 of <100. RV, recombinant virus; PSV, pseudotyped virus; NA, not available; n.d., not done.
Twenty-five-microliter aliquots of the corresponding serum 2-fold dilution (1/100 to 1/6,400) in DMEM–10% fetal bovine serum was added. All serum samples were heat inactivated at 56°C for 30 min before use in neutralization assays. Each virus in a total volume of 75 μl then was added to the corresponding wells. The amount of each virus chosen was the lowest level of viral input sufficient to give a clear luciferase signal within the linear range for each virus. The plate was incubated for 1 h at 37°C. After incubation, 104 target cells (TZM-bl) in a volume of 100 μl were added to each well. The plate then was placed in a humidified chamber within a CO2 incubator at 37°C. Luciferase activity in cell lysates was assessed 72 h after infection for recombinant viruses and 48 h after infection for pseudotyped viruses. Luciferase activities in cell lysate infected with nonneutralized viruses were considered 100%. We defined cross-reactive neutralizing responses as the neutralization of at least one virus included in the previously described minipanel from at least three different subtypes and bNA as the ability of serum to neutralize viruses from different clades with a geometric mean 50% infective dose (ID50) titer between 100 and 500 as previously described (29, 30).
Epitope mapping. (i) Neutralization assays.
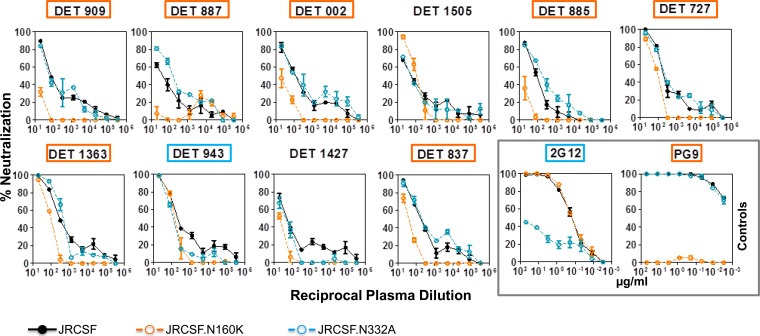
For the characterization of neutralizing antibodies, neutralization assays with single-round HIV-1 Env pseudovirus infection were performed using a luciferase-based assay in TZM-bl cells (38). To determine the serum concentration causing a 50% reduction of relative light unit value, 4-fold dilutions (1/20 to 1/327,680) were made. The neutralization dose-response curves were fitted by nonlinear regression using a four-parameter hill slope equation. For the assessment of CD4 binding site (CD4bs)-directed neutralization, serum neutralization was blocked with specific protein probes (RSC3 or RSC3 371I/P363N) in a competition assay as previously described (39). For the mapping of neutralizing antibodies directed to glycan structures in the variable region (V1V2 and V3), neutralization assays were performed using JRCSF virus with the N160K mutation and the N332A mutation, respectively (3, 40). For the mapping of NAbs to the MPER of gp41, sera were tested for neutralizing activity against a chimeric HIV-2 virus containing the MPER of HIV-1 (71312.C1) and the parental HIV-2 7312A clone (41, 42). The serum neutralizing antibodies also were mapped to the MPER region by a method of selective peptide inhibition of neutralization (43).
(ii) ELISAs.
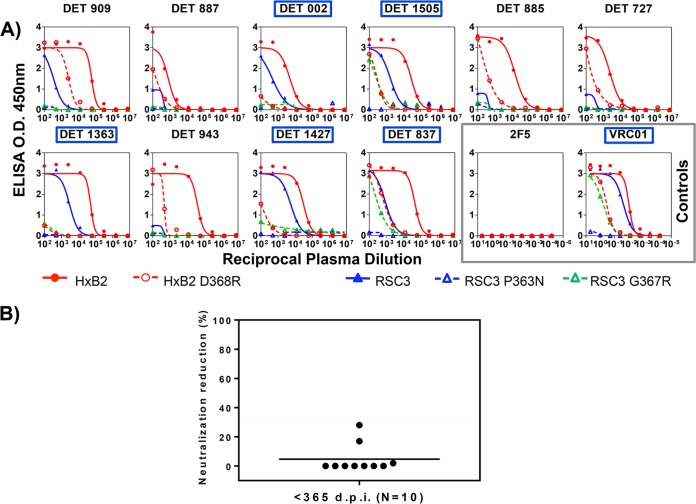
Enzyme-linked immunosorbent assays (ELISAs) were performed as previously described (39, 44). The ELISA plates were coated with the following probes: HxB2 gp120wt and HxB2 gp120 D368R proteins, the antigenically resurfaced glycoprotein RSC3 containing the CD4bs, the RSC3 P363N mutant, and the RSC3 G367R probe. Sera that showed a loss of reactivity to the CD4bs mutants (HxB2 D368R and RSC3 P363N) and reacted to both RSC3 and RSC3 G367R were classified as containing CD4bs antibodies.
Statistics.
Analyses were performed using GraphPad Prism 5 (GraphPad Software). Mann-Whitney U test was used for comparisons of continuous variables between groups. Simple comparisons were made with the use of a two-sided alpha level of 0.05.
RESULTS
Demographic characteristics.
In the <365-dpi group, 95.5% of participants were male and 4.5% were female. Heterosexual transmission was reported for 8.9% of the patients, while men who have sex with men (MSM) accounted for 86.6% of the study population. Intravenous drug use (IDU) accounted for 1.3% of the study population. In the >365-dpi group, 77.6% of participants were male and 22.4% were female. Heterosexual transmission was reported for 24.7% of the patients, while men who have sex with men accounted for 65.9% of the study population. Intravenous drug use accounted for 7.1% of the study population (Table 1). In the <365-dpi group, all patients were treatment naive. The chronically infected individuals (>365 dpi) with a history of treatment at the time of sampling had been off treatment for at least 3 years. Clinical characteristics of the patients are shown in Table 1. The median and range for each patient group for CD4+ T cell count, viral load (VL), and number of years since HIV diagnosis at the time of screening are shown.
TABLE 1.
Clinical characteristics of patient groups
| Parameter | Value(s) for group: |
P valued | |
|---|---|---|---|
| <365 dpi (n = 157) | >365 dpi (n = 170) | ||
| Gender (no. [%]) | |||
| Male | 150 (95.5) | 132 (77.6) | |
| Female | 7 (4.5) | 38 (22.4) | <0.001 |
| Age (median yr [IQR]) | 34 (28, 39) | 41 (34, 46) | |
| Origin (no. [%]) | |||
| Western Europe | 106 (67.5) | 130 (76.5) | 0.065 |
| Eastern Europe | 1 (0.6) | ||
| South America | 37 (23.6) | 22 (12.9) | |
| Caribbean | 3 (1.9) | 6 (3.5) | |
| North America | 4 (2.5) | 1 (0.6) | |
| Sub-Saharan Africa | 1 (0.6) | ||
| Northern Africa | 1 (0.6) | ||
| Other | 5 (3.2) | 10 (1.7) | |
| Transmission route (no. [%]) | |||
| MSM | 136 (86.6) | 112 (65.9) | 0.003 |
| Heterosexual | 14 (8.9) | 42 (24.7) | |
| Bisexual | 5 (3.2) | 2 (1.2) | |
| IDU | 2 (1.3) | 12 (7.1) | |
| Time since infectiona (median days [IQR]) | 94 (52, 137) | 1,962 (887, 3,607) | <0.001 |
| Early recent (n = 88) | 56 (37, 73) | ||
| Late recent (n = 43) | 131 (111, 145) | ||
| Early chronic (n = 26) | 203 (182, 222) | ||
| Symptomatic primary infection (no. [%]) | 89 (56.7) | ||
| Fiebig stageb (no. [%]) | |||
| I | |||
| II | 2 (1.3) | ||
| III | 4 (2.5) | ||
| IV | 6 (3.8) | ||
| V | 75 (47.8) | ||
| VI | 70 (44.6) | 170 (100) | |
| Subtypec (no. [%]) | |||
| B | 125 (79.6) | 57 (33.5) | 0.086 |
| Non-B | 21 (13.4) | 4 (2.4) | |
| Unknown | 11 (7) | 109 (64.1) | |
| CD4b (median no. of cells/μl [IQR]) | 479 (366, 629.5) | 559.50 (435.75, 753.75) | 0.489 |
| CD8b (median no. of cells/μl [IQR]) | 905 (605.3, 1,415.3) | 1094 (859.75, 1,591.5) | 0.041 |
| CD4+/CD8+b (median no. of cells/μl [IQR]) | 0.4 (0.19, 2.28) | 0.5 (0.36, 0.71) | <0.001 |
| Plasma VLb (median no. of copies [IQR]) | 55,466 (16,652, 178,436) | 8,898 (2,427, 24,310) | <0.001 |
| Plasma VL log10b (median [IQR]) | 4.73 (4.22, 5.27) | 3.94 (3.38. 4.38) | <0.001 |
Estimated days of infection.
At the time of sample collection.
According to protease and retrotranscriptase sequences.
Mann-Whitney U test was used for continuous variables, and for simple comparisons a two-sided alpha level of 0.05 was used.
Identification and characterization of patients infected with HIV for less than 1 year.
We studied neutralization breadth in a group of 157 patients with recent HIV-1 infection. Within these patients, 88 were infected for less than 100 days (termed early recent infection), 43 were infected for more than 100 days but less than 180 days (termed late recent infection), and 26 patients were infected for more than 180 days but less than 365 days (termed early chronic infection). Regarding Fiebig stages of infection (37), the majority of patients were classified as stages V and VI (47.8% and 44.6%, respectively), and a small proportion were recently infected and classified as stages II, III, and IV (1.3%, 2.5%, and 3.8%, respectively) (Table 1).
We next calculated the estimated day of infection for all samples from the <365-dpi group as 20 days before the symptom onset date for symptomatic subjects or the midpoint between the last negative and first positive HIV test for those lacking symptoms (28, 45). The median estimated duration of infection for the patients with less than 1 year of infection was 94 days (interquartile range [IQR], 52 to 138). Furthermore, according to the three groups established for patients infected for less than 1 year, the median estimated duration of the infection in early recent infection was 56 days (IQR, 37 to 73); the median estimated duration for patients with late recent infection was 131 days (IQR, 111 to 145), and the median estimated duration of infection for early chronic patients was 203 days (IQR, 184 to 222) (Table 1).
As a control group, we selected 170 individuals with evidence of chronic HIV-1 infection (more than 365 dpi since the first HIV-positive sample) from a previous cross-sectional study (33). At the time of sampling, these samples had a median estimated duration of infection, calculated as the time since the first positive HIV serology to the time of sampling, of 1,962 days, or 5.4 years (IQR, 887 to 3,607 days) (Table 1).
Initial screen for cross-neutralizing sera.
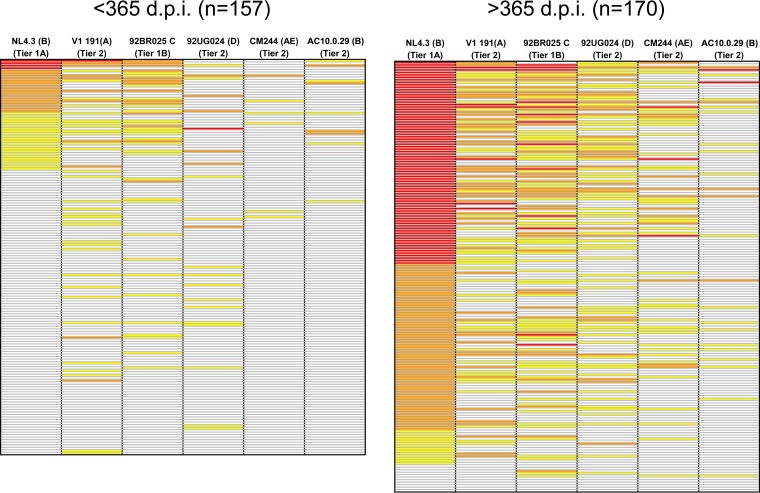
We next sought to use the screening panel to survey all of the serum samples (157 from the <365-dpi group and 170 from the >365-dpi group) for cross-neutralizing activity. All of the samples were tested against a previously described 6-virus minipanel (Fig. 2). This minipanel includes 6 viruses from 5 different subtypes (33). Each virus-serum sample combination was analyzed in triplicate at 1/200 dilution. Figure 2 summarizes the neutralization results for the 331 serum samples.
FIG 2.
Neutralization data for patients infected for less than 365 days and patients infected more than 365 days against the minipanel of viruses. Shown are percentages of neutralization at a 1/200 serum dilution. A white box indicates <50% neutralization, a yellow box indicates ≥50% and <70% neutralization, an orange box indicates ≥70% and <90%, and a red box indicates ≥90% neutralization.
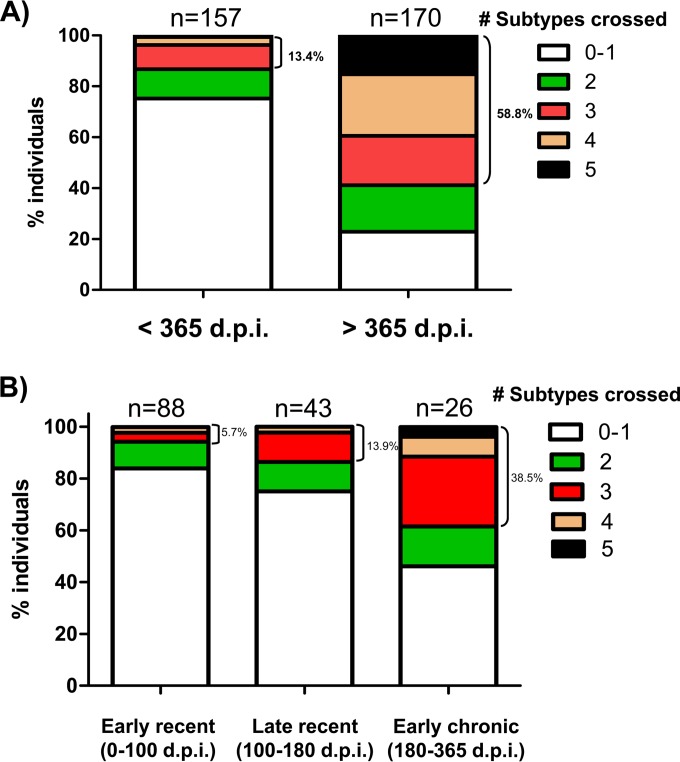
Detectable cross-neutralizing responses, defined as neutralization of at least one virus from at least three different subtypes, were observed in sera from 21 patients infected for less than 1 year. The selection criterion for identification of cross-neutralizing responses used in the present study was less restrictive than that of previous studies done with the same virus panel due to the relevance and rarity of these responses (24, 33). Significant differences in neutralization breadth were observed between both groups (<365 dpi and >365 dpi) (Fig. 3A). A total of 13.4% (21 out of 157) of the samples from <365-dpi patients achieved 50% neutralization of at least one recombinant virus from three different subtypes at a ≥1/200 dilution. In contrast, the percentage of samples from >365-dpi patients capable of neutralizing at least one recombinant virus from three different subtypes was 58.8% (100 out of 170). No significant neutralization of the VSV pseudotyped control was observed in any of the samples analyzed. It is important to note that, despite being much less frequent (P = 0.001), we have detected cross-neutralizing responses in patients infected for less than 1 year. Remarkably, 3.8% of the samples from <365-dpi patients were capable of neutralizing viruses from at least 4 different subtypes (6 out of 157) (Fig. 3A).
FIG 3.
Differences in neutralization breadth in patients from different times postinfection. The percentages of patients capable of neutralizing at least one virus from different subtypes out of a previously described minipanel in each patient group are shown. The number of subtypes crossed are indicated with different colors. The numbers of patients per group are indicated. The percentages of patients capable of neutralizing at least one virus from 3 different subtypes also are indicated. (A) Comparison between <365-dpi and >365-dpi patient groups. (B) Comparison among three patient groups within the first year of infection (0 to 100, 100 to 180, and 180 to 365 dpi).
We then analyzed differences in neutralization breadth among the three groups of patients infected for less than 1 year (early recent, late recent, and early chronic infection) (Fig. 3B). The percentage of patients capable of neutralizing at least one virus from three different subtypes was 5.7% (5 out of 88) in early recent infection (<100 dpi), 13.9% (6 out of 43) in late recent infection (100 to 180 dpi), and 38.5% (10 out of 26) in early chronic infection (180 to 365 dpi). These results agree with previous reports that showed an increase in the frequency of broadly neutralizing responses over time in chronically infected patients, but to our knowledge, this is the first time that this effect has been described in patients infected with HIV for less than 1 year (28, 46). It is important to note that 11 of the 21 patients (52.3%) that showed a significant neutralization breadth within the first year of infection (5 in early recent and 6 in late recent infection) showed detectable broad cross-neutralizing responses within the first 6 months of infection.
Confirmation of neutralization breadth in patients that showed cross-neutralizing responses within the first 6 months of infection.
Due to the potential interest of bNA responses induced so early after seroconversion, we next further characterized the cross-neutralizing activities detected within the first 6 months of infection against both the minipanel and an extended virus panel by making complete neutralization curves and by calculating the ID50 values. In this study, we have used the definition of bNA previously used by van den Kerkhof et al. (“the ability of serum to neutralize viruses from different clades with a geometric mean IC50 titer between 100 and 500”) (30).
From the available sera from patients within the first 6 months of infection, we selected 2 patients infected for <100 dpi (DET 887 and DET 909) and 3 patients infected for >100 but <180 dpi (DET 002, DET 802, and DET 1505) capable of neutralizing at least one virus from three different subtypes from the minipanel of recombinant viruses. Clinical characteristics of the selected patients are shown in Table 2. The other 6 samples with cross-neutralizing activity within the first 6 months of infection could not be further characterized for sample availability reasons. The selected samples also were tested against a new panel with 20 additional pseudotyped viruses from 4 different subtypes and recombinant forms. In order to increase the stringency of our analysis, we chose preferably difficult-to-neutralize viruses (2 tier 3, 14 tier 2, and 4 tier 1). Complete neutralization curves were done with these sera and viruses from both panels (6-recombinant-virus minipanel and 20-pseudovirus panel). In total, 5 selected serum samples were tested against 26 viruses from 6 different subtypes and recombinant forms, different states of infection (Fiebig classification [34]), different tropisms, and different sensitivities to neutralization (tier classification [33]). The characteristics of each virus are shown in Fig. 4. Reciprocal dilutions of sera that reduce infectivity by 50% (ID50s) calculated from these curves also are depicted in Fig. 4. This new panel confirmed that the neutralizing activity of the analyzed viruses (previously selected with the 6-virus panel) was broadly reactive.
TABLE 2.
Clinical characteristics of 5 of the patients that showed a significant cross-neutralizing response in the first 6 months of infection
| Parameter | Value fora: |
||||
|---|---|---|---|---|---|
| DET 887 | DET 909 | DET 002 | DET 802 | DET 1505 | |
| Time since infection (days) | 96 | 77 | 174 | 180 | 119 |
| Infection subgroup | Early recent | Early recent | Late recent | Late recent | Late recent |
| % viruses neutralized (extended panel) | 34.6 | 15.3 | 19.2 | 34.6 | 26.9 |
| No. of subtypes neutralized | 6 | 3 | 4 | 4 | 4 |
| Gender | Male | Male | Male | Male | Male |
| Age (yr) | 42 | 26 | 43 | 31 | 26 |
| Origin | Western Europe | Western Europe | Western Europe | Western Europe | Western Europe |
| Transmission route | MSM | MSM | IDU | Bisexual | Heterosexual |
| Test for recent infection (<180 days) | Positive | Positive | Positive | Positive | ND |
| Western blot (p31 band) result | Negative | Negative | ND | ND | ND |
| Symptomatic infection | Yes | Yes | No | No | No |
| Fiebig stage | V | V | VI | VI | VI |
| Subtype (retrotranscriptase/protease) | B/B | B/B | B/CRF02AG | B/B | B/B |
| Viral tropism | Dual | R5 | ND | R5 | R5 |
| CD4 (cells/μl) | 653 | 234 | 536 | 780 | 216 |
| CD8 (cells/μl) | 658 | 1,458 | 2,642 | ND | 445 |
| CD4+/CD8+ | 0.99 | 0.16 | 0.20 | ND | 0.49 |
| VL (copies/ml) | 76,311 | 111,008 | 148,000 | 5,271 | 24,630 |
| Log10 VL | 4.88 | 5.05 | 5.17 | 3.72 | 4.39 |
ND, not determined.
Impact of plasma viral load levels on neutralization breadth in the first year of infection.
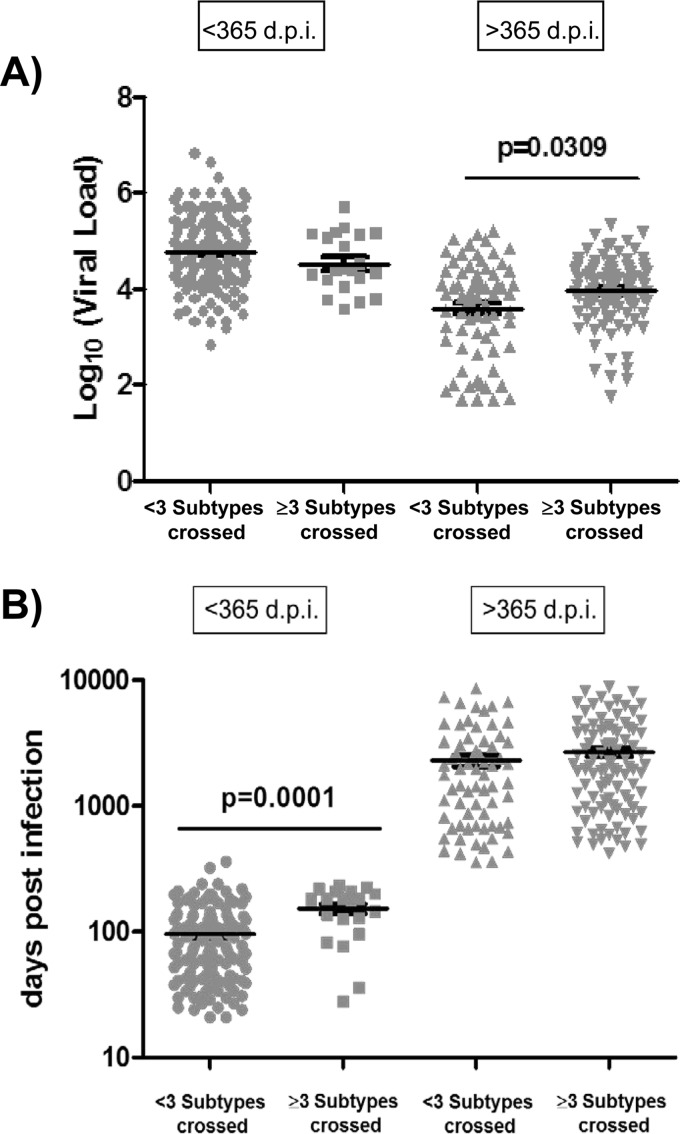
In order to investigate in our patients the previously reported correlation between neutralization breadth and plasma viral load levels, we evaluated the levels of viremia in patients with cross-neutralizing responses and patients with less neutralization breadth. Data on all patients, infected for more or less than 1 year, were compiled and analyzed by comparing samples from patients capable of neutralizing viruses across 3 or more subtypes (cross-neutralizing response) and samples from patients that neutralized viruses from fewer than 3 subtypes. Our analysis revealed that the levels of viremia were significantly higher for patients infected for more than 1 year and showing cross-neutralizing responses (P = 0.0309) (Fig. 5A). However, no differences were observed when we compared the levels of viremia between patients capable of neutralizing three or more subtypes and patients that neutralized fewer than three subtypes within the first year of infection (Fig. 5A).
FIG 5.
Viral loads and days postinfection in patients with different neutralization breadths (<3 subtypes crossed and ≥3 subtypes crossed). Statistical analysis of the viral load levels (A) and the days postinfection (B) is shown. Horizontal bars within the point plots indicate the median for each group ± standard errors of the means (SEM). Significance between groups is indicated above groups. Each symbol indicated a different group of patients. Gray circles indicate <3 subtypes crossed within the <365-dpi group, gray squares indicate ≥3 subtypes crossed within the <365-dpi group, gray triangles indicate <3 subtypes crossed within the >365-dpi group, and gray inverted triangles indicate ≥3 subtypes crossed within the >365-dpi group. Mann-Whitney U tests were used for comparisons between groups. Simple comparisons were made with the use of a two-sided alpha level of 0.05.
Impact of the time post-HIV-1 infection on neutralization breadth within the first year of infection.
We next investigated the potential relationship between neutralization breadth and time postinfection in our patients. A univariate model analysis showed that, in patients infected for more than 1 year, the number of days postinfection were similar in patients with broad neutralization and patients with less neutralization breadth (Fig. 5B). In contrast, the number of days postinfection was significantly higher for patients with cross-neutralizing responses and less than 1 year of infection (P = 0.0001). Our results showed that there is a strong correlation between neutralization breadth and time postinfection during the first year of HIV infection. However, this correlation was not observed when patients were infected for more than 1 year (Fig. 5B).
Impact of CD4+/CD8+ T lymphocyte ratios and the presence of symptomatic primary infection on neutralization breadth within the first year of infection.
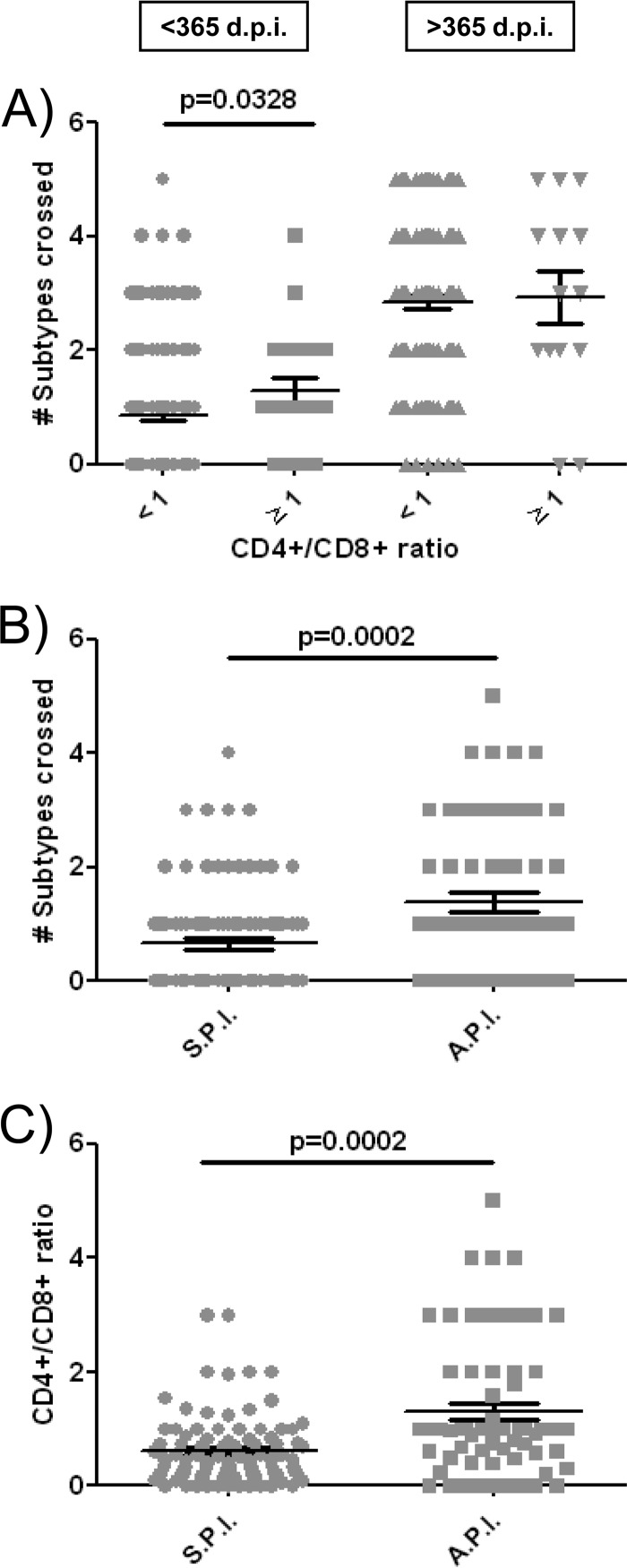
We also investigated the potential association of neutralization breadth with the CD4+/CD8+ ratio. It has been suggested that the cutoff ratio of <1.0 is associated with immunosenescence and mortality (47–49). We therefore evaluated the levels of neutralization breadth (number of subtypes crossed) in patients with CD4+/CD8+ ratios of <1 and ≥1. Data on all patients from <365- and ≥365-dpi groups were compiled and analyzed by comparing samples from patients with CD4+/CD8+ ratios of ≥1 and patients with CD4+/CD8+ ratios of <1. Our analysis revealed that neutralization breadth was significantly higher in patients with a healthy CD4+/CD8+ ratio (≥1) within the first year of infection (P = 0.0328) (Fig. 6A). In addition, the breadth of the humoral immune response in patients with an asymptomatic primary infection is also significantly higher (P = 0.0002) (Fig. 6B). Both the absence of symptoms during primary infection and the maintenance of CD4+/CD8+ ratios above 1 can be interpreted as a consequence of a lower level of immune damage during recent infection. In fact, in the present study we have observed an asymptomatic primary infection is significantly associated with higher CD4+/CD8+ ratios during the first year of HIV infection (P = 0.0002) (Fig. 6C). Contrary to what was observed during the first year of infection, we found no differences in neutralization breadth between patients with CD4+/CD8+ ratios of ≥1 or <1 in chronic infection (Fig. 6A).
FIG 6.
Neutralization breadths in patients with different CD4+/CD8+ ratios and, within the first year of infection, with or without symptomatic primary infection. (A) Statistical analysis of CD4+/CD8+ ratios, (B) the presence or absence of symptomatic primary infection in the <365-dpi group, and (C) CD4+/CD8+ ratios in patients with or without primary infection within the <365-dpi group. Horizontal bars within the point plots indicate the medians for each group ± SEM. Significance between groups is indicated above groups. Each symbol indicated a different group of patients. (A) Gray circles indicate CD4+/CD8+ ratios of <1 and <365 dpi, gray squares indicate CD4+/CD8+ ratios of ≥1 and <365 dpi, gray triangles indicate CD4+/CD8+ ratios of <1 and >365 dpi, and gray inverted triangles indicate CD4+/CD8+ ratios of ≥1 and >365 dpi. (B and C) Gray circles indicate symptomatic primary infection (S.P.I.), and gray squares indicate asymptomatic primary infection (A.P.I.). Mann-Whitney U tests were used for comparison between groups. Simple comparisons were made with the use of a two-sided alpha level of 0.05.
We also compared some additional clinical and demographic data for all patients (<365-dpi group and >365-dpi group) to the presence of cross-neutralizing activity and observed no association with gender, age, origin, transmission route, or subtype (data not shown). We cannot rule out that some of the clinical or demographic variables analyzed are associated with the presence of cross-neutralizing activity, but we have not been able to establish these associations with the group of patients included in our study.
Epitope mapping.
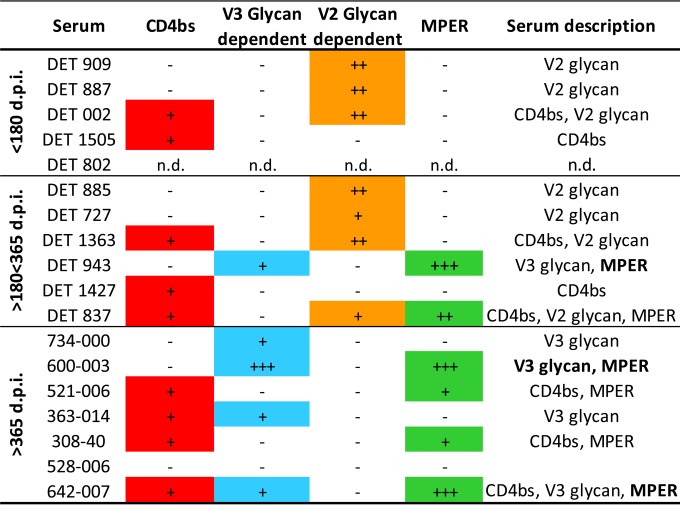
The epitopes targeted by the neutralizing antibodies present in sera from 4 patients with recent infection (<180 dpi) and 6 early chronic patients (180 to 365 dpi) with cross-neutralizing profiles (capable of neutralizing at least one virus from 3 subtypes from the minipanel) were mapped (Fig. 7 to 10). The remaining 11 sera with cross-neutralizing profiles were not further characterized for sample availability reasons. The presence of antibodies directed against CD4bs, glycans in the V2 loop region, glycans in the V3 loop, and MPER epitopes also was analyzed in samples from seven patients with cross-neutralizing profiles chronically infected for more than 365 dpi that were previously characterized with the same minipanel of viruses (Fig. 7).
FIG 7.
Summary of the serum specificities of the sera from patients with a broad neutralization profile in recent (less than 180 dpi), early chronic (180 to 365 dpi), and chronic (>365 dpi) infection. The presence of antibodies against the four epitopes (CD4bs, V3 glycans, V2 glycans, and MPER) is represented by plus signs as follows. For CD4bs, + indicates RSC3 protein competition with <30% reduction in ID50 but ELISA differences in binding RSC3/DRSC3 and/or differences in binding G367R/DRSC3. Binding was categorized based on the optical density at 450 nm (OD450) at the highest dilution tested (1/100) and 50% effective concentration (EC50) values: ++++, OD450 > 3.0 and EC50 > 5,000; +++, OD450 > 3.0 and EC50 < 5,000; ++, 1.0 < OD450 < 3.0; +, 0.2 < OD450 < 1.0; −, OD450 < 0.2. For the V3 glycan/V2 glycan, +++ indicates ≥50% effect on ID50 and ID80 of JRCSF.N332A/JRCSF.N160K, ++ indicates ≥70% effect on ID50 JRCSF.N332A/JRCSF.N160K, and + indicates ≥50% effect on ID50 JRCSF.N332A/JRCSF.N160K. For MPER, +++ indicates ≥50% neutralization inhibited by MPER peptide competition (MPR.03) and ≥10-fold 7312.C1.Y720S/7312.Y720S reduction in neutralization, ++ indicates ≥10-fold 7312.C1.Y720S/7312.Y720S reduction in neutralization, and + indicates ≥4-fold 7312.C1.Y720S/7312.Y720S reduction in neutralization.
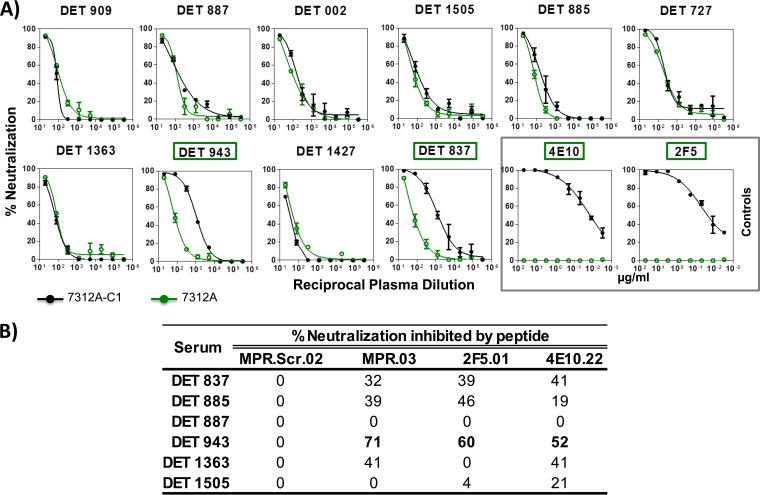
FIG 10.
Detection of antibodies specific for the membrane-proximal region in the sera from patients within the first year of HIV-1 infection. (A) Sera with neutralizing antibodies against the MPER are detected by neutralization assays with viruses 7312A-C1 and 7312A. Sera with reactivity to the MPER domain are indicated in green. (B) ID50 titers in the presence of MPER-derived peptides. Selected sera were tested against 7312.C1 virus competing with soluble MPER peptides (MPR.Scr.02, MPR.03, 2F5.01, and 4E10.22). The peptide-specific neutralizing antibody response was calculated with the equation [1 − (ID50 with peptide/ID50 with mock peptide)] × 100. Values greater than 50% are shown in boldface.
Sera with antibodies directed against CD4bs were detected by ELISA in all groups of patients (Fig. 7 and 8 and N. Gonzalez, K. McKee, R. M. Lynch, I. S. Georgiev, L. Jimenez, E. Grau, E. Yuste, P. D. Kwong, J. R. Mascola, and J. Alcami, submitted for publication). To determine whether virus neutralization was mediated by CD4bs antibodies contained in sera, RSC3 competition neutralization assays were carried out (Fig. 7 and 8B). None of the sera contained detectable CD4bs-directed neutralizing antibodies capable of being blocked by RSC3. Regarding the identification of V2 and V3 glycan-dependent HIV-1 neutralizing antibodies, a decrease in serum neutralization against JRCSF.N160K or JRCSF.N332A compared to the neutralization of wild-type JRCSF suggests the presence of neutralizing antibodies directed to glycans in V1V2 or V3, respectively (Fig. 7 and 9). V2 glycan-dependent HIV-1 NAbs were abundantly found in serum samples from individuals infected with HIV for less than 365 dpi (7 out of 10). However, no antibodies against this region were detected in the samples from chronic individuals (0 out of 7), suggesting that V2 glycan epitopes are targeted predominantly by antibodies from early neutralizing responses in the studied patients. Nevertheless, V3 glycan-dependent antibodies were not detected in the samples from patients with recent infection (<180 dpi), while antibodies against that epitope were detected in chronic patients (4 out of 7) (Fig. 7 and Gonzalez et al., submitted).
FIG 8.
CD4 binding site neutralizing antibody specificity of the sera from patients with a broad neutralization profile within the first year of infection. (A) ELISA antigen binding profile of the sera from 10 patients capable of neutralizing at least one virus from 3 different subtypes from a previously described minipanel. Sera with antibodies against CD4bs were detected by ELISA with the following probes: HxB2 gp120wt, D368R, RSC3 containing the CD4bs, and the corresponding P363N and G367R mutants. Sera with reactivity to the CD4bs are indicated in blue. Monoclonal antibodies 2F5 and VRC01 have been used as controls and are indicated by a gray box. (B) Effect of resurfaced stabilized cores RSC3 and RSC3 P363N on the neutralization of RW020 by the selected sera. The net percent reduction in neutralization of RW020 attributed to RSC3 compared to RSC3 P363N is plotted (y axis). A reduction greater than 30% is considered positive.
FIG 9.
Detection of glycan-dependent HIV-1 neutralizing antibodies in sera from patients within the first year of HIV-1 infection. Sera with neutralizing antibodies against glycan-dependent motifs in the V2 and V3 loops are detected by neutralization assays with JRCSFwt and the corresponding N160K and N332A mutants (V2 and V3, respectively). Percent serum neutralizing activity sensitive to the indicated residue replacement was calculated using the equation [1 − (IC50 variant/IC50 wild type)] × 100. A sample is considered positive if there is a decrease in ID50 greater than or equal to 50% for the mutant relative to the wild-type virus. Reactivities to glycan-dependent motifs in V2 and V3 are indicated in orange and blue, respectively.
For the mapping of antibodies directed against the MPER of gp41 (Fig. 7 and 10A), sera were analyzed assessing neutralizing activity against 7312A and 7312A-C1 viruses, and 2 sera out of 6 from early chronic patients and 4 sera out of 7 from chronic patients appeared to contain MPER-reactive NAbs. In 3 out of these 6 samples (serum DET 943 from an early chronic patient and sera 600-003 and 642-007 from chronically infected individuals), the specificity of the antibodies against MPER was inhibited with MPR.03 peptide, which covers the complete MPER domain (data not shown). Similar to what has been observed in V3, the MPER epitope was not targeted by antibodies in samples from patients infected for less than 180 dpi.
Overall, in samples from patients with recent infection (<180 dpi), cross-neutralization was mediated mainly by a single specificity among the known regions of susceptibility, whereas in most of the samples with early chronic infection (>180 dpi) and chronic infection (>365 dpi) it was associated with different specificities (Fig. 7).
DISCUSSION
It is now recognized that up to 50% of HIV-1 chronically infected subjects are able to generate cross-neutralizing antibody responses of significant breadth, and such responses are detectable in their plasma samples, on average, 2.5 years after HIV-1 infection (6, 50). However, on very rare occasions, cross-neutralizing antibodies have been detected close to the first year of infection (29, 30, 50). Still, it is currently unknown whether these responses can be detected even closer to the time of infection. In order to answer this question, here we screened for patients with cross-neutralizing responses within the first year of infection. Remarkably, we have identified 5 patients with a broad neutralizing response within the first 6 months of infection. In fact, one of these patients serum neutralized tier 1A, 1B, and 2 viruses from 3 different subtypes and 2 recombinant forms just 96 days postinfection. To our knowledge, this is the first time that bNA has been detected in such a relatively short time frame postinfection. Our data, together with the publications mentioned above showing bNA close to the first year of infection (29, 30, 50), serve as evidence that the human immune system, under certain circumstances, is capable of developing neutralizing responses against the HIV-1 envelope glycoprotein from a diverse group of HIV-1 isolates earlier than previously thought.
It is currently unknown whether the rare early generation of bNA is the result of a stochastic event or could be attributed to viral factors, host factors, or an interaction between both. For instance, patients capable of inducing broad neutralizing responses could have been infected with viruses with special envelope signatures that allow them to better engage bNAb precursors, such as shorter V1-V4 envelope loops, specific signatures in the envelope CD4 coreceptor binding site, a shorter V1 envelope region, lower general envelope glycosylation, and a lower probability of glycosylation at the gp160 332 position (51–53).
Another viral factor that could contribute to the generation of an early bNA is viral diversity. The role of viral diversity driving bNAb induction has been previously described in chronically infected patients (54–56). In the present study, we have observed in patients within the first year of HIV-1 infection a significant correlation between neutralization breadth and time postinfection. Considering that early in infection viral genetic diversity increases in an approximately linear fashion (57, 58), we hypothesize that one of the factors that contributed to an improved neutralization breadth in recent infection is the accumulated viral diversity.
In our study, we have identified five individuals who developed bNA within the first 6 months of infection (2 as early as 77 and 96 days postinfection). Unfortunately, the small number of patients of this type and limitations in sample availability that we have been able to identify makes the association of certain viral factors with the ability to induce bNA highly unlikely. Further studies with larger and more diverse patient cohorts would be necessary in order to determine the association between different viral factors, such as specific envelope signatures or the diversity of the viral quasispecies and the ability to induce broadly neutralizing responses in recent infection.
Among the host factors that could facilitate the induction of a broader neutralizing response are certain characteristics of the immune status of the patient. In fact, there are several studies that show a correlation between certain B cell profiles and an improved ability to induce broad humoral immune responses against HIV in chronic patients. A study published in 2013 by our group showed an association between a broad neutralizing response and a partially restored B cell phenotype evidenced by increased frequency of naive B cells and a lower frequency of tissue-like and activated B cells (24). A recent report has also shown that an early preservation of CXCR5+ PD-1+ helper T cells and B cell activation was associated with induction of broadly neutralizing responses in chronic infection (59). These observations allow us to be more optimistic about the possibilities of the development of vaccination strategies based on the induction of bNAbs, since they indicate that while this type of antibody is very difficult to induce in infected patients, they would be favored in healthy individuals with an intact B cell phenotype. In the present study, we found a similar effect in comparisons of the amplitude of the neutralizing response in patients with healthy CD4+/CD8+ ratios (≥1) (47–49), within the first year of HIV infection, and with patients with lower ratios. Neutralization breadth was significantly higher in patients with CD4+/CD8+ ratios similar to those of healthy individuals. In contrast, these differences were not observed in chronically infected patients. Furthermore, several studies have suggested that symptomatic primary infection is associated with poorer subsequent outcomes and higher risk of disease progression (60–62). Consistent with this observation, we found that patients with an asymptomatic primary infection had significantly increased neutralization breadth that could be attributed to minor immune damage, also evidenced by higher CD4+/CD8+ ratios (Fig. 6).
Another important aspect on which we have focused in the present study is the mapping of the epitopes recognized by patients who show improved neutralization breadth in recent infection. While it has been described that early cross-neutralizing antibody responses (on average, at 2.5 years after infection) targeted mainly epitopes within and around the CD4-binding site or epitopes present on the virion-associated trimeric envelope but not glycopeptide residues in the V1/V2 region (49), early development of broadly neutralizing antibodies in HIV-1-infected infants (at approximately 1 year after transmission from their mothers) has not been associated with any epitope specificity (63). Moreover, there is no information about epitope specificities of HIV-1-cross-neutralizing antibodies earlier than 1 year after infection.
In our study, we have observed that most of the cross-neutralizing responses during the first year of infection were highly associated with antibodies that recognize glycans in V2, while in chronic infection these responses were more related to the recognition of epitopes located on the CD4 binding site, in the glycan-dependent V3 site of vulnerability, and the MPER. In fact, no recognition of V2 glycans was found in patients with cross-neutralizing responses in chronic infection when the same characterization was carried out (Gonzalez et al., submitted). However, although a significant trend is observed, these data are not statistically significant. A much larger screening, incorporating individuals with no bNA within the first year of infection, would be necessary to confirm this observation. RSC3-reactive antibodies identified by ELISA were not able to display neutralizing activity as confirmed by competition neutralization assays. In these samples the levels of antibodies directed against CD4bs may be too low to mediate neutralization; alternatively, the maturation of these antibodies could be insufficient to elicit strong avidity toward the CD4bs. Overall, in samples from patients with early recent and late recent infections (<180 dpi), cross-neutralization was mainly mediated by a single specificity (glycans in V2). In contrast, in most of the samples with early chronic (180 to 365 dpi) (Fig. 7) and chronic infections (>365 dpi; Gonzalez et al., submitted), it was associated with 1 to 3 different specificities (CD4 binding site, glycan-dependent V3 site of vulnerability single specificity, and the MPER) according to what has been previously described in chronically infected individuals (3, 46).
Our observation that cross-neutralizing antibodies induced within the first months after infection targeted mainly glycans in the V2 region is not surprising considering that neutralizing antibodies to the V1V2 region are among the most prevalent cross-reactive neutralizing antibodies elicited by natural infection, and they can be elucidated relatively rapidly through initial selection of B cells with a long complementarity-determining region H3 segment and limited somatic hypermutation (3, 46, 55, 64–66).
The results of this study show that, contrary to what had been previously thought, the induction of neutralizing responses of significant breadth during the first 6 months of infection is feasible. We have also determined that this type of response is favored when the immune system of the patient has not undergone a major deterioration during the primary infection and is associated with the recognition of V1-V2 glycan-dependent motives. The study of the viral envelope proteins responsible for the induction of antibodies with improved neutralization breadth in recent infection, along with the characterization of bNAbs isolated from these patients, would provide valuable information for the design of an HIV-1 vaccine.
ACKNOWLEDGMENTS
We thank the study participants. We also thank the Collaboration for AIDS Vaccine Discovery (CAVD)/HIV Specimen Cryorepository (HSC) at the Fraunhofer IBMT and their contributors, D. Montefiori (HIV-Bx08.16, HIV-QH0692.42, HIV-PVO.4, HIV-TRO.11, HIV-Du422.1, HIV-TV1.21, HIV-16055_2.3, and HIV-25711_2.4), B. Hahn and J. Salazar (HIV-RHPA4259.7), B. Hahn (HIV-SC422661.8), B. Hahn and X. Wei (HIV-TRJO4551.58), B. Hahn and D. Kothe (HIV-THRO4156.18), L. Morris (HIV-CAP45.2.00.G3), J. Overbaugh (HIV-Q23.17, HIV-Q769.d22, HIV-Q259.d2.17, and HIV-Q842.d12), and K. Hong and Y. Shao (HIV-CH110.2, HIV-CH117.4, and HIV-CH181.12), for providing the respective HIV-1 Env clones. TZM-bl cells were obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
REFERENCES
- 1.Mascola JR, Montefiori DC. 2010. The role of antibodies in HIV vaccines. Annu Rev Immunol 28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 2.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog 6:e1001028. doi: 10.1371/journal.ppat.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley JM, Lybarger EA, Crooks ET, Seaman MS, Gray E, Davis KL, Decker JM, Wycuff D, Harris L, Hawkins N, Wood B, Nathe C, Richman D, Tomaras GD, Bibollet-Ruche F, Robinson JE, Morris L, Shaw GM, Montefiori DC, Mascola JR. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J Virol 82:11651–11668. doi: 10.1128/JVI.01762-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doria-Rose NA, Klein RM, Manion MM, O'Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. 2014. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter CC, Wagner GA, Hightower GK, Caballero G, Phung P, Richman DD, Pond SL, Smith DM. 2015. HIV-1 neutralizing antibody response and viral genetic diversity characterized with next generation sequencing. Virology 474:34–40. doi: 10.1016/j.virol.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, Wang C, O'Dell S, Li Y, Keele BF, Yang Z, Imamichi H, Doria-Rose N, Hoxie JA, Connors M, Shaw GM, Wyatt RT, Mascola JR. 2012. Selection pressure on HIV-1 envelope by broadly neutralizing antibodies to the conserved CD4-binding site. J Virol 86:5844–5856. doi: 10.1128/JVI.07139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 10.Mascola JR, Lewis MG, Stiegler G, Harris D, VanCott TC, Hayes D, Louder MK, Brown CR, Sapan CV, Frankel SS, Lu Y, Robb ML, Katinger H, Birx DL. 1999. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J Virol 73:4009–4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 14.Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. 2003. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med 9:343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- 15.Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. 2010. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med 16:1117–1119. doi: 10.1038/nm.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M Jr, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto Y, Eda Y, Ogura A, Shibata S, Amagai T, Katsura Y, Asano T, Kimachi K, Makizumi K, Honda M. 1998. In SCID-hu mice, passive transfer of a humanized antibody prevents infection and atrophic change of medulla in human thymic implant due to intravenous inoculation of primary HIV-1 isolate. J Immunol 160:69–76. [PubMed] [Google Scholar]
- 18.Pietzsch J, Gruell H, Bournazos S, Donovan BM, Klein F, Diskin R, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. A mouse model for HIV-1 entry. Proc Natl Acad Sci U S A 109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. 2002. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol 76:8769–8775. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Neale ES Jr, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M Jr, Lifson JD, Gao G, Desrosiers RC, Evans DT, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. 2015. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O'Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun TW, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE. 2015. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caskey M, Klein F, Lorenzi JC, Seaman MS, West AP Jr, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fatkenheuer G, Schlesinger SJ, Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doria-Rose NA, Klein RM, Daniels MG, O'Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol 84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira CB, Merino-Mansilla A, Llano A, Perez I, Crespo I, Llinas L, Garcia F, Gatell JM, Yuste E, Sanchez-Merino V. 2013. Evolution of broadly cross-reactive HIV-1-neutralizing activity: therapy-associated decline, positive association with detectable viremia, and partial restoration of B-cell subpopulations. J Virol 87:12227–12236. doi: 10.1128/JVI.02155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overbaugh J, Morris L. 2012. The antibody response against HIV-1. Cold Spring Harb Perspect Med 2:a007039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doria-Rose NA, Joyce MG. 2015. Strategies to guide the antibody affinity maturation process. Curr Opin Virol 11:137–147. doi: 10.1016/j.coviro.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola JR, Haynes BF. 2013. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev 254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. 2010. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Euler Z, van den Kerkhof TL, van Gils MJ, Burger JA, Edo-Matas D, Phung P, Wrin T, Schuitemaker H. 2012. Longitudinal analysis of early HIV-1-specific neutralizing activity in an elite neutralizer and in five patients who developed cross-reactive neutralizing activity. J Virol 86:2045–2055. doi: 10.1128/JVI.06091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Kerkhof TL, Euler Z, van Gils MJ, Boeser-Nunnink BD, Schuitemaker H, Sanders RW. 2014. Early development of broadly reactive HIV-1 neutralizing activity in elite neutralizers. AIDS 28:1237–1240. doi: 10.1097/QAD.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 31.Romero A, Gonzalez V, Esteve A, Martro E, Matas L, Tural C, Pumarola T, Casanova A, Ferrer E, Caballero E, Ribera E, Margall N, Domingo P, Farre J, Puig T, Sauca MG, Barrufet P, Amengual MJ, Navarro G, Navarro M, Vilaro J, Ortin X, Orti A, Pujol F, Prat JM, Massabeu A, Simo JM, Villaverde CA, Benitez MA, Garcia I, Diaz O, Becerra J, Ros R, Sala R, Rodrigo I, Miro JM, Casabona J. 2012. Identification of recent HIV-1 infection among newly diagnosed cases in Catalonia, Spain (2006-08). Eur J Public Health 22:802–808. doi: 10.1093/eurpub/ckr179. [DOI] [PubMed] [Google Scholar]
- 32.Ambrosioni J, Sued O, Nicolas D, Parera M, Lopez-Dieguez M, Romero A, Aguero F, Marcos MA, Manzardo C, Zamora L, Gomez-Carrillo M, Gatell JM, Pumarola T, Miro JM. 2015. Trends in transmission of drug resistance and prevalence of non-B subtypes in patients with acute or recent HIV-1 infection in Barcelona in the last 16 years (1997-2012). PLoS One 10:e0125837. doi: 10.1371/journal.pone.0125837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina-Ramirez M, Sanchez-Merino V, Sanchez-Palomino S, Merino-Mansilla A, Ferreira CB, Perez I, Gonzalez N, Alvarez A, Alcocer-Gonzalez JM, Garcia F, Gatell JM, Alcami J, Yuste E. 2011. Broadly cross-neutralizing antibodies in HIV-1 patients with undetectable viremia. J Virol 85:5804–5813. doi: 10.1128/JVI.02482-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz A, Koch S, Fuss M, Mazzotta AS, Sarzotti-Kelsoe M, Ozaki DA, Montefiori DC, von Briesen H, Zimmermann H, Meyerhans A. 2012. An automated HIV-1 Env-pseudotyped virus production for global HIV vaccine trials. PLoS One 7:e51715. doi: 10.1371/journal.pone.0051715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozaki DA, Gao H, Todd CA, Greene KM, Montefiori DC, Sarzotti-Kelsoe M. 2012. International technology transfer of a GCLP-compliant HIV-1 neutralizing antibody assay for human clinical trials. PLoS One 7:e30963. doi: 10.1371/journal.pone.0030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scanlan CN, Pantophlet R, Wormald MR, Ollmann SE, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1–>2 mannose residues on the outer face of gp120. J Virol 76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gray ES, Moore PL, Choge IA, Decker JM, Bibollet-Ruche F, Li H, Leseka N, Treurnicht F, Mlisana K, Shaw GM, Karim SS, Williamson C, Morris L. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J Virol 81:6187–6196. doi: 10.1128/JVI.00239-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR, Wyatt R. 2009. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol 83:1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynch RM, Tran L, Louder MK, Schmidt SD, Cohen M, Dersimonian R, Euler Z, Gray ES, Abdool KS, Kirchherr J, Montefiori DC, Sibeko S, Soderberg K, Tomaras G, Yang ZY, Nabel GJ, Schuitemaker H, Morris L, Haynes BF, Mascola JR. 2012. The development of CD4 binding site antibodies during HIV-1 infection. J Virol 86:7588–7595. doi: 10.1128/JVI.00734-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunwar P, Hawkins N, Dinges WL, Liu Y, Gabriel EE, Swan DA, Stevens CE, Maenza J, Collier AC, Mullins JI, Hertz T, Yu X, Horton H. 2013. Superior control of HIV-1 replication by CD8+ T cells targeting conserved epitopes: implications for HIV vaccine design. PLoS One 8:e64405. doi: 10.1371/journal.pone.0064405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray ES, Madiga MC, Hermanus T, Moore PL, Wibmer CK, Tumba NL, Werner L, Mlisana K, Sibeko S, Williamson C, Abdool Karim SS, Morris L. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J Virol 85:4828–4840. doi: 10.1128/JVI.00198-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, Van Natta ML, Meinert CL, Lederman MM, Hatano H, Jain V, Huang Y, Hecht FM, Martin JN, McCune JM, Moreno S, Deeks SG. 2014. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 10:e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torti C, Prosperi M, Motta D, Digiambenedetto S, Maggiolo F, Paraninfo G, Ripamonti D, Cologni G, Fabbiani M, Caputo SL, Sighinolfi L, Ladisa N, El-Hamad I, Quiros-Roldan E, Frank I. 2012. Factors influencing the normalization of CD4+ T-cell count, percentage and CD4+/CD8+ T-cell ratio in HIV-infected patients on long-term suppressive antiretroviral therapy. Clin Microbiol Infect 18:449–458. doi: 10.1111/j.1469-0691.2011.03650.x. [DOI] [PubMed] [Google Scholar]
- 49.Wikby A, Maxson P, Olsson J, Johansson B, Ferguson FG. 1998. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev 102:187–198. doi: 10.1016/S0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 50.Mikell I, Sather DN, Kalams SA, Altfeld M, Alter G, Stamatatos L. 2011. Characteristics of the earliest cross-neutralizing antibody response to HIV-1. PLoS Pathog 7:e1001251. doi: 10.1371/journal.ppat.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rademeyer C, Moore PL, Taylor N, Martin DP, Choge IA, Gray ES, Sheppard HW, Gray C, Morris L, Williamson C. 2007. Genetic characteristics of HIV-1 subtype C envelopes inducing cross-neutralizing antibodies. Virology 368:172–181. doi: 10.1016/j.virol.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Gnanakaran S, Daniels MG, Bhattacharya T, Lapedes AS, Sethi A, Li M, Tang H, Greene K, Gao H, Haynes BF, Cohen MS, Shaw GM, Seaman MS, Kumar A, Gao F, Montefiori DC, Korber B. 2010. Genetic signatures in the envelope glycoproteins of HIV-1 that associate with broadly neutralizing antibodies. PLoS Comput Biol 6:e1000955. doi: 10.1371/journal.pcbi.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van den Kerkhof TL, Feenstra KA, Euler Z, van Gils MJ, Rijsdijk LW, Boeser-Nunnink BD, Heringa J, Schuitemaker H, Sanders RW. 2013. HIV-1 envelope glycoprotein signatures that correlate with the development of cross-reactive neutralizing activity. Retrovirology 10:102. doi: 10.1186/1742-4690-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, Mullikin JC, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BT, Kwong PD, Mascola JR, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, Ernandes MJ, Georgiev IS, Kim HJ, Pancera M, Staupe RP, Altae-Tran HR, Bailer RT, Crooks ET, Cupo A, Druz A, Garrett NJ, Hoi KH, Kong R, Louder MK, Longo NS, McKee K, Nonyane M, O'Dell S, Roark RS, Rudicell RS, Schmidt SD, Sheward DJ, Soto C, Wibmer CK, Yang Y, Zhang Z, NISC Comparative Sequencing Program, Mullikin JC, Binley JM, Sanders RW, Wilson IA, Moore JP, Ward AB, Georgiou G, Williamson C, Abdool Karim SS, Morris L, Kwong PD, Shapiro L, Mascola JR. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509:55–62. doi: 10.1038/nature13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pernas M, Sanchez-Merino V, Casado C, Merino-Mansilla A, Olivares I, Yuste E, Lopez-Galindez C. 2015. HIV-1 dual infected LTNP-EC patients developed an unexpected antibody cross-neutralizing activity. PLoS One 10:e0134054. doi: 10.1371/journal.pone.0134054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shankarappa R, Margolick JB, Gange SJ, Rodrigo AG, Upchurch D, Farzadegan H, Gupta P, Rinaldo CR, Learn GH, He X, Huang XL, Mullins JI. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol 73:10489–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kearney M, Maldarelli F, Shao W, Margolick JB, Daar ES, Mellors JW, Rao V, Coffin JM, Palmer S. 2009. Human immunodeficiency virus type 1 population genetics and adaptation in newly infected individuals. J Virol 83:2715–2727. doi: 10.1128/JVI.01960-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen K, Altfeld M, Alter G, Stamatatos L. 2014. Early preservation of CXCR5+ PD-1+ helper T cells and B cell activation predict the breadth of neutralizing antibody responses in chronic HIV-1 infection. J Virol 88:13310–13321. doi: 10.1128/JVI.02186-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lodi S, Fisher M, Phillips A, De Luca A, Ghosn J, Malyuta R, Zangerle R, Moreno S, Vanhems P, Boufassa F, Guiguet M, Porter K. 2013. Symptomatic illness and low CD4 cell count at HIV seroconversion as markers of severe primary HIV infection. PLoS One 8:e78642. doi: 10.1371/journal.pone.0078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindback S, Brostrom C, Karlsson A, Gaines H. 1994. Does symptomatic primary HIV-1 infection accelerate progression to CDC stage IV disease, CD4 count below 200 × 106/l, AIDS, and death from AIDS? BMJ 309:1535–1537. doi: 10.1136/bmj.309.6968.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanhems P, Lambert J, Cooper DA, Perrin L, Carr A, Hirschel B, Vizzard J, de Kinloch LS, Allard R. 1998. Severity and prognosis of acute human immunodeficiency virus type 1 illness: a dose-response relationship. Clin Infect Dis 26:323–329. doi: 10.1086/516289. [DOI] [PubMed] [Google Scholar]
- 63.Goo L, Chohan V, Nduati R, Overbaugh J. 2014. Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat Med 20:655–658. doi: 10.1038/nm.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynch RM, Rong R, Boliar S, Sethi A, Li B, Mulenga J, Allen S, Robinson JE, Gnanakaran S, Derdeyn CA. 2011. The B cell response is redundant and highly focused on V1V2 during early subtype C infection in a Zambian seroconverter. J Virol 85:905–915. doi: 10.1128/JVI.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georgiev IS, Doria-Rose NA, Zhou T, Kwon YD, Staupe RP, Moquin S, Chuang GY, Louder MK, Schmidt SD, Altae-Tran HR, Bailer RT, McKee K, Nason M, O'Dell S, Ofek G, Pancera M, Srivatsan S, Shapiro L, Connors M, Migueles SA, Morris L, Nishimura Y, Martin MA, Mascola JR, Kwong PD. 2013. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science 340:751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 66.Gorman J, Soto C, Yang MM, Davenport TM, Guttman M, Bailer RT, Chambers M, Chuang GY, DeKosky BJ, Doria-Rose NA, Druz A, Ernandes MJ, Georgiev IS, Jarosinski MC, Joyce MG, Lemmin TM, Leung S, Louder MK, McDaniel JR, Narpala S, Pancera M, Stuckey J, Wu X, Yang Y, Zhang B, Zhou T, Program NC, Mullikin JC, Baxa U, Georgiou G, McDermott AB, Bonsignori M, Haynes BF, Moore PL, Morris L, Lee KK, Shapiro L, Mascola JR, Kwong PD. 2016. Structures of HIV-1 Env V1V2 with broadly neutralizing antibodies reveal commonalities that enable vaccine design. Nat Struct Mol Biol 23:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]