Abstract
Background
Accumulating evidence has demonstrated that renal hypoxia has a crucial role in the pathogenesis of acute kidney injury (AKI), chronic kidney disease (CKD), and AKI-to-CKD transition, ultimately culminating in end-stage kidney disease. Renal hypoxia in progressive CKD is intricately linked to persisting capillary loss, which is mainly due to dysregulated angiogenesis.
Summary
In CKD, hypoxia-inducible factor (HIF) accumulates in the ischemic tubulointerstitium but fails to sufficiently stimulate angiogenic responses, partly because of blunted activation of HIF, which is best exemplified in diabetic kidney disease. In addition, vascular endothelial growth factor (VEGF) expression is downregulated, possibly because injured tubules are not able to express sufficient VEGF and inflammatory circumstances inhibit VEGF expression. The upregulation of antiangiogenic factors and the incompetence of endothelial progenitor cells (EPCs) may also play some roles in the inadequacy of capillary restoration. Administration of VEGF or angiopoietin-1 maintains peritubular capillaries in several kidney diseases; however, administration of a single angiogenic factor may lead to the formation of abnormal vessels and induce inflammation, resulting in worsening of hypoxia and tubulointerstitial fibrosis. HIF stabilization, which aims to achieve the formation of mature and stable vessels by inducing coordinated angiogenesis, is a promising strategy. Given that the effect of systemic HIF activation is highly context-dependent, further studies are needed to elucidate the precise roles of HIF in various kidney diseases. The adoptive transfer of EPCs or mesenchymal stem cells (MSCs) is a fascinating alternative strategy to restore the peritubular capillaries.
Key Message
Suppressed HIF activation and VEGF expression may be responsible for the dysregulated angiogenesis in progressive CKD. Administration of a single angiogenic factor can cause abnormal vessel formation and inflammation, leading to a detrimental result. Although further studies are warranted, HIF stabilization and adoptive transfer of EPCs or MSCs appear to be promising strategies to restore normal capillaries.
Key Words: Acute kidney injury, Angiogenesis, Chronic kidney disease, Hypoxia, Hypoxia-inducible factor
Introduction
The incidence of chronic kidney disease (CKD) is increasing, and the medical, social, and economic burden of CKD is now recognized as a critical issue around the world. Chronic renal hypoxia, which is closely linked to capillary rarefaction, has been broadly accepted as a final common pathway by which CKD progresses to end-stage kidney disease (ESKD) irrespective of the underlying disease [1]. Accumulating evidence has also suggested that renal hypoxia is a key player and can be a therapeutic target in acute kidney injury (AKI) and AKI-to-CKD transition [2]. In progressive CKD, dysregulated angiogenesis plays an important role in persisting capillary loss and hypoxia. In this review, we discuss the roles of dysregulated angiogenesis and hypoxia in kidney disease, focusing on the therapeutic potential of angiogenic strategies to restore the capillary and oxygenation in the kidney.
Vascular Architecture and Oxygenation of the Kidney
The complicated structure of the renal vascular system is crucial for the proper functioning of the kidney. Cortical peritubular capillaries arise from the efferent arterioles of the superficial glomeruli. The vasa recta arise from those of the juxtamedullary glomeruli, while the descending and ascending vasa recta are connected by the peritubular capillaries within the medulla. These peritubular capillaries deliver oxygen and nutrients to the tubules. Despite such an abundance of vessels and the fact that the organ takes as much as 20% of the cardiac output in humans, the kidney is known to be physiologically hypoxic. The partial pressure of oxygen in various animals has been reported to be 20-60 and 10-30 mm Hg in the cortex and medulla, respectively, although some recent studies have reported lower values [2]. Such a hypoxic state of the kidney is primarily because of the presence of oxygen shunt diffusion between arterial and venous vessels running in close parallel contact [1]. Moreover, in kidney disease, a decreased blood (oxygen) supply and increased oxygen demand easily occurs, which together with a physiologically hypoxic state predisposes the kidney to hypoxic damage.
Angiogenesis
Quiescent endothelial cells have long half-lives and are protected by autocrine signals such as vascular endothelial growth factor (VEGF), angiopoietin (Ang)-1, and Notch [3]. These endothelial cells are surrounded by pericytes, which secrete paracrine signals such as VEGF and Ang-1 to maintain endothelial cell homeostasis. When quiescent endothelial cells are exposed to angiogenic stimuli, such as VEGF and Ang-2, pericytes detach from the vessel walls via the antagonistic role of Ang-2 against Ang-1, permitting endothelial cell sprouting. Under VEGF exposure, which upregulates the Notch ligand Delta-like protein 4 (DLL4) in all endothelial cells, endothelial cells that express DLL4 most quickly or at highest levels can become tip cells, those that lead the tip of sprouting vessels. The upregulated DLL4 in tip cells activates Notch signaling in neighboring cells (stalk cells), promotes the elongation of these stalk cells, and downregulates their VEGF receptor 2 (VEGFR2) expression, making the stalk cells less responsive to VEGF and enabling only the tip cells to take the lead. DLL4 expression should be dynamically regulated because the shuffling of tip-stalk position of endothelial cells occurs [4]. Sprouting endothelial cells release Ang-2 to enhance pericyte detachment and further sprouting. Finally, proliferating stalk cells become covered by pericytes via several signals, such as Ang-1 and platelet-derived growth factor B, which lead to the formation of mature and stable vessels.
The Importance of VEGF in Maintaining Peritubular Capillaries
Among several angiogenic factors, VEGF is thought to play a critical role in the maintenance of peritubular capillaries. In normal kidneys, VEGF is mainly expressed in podocytes and the thick ascending limb (TAL), and to a lesser extent in the proximal and distal tubules, whereas VEGF receptors (VEGFR1 and VEGFR2) are localized to endothelial cells in the peritubular capillaries and glomerular capillary loops [5]. Kamba et al. [6] reported reductions in peritubular and glomerular capillaries (30 and 10%, respectively) in mouse kidneys after treatment with a small-molecule VEGFR tyrosine kinase inhibitor. Mice in which VEGF was ablated in the renal tubules during development had smaller but apparently normal kidneys [5]. However, the peritubular capillary surface area was reduced by 60% in these mice and the vessel permeability in the kidneys was decreased. Moreover, remarkable polycythemia with elevated serum and renal erythropoietin (Epo) levels was also observed in these mice. This may suggest that capillary rarefaction resulted in renal hypoxia, which was detected by renal Epo-producing cells.
In contrast, excessive VEGF expression in the tubular cells also appears to be detrimental because the kidneys of transgenic mice with increased tubular VEGF expression demonstrated significant fibrosis and cyst formation [7]. These studies clearly show that, similar to the relationship between podocytes and endothelial cells within the glomerulus, an appropriate range of VEGF production by tubules is essential for maintaining peritubular capillary health.
Under hypoxic conditions, VEGF expression is increased via hypoxia-inducible factor (HIF)-mediated transcriptional upregulation as will be discussed below. In addition, the hypoxic milieu upregulates VEGF in other ways, such as by increased posttranscriptional stabilization [8].
HIF in the Kidney
HIF is a master regulator of cellular adaptation to hypoxia that upregulates the transcription of >100 genes such as Epo, VEGF, glucose transporters, and glycolytic enzymes. This transcriptional factor is a heterodimer that consists of HIF-α and HIF-β. HIF-β is constitutively expressed regardless of oxygen tension, whereas HIF-α expression is tightly regulated by oxygen tension. HIF-α has two conserved proline residues that are hydroxylated by prolyl hydroxylase domain-containing proteins (PHDs) under normoxic conditions. Hydroxylated HIF-α is recognized by the von Hippel-Lindau tumor suppressor protein (pVHL), resulting in polyubiquitination and proteasomal degradation. However, under hypoxic conditions, HIF-α escapes hydroxylation by PHDs and degradation thereafter, which enables HIF-α to enter the nucleus, where it binds with HIF-β to form a heterodimer. HIF heterodimers then bind to hypoxia response elements, existing in the regulatory regions of target genes, and activate transcription [1,9]. HIF-α also has one asparagine residue which is hydroxylated by an asparaginyl hydroxylase (factor-inhibiting HIF-1, FIH-1). Oxygen-dependent hydroxylation of HIF-1α by FIH-1 blocks the binding of the coactivators p300 and CREB-binding protein, leading to repression of transactivation [10]. In addition to these post-translational modifications of HIF-α by oxygen levels, HIF-1α mRNA levels have been reported to increase in response to hypoxia, which contributes to augmenting the HIF-1 response [11].
HIF-α has two major active isoforms, HIF-1α and HIF-2α, which play nonredundant roles in the adaptation to hypoxia. In the hypoxic kidney, HIF-1α is predominantly expressed in tubular epithelial cells, whereas HIF-2α is expressed in endothelial cells and interstitial fibroblasts. Recent evidence shows that Epo is produced by fibroblast-like Epo-producing cells in the kidney, and HIF-2α is a major regulator of its expression [12].
The Role of HIF in Each Cell Type of the Kidney
Recent cell type-specific gene manipulation studies have provided a deeper understanding of the role of HIF in each cell type (table 1). The specific deletion of HIF-1α in proximal tubular cells (PTCs) attenuated kidney fibrosis and macrophage infiltration in a mouse unilateral ureteral obstruction (UUO) model, suggesting that HIF-1α in PTCs can promote kidney fibrosis [13]. Theilig et al. [14] generated mice with tubular cell-specific ablation of VHL, which resulted in significant HIF-1α and HIF-2α accumulation in these cells. After nephrotoxic nephritis (NTN) induction, mutant mice showed lower blood urea nitrogen and urinary protein excretion with attenuated glomerular and tubulointerstitial damage but similar tubulointerstitial fibrosis. In contrast, only ablation of VHL (without NTN induction) resulted in increased tubulointerstitial fibrosis. These studies caution that inappropriate HIF activation can lead to a detrimental result, although another study demonstrated that tubular cell-specific ablation of VHL was beneficial in rhabdomyolysis-induced AKI [15].
Table 1.
Cell type-specific gene manipulation studies
| Cell type | Deleted gene | Principal findings | Ref. |
|---|---|---|---|
| PTC | HIF1ɑ | Fibrosis ↓ in UUO | 13 |
| Tubular epithelial cell | VHL | Fibrosis → at baseline | 14 |
| Tubular injury ↓/fibrosis → in NTN | |||
| Tubular epithelial cell | VHL | Injury ↓ in rhabdomyolysis-induced AKI | 15 |
| TAL cell | VHL | Injury ↓ in IR | 16 |
| Myeloid cell | HIF1ɑ/2ɑ | Macrophage infiltration → in UUO | 17 |
| VHL | Macrophage infiltration ↓ in UUO | ||
| Endothelial cell | HIF1ɑ | Injury → in IR | 18 |
| HIF2ɑ | Injury → in IR | ||
| PHD2 | Injury ↓ in IR | ||
TAL-specific ablation of VHL, which resulted in significant HIF-1α accumulation in TAL, ameliorated kidney injury in a bilateral renal ischemia-reperfusion (IR) model without affecting baseline renal function or morphology [16]. Myeloid cell-specific VHL and HIF-1α/2α deletion demonstrated decreased and increased macrophage infiltration in a UUO model, respectively, without affecting fibrosis [17]. Recently, Kapitsinou et al. [18] generated mice with endothelial cell-specific ablation of HIF-1α or HIF-2α. Three days after unilateral IR, tubular injury worsened in HIF-2α−/− mice, whereas HIF-1α ablation did not affect the extent of the injury. They also showed that inactivation of endothelial PHD2 ameliorated renal IR injury. Although a pharmacological approach to HIF stabilization using a PHD inhibitor successfully attenuated renal IR injury in wild-type mice, this beneficial effect was diminished in HIF-2α−/− mice. These results suggest that endothelial HIF-2α plays an important role in protecting the kidney against ischemic injury, in keeping with a previous study using HIF-2α hypomorph mice with endothelial reconstitution [19].
Hypoxia and HIF Accumulation in Kidney Disease
AKI
Renal hypoxia, as demonstrated by positive staining for pimonidazole, a marker of deep hypoxia (oxygen tension <10 mm Hg), has been observed in many AKI models in which AKI was induced by radiocontrast agents, cisplatin, and rhabdomyolysis as well as cross clamp with or without reperfusion. HIF, which accumulates in the instant phase of hypoxia, is also expressed during the recovery phase of AKI [20], which may indicate that tissue repair after injury requires much oxygen and results in prolonged or recurrent hypoxia. Evidence of hypoxia in human AKI is scarce, but an increased renal oxygen extraction ratio was confirmed in patients with AKI undergoing cardiac surgery [21].
AKI-to-CKD Transition
Recent epidemiological data and supporting animal studies have shown that AKI can eventually result in CKD. Renal hypoxia, which is mainly the result of capillary rarefaction, is gathering attention as a key player in the pathophysiology of the AKI-to-CKD transition [2]. In several studies on rodent IR injury with or without renal mass reduction, renal hypoxia was accompanied by capillary rarefaction in the recovery phase, which was followed by tubulointerstitial fibrosis [22]. Although the precise mechanisms of capillary loss after AKI remain elusive, loss of angiogenic factors, particularly VEGF, has been reported to have an important role [2]. VEGF expression was decreased during the early phase of renal IR injury [23], and VEGF-121 administration ameliorated capillary rarefaction and the subsequent tubulointerstitial fibrosis [22].
In the AKI-to-CKD transition, dedifferentiated tubular cells with failed redifferentiation after AKI are thought to continuously produce profibrotic peptides, which promote subsequent fibrosis. Polichnowski et al. [24] performed renal IR in rats with renal mass reduction. There were strong correlations among vimentin (a marker of dedifferentiation) positivity, decreased tubular VEGF expression, loss of capillary density, and tubulointerstitial fibrosis 4 weeks after injury. This result possibly suggests that hypoxia, having been mainly caused by a loss of peritubular capillaries, prevents the redifferentiation of regenerating tubules; this in turn decreases VEGF expression in these tubules and maintains the hypoxic state through impaired angiogenesis, forming a vicious cycle between dedifferentiated tubules with decreased VEGF expression and hypoxia due to capillary rarefaction (fig. 1).
Fig. 1.
A possible vicious cycle between damaged tubules and hypoxia in CKD progression. Hypoxia, mainly due to loss of peritubular capillaries, prevents damaged tubules from recovery. Decreased VEGF expression in these tubules mediates capillary rarefaction and hypoxia through impaired angiogenesis. Thus, damaged tubules with decreased VEGF and hypoxia due to capillary rarefaction can form a vicious cycle.
In addition to the direct effect of angiogenic factors on capillary endothelium, recent studies by Duffield and colleagues have emphasized pericyte detachment from capillaries as a key step of capillary rarefaction [25]. Although the precise mechanisms of the pericyte detachment in kidney disease are currently under investigation, dysregulated activation of endothelial VEGFR2 may play an important role. Blockade of endothelial VEGFR2 signaling with circulating soluble receptor ectodomains leads to the amelioration of pericyte detachment from capillaries, capillary loss, and tubulointerstitial fibrosis in mouse UUO and IR injury models [26]. Thus, the role of VEGF signaling in maintaining renal microvasculature is complicated and needs further investigation, including studies on possibly different roles of VEGF isoforms and distinct VEGF receptors [26].
CKD
Hypoxia has been proven in many animal models of CKD [10], and chronic hypoxia in the tubulointerstitium is generally accepted as a final common pathway in the development of ESKD [1]. However, detection of hypoxia in human CKD has long been challenging because of the limitations of available noninvasive methodology in humans. Blood oxygen level-dependent magnetic resonance imaging, which can visualize the tissue oxygen status in vivo by determining deoxyhemoglobin levels, has recently become available to detect hypoxia in human CKD. In one study, a correlation was established between estimated glomerular filtration rate and renal oxygenation levels [27]. HIF accumulation is also a frequent observation in various animal models of CKD as well as in human nephropathies, although the expression of HIF may be inappropriately suppressed in some animal models [10,12].
Capillary rarefaction, as discussed in the AKI-to-CKD Transition section, is also a common feature closely linked to hypoxia in CKD [12]. Significant correlations have been reported between capillary densities in kidney biopsy samples and renal function. Injured/atrophic tubules with decreased production of vascular factors such as VEGF mediate insufficient angiogenic response and hypoxia, and further aggravate injury; thus, forming a vicious cycle in the progression of CKD to ESKD (fig. 1). Hypoxia is deleterious to tubular cells (e.g., apoptosis), is intricately linked to inflammation (e.g., enhanced leukocyte infiltration), and can activate fibroblasts, all of which contribute to fibrogenesis. Fibrosis, in turn, aggravates hypoxia via reduced oxygen diffusion efficiency by increasing the distance from capillaries to tubular cells. Again, hypoxia and tubulointerstitial fibrosis form a vicious cycle that progresses to ESKD [9].
Why Does Capillary Rarefaction Persist and Progress despite HIF Activation in CKD?
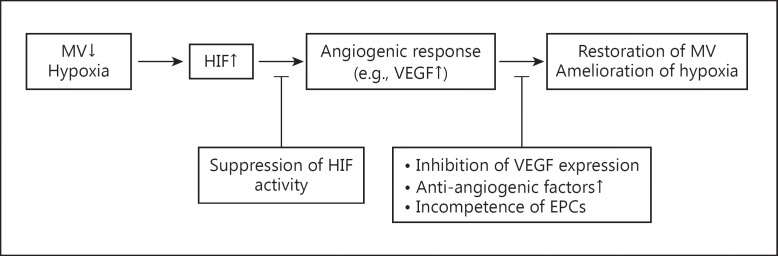
As discussed above, hypoxia is observed in both AKI and CKD. While HIF, as envisaged by capillary rarefaction and concomitant hypoxia, triggers the induction of multiple angiogenic factors such as VEGF and likely stimulates angiogenic responses, this adaptive response almost always fails to ensue; capillary rarefaction and hypoxia continue to worsen in progressive CKD, ultimately leading to ESKD. Several mechanisms have been reported to explain this phenomenon (fig. 2).
Fig. 2.
Possible mechanisms behind the inadequate angiogenic response in CKD. HIF is upregulated by hypoxia following loss of microvessels (MV) in CKD. Accumulated HIF would stimulate angiogenic responses, e.g., by increasing VEGF expression, which mechanistically leads to the restoration of MV and amelioration of renal hypoxia. However, in most CKD settings, capillary rarefaction and hypoxia persist as the disease progresses, ultimately leading to ESKD. Several mechanisms including suppressed HIF activity, inhibition of VEGF expression, upregulation of antiangiogenic factors, and the incompetence of EPCs may be responsible for this observation.
First, the extent of HIF activation may be inadequate. In diabetic kidney disease, blunted HIF activation via several pathways, including oxidative stress, has been reported [28]. Indoxyl sulfate, a representative uremic toxin, also impairs the HIF-mediated cellular response, which suggests that suboptimal HIF activation can occur in advanced CKD [29]. Second, as discussed above, VEGF expression becomes rather downregulated, despite hypoxia in various kidney diseases. Damaged or atrophic tubular cells in CKD may not be able to express a sufficient amount of VEGF to restore peritubular capillaries. In addition, the inflammatory environment in CKD probably suppresses VEGF expression [30]. Third, antiangiogenic factors such as thrombospondin-1 and endostatin are upregulated in kidney disease [30,31,32]. Fourth, the incompetence of endothelial progenitor cells (EPCs), which has been shown in human CKD and various kidney disease models, may be responsible for the inadequacy of capillary restoration [33]. Finally, epigenetic changes may also have some roles [34]. Recent data suggest that epigenetic changes induced by hypoxia can promote proinflammatory and profibrotic gene expression, which are closely linked to capillary rarefaction in vivo [35].
Angiogenesis-Based Treatment of Kidney Disease
Administration of Angiogenic Factors
VEGF
In the rat remnant kidney model, VEGF-121 administration preserved peritubular capillaries, restored renal function, and ameliorated tubulointerstitial fibrosis [36]. The intrarenal administration of VEGF also preserved microvascular density and decreased fibrosis in a pig unilateral renal artery stenosis (RAS) model [37]. The AKI-to-CKD transition was also blocked by the treatment with VEGF-121, which ameliorated the capillary rarefaction after IR injury [22].
In contrast to most CKD settings, the early manifestation of diabetic kidney is characterized by an excess of vascular factors such as VEGF, which provides us with a cautious view on the strategy of instant VEGF administration. The intrarenal administration of VEGF in pig RAS kidneys resulted in a tendency toward larger microvascular densities in both the peritubular and glomerular areas compared with normal controls, suggesting overgrowth of microvessels [37]. Moreover, angiogenesis attained by VEGF alone results in distorted, leaky vessels connected by loose junctions, which presents with insufficient perfusion. Extravasation from these abnormal vessels can increase interstitial pressure and induce tubulointerstitial inflammation, leading to decreased capillary flow and hypoxia [12].
Ang-1
In humans, CKD was associated with decreased Ang-1 levels in the blood [38]. The same tendency was reported in a mouse UUO model [39], although not all studies in distinct models support this view. Supplementation of Ang-1, which was achieved by treatment with an adenovirus carrying an engineered variant of native Ang-1 with higher activity, successfully preserved peritubular capillaries and ameliorated tubulointerstitial fibrosis in a mouse UUO model [39]. The same treatment was also examined in the AKI-to-CKD transition in a mouse IR injury model. The authors observed preserved peritubular capillaries, better renal function, and higher renal blood flow during the acute phase, as well as milder tubulointerstitial fibrosis 30 days after injury in the treatment group [40]. However, similar to VEGF, it should be noted that Ang-1 treatment could induce inflammation. Ang-1 delivery by adenoviral vectors was significantly associated with enhanced neutrophil/macrophage infiltration and tubulointerstitial fibrosis, despite capillary preservation in mice with folic acid-induced nephropathy [41]. This result may be related to the fact that Ang-1 could promote the recruitment of Tie-2-expressing neutrophils and macrophages [42,43].
HIF Stabilization
To attain normal and mature vessel formation, treatments such as VEGF in combination with Ang-1 have been tested, with promising results [44]. On the other hand, coordinated angiogenesis by HIF activation can also achieve the same goal. HIF accumulation by PHD inhibition has been shown to form mature vessels (covered by pericytes) in in vivo studies [45]. In tumors, endothelial deletion of HIF-1α results in loss of angiogenic potential [46], while endothelial deletion of HIF-2α leads to immature and poorly functional vasculature, suggesting that HIF-2α inhibits vessel sprouting; however, it still promotes capillary maturation [47,48]. These contrasting properties of HIF-1 and HIF-2 on vascular formation also highlight the potential advantage of PHD inhibition as a means of therapeutic angiogenesis.
It has been broadly accepted that HIF has a beneficial role in AKI. In a mouse bilateral IR injury model, preconditioning with a PHD inhibitor not only decreased the severity of AKI but also blocked the mechanisms of the AKI-to-CKD transition itself, probably by enhancing the repair process [49]. Some promising results with HIF activation have also been reported in CKD. The systemic activation of HIF by cobalt resulted in ameliorated capillary rarefaction and tubulointerstitial damage in a rat remnant kidney model [50]. However, there remain some concerns, particularly related to the profibrotic effect of HIF, over the HIF activation strategy generally used in CKD [13]. This controversy appears to originate, at least in part, from the differences in when and how much HIF is activated. Genetic manipulation usually results in stronger and permanent changes than pharmacological intervention. Recent studies have revealed that the timing of HIF activation is critical to its renoprotective effect [49,51]. In addition, because HIF appears to play different roles in each kidney cell as discussed above, the effect of systemic HIF activation should be highly context-dependent. Therefore, it is essential to clarify the context in which HIF stabilization is safe and plays a beneficial role before utilizing this strategy.
Cell Therapy
EPCs
The renal vasculature is formed by both angiogenesis (vessel sprouting from an existing vessel) and vasculogenesis (new vessel formation from EPCs) [52]. In adulthood, EPCs have been described to reside in the bone marrow, circulating blood, and blood vessels, whereas incompetence of resident EPCs has been reported in human CKD and several kidney injury models [33]. Therefore, it should be reasonable to consider enhancing vasculogenesis, which could be attained by the adoptive transfer of EPCs to restore the capillaries in injured kidneys. Although studies with EPCs show many varieties in markers to identify and isolate EPCs, the adoptive transfer of these cells has been associated with preserved microvascular densities and the amelioration of kidney damage in various animal models [31].
What is the mechanism through which EPCs preserve capillaries and enhance new vessel formation? Recent studies have questioned the direct involvement of bone marrow-derived or circulating stem/progenitor cells in blood vessel regeneration, and have suggested that angiogenesis during blood vessel regeneration is derived from resident endothelial progenitors. For example, using transplantation of fluorescence-labeled hematopoietic stem cells (HSCs) and pairs of genetically marked parabiotic mice, Rinkevich et al. [53] demonstrated that HSCs or circulating cells did not contribute to angiogenesis in the regeneration of the distal mouse digit. EPCs have been demonstrated to contribute to the regenerative process, including angiogenesis by homing to the kidney after injury and paracrine secretion of growth factors (e.g., VEGF) [12].
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are adult stem cells of mesodermal origin with the differentiation capacity toward mesenchymal cell types including adipocytes, osteoblasts, and chondrocytes. MSCs have been demonstrated to reside in many tissues including the bone marrow and adipose tissue. Similar to EPCs, the administration of MSCs derived from these tissues has been reported to preserve microvascular density and ameliorate kidney injury in a variety of kidney disease models. However, MSCs appear to have different mechanisms of capillary preservation compared with EPCs. Zhu et al. [54] investigated the effects of EPCs derived from the peripheral blood and adipose-derived MSCs in a swine RAS model. The administration of EPCs or MSCs via the renal arteries improved the microvascular density in the cortex, GFR, and fibrosis. Although MSCs secreted more VEGF than EPCs in the culture medium in vitro, impaired VEGF expression in RAS kidneys was restored by treatment with EPCs but not MSCs, which may suggest that EPCs may stimulate surrounding tubular epithelial cells to express VEGF in addition to their own paracrine secretion. On the other hand, treatment with MSCs attenuated inflammation, apoptosis, and endoplasmic reticulum stress in the RAS kidney more significantly than treatment with EPCs.
Although the administration of MSCs should be a promising treatment strategy against kidney disease, there remain some important concerns about it. Kunter et al. [55] examined the effectiveness and safety of MSCs administration in a rat anti-Thy1.1 mesangioproliferative glomerulonephritis model. In this study, bone marrow-derived MSCs were injected intra-arterially into the kidney 2 days after disease induction with uninephrectomy. Although fluorescently labeled MSCs were detected within many glomeruli on day 10, and treatment with MSCs successfully ameliorated kidney injury, glomeruli in MSC-treated animals showed an increased expression of α-smooth muscle actin and collagen types I, III, and IV compared with those in controls on day 60. Approximately 20% of the glomeruli of MSC-treated animals contained adipocytes surrounded by fibrosis on day 60, whereas none of the glomeruli of control animals throughout the course of treatment and MSC-treated animals on day 10 contained adipocytes. These adipocytes exhibited fluorescence, which clearly indicates that exogenously adopted MSCs underwent adipogenic differentiation within the glomerulus.
On the other hand, Humphreys and colleagues [56] have recently shown that a certain population of perivascular MSCs (Gli1+ cells) is a key player in organ fibrosis. These cells represented only a small fraction of the total pericyte population within the kidney. In a UUO and bilateral IR injury model, resident Gli1+ cells in the kidney, which were neither bone marrow-derived nor circulating Gli1+ cells, significantly proliferated and became myofibroblasts (occupying approximately half of all myofibroblasts), resulting in renal fibrosis. Moreover, genetic ablation of Gli1+ cells decreased renal fibrosis in a UUO by 50%. The pathogenic importance of Gli1+ cells in fibrosis was also shown in other major organs (heart, lung, and liver). These studies emphasize that MSCs are not always beneficial and can cause a detrimental effect.
Conclusions
Dysregulated angiogenesis, capillary loss, and hypoxia are intricately associated with each other in the progression of kidney disease. Despite HIF activation, which would enhance angiogenic responses such as increased VEGF expression and maintain peritubular capillaries, capillary loss and hypoxia persist and progress, resulting in ESKD. Several mechanisms, including blunted HIF activation in CKD, particularly diabetic kidney disease, downregulated VEGF expression, upregulated antiangiogenic factors, and the incompetence of EPCs can explain the dysregulated angiogenesis. Although administration of angiogenic factors, such as VEGF and Ang-1, maintains peritubular capillaries in various kidney diseases, administration of a single factor may result in abnormal vessel formation and inflammation, leading to worsening of hypoxia and tubulointerstitial fibrosis. HIF stabilization is a promising strategy to achieve the formation of mature and stable vessels by inducing coordinated angiogenesis; however, the roles of HIF in CKD remain controversial. Understanding the precise roles of HIF in kidney disease is essential to safely use this strategy in clinical settings. Although we should be cautious about the adverse effects including maldifferentiation, the adoptive transfer of EPCs or MSCs is emerging as an alternative strategy to restore the capillaries.
Disclosure Statement
The authors have no conflicts of interest to declare.
Acknowledgements
The work of the authors is supported by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (26111003; M.N.) and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (24390213; M.N., and 26461215; T.T.).
References
- 1.Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25. doi: 10.1681/ASN.2005070757. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka S, Tanaka T, Nangaku M. Hypoxia as a key player in the AKI-to-CKD transition. Am J Physiol Renal Physiol. 2014;307:F1187–F1195. doi: 10.1152/ajprenal.00425.2014. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE. Tubulovascular cross-talk by vascular endothelial growth factor A maintains peritubular microvasculature in kidney. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2014010060. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 7.Hakroush S, Moeller MJ, Theilig F, Kaissling B, Sijmonsma TP, Jugold M, Akeson AL, Traykova-Brauch M, Hosser H, Hähnel B, Gröne HJ, Koesters R, Kriz W. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am J Pathol. 2009;175:1883–1895. doi: 10.2353/ajpath.2009.080792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996;271:2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 9.Mimura I, Nangaku M. The suffocating kidney: tubulointerstitial hypoxia in end-stage renal disease. Nat Rev Nephrol. 2010;6:667–678. doi: 10.1038/nrneph.2010.124. [DOI] [PubMed] [Google Scholar]
- 10.Nangaku M, Rosenberger C, Heyman SN, Eckardt KU. Regulation of hypoxia-inducible factor in kidney disease. Clin Exp Pharmacol Physiol. 2013;40:148–157. doi: 10.1111/1440-1681.12005. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi J, Tanaka T, Eto N, Nangaku M. Inflammation and hypoxia linked to renal injury by CCAAT/enhancer-binding protein δ. Kidney Int. 2015 doi: 10.1038/ki.2015.21. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka T, Nangaku M. Angiogenesis and hypoxia in the kidney. Nat Rev Nephrol. 2013;9:211–222. doi: 10.1038/nrneph.2013.35. [DOI] [PubMed] [Google Scholar]
- 13.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Theilig F, Enke AK, Scolari B, Polzin D, Bachmann S, Koesters R. Tubular deficiency of von Hippel-Lindau attenuates renal disease progression in anti-GBM glomerulonephritis. Am J Pathol. 2011;179:2177–2188. doi: 10.1016/j.ajpath.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fähling M, Mathia S, Paliege A, Koesters R, Mrowka R, Peters H, Persson PB, Neumayer HH, Bachmann S, Rosenberger C. Tubular von Hippel-Lindau knockout protects against rhabdomyolysis-induced AKI. J Am Soc Nephrol. 2013;24:1806–1819. doi: 10.1681/ASN.2013030281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schley G, Klanke B, Schödel J, Forstreuter F, Shukla D, Kurtz A, Amann K, Wiesener MS, Rosen S, Eckardt KU, Maxwell PH, Willam C. Hypoxia-inducible transcription factors stabilization in the thick ascending limb protects against ischemic acute kidney injury. J Am Soc Nephrol. 2011;22:2004–2015. doi: 10.1681/ASN.2010121249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi H, Gilbert V, Liu Q, Kapitsinou PP, Unger TL, Rha J, Rivella S, Schlöndorff D, Haase VH. Myeloid cell-derived hypoxia-inducible factor attenuates inflammation in unilateral ureteral obstruction-induced kidney injury. J Immunol. 2012;188:5106–5115. doi: 10.4049/jimmunol.1103377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapitsinou PP, Sano H, Michael M, Kobayashi H, Davidoff O, Bian A, Yao B, Zhang MZ, Harris RC, Duffy KJ, Erickson-Miller CL, Sutton TA, Haase VH. Endothelial HIF-2 mediates protection and recovery from ischemic kidney injury. J Clin Invest. 2014;124:2396–2409. doi: 10.1172/JCI69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, Ohneda O, Takeda N, Sata M, Miyata T, Fujita T, Nangaku M. Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol. 2007;18:1218–1226. doi: 10.1681/ASN.2006060639. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger C, Pratschke J, Rudolph B, Heyman SN, Schindler R, Babel N, Eckardt KU, Frei U, Rosen S, Reinke P. Immunohistochemical detection of hypoxia-inducible factor-1alpha in human renal allograft biopsies. J Am Soc Nephrol. 2007;18:343–351. doi: 10.1681/ASN.2006070792. [DOI] [PubMed] [Google Scholar]
- 21.Redfors B, Bragadottir G, Sellgren J, Swärd K, Ricksten SE. Acute renal failure is NOT an ‘acute renal success’ - a clinical study on the renal oxygen supply/demand relationship in acute kidney injury. Crit Care Med. 2010;38:1695–1701. doi: 10.1097/CCM.0b013e3181e61911. [DOI] [PubMed] [Google Scholar]
- 22.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F1648–F1657. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basile DP, Fredrich K, Chelladurai B, Leonard EC, Parrish AR. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294:F928–F936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 24.Polichnowski AJ, Lan R, Geng H, Griffin KA, Venkatachalam MA, Bidani AK. Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. J Am Soc Nephrol. 2014;25:1496–1507. doi: 10.1681/ASN.2013040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol. 2011;178:911–923. doi: 10.1016/j.ajpath.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue T, Kozawa E, Okada H, Inukai K, Watanabe S, Kikuta T, Watanabe Y, Takenaka T, Katayama S, Tanaka J, Suzuki H. Noninvasive evaluation of kidney hypoxia and fibrosis using magnetic resonance imaging. J Am Soc Nephrol. 2011;22:1429–1434. doi: 10.1681/ASN.2010111143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bento CF, Pereira P. Regulation of hypoxia-inducible factor 1 and the loss of the cellular response to hypoxia in diabetes. Diabetologia. 2011;54:1946–1956. doi: 10.1007/s00125-011-2191-8. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka T, Yamaguchi J, Higashijima Y, Nangaku M. Indoxyl sulfate signals for rapid mRNA stabilization of Cbp/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domain 2 (CITED2) and suppresses the expression of hypoxia-inducible genes in experimental CKD and uremia. FASEB J. 2013;27:4059–4075. doi: 10.1096/fj.13-231837. [DOI] [PubMed] [Google Scholar]
- 30.Kang DH, Joly AH, Oh SW, Hugo C, Kerjaschki D, Gordon KL, Mazzali M, Jefferson JA, Hughes J, Madsen KM, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: I. Potential role of vascular endothelial growth factor and thrombospondin-1. J Am Soc Nephrol. 2001;12:1434–1447. doi: 10.1681/ASN.V1271434. [DOI] [PubMed] [Google Scholar]
- 31.Long DA, Norman JT, Fine LG. Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol. 2012;8:244–250. doi: 10.1038/nrneph.2011.219. [DOI] [PubMed] [Google Scholar]
- 32.Maciel TT, Coutinho EL, Soares D, Achar E, Schor N, Bellini MH. Endostatin, an antiangiogenic protein, is expressed in the unilateral ureteral obstruction mice model. J Nephrol. 2008;21:753–760. [PubMed] [Google Scholar]
- 33.Goligorsky MS, Yasuda K, Ratliff B. Dysfunctional endothelial progenitor cells in chronic kidney disease. J Am Soc Nephrol. 2010;21:911–919. doi: 10.1681/ASN.2009111119. [DOI] [PubMed] [Google Scholar]
- 34.Mimura I, Kanki Y, Kodama T, Nangaku M. Revolution of nephrology research by deep sequencing: ChIP-seq and RNA-seq. Kidney Int. 2014;85:31–38. doi: 10.1038/ki.2013.321. [DOI] [PubMed] [Google Scholar]
- 35.Mimura I, Tanaka T, Nangaku M. Novel therapeutic strategy with hypoxia-inducible factors via reversible epigenetic regulation mechanisms in progressive tubulointerstitial fibrosis. Semin Nephrol. 2013;33:375–382. doi: 10.1016/j.semnephrol.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 36.Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model. II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–1457. doi: 10.1681/ASN.V1271448. [DOI] [PubMed] [Google Scholar]
- 37.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant. 2010;25:1079–1087. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Futrakul N, Butthep P, Futrakul P. Altered vascular homeostasis in chronic kidney disease. Clin Hemorheol Microcirc. 2008;38:201–207. [PubMed] [Google Scholar]
- 39.Kim W, Moon SO, Lee SY, Jang KY, Cho CH, Koh GY, Choi KS, Yoon KH, Sung MJ, Kim DH, Lee S, Kang KP, Park SK. COMP-angiopoietin-1 ameliorates renal fibrosis in a unilateral ureteral obstruction model. J Am Soc Nephrol. 2006;17:2474–2483. doi: 10.1681/ASN.2006020109. [DOI] [PubMed] [Google Scholar]
- 40.Jung YJ, Kim DH, Lee AS, Lee S, Kang KP, Lee SY, Jang KY, Sung MJ, Park SK, Kim W. Peritubular capillary preservation with COMP-angiopoietin-1 decreases ischemia-reperfusion-induced acute kidney injury. Am J Physiol Renal Physiol. 2009;297:F952–F960. doi: 10.1152/ajprenal.00064.2009. [DOI] [PubMed] [Google Scholar]
- 41.Long DA, Price KL, Ioffe E, Gannon CM, Gnudi L, White KE, Yancopoulos GD, Rudge JS, Woolf AS. Angiopoietin-1 therapy enhances fibrosis and inflammation following folic acid-induced acute renal injury. Kidney Int. 2008;74:300–309. doi: 10.1038/ki.2008.179. [DOI] [PubMed] [Google Scholar]
- 42.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Lemieux C, Maliba R, Favier J, Théorêt JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 44.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Leite de Oliveira R, Deschoemaeker S, Henze AT, Debackere K, Finisguerra V, Takeda Y, Roncal C, Dettori D, Tack E, Jönsson Y, Veschini L, Peeters A, Anisimov A, Hofmann M, Alitalo K, Baes M, D'hooge J, Carmeliet P, Mazzone M. Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell. 2012;22:263–277. doi: 10.1016/j.ccr.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 46.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Skuli N, Liu L, Runge A, Wang T, Yuan L, Patel S, Iruela-Arispe L, Simon MC, Keith B. Endothelial deletion of hypoxia-inducible factor-2alpha (HIF-2alpha) alters vascular function and tumor angiogenesis. Blood. 2009;114:469–477. doi: 10.1182/blood-2008-12-193581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, Runge A, Liu L, Kim MN, Liang J, Schenkel S, Yodh AG, Keith B, Simon MC. Endothelial HIF-2α regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122:1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kapitsinou PP, Jaffe J, Michael M, Swan CE, Duffy KJ, Erickson-Miller CL, Haase VH. Preischemic targeting of HIF prolyl hydroxylation inhibits fibrosis associated with acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F1172–F1179. doi: 10.1152/ajprenal.00667.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanaka T, Kojima I, Ohse T, Ingelfinger JR, Adler S, Fujita T, Nangaku M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab Invest. 2005;85:1292–1307. doi: 10.1038/labinvest.3700328. [DOI] [PubMed] [Google Scholar]
- 51.Yu X, Fang Y, Liu H, Zhu J, Zou J, Xu X, Jiang S, Ding X. The balance of beneficial and deleterious effects of hypoxia-inducible factor activation by prolyl hydroxylase inhibitor in rat remnant kidney depends on the timing of administration. Nephrol Dial Transplant. 2012;27:3110–3119. doi: 10.1093/ndt/gfr754. [DOI] [PubMed] [Google Scholar]
- 52.Stolz DB, Sims-Lucas S. Unwrapping the origins and roles of the renal endothelium. Pediatr Nephrol. 2014 doi: 10.1007/s00467-014-2798-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rinkevich Y, Lindau P, Ueno H, Longaker MT, Weissman IL. Germ-layer and lineage-restricted stem/progenitors regenerate the mouse digit tip. Nature. 2011;476:409–413. doi: 10.1038/nature10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu XY, Urbieta-Caceres V, Krier JD, Textor SC, Lerman A, Lerman LO. Mesenchymal stem cells and endothelial progenitor cells decrease renal injury in experimental swine renal artery stenosis through different mechanisms. Stem Cells. 2013;31:117–125. doi: 10.1002/stem.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J. Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007;18:1754–1764. doi: 10.1681/ASN.2007010044. [DOI] [PubMed] [Google Scholar]
- 56.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]