Abstract
Background
Idiopathic membranous nephropathy (IMN) is a common cause of nephrotic syndrome (NS) in adults in Western countries. In 2012, the KDIGO (Kidney Disease: Improving Global Outcomes) working group published guidelines for the management of glomerulonephritis, thus providing a template for the treatment of this condition. While being aware of the impact of the clinicians' acumen and that patients may choose a different therapeutic option due to the risks of specific drugs and also of the evolving guidelines, this review details our approach to the management of patients with IMN in a Western center (Toronto).
Summary
Based on studies published in Europe and North America, we included recent advances in the diagnosis and management of patients with membranous nephropathy similar to our practice population. We highlight the importance of establishing the idiopathic nature of this condition before initiating immunosuppressive therapy, which should include the screening for secondary causes, especially malignancy in the elderly population. The expected outcomes with and without treatment for patients with different risks of progression will be discussed to help guide clinicians in choosing the appropriate course of treatment. The role of conservative therapy as well as of established immunosuppressive treatment, such as the combination of cyclophosphamide and prednisone, and calcineurin inhibitors (CNIs), as well as of newer agents such as rituximab will be reviewed.
Key Messages
Appropriate assessment is required to exclude secondary conditions causing membranous glomerulonephritis. The role of antibodies to phospholipase A2 receptor (anti-PLA2R) in establishing the primary disease is growing, though more data are required. The increase in therapeutic options supports treatment individualization, taking into account the availability, benefits and risks, as well as patient preference.
Facts from East and West
(1) The prevalence of IMN is increasing worldwide, particularly in elderly patients, and has been reported in 20.0–36.8% of adult-onset NS cases. The presence of anti-PLA2R antibodies in serum or PLA2R on renal biopsy is the most predictive feature for the diagnosis of IMN and is used in both the East and West; however, appropriate screening to rule out secondary causes should still be performed. (2) Several observational (nonrandomized) Asian studies indicate a good response to corticosteroids alone in IMN patients, although no randomized controlled trials (RCTs) have been done in Asian membranous patients at high risk of progression. Corticosteroid monotherapy has failed in randomized controlled studies in Western countries and is therefore not recommended. (3) Cyclophosphamide is the most commonly prescribed alkylating agent in Europe and China. Also, chlorambucil is still used in some Western countries, particularly in Europe. In North America, CNIs are the more common first-line treatment. (4) Cyclosporine is predominantly used as monotherapy in North America, although KDIGO and Japanese guidelines still recommend a combination with low-dose corticosteroids. Clinical studies both in Asia and Europe showed no or little effects of monotherapy with mycophenolate mofetil compared to standard therapies. (5) There are encouraging data from nonrandomized Western studies for the use of rituximab and a few small studies using adrenocorticotropic hormone. Clinical trials are ongoing in North America to confirm these observations. These drugs are rarely used in Asia. (6) A Chinese study reported that 36% of IMN patients suffered from venous thromboembolism versus 7.3% in a North American study. Prophylactic anticoagulation therapy is usually added to IMN patients with a low risk of bleeding in both Eastern and Western countries. (7) The Chinese traditional medicine herb triptolide, which might have podocyte-protective properties, is used in China to treat IMN. An open-label, multicenter RCT showed that Shenqi, a mixture of 13 herbs, was superior to corticosteroids plus cyclophosphamide therapy to restore epidermal growth factor receptor in IMN patients, although proteinuria improvement was equal in the two groups. Importantly, Shenqi treatment induced no severe adverse events while standard therapy did.
Key Words: Nephrotic syndrome, Membranous nephropathy, Glomerulonephritis
Introduction
Idiopathic membranous nephropathy (IMN) remains one of the most common causes of adult-onset nephrotic syndrome (NS) in Western countries [1,2,3]. Since membranous nephropathy (MN) has originally been described over half a century ago [4], considerable information has been collected to help guide us with patient management. Some of these data relate to an understanding of the natural history and pathophysiology of the disease, while other studies are about new treatments and an increased awareness of the risks as well as of the benefits of these therapeutic regimens. Today, we have the capacity to reduce proteinuria and ameliorate the consequences of IMN.
Guidelines for all common histologic variants of IMN have recently been published under the auspices of the KDIGO (Kidney Disease: Improving Global Outcomes) initiative [5]. These guidelines, graded by the quality of evidence, were established to help supplement the clinician's decision-making process, but not to replace physician judgment [6]. The highest quality of evidence (grade A) is uncommon in IMN, leaving disease management still highly dependent on the individual case characteristics and the integration of both the physician's judgment and the patient's preference of treatment. The latter are particularly relevant in Western countries and commonly relate to adverse events (AE) that can be associated with immunotherapy, which are in turn often impacted by the patient's age and sex. In this review, we present our approach to the management of IMN that has evolved not only based on published data such as the KDIGO glomerulonephritis guidelines but also on our clinical experience, taking into account these factors and reflecting our current practice in the Glomerulonephritis Clinic and Registry at the University of Toronto in Canada.
Confirming the Diagnosis
Usually, IMN patients present with features of NS, which, however, is not specific to this condition and overlaps with many other primary and secondary glomerular diseases. Thus, the diagnosis of MN can only be confirmed by a kidney biopsy and appropriate histopathologic examination. Along with this histologic confirmation, screening for other causes of NS and for secondary causes of the membranous lesion must be undertaken (table 1).
Table 1.
Common causes of SMN and routine tests to rule out these possible conditions
| Causes | Tests | |
|---|---|---|
| Autoimmune diseases | lupus nephritis | ANA, ENA, C3, C4 |
| rheumatoid arthritis | rheumatoid factor, anti-CCP | |
| Infections | HBV | HBsAg, anti-HBc, anti-HBs |
| HCV | anti-HCV, HCV RNAa | |
| HIV | HIV screeningb | |
| Medications | NSAIDs | medication review |
| classic: gold, penicillamine, captopril | ||
| recent: anti-TNF-ɑ | ||
| Malignancyb | lung | low-dose chest CT |
| prostate | PSA | |
| colon | FOBT, colonoscopy | |
| breast | mammogram, US | |
| plasma cell dyscrasias | SPEP, serum FLC | |
ANA = Antinuclear antibody; ENA = extractable nuclear antigens antibodies; CCP = cyclic citrullinated peptide; HBV = hepatitis B virus; HCV = hepatitis C virus; HBsAG = hepatitis B surface antigen; anti-HBc = hepatitis B core antibody; anti-HBs = hepatitis B surface antibody; HIV = human immunodeficiency virus; NSAIDs = nonsteroidal anti-inflammatory drugs; TNF = tumor necrosis factor; CT = computerized tomography; PSA = prostate-specific antigen; FOBT = fecal occult blood test; US = ultrasonography; SPEP = serum protein electrophoresis; FLC = free light chains.
If anti-HCV antibodies are positive.
Recommendations are under review by Centers for Disease Control and Prevention, and specific recommendations from local authorities should be followed.
Pathology
The diagnosis of IMN is suspected by the stiffening and thickening of the glomerular basement membrane by light microscopy; however, the deposition of immunoglobulin G (IgG) and complement 3 (C3) along the glomerular basement membrane by immunofluorescence (IF) and subepithelial electron-dense deposits by electron microscopy are the sine qua non of the MN pattern [7,8]. Secondary etiologies (SMN) share these features, along with additional findings such as mesangial or endocapillary cell proliferation by light microscopy and tubuloreticular inclusions; also, electron-dense deposits in other regions (mesangial or subendothelial) by electron microscopy or a ‘full-house’ pattern by IF staining for immunoglobulins and complements (IgG, IgA and IgM plus C3 and C1q) strongly suggest lupus nephritis [9,10]. If an increased number of inflammatory cells (>8 per glomerulus) are seen, MN associated with malignancy should also be considered [11].
A recently suggested feature distinguishing IMN from SMN is the staining pattern for the IgG subclasses, with predominantly IgG4 subclass deposition for IMN versus an IgG1 and IgG3 staining pattern in the case of lupus MN [12,13]; IgG1 and IgG2 dominance was associated with malignancy-associated MN [14]. Although of interest, the overlap in the deposition pattern of the IgG subclasses currently limits our ability to discriminate primary from secondary disease [15]. Although our nephropathologists use this approach in their tissue examination and believe it will eventually provide valuable information, its application is currently at the confirmatory rather than at the diagnostic level.
Preliminary Screening
The prevalence of secondary causes of MN is highly variable in the published literature, but approximately 30% of adults in the cohorts of Western countries have an identifiable etiologic agent leading to the injury pattern of MN [16,17,18]. This underlines the critical need for a proper diagnosis and dictates subsequent management. A growing concern is the increased rate of malignancy among MN patients older than 60 years, where the incidence may reach 20% [19,20]. Investigation of these patients should closely follow appropriate age and sex malignancy screening as recommended by regional guidelines. In our clinic, appropriate screening is driven not only by these guidelines, but also by clinical suspicion (table 1). An elderly patient, for example, with a significant history of smoking, even if remote, would dictate a chest CT to rule out lung cancer, versus a young woman recently emigrated from East Asia, for whom screening for viral hepatitis and autoimmune disease would be deemed appropriate even without a documented history of systemic involvement.
Anti-Phospholipase A2 Receptor Antibodies
Antibodies to phospholipase A2 receptor (anti-PLA2R), a transmembrane protein found in podocytes, were originally detected in the serum of 70% of IMN patients [21]. These antibodies can also be localized in glomerular tissue by IF staining, further implicating their role in IMN pathobiology [21,22]. Following the initial description, publications from Western countries have reported variable prevalence, with a positivity of these circulating antibodies in IMN cases ranging from 52 to 78% [23,24]. These antibodies are rarely detected in SMN, offering high specificity. Unfortunately, in many of the IMN studies, it has not been possible to assess the relationship between circulating and kidney-bound antibodies due to the absence of concurrent serum and tissue samples [22], making this currently an association rather than a cause-and-effect relationship. Perhaps equally concerning, a few patients with malignancy and presumed SMN have demonstrated anti-PLA2R in the serum [25], reinforcing the need to screen for malignancy in patients at risk, regardless of their anti-PLA2R status. In our clinic, we routinely test both serum and tissue anti-PLA2R but do not rely on them as the sole discriminator between IMN and SMN, given the above overlap, lack of sensitivity and, potentially, lack of specificity. There are several other outstanding issues that need resolution, including the significant percentage of IMN patients with PLA2R negativity. Initially, this was thought to possibly represent patients about to enter spontaneous remission but, with time, it has been noted that it does not explain the great percentage of the negative-antibody cohort. In a series of patients treated with rituximab, among those with detectable serum anti-PLA2R antibodies (approx. 80% of those screened), lower circulating anti-PLA2R antibody titers at baseline and depletion of anti-PLA2R within 6 months of therapy predicted remission, and re-emergence of circulating antibodies predicted relapse. However, when compared to patients who were anti-PLA2R negative at screening, the treatment response was similar [26]. Therefore, although helpful in finding both tissue and circulating anti-PLA2R, more prospective data are needed to guide the use of antibodies during follow-up and also guide the monitoring of antibody-negative patients.
Assessing the Risk of Progression
The variability in the natural history of IMN makes the assessment of prognosis a critical element in the management of the patient. Although this assessment usually requires observing the development of protein excretion over time, the evaluation of immediate morbidity risks is also warranted. This includes the evaluation of the risk of complications arising from severe nephrosis (such as anasarca and the risk of infection and thromboembolism) since they can act as indicators to initiate therapy earlier than the severity of proteinuria alone might dictate.
Risk Factors for Progression to End-Stage Kidney Disease
Proteinuria
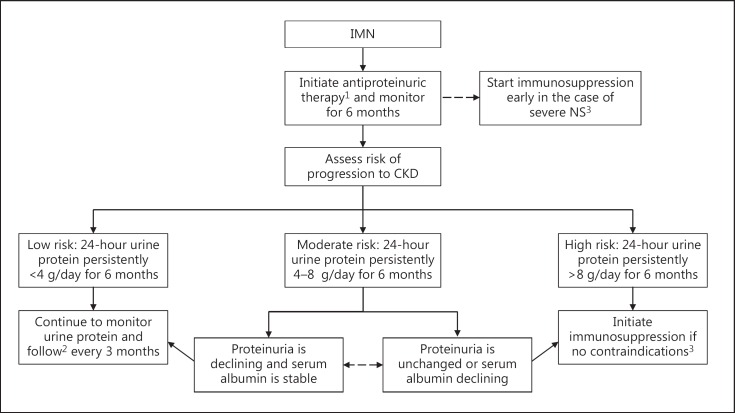
Currently, a prolonged duration of high-grade proteinuria is the strongest predictor of progression to renal impairment and remains the best evidence-based guide to a long-term outcome (fig. 1). This is the method we employ to assess prognosis in the great majority of patients with IMN evaluated in our Glomerulonephritis Clinic. European studies have found that high levels of urinary IgG and low-molecular-weight (LMW) proteins (such as β2- and α1-microglobulins) were associated with worse renal survival [27,28,29]. However, LMW proteins do not appear to predict either long-term outcome or a response to cyclophosphamide [30], and a recent North American study found that although LMW proteins were predictive of a response to rituximab at 12 months, they did not predict long-term outcome (24 months) any better than changes in the 24-hour urine protein excretion rate [31]. High levels of anti-PLA2R have also been suggested as indicative of a poor prognosis, though more experience is needed in this respect [32]. Potentially, the combination of several of these factors may be better than any single one to guide prognosis.
Fig. 1.
Suggested treatment algorithm based on risk assessment and degree of protein excretion. 1 Initiate low-salt diet; start ACE inhibitors/ARB (target blood pressure <130/80 mm Hg) as tolerated. 2 If the patient relapses, consider immunosuppressive therapy. 3 Initial treatment with an appropriate immunosuppressive agent after excluding contraindications to treatment, such as infections, malignancy and impaired GFR.
Age
MN is the most common cause of NS in patients over the age of 60 years [33]. This age group accounts for 23% of patients diagnosed with IMN [34]. Although increased age is reported to be a risk factor for progression, it likely reflects the natural decline in glomerular filtration rate (GFR) associated with normal aging (i.e. the loss of functional nephrons). Indeed, when compared to younger IMN patients, the rate of decline in GFR in the elderly was similar, and the higher end-stage kidney disease rate was due to a lower GFR at therapy start [34]. Thus, older individuals will require careful attention given their lower reserve of kidney function at baseline, but age alone should not preclude therapy. A heightened awareness must be maintained both before and after initiating potent immunosuppressive treatment, given that AE of immunosuppression are likely to be greater.
Sex
A number of studies have reported worse outcomes in men compared to women with IMN. A review of the data from our registry did find that, on average, men had a higher blood pressure (2 mm Hg) than women and higher mean proteinuria. However, after adjusting for these two factors, the rate of decline in GFR was almost the same, making our approach to treatment, after these parameters had been assessed, independent of the patient's sex [35].
Kidney Function
A reduced kidney function at presentation (as determined by increased serum creatinine or reduced creatinine clearance) has been shown to correlate with reduced renal survival. In addition, a progressive decline in renal function over time (the slope of creatinine clearance) is strongly predictive of a poor renal outcome. Our concern in this cohort is that the former, at presentation, is often not related to the disease process per se and that the time span needed before a decline in the slope as the only parameter of progression can clearly be determined results in an irreversible loss of a substantial number of functioning nephrons [36].
Choosing the Right Therapy
The natural history of untreated IMN is variable. Based on Western studies [37,38], a third of the patients will have spontaneous and complete remission, another third will have persistent proteinuria, and another third will have persistent proteinuria and progress to end-stage renal disease. This illustrates the relevance of establishing, as early as possible, those patients who will achieve spontaneous remission and thus be spared the potential risks of immunosuppression. On the other end of the spectrum, there exists an IMN group with an increased risk of developing renal impairment, including patients with persistent high-grade proteinuria and those who present with either a low GFR or with evidence of progressive decline in renal function. This group represents a relatively small percentage of IMN patients, but they may warrant immunosuppressive therapy earlier than the standard patient, given that their renal reserve is low and their risk of NS complications is high. Previously, we published a validated tool using daily protein excretion and GFR over time to predict the risk of progression [36]. In our practice, we use a modified version of this algorithm to determine the overall risk of progression to help guide our treatment in the majority of IMN patients (fig. 1).
Low-Risk Group
Patients in this group have low-grade proteinuria (≤4 g/day) and a normal renal function that persists over a 6-month period of observation. They have a good prognosis, with only a 5% risk of progression to chronic kidney disease (CKD) over 5 years. Conservative therapy only is our approach in this cohort and should include dietary salt restriction and blood pressure control, preferably with angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB), which may also contribute to proteinuria reduction.
Medium-Risk Group
This group is defined by persistent proteinuria between 4 and 8 g/day over 6 months, despite maximum conservative therapy; the patients have a >50% probability of developing CKD within 5 years. In general, we follow the KDIGO guidelines that recommend the initiation of immunosuppressive therapy in conjunction with conservative therapy at the end of that observation period. The exceptions are patients with advanced CKD (too late to treat) or life-threatening infections (too risky to treat). In our clinic, we take these factors into consideration, along with age and other comorbidities like diabetes that may significantly elevate the risk of treatment, thus favoring further observation. This approach is used particularly if proteinuria is showing a progressive decline and GFR is stable over the 6-month span [37].
High-Risk Group
Patients in this group have persistent proteinuria >8 g/day and/or a deteriorating kidney function. They have a 65–80% risk of progression to CKD in 5 years and, in these patients, we may choose to start therapy earlier than the 6 months. Additionally, ruling out complications of NS as additional factors in this clinical state (such as renal vein thrombosis) or therapy (such as over-diuresis or drug-induced interstitial nephritis) is critical. Repeated assessment is required especially if the GFR trajectory has changed or the patient's clinical status is deteriorating, since there is a radical difference in management if the deterioration is due to a complication rather than the disease process itself. In the past, those with a GFR <40 ml/min/1.73 m2, but still assessed as having a reasonable chance of response, might receive either cyclical cyclophosphamide/prednisone or cyclosporine. Although a recent randomized controlled trial (RCT) points out that the risk-benefit ratio is substantially altered in this scenario and any therapy must be carefully weighed against the risk, we occasionally consider these therapies under case-specific conditions including the use of rituximab [39].
Conservative Therapy
Dietary Modifications
We strongly recommend salt restriction in our patients with proteinuria with or without hypertension. The average salt consumption in most Western countries is well beyond the recommended daily intake per person (<2 g of sodium/day based on WHO recommendations) [40]. We often refer patients to a dietitian for education and reinforcement of the necessary dietary changes. It has also been shown that the effect of a renin-angiotensin system blockade is diminished in patients who have high urinary sodium excretion, which is another reason for paying careful attention to sodium intake [41]. In terms of dietary protein intake, we advise adults >18 years to take 0.8 g/kg body weight/day plus replacing the protein excreted in the urine on a gram-for-gram basis [42]. Diets low in fat and cholesterol are also advised in nephrotic patients [5].
ACE Inhibitors/ARB
The ideal target blood pressure in IMN is not clear. A blood pressure of <130/80 mm Hg among those with >1 g/day protein excretion is a reasonable target. Given the positive effect on proteinuria in other renal diseases, unless there is a specific contraindication, we prescribe ACE inhibitors or ARB in this setting. Their benefit has been suggested in a recent study, which showed that patients who achieved spontaneous partial or complete remission in proteinuria were more likely to have received ACE inhibitor therapy prior to achieving this state [37]. We do not combine ACE inhibitors and ARB, given the lack of any evidence of benefit in IMN and the potential risk of side effects such as acute kidney injury, hyperkalemia and hypotension [43]. We also advise patients experiencing intercurrent illness, especially associated with volume depletion (vomiting or diarrhea), to discontinue their renin-angiotensin system blockade therapy temporarily to avoid acute kidney injury.
Hyperlipidemia
Hypercholesterolemia and hypertriglyceridemia are known complications of NS and almost always accompany IMN patients with nephrotic-range proteinuria. The most effective way to improve hyperlipidemia associated with NS is to cure the underlying IMN or at least reduce proteinuria to the subnephrotic range. However, for patients where this cannot be accomplished and where there is a projected long duration of nephrosis, we prescribe statins to help retard one of the factors known to be associated with accelerated cardiovascular disease [44].
Thromboembolism
Compared with other primary glomerulonephritis, patients with IMN accompanied by NS have a higher risk of developing renal vein thrombosis and thromboembolism [45]. Chronic prophylactic anticoagulation has long been debated [46]. In a cohort of 898 patients with MN in North America, the risk of venous thromboembolism (VTE) was 7.2%, with most cases diagnosed within the first 2 years of onset of nephrosis [47]. Low serum albumin (<28 g/l) in multivariate analysis was the dominant factor associated with a higher incidence of VTE. Recently, a risk-benefit tool has been developed to help clinicians in their decision-making based on the data surrounding the question of prophylactic anticoagulation, which takes into account not only the risks of thromboembolism but also the risks of bleeding (www.gntools.com) [48]. Interestingly, episodes of VTE were not shown to affect renal survival. Earlier reports have suggested higher incidences of VTE [49,50], especially when routine screening was employed; however, there are no trials that have shown improved outcomes with screening. In our clinic, we often prescribe prophylactic anticoagulation in patients with serum albumin levels of <25 g/l but only after very careful assessment and after determining that the risk of bleeding is low.
Immunosuppressive Therapy
In patients in whom immunosuppressive therapy is warranted, treatment with either an alkylating agent combined with prednisone or cyclosporine is recommended by the KDIGO glomerulonephritis guidelines. Recently, a systematic review of RCTs (36 trials, 1,762 patients) of immunosuppressive treatments showed a reduction in short- and long-term complications of IMN in patients treated with a combination of prednisone and alkylating agents, however, at the cost of increased side effects. The benefit of other immunosuppressive agents was less clear in their relationship with the standard hard outcomes of renal survival primarily due to sample sizes (not powered to detect these outcomes) and the short follow-up period in relation to the usual slow progression over years of MN [51]. Which of these therapeutic regimens is used initially depends on the careful assessment of the individual patient. Comorbidities such as obesity and diabetes may deter clinicians and patients from alkylating agent-based regimens, given the significant steroid exposure. Additionally, the risk of complications from cyclophosphamide may be higher in older individuals and/or in those with a reduced GFR. On the other hand, cyclosporine is often difficult to manage and not as well tolerated in patients with reduced GFR and/or in those with severe underlying vascular damage on kidney biopsy, which tends to accentuate the calcineurin inhibitor (CNI) nephrotoxicity. Recently, there has been evidence suggesting that treatment with rituximab can be an effective alternative to these agents, although the published data to date are limited, and long-term results are lacking in direct comparison to these other agents. We have summarized the established immunosuppressive regimens in table 2.
Table 2.
Common immunosuppressive regimens used for the treatment of MN
| Group | Dosage | Special considerations |
|---|---|---|
| Alkylating agents | Ponticelli-based regimens: | – Follow WBC, serum creatinine and urine protein excretion every 2 weeks for the first 2 months, then every month while on therapy; monitor serum glucose while on prednisone |
| – Methylprednisolone 1 g i.v. for 3 days, then prednisone 0.4 mg/kg daily for 27 days in months 1, 3 and 5, alternating with chlorambucil 0.2 mg/kg/day for 30 days in months 2, 4 and 6 | – Hold chlorambucil or cyclophosphamide if WBC ≤3,500/mm3 until recovery to >4,000/ mm3, then adjust the dose | |
| – Methylprednisolone 1 g i.v. for 3 days, then prednisone 0.4 mg/kg daily for 27 days in months 1, 3 and 5, alternating with cyclophosphamide 2.5 mg/kg/day for 30 days in months 2, 4 and 6 | – Consider PJP prophylaxis for patients on cyclophosphamide | |
| – Methylprednisolone 1 g i.v. for 3 consecutive days, then prednisone 0.5 mg/kg every other day for 6 months with subsequent tapering plus cyclophosphamide 1.5–2 mg/day for 1 yeara | – Consider gastric (PPI) and bone protection (calcium and vitamin D ± bisphosphonate) for patients on prednisone | |
| CNIs | – Cyclosporine 3.5 mg/kg/day given in 2 equally divided doses 12 h apart, with or without prednisone (0.15 mg/kg/day) for 6 months | – Start at lower dose: target cyclosporine 12 h trough level of 110–170 μg/1 |
| – Tacrolimus 0.05–0.075 mg/kg/day given in 2 equally divided doses 12 h apart, without prednisone for 6–12 months | – Start at lower dose: target tacrolimus 12 h trough level of 4–8 μg/l | |
| –Consider extending the course if the patient is in partial remission, with slow tapering over 6–18 months | ||
| Rituximab | – Either 375 mg/m2 i.v. at 4 weekly doses or 1 g i.v. at 2 doses 15 days apart | –Given in a monitored setting; caution of severe infusion reactions |
| – A repeated course may be necessary if only partial reduction at 6 months is observed | – Consider PJP prophylaxis | |
| – Monitoring of the CD19/20 count may be usefulb | ||
PJP = Pneumocystis jirovecii pneumonia; PPI = proton pump inhibitor.
More recently, a Dutch group has proposed a cyclophosphamide course of 6 months duration to lower the risk of long-term toxicity.
More evidence is needed regarding the utility and frequency of monitoring CD19/20 counts.
Alkylating Agent Regimens
One of the first immunosuppressive regimens proven to be effective against IMN was the combination of chlorambucil and prednisone [52]. Ponticelli et al. [53] showed a significant increase in complete and partial remissions compared to symptomatic management (72 vs. 30%). Subsequently, cyclophosphamide was compared to chlorambucil, with similar rates of remission but a decreased incidence of side effects. A smaller RCT (15 patients treated with chlorambucil plus steroids and 17 patients with cyclophosphamide plus steroids) was stopped prematurely due to increased side effects in the chlorambucil-treated group [54]. Although these agents are effective in Western and Eastern populations [55,56], they do have a significant rate of late relapse. In our clinic, we usually prescribe these regimens with some modification, i.e. specifically eliminating the pulses of steroids, given the technical difficulty in arranging intravenous methylprednisolone and our concern that the additional steroids add considerably to the AE profile based on cumulative exposure. Furthermore, patients often do not tolerate their ‘off prednisone’ months, commonly experiencing symptoms of fatigue or feeling ‘low’. Although not as highly recommended by KDIGO, the cytotoxic regimen from the Dutch group has less steroid exposure but a higher cumulative dose of cyclophosphamide, though the short-term efficacy seems comparable [56]. These authors have recently suggested that a reduction from a 12- to 6-month course of cyclophosphamide is as effective as the standard 12-month course, but long-term data are lacking [57].
Calcineurin Inhibitors
Based on both RCTs and observational studies [58,59,60], cyclosporine has been shown to be effective in inducing remission among patients with steroid-resistant nephrotic IMN (75% remission vs. 22% remission in the placebo group) and, in a small RCT, among those with a progressive decline in renal function and high-grade proteinuria. Although there was a high rate of relapse during follow-up in this 6-month study (30–47%), a significantly lower rate of relapse has been seen when therapy was more prolonged [58]. It is suggested that cyclosporine monotherapy is as effective as the combination of cyclosporine and prednisone in inducing remission after 1 year [58]. Based on these data, we assess treatment response in our clinic after a minimum of 6 months and, if the patients have achieved complete remission, cyclosporine is slowly tapered off over a 6-month follow-up, but, if only partial remission has been achieved, we continue treatment with cyclosporine for a minimum of an additional 6 months, titrating the cyclosporine down if possible to reduce the likelihood of nephrotoxicity while still maintaining partial remission. If partial remission has been difficult to achieve or the patient has had complications due to NS in the past, we often maintain treatment with a low level of cyclosporine for a prolonged period (years) to maintain control of the disease. In patients who do not achieve at least partial remission by 6 months, we discontinue cyclosporine therapy and start with an alternative agent if the benefit-risk ratio remains positive. Patients on any CNI may have periods of renal impairment, often transient and dose related, and, therefore, we do monitor both serum creatinine and drug levels during the initial period, during any up-titration and when there is an unexpected change in the serum creatinine level.
The other CNI, tacrolimus, appears to be as effective as cyclosporine [61]. In an RCT from Spain, patients on tacrolimus monotherapy had significantly higher combined partial and complete remission rates at 18 months compared to controls (76 vs. 34%) but with similar relapse rates to cyclosporine therapy following its withdrawal [61]. In our clinic, cyclosporine is the predominant CNI used, but only because of its availability through government programs and its lower cost; however, given its AE profile and convenience, if tacrolimus is covered by the patient's health insurance, it is often preferred. In our experience, the two CNI options are equally efficacious.
Rituximab
Several prospective but uncontrolled trials have shown rituximab to be an effective therapy for patients with IMN [62,63,64,65,66]. Recently, 100 consecutive IMN patients were treated with rituximab over a median follow-up time of 29 months, and partial or complete remission was achieved in 65 patients [65]. AE aside from infusion reactions were uncommon. There was a 28% relapse rate, similar to other IMN drug regimens, but relapse tended to occur later than in CNI-based regimens. Also, in parallel to the other major therapeutic options, the response time usually takes months (a median time of 7 months), and most patients with relapse respond to a repeat course of their primary treatment. A single infusion with monitoring of B-cell depletion, low-dose weekly infusions over 4 weeks or a larger 2-dose regimen have all been shown to be effective. Based on these pilot studies, with its ease of use and at least a short-term low AE profile, rituximab is an evolving candidate for the treatment of IMN. Results from ongoing RCTs such as the MENTOR trial (NCT01180036) comparing rituximab to cyclosporine treatment are awaited and, if the earlier results are confirmed, they will substantially raise evidence supporting this approach.
Adrenocorticotropic Hormone
Two forms of the adrenocorticotropic hormone (ACTH) drug are available. First, the natural form of ACTH derived from the porcine pituitary gland was used. It was demonstrated to be effective in the treatment of NS in children in the early 1950s [67,68]. Although there are no RCTs evaluating this form of ACTH, it remains the only FDA drug approved in the United States for the treatment of NS, based on these historical data. This medication has recently reemerged as therapy in nephrotic IMN patients. In a 20-patient, dose-response prospective study, a >50% proteinuria reduction was seen in 65% of the patients at 12 months, and the response was proportionate to cumulative drug exposure [69]. The second ACTH is a synthetic form that has been available in Europe for more than 2 decades. A small randomized trial in patients with moderate IMN disease and no previous immunosuppression showed that a year of this therapy was equivalent to the Ponticelli steroids/cytotoxic combination in achieving remission [70]. Although its mechanism of action is unknown, it is postulated that ACTH may exert its effect by interacting with melanocortin receptors in the podocytes, which may stabilize these cells [71]. We do not prescribe this medication as an initial agent, given its limited evidence base, common side effects and high cost, but our initial dose-response study did demonstrate its potential. An ongoing trial comparing naturally occurring ACTH (Acthar) to placebo for resistant IMN is ongoing (NCT01386554).
Mycophenolic Acid
Studies assessing mycophenolate mofetil (MMF) for the treatment of IMN have reported variable results. In a Dutch cohort, compared to a historic control group receiving cyclophosphamide, 32 patients receiving MMF plus corticosteroids had a proteinuria remission rate of 66% at 1 year [72]. The relapse rate was significant (38%) and, perhaps most importantly, it was noted in some even before the trial of therapy was completed. In contrast, in an open-label RCT from France, MMF monotherapy showed no significant difference in remission rates compared to placebo therapy [73]. We occasionally use MMF as adjunct therapy in patients who are intolerant of full-dose, first-line therapy.
Azathioprine
There are very little data that indicate a benefit of azathioprine with or without corticosteroids for the treatment of IMN. We do not use this agent in our glomerulonephritis clinic for IMN.
Prednisone
Although prednisone has been shown to be effective in combination with other agents, there is substantial evidence to suggest it is ineffective as monotherapy in the treatment of IMN in Western countries [74,75].
Progressive and Resistant Disease
Most of the above treatment regimens were established in patients with nephrotic-range proteinuria and generally well-preserved GFR. Evidence of an efficacy in patients with severe or progressive renal insufficiency is scarce. A recent 3-armed RCT from the United Kingdom compared the cyclical regimen of chlorambucil/prednisone to cyclosporine or supportive therapy only in patients with documented rapidly progressive IMN and a mean creatinine clearance of 50 ml/min at entry [39]. Those in the chlorambucil group showed a modest slowing in the rate of decline in GFR, but with a higher percentage of AE than those in the other two arms, suggesting that this rare category of IMN patients needs to be carefully assessed before any therapy is tried.
In the nonresponsive patient, adherence to medications and to sodium dietary restrictions as well as ruling out specific IMN complications such as renal vein thrombosis should be entertained. It is also important to differentiate primary nonresponse from relapse, given that the latter is likely to respond to retreatment. An additional important caveat is that clinical response to immunosuppressive therapy may lag by months following a treatment course, especially in the case of alkylating agents and rituximab; thus, after the initial course of therapy has been completed, it is recommended to wait for up to 6 months before determining failure and embarking on a different immunosuppressive regimen.
Follow-up
Long-term follow-up is necessary in all patients with IMN. The frequency of follow-up is often determined by the patients’ remission status as defined by KDIGO (table 3). Even patients with complete remission should be made aware of the clinical signs associated with disease relapse and have regular monitoring of urine protein excretion, since recurrence of low-grade proteinuria can precede the full-blown clinical features of NS by months.
Table 3.
Definitions of the response to observation, conservative or immunosuppressive treatment
| Definition | Criteria |
|---|---|
| Complete remission | Reduction in urine protein to <0.3 g/day (urine protein-creatinine ratio <300 mg/g or 30 mg/mmol) confirmed by two values at least 1 week apart, with normal serum albumin and renal function |
| Partial remission | Reduction in urine protein to <3.5 g/day (urine protein-creatinine ratio <3,500 mg/g or 350 mg/mmol) and ≥50% reduction from peak values, confirmed by two values at least 1 week apart, accompanied by an improvement or normalization of serum albumin, and stable renal function |
| No response | Not fitting either criteria (note: response can be delayed, and this should only be confirmed after 6 months of observation from the end of an appropriate regimen, especially with cytotoxic agents and rituximab) |
| Relapse | NS alter achieving either complete or partial remission |
Summary
This review provides an approach to the management of IMN but does not cover all patient scenarios such as pregnancy and recurrent or de novo disease in renal transplants. It does specifically reflect the Toronto approach as well as, in general, the approach of Western countries for patients once IMN has been established. It largely follows the published KDIGO glomerulonephritis guidelines but also highlights the changes that have occurred since their publication and emphasizes the ongoing need for integrating physician judgment and patient preferences into the management algorithm.
Disclosure Statement
Both authors have no conflicts of interest to disclose.
References
- 1.Swaminathan S, et al. Changing incidence of glomerular disease in Olmsted County, Minnesota: a 30-year renal biopsy study. Clin J Am Soc Nephrol. 2006;1:483–487. doi: 10.2215/CJN.00710805. [DOI] [PubMed] [Google Scholar]
- 2.Hanko JB, et al. The changing pattern of adult primary glomerular disease. Nephrol Dial Transplant. 2009;24:3050–3054. doi: 10.1093/ndt/gfp254. [DOI] [PubMed] [Google Scholar]
- 3.Haas M, Meehan SM, Karrison TG, Spargo BH. Changing etiologies of unexplained adult nephrotic syndrome: a comparison of renal biopsy findings from 1976-1979 and 1995-1997. Am J Kidney Dis. 1997;30:621–631. doi: 10.1016/s0272-6386(97)90485-6. [DOI] [PubMed] [Google Scholar]
- 4.Jones DB. Nephrotic glomerulonephritis. Am J Pathol. 1957;33:313–329. [PMC free article] [PubMed] [Google Scholar]
- 5.Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO Clinical Practice Guideline for Glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 6.Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines - application to the individual patient. Kidney Int. 2012;82:840–856. doi: 10.1038/ki.2012.280. [DOI] [PubMed] [Google Scholar]
- 7.Gluck MC, Gallo G, Lowenstein J, Baldwin DS. Membranous glomerulonephritis. Evolution of clinical and pathologic features. Ann Intern Med. 1973;78:1–12. doi: 10.7326/0003-4819-78-1-1. [DOI] [PubMed] [Google Scholar]
- 8.Austin HA, Antonovych TT, MacKay K, Boumpas DT, Balow JE. NIH conference. Membranous nephropathy. Ann Intern Med. 1992;116:672–682. doi: 10.7326/0003-4819-116-8-672. [DOI] [PubMed] [Google Scholar]
- 9.Jennette JC, Iskandar SS, Dalldorf FG. Pathologic differentiation between lupus and nonlupus membranous glomerulopathy. Kidney Int. 1983;24:377–385. doi: 10.1038/ki.1983.170. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz GS. Membranous glomerulopathy: emphasis on secondary forms and disease variants. Adv Anat Pathol. 2001;8:119–125. doi: 10.1097/00125480-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Lefaucheur C, et al. Membranous nephropathy and cancer: epidemiologic evidence and determinants of high-risk cancer association. Kidney Int. 2006;70:1510–1517. doi: 10.1038/sj.ki.5001790. [DOI] [PubMed] [Google Scholar]
- 12.Haas M. IgG subclass deposits in glomeruli of lupus and nonlupus membranous nephropathies. Am J Kidney Dis. 1994;23:358–364. doi: 10.1016/s0272-6386(12)80997-8. [DOI] [PubMed] [Google Scholar]
- 13.Kuroki A, et al. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern Med. 2002;41:936–942. doi: 10.2169/internalmedicine.41.936. [DOI] [PubMed] [Google Scholar]
- 14.Ohtani H, et al. Distribution of glomerular IgG subclass deposits in malignancy-associated membranous nephropathy. Nephrol Dial Transplant. 2004;19:574–579. doi: 10.1093/ndt/gfg616. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–715. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 16.Ehrenreich T, et al. Treatment of idiopathic membranous nephropathy. N Engl J Med. 1976;295:741–746. doi: 10.1056/NEJM197609302951401. [DOI] [PubMed] [Google Scholar]
- 17.Cahen R, Francois B, Trolliet P, Gilly J, Parchoux B. Aetiology of membranous glomerulonephritis: a prospective study of 82 adult patients. Nephrol Dial Transplant. 1989;4:172–180. doi: 10.1093/oxfordjournals.ndt.a091852. [DOI] [PubMed] [Google Scholar]
- 18.Honkanen E. Survival in idiopathic membranous glomerulonephritis. Clin Nephrol. 1986;25:122–128. [PubMed] [Google Scholar]
- 19.Glassock RJ. Secondary membranous glomerulonephritis. Nephrol Dial Transplant. 1992;7(suppl 1):64–71. [PubMed] [Google Scholar]
- 20.Zech P, et al. The nephrotic syndrome in adults aged over 60: etiology, evolution and treatment of 76 cases. Clin Nephrol. 1982;17:232–236. [PubMed] [Google Scholar]
- 21.Beck LH, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–690. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 23.Hoxha E, et al. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 24.Hofstra JM, Beck LH, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–1291. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin W, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–1143. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruggenenti P, et al. Anti-phospholipase A2 receptor antibody titer predicts post-rituximab outcome of membranous nephropathy. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014070640. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branten AJW, et al. Urinary excretion of beta2-microglobulin and IgG predict prognosis in idiopathic membranous nephropathy: a validation study. J Am Soc Nephrol. 2005;16:169–174. doi: 10.1681/ASN.2004040287. [DOI] [PubMed] [Google Scholar]
- 28.Reichert LJ, Koene RA, Wetzels JF. Urinary excretion of beta 2-microglobulin predicts renal outcome in patients with idiopathic membranous nephropathy. J Am Soc Nephrol. 1995;6:1666–1669. doi: 10.1681/ASN.V661666. [DOI] [PubMed] [Google Scholar]
- 29.Bazzi C, et al. Urinary excretion of IgG and alpha(1)-microglobulin predicts clinical course better than extent of proteinuria in membranous nephropathy. Am J Kidney Dis. 2001;38:240–248. doi: 10.1053/ajkd.2001.26080. [DOI] [PubMed] [Google Scholar]
- 30.Du Buf-Vereijken PWG, Wetzels JFM. Treatment-related changes in urinary excretion of high and low molecular weight proteins in patients with idiopathic membranous nephropathy and renal insufficiency. Nephrol Dial Transplant. 2006;21:389–396. doi: 10.1093/ndt/gfi219. [DOI] [PubMed] [Google Scholar]
- 31.Irazabal MV, et al. Low- and high-molecular-weight urinary proteins as predictors of response to rituximab in patients with membranous nephropathy: a prospective study. Nephrol Dial Transplant. 2013;28:137–146. doi: 10.1093/ndt/gfs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoxha E, et al. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–1366. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron JS. Nephrotic syndrome in the elderly. Semin Nephrol. 1996;16:319–329. [PubMed] [Google Scholar]
- 34.Zent R, Nagai R, Cattran DC. Idiopathic membranous nephropathy in the elderly: a comparative study. Am J Kidney Dis. 1997;29:200–206. doi: 10.1016/s0272-6386(97)90030-5. [DOI] [PubMed] [Google Scholar]
- 35.Cattran DC, et al. The impact of sex in primary glomerulonephritis. Nephrol Dial Transplant. 2008;23:2247–2253. doi: 10.1093/ndt/gfm919. [DOI] [PubMed] [Google Scholar]
- 36.Cattran DC, et al. Validation of a predictive model of idiopathic membranous nephropathy: its clinical and research implications. Kidney Int. 1997;51:901–907. doi: 10.1038/ki.1997.127. [DOI] [PubMed] [Google Scholar]
- 37.Polanco N, et al. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schieppati A, et al. Prognosis of untreated patients with idiopathic membranous nephropathy. N Engl J Med. 1993;329:85–89. doi: 10.1056/NEJM199307083290203. [DOI] [PubMed] [Google Scholar]
- 39.Howman A, et al. Immunosuppression for progressive membranous nephropathy: a UK randomised controlled trial. Lancet. 2013;381:744–751. doi: 10.1016/S0140-6736(12)61566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Sodium intake for adults and children. http://www.who.int/nutrition/publications/guidelines/sodium_intake/en/
- 41.Vegter S, et al. Sodium intake, ACE inhibition, and progression to ESRD. J Am Soc Nephrol. 2012;23:165–173. doi: 10.1681/ASN.2011040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The National Academies Press Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) http://www.nap.edu/catalog/10490/dietary-reference-intakes-for-energy-carbohydrate-fiber-fat-fatty-acids-cholesterol-protein-and-amino-acids-macronutrients [DOI] [PubMed]
- 43.Mann JFE, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 44.Taylor F, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barbour SJ, et al. Disease-specific risk of venous thromboembolic events is increased in idiopathic glomerulonephritis. Kidney Int. 2012;81:190–195. doi: 10.1038/ki.2011.312. [DOI] [PubMed] [Google Scholar]
- 46.Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18:2221–2225. doi: 10.1681/ASN.2006111300. [DOI] [PubMed] [Google Scholar]
- 47.Lionaki S, et al. Venous thromboembolism in patients with membranous nephropathy. Clin J Am Soc Nephrol. 2011;7:43–51. doi: 10.2215/CJN.04250511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee T, et al. Personalized prophylactic anticoagulation decision analysis in patients with membranous nephropathy. Kidney Int. 2014;85:1412–1420. doi: 10.1038/ki.2013.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagoner RD, Stanson AW, Holley KE, Winter CS. Renal vein thrombosis in idiopathic membranous glomerulopathy and nephrotic syndrome: incidence and significance. Kidney Int. 1983;23:368–374. doi: 10.1038/ki.1983.28. [DOI] [PubMed] [Google Scholar]
- 50.Llach F, Papper S, Massry SG. The clinical spectrum of renal vein thrombosis: acute and chronic. Am J Med. 1980;69:819–827. doi: 10.1016/s0002-9343(80)80006-4. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y, et al. Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol. 2013;8:787–796. doi: 10.2215/CJN.07570712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponticelli C, et al. A 10-year follow-up of a randomized study with methylprednisolone and chlorambucil in membranous nephropathy. Kidney Int. 1995;48:1600–1604. doi: 10.1038/ki.1995.453. [DOI] [PubMed] [Google Scholar]
- 53.Ponticelli C, et al. A randomized study comparing methylprednisolone plus chlorambucil versus methylprednisolone plus cyclophosphamide in idiopathic membranous nephropathy. J Am Soc Nephrol. 1998;9:444–450. doi: 10.1681/ASN.V93444. [DOI] [PubMed] [Google Scholar]
- 54.Branten AJ, Reichert LJ, Koene RA, Wetzels JF. Oral cyclophosphamide versus chlorambucil in the treatment of patients with membranous nephropathy and renal insufficiency. Q J Med. 1998;91:359–366. doi: 10.1093/qjmed/91.5.359. [DOI] [PubMed] [Google Scholar]
- 55.Jha V, et al. A randomized, controlled trial of steroids and cyclophosphamide in adults with nephrotic syndrome caused by idiopathic membranous nephropathy. J Am Soc Nephrol. 2007;18:1899–1904. doi: 10.1681/ASN.2007020166. [DOI] [PubMed] [Google Scholar]
- 56.Du Buf-Vereijken PWG, et al. Cytotoxic therapy for membranous nephropathy and renal insufficiency: improved renal survival but high relapse rate. Nephrol Dial Transplant. 2004;19:1142–1148. doi: 10.1093/ndt/gfh036. [DOI] [PubMed] [Google Scholar]
- 57.Hofstra JM, Wetzels JFM. Alkylating agents in membranous nephropathy: efficacy proven beyond doubt. Nephrol Dial Transplant. 2010;25:1760–1766. doi: 10.1093/ndt/gfq017. [DOI] [PubMed] [Google Scholar]
- 58.Alexopoulos E, Papagianni A, Tsamelashvili M, Leontsini M, Memmos D. Induction and long-term treatment with cyclosporine in membranous nephropathy with the nephrotic syndrome. Nephrol Dial Transplant. 2006;21:3127–3132. doi: 10.1093/ndt/gfl360. [DOI] [PubMed] [Google Scholar]
- 59.Cattran DC, et al. Cyclosporine in patients with steroid-resistant membranous nephropathy: a randomized trial. Kidney Int. 2001;59:1484–1490. doi: 10.1046/j.1523-1755.2001.0590041484.x. [DOI] [PubMed] [Google Scholar]
- 60.Cattran DC, et al. A controlled trial of cyclosporine in patients with progressive membranous nephropathy. Canadian Glomerulonephritis Study Group. Kidney Int. 1995;47:1130–1135. doi: 10.1038/ki.1995.161. [DOI] [PubMed] [Google Scholar]
- 61.Praga M, Barrio V, Juárez GF, Luño J. Tacrolimus monotherapy in membranous nephropathy: a randomized controlled trial. Kidney Int. 2007;71:924–930. doi: 10.1038/sj.ki.5002215. [DOI] [PubMed] [Google Scholar]
- 62.Fervenza FC, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 63.Fervenza FC, et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bomback AS, et al. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009;4:734–744. doi: 10.2215/CJN.05231008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruggenenti P, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1416–1425. doi: 10.1681/ASN.2012020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Segarra A, et al. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin J Am Soc Nephrol. 2009;4:1083–1088. doi: 10.2215/CJN.06041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Metcoff J, Rance CP, Kelsey WM, Nakasone N, Janeway CA. Adrenocorticotrophic hormone (ACTH) therapy of the nephrotic syndrome in children. Pediatrics. 1952;10:543–566. [PubMed] [Google Scholar]
- 68.Rapoport M, McCrory WW, Barbero G, Barnett HL, Forman CW. Effect of corticotropin (ACTH) on children with the nephrotic syndrome. J Am Med Assoc. 1951;147:1101–1106. doi: 10.1001/jama.1951.03670290009004. [DOI] [PubMed] [Google Scholar]
- 69.Hladunewich MA, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29:1570–1577. doi: 10.1093/ndt/gfu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ponticelli C, et al. A randomized pilot trial comparing methylprednisolone plus a cytotoxic agent versus synthetic adrenocorticotropic hormone in idiopathic membranous nephropathy. Am J Kidney Dis. 2006;47:233–240. doi: 10.1053/j.ajkd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Lindskog A, et al. Melanocortin 1 receptor agonists reduce proteinuria. J Am Soc Nephrol. 2010;21:1290–1298. doi: 10.1681/ASN.2009101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Branten AJ, du Buf-Vereijken PW, Vervloet M, Wetzels JF. Mycophenolate mofetil in idiopathic membranous nephropathy: a clinical trial with comparison to a historic control group treated with cyclophosphamide. Am J Kidney Dis. 2007;50:248–256. doi: 10.1053/j.ajkd.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 73.Dussol B, et al. Mycophenolate mofetil monotherapy in membranous nephropathy: a 1-year randomized controlled trial. Am J Kidney Dis. 2008;52:699–705. doi: 10.1053/j.ajkd.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 74.Cattran DC, et al. A randomized controlled trial of prednisone in patients with idiopathic membranous nephropathy. N Engl J Med. 1989;320:210–215. doi: 10.1056/NEJM198901263200403. [DOI] [PubMed] [Google Scholar]
- 75.Cameron JS, Healy MJR, Adu D; on behalf of the MRC glomerulonephritis working party. The Medical Research Council trial of short-term high-dose alternate day prednisolone in idiopathic membranous nephropathy with nephrotic syndrome in adults. Q J Med. 1990;74:133–156. [PubMed] [Google Scholar]