Abstract
Background
Renal impairment (RI) is a common complication of multiple myeloma (MM). Around 50% of patients with MM have RI at presentation, and up to 5% require dialysis treatment. Severe acute kidney injury (AKI) as a cause of RI is a particular challenge as historically the survival of patients who sustain this complication and require dialysis is very poor. However, in this current period, survival is improving and the focus is on optimum use of novel chemotherapies and the evaluation of extra-corporeal therapies for removal of serum immunoglobulin light chains.
Summary
RI in patients with MM is commonly associated with excess monoclonal free light chain (FLC) production; myeloma cast nephropathy is the predominant renal pathology in patients presenting with severe RI secondary to AKI. The majority of patients have mild to moderate RI and recover renal function. However, patients with more severe RI, in particular those with a requirement for dialysis, are less likely to recover renal function. Rapid diagnosis and prompt institution of anti-myeloma therapy is an important determinant of renal function recovery, through targeting early and sustained reduction of involved monoclonal FLC. Novel agents are associated with excellent disease response, and bortezomib is now widely used as a first-line agent in the management of MM in patients with severe RI. Extended haemodialysis using high cut-off dialysers is more effective for extracorporeal removal of FLC than plasma exchange, and clinical trials are in process. High-dose chemotherapy with autologous stem cell transplantation does have a role in patients with severe RI but requires careful patient selection.
Key Messages
RI is very common in patients with MM, and renal function recovery is associated with improved clinical outcomes. We summarise the epidemiology of MM in the UK, present the impact of RI and renal function recovery on patient outcome, and describe the current management of MM in western countries.
Facts from East and West
(1) A serum creatinine level >2 mg/dl has been reported in 16, 21, 24, and 33% of patients with MM in cohort studies from Japan, Europe, China, and Korea, respectively. A creatinine clearance rate <30 ml/min was observed in 30 and 15% of patients in Chinese and Western MM cohorts, respectively. The commonest cause of severe RI in patients with MM is myeloma cast nephropathy. (2) The efficacy of novel treatments (bortezomib, carfilzomib, thalidomide, and lenalidomide) has predominantly been assessed in Western patients. Bortezomib and dexamethasone are the current standard of care for MM and severe RI in the West. Severe RI is not a contraindication to autologous stem cell transplantation (ASCT). Most of the data are from the West; there are case reports from China describing good outcomes with ASCT. The removal of FLC by high-cut-off hemodialysis is under evaluation in randomized controlled trials (RCTs) in the West. Studies in this area are not yet conducted in China. In China, new treatments, such as bortezomib, are more widely used than before, and favorable results are being reported; however, RCT studies are still needed in this area to confirm the efficacy and safety of this and other novel treatments.
Key Words: Multiple myeloma, Renal impairment, Haematological response, Renal response/survival
Introduction
Multiple myeloma (MM) is a cancer of plasma cells and has an incidence of around 50 cases per million population (pmp)/year. Fifty percent of patients have renal impairment (RI) at presentation, and up to 20% have severe acute kidney injury (AKI). Severe AKI is usually a consequence of myeloma cast nephropathy (MCN), caused by high levels of immunoglobulin free light chain (FLC). The presence of severe AKI is an important complication of MM as it is associated with an increased risk of early mortality; however, the impact of mild-to-moderate RI at presentation on patient outcome is unclear. Recent advances in chemotherapy have led to better overall survival (OS) and for patients who require dialysis as a consequence of MM, increased recovery rates of independent renal function are being reported, and these improved renal outcomes are associated with better OS.
Epidemiology
MM is the 23rd most common cancer worldwide and accounted for 0.8% of all cancer diagnoses and 1.0% of all cancer-related mortality in 2012 [1]. In the UK, it is the 17th most common cancer and accounted for 1% of all new cases in 2011, with 4,792 patients with a new diagnosis of MM. The overall UK age-standardised annual incidence is 55 pmp [2]. The incidence rises with age, and in the UK 43% of all new cases were diagnosed in individuals ≥75 years old [2]. MM is more common in males (56%) than females (44%), with a male-to-female ratio of 6:5. The cancer is twice as common in African-Americans than Caucasians, and a similar ethnic pattern is also noted in the UK [3]. The UK age-standardised mortality rate for MM is 28 deaths pmp/year. The age-standardised mortality rates decreased by 19% in the periods from 1987 to 1989 and 2010 to 2012 for males and 16% for females. Most of these improvements in outcome have occurred in the last decade; this in part reflects better diagnostics, more effective chemotherapy and improved coding practices [2].
The Definition of RI in MM
In 2003, the CRAB criteria (elevated Calcium level, Renal insufficiency, Anaemia and Bone lesions) were introduced to standardise the definition of end-organ damage associated with symptomatic MM. A serum creatinine level of >173 µmol/l (or >2 mg/dl) was the cut-off for RI [4]. The Greek Myeloma Study Group subsequently utilised this threshold to report an incidence of 21% in a cohort of 756 patients presenting with MM [5]. However, this threshold does not include the majority of patients if conventional criteria to classify RI are used. For reporting RI in clinical outcome and clinical intervention studies, a number of definitions have been used. These are summarised in table 1.
Table 1.
Historic definitions and incidence of RI in published studies
| Study | Year | Cases | Definition and incidence of RI |
|---|---|---|---|
| Dawson et al. [130] | 1971 | 166 | out of 128 patients where blood urea level was available at diagnosis, 57 (44.5%) had serum urea levels >50 mg/dl |
| Kyle [131] | 1975 | 869 | serum creatinine >1.3 mg/dl in 55% of patients |
| MacLennan et al. [18] | 1989 | 1,205 | serum creatinine >130 μmol/l in 42% of patients that included 12% with serum creatinine >200 μmol/l and 10% with serum creatinine >300 μmol/l |
| Rayner et al. [132] | 1991 | 141 | serum creatinine ≥120 μmol/l in 65 (46%) patients that included 52 (80%) with serum creatinine 120–500 μmol/l and 13 (20%) with serum creatinine >500 μmol/l |
| Knudsen et al. [133] | 1994 | 1,353 | serum creatinine ≥130 μmol/l in 416 (31%) patients that included 16% with severe RI defined as a serum creatinine >200 μmol/l; CrCl was calculated in 1,206 patients – mild RI was observed in 296 (25%), moderate in 175 (15%) and severe in 115 (9%) |
| Kyle et al. [134] | 2003 | 1,027 | serum creatinine >1.3 mg/dl in 48% of patients that included 19% with serum creatinine ≥2 mg/dl |
| Eleutherakis-Papaiakovou et al. [5] | 2007 | 756 | serum creatinine ≥2 mg/dl in 160 (21%) of the 756 of patients that included 7% of patients with serum creatinine ≥4 mg/dl, 4% with serum creatinine ≥6 mg/dl, and 1% with serum creatinine ≥8 mg/dl |
The lack of standardisation of reporting of renal function in patients with MM is an important shortfall. Converting serum creatinine into estimated glomerular filtration rate (eGFR) illuminates the potential for inaccuracy. For example, a 65-year-old female with a creatinine level above 87 µmol/l will have RI as defined by an eGFR <60 ml/min [stage 3 chronic kidney disease (CKD)]; for a male of the same age, the stage 3 CKD threshold is 113 µmol/l. When RI is defined based on the threshold set by the CRAB criteria, the same 65-year-old female will have an eGFR <25 ml/min that is equivalent to stage 4 CKD; whereas for a male of the same age, the eGFR cut-off is <34 ml/min.
A major additional confounder is that equations that have been developed for renal function estimation in CKD are being used for measurement of renal function in patients with AKI, with the International Myeloma Working Group recommending criteria for the diagnosis of RI in MM based on the four-variable Modification of Diet in Renal Disease (MDRD) [6] or the CKD-Epidemiology Collaboration 2009 (CKD-EPI 2009) equation [7,8]. The large majority of patients with MM and RI at presentation have AKI not CKD.
Furthermore, creatinine-derived equations can be confounded by other factors relevant to patients with MM [9,10,11,12]; these include changes in muscles mass, dietary intake and proximal tubular dysfunction [13,14,15]. Recent studies have indicated that serum cystatin-C is superior to creatinine in assessing early RI, including patients with MM [16,17]. An elevated cystatin-C level (>0.95 mg/l) was reported in 90 (57.3%) of 157 newly diagnosed patients with MM, whereas a high creatinine level (>1.4 mg/dl in men and >1.2 mg/dl in women) was detected only in 37 (23.5%) patients, with 97 (61.7%) patients demonstrating a creatinine clearance (CrCl) <80 ml/min [17].
The Relationship between Renal Function and Survival in MM
An early Medical Research Council (MRC) myelomatosis trial reported a higher number of deaths (39%) within 100 days of recruitment in patients with a serum urea ≥15 µmol/l, or creatinine ≥200 µmol/l if the urea was <15 µmol/l, at diagnosis. Patients presenting with RI had an OS of 380 days with 28% survival at 2 years, compared to 52% for the entire study population at the same time point [18]. RI was responsible for 28% of the early deaths in a subsequent analysis that combined data from the MRC IV to MRC VIII trials that excluded a large proportion of patients presenting with severe AKI [19]. A total of 3,107 patients from the MRC myelomatosis trials between 1980 and 2002 were eligible to participate in this study.
A recent retrospective study from 15 Swedish hospitals reported a significantly inferior OS in patients with RI (eGFR <60 ml/min) compared to those who did not have RI. The survival of patients with RI who were treated with bortezomib-based chemotherapy (60 months) was better than survival of those who received conventional agents (27 months) [20]. Mayo Clinic investigators reported a complete renal response rate in a higher proportion of patients treated with novel agents compared to conventional treatment. Improvement of renal function was associated with better OS, although still inferior to patients with no RI at diagnosis [21].
A number of studies have reported on outcomes in patients with MM who required dialysis, a complication sustained by approximately 5% of patients at diagnosis [22,23,24]. The frequency of this complication may be decreasing. In a recent study that reported on 1,773 consecutive unselected patients with newly diagnosed MM between 1991 and 2011, the incidence of patients requiring dialysis was lower (3.5%) for those diagnosed after 2005 compared to the other three time cohorts: 5.5% for 2000–2004, 6% for 1995–1999, and 5% for 1990–1994 [25].
Dialysis has a major impact on the prognosis of patients with MM. A median survival of 10.2 months was reported in a single-centre study, where 82% of patients received dialysis at presentation; only 17% subsequently recovered independent renal function, these patients had a significantly better OS compared to those who remained dialysis dependent [26]. These results are comparable to data from the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA), with a median survival of 0.91 years for patients with MM or light chain deposition disease, compared to 4.46 years for those who did not have either of these diagnoses [27].
Recent single-centre studies are reporting improved renal recovery rates of around 60% in patients who require dialysis, and an associated improvement in survival [28]. These reports are not yet confirmed in larger prospective multicentre studies.
Pathogenesis of RI in MM
The association of MM with infection, dehydration, hypercalcaemia and bone pain leading to prescription of non-steroidal anti-inflammatory drugs, produces factors either singly or in combination that can cause AKI stage 1 and stage 2 [29]. The large majority of patients with this level of RI at presentation are likely to recover renal function. Whether this recovery is to the previous baseline is not known; we are not aware of any studies that have assessed this.
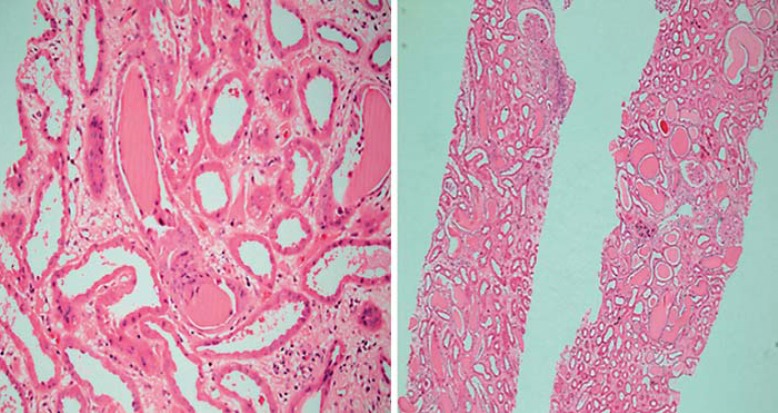
An involved FLC clone with a serum level in excess of 500 mg/l has the potential to cause severe RI secondary to MCN [28,30,31]. Where patients with MM and severe AKI have undergone renal biopsy, up to 90% are reported as showing MCN [30]; this is the characteristic disease-specific renal lesion. The typical histopathologic appearance is of an eosinophilic, fractured, proteinaceous distal tubular cast consisting of monoclonal FLC and Tamm-Horsfall protein (uromodulin) along with tubulointerstitial inflammation often accompanied by a proximal tubular injury (fig. 1).
Fig. 1.
Typical appearance of an MCN: tubules containing eosinophilic proteinaceous cast with a fractured appearance and surrounding cellular reaction.
However, many patients with a serum FLC (sFLC) >500 mg/l have normal renal function, highlighting the heterogeneity of the involved monoclonal light chain for cast formation. This reflects the variability in the complementarity-determining region-3 (CDR3) of the light chain in binding Tamm-Horsfall protein [32]. In some patients, MCN is triggered by a co-factor consistent with animal models that demonstrated that hypercalcaemia, acidosis and use of diuretics all lowered the threshold for cast formation.
There are a number of other renal lesions that can be associated with MM, including AL amyloidosis, monoclonal immunoglobulin deposition disease and light-chain proximal tubulopathy presenting as Fanconi syndrome [29,33,34]. There is a major overlap amongst these disorders, and there are reports of some patients with MM having multiple renal pathologies.
Recovery of Renal Function and Clinical Outcomes
In an early study that included 94 patients with RI defined as a creatinine level ≥177 μmol/l, renal recovery occurred in 26% and was associated with improved survival compared to those who did not recover renal function. A creatinine level <354 μmol/l, calcium level ≥2.88 mmol/l and a lower urinary light chain excretion (<1 g/day) were independently associated with renal function recovery [22].
Since the widespread adoption of dexamethasone as a standard component of chemotherapy regimens for patients with MM and RI, better rates of renal recovery are being reported. A single-centre series that consisted of 41 patients with newly diagnosed MM reported renal function improvement in 73% at a median of 1.9 months in patients who received high-dose dexamethasone, either with or without a novel agent. The introduction of novel agents has further improved the potential for renal recovery. Reversibility of RI was seen in 80% at a median of 0.8 months in patients who were treated with dexamethasone and a novel agent [35].
The relationship between disease-specific response as measured by sFLC levels and renal recovery is of great interest. A study from two specialist centres reported a cohort of patients presenting with a median eGFR of 9 ml/min with 62% of patients requiring dialysis. A 60% reduction in sFLC at day 21 was associated with increased likelihood of renal recovery; 71% of patients either became independent of dialysis or had an improvement in renal function from presentation. The median survival in patients who recovered independent renal function was 42.7 months compared to 7.8 months in patients who did not recover renal function [28].
The current consensus definition for a renal response in patients presenting with less severe RI was introduced by Ludwig and colleagues [36]. These criteria are based on changes in eGFR calculated by the 4-variable MDRD equation. A complete renal response (CRrenal) is defined as a sustained (at least 2-month) improvement in baseline eGFR from <50 to ≥60 ml/min; partial renal response (PRrenal), a sustained improvement in baseline GFR from <15 to 30–59 ml/min; minor renal response (MRrenal), a sustained improvement in baseline GFR from <15 to 15–29 ml/min or from 15–29 to 30–59 ml/min.
The renal response criteria were validated in a study which compared the efficacy of a bortezomib-based chemotherapy regimen in 46 consecutive patients with MM and RI. A renal response was reported in 59%, with 30% achieving a CRrenal and with a higher probability of response amongst those with light chain-only MM. When cystatin-C was used to measure renal function, patients with levels of >2 mg/l or a cystatin-C calculated eGFR <30 ml/min had a lower probability of achieving a CRrenal response [37].
A single-centre study reported on 96 previously untreated MM patients, who were divided into three groups based on their chemotherapy regimen. Thirty-seven patients were treated with conventional agents, 47 received a thalidomide/lenalidomide-based regimen and 17 received a bortezomib-based regimen. A sustained renal response was recorded in 94% of patients treated with a bortezomib-based regimen, 79% treated with thalidomide/lenalidomide regimen and 59% treated with conventional chemotherapy [38]. Several other studies have reported on the efficacy of novel agents when used as first-line chemotherapy for the management of patients with MM and RI [30,39,40,41].
Management
Medical Management
AKI in MM is a medical emergency. Optimal management comprises early institution of anti-myeloma therapy along with good supportive care. Accurate assessment of fluid balance is required; if volume depleted, treatment with intravenous fluids should be commenced immediately. Intravenous hydration along with alkalinisation of urine has shown to increase the solubility of the involved light chain clone and prevent the formation of intratubular casts [18,42].
NSAIDs, aminoglycosides, contrast agents, angiotensin-converting enzyme inhibitor, angiotensin-II receptor blocker and loop diuretics should be stopped and further prescriptions avoided; these agents are associated with an increased risk of developing MCN. Hypercalcaemia is very common, and bisphosphonates can be used for the treatment of elevated calcium levels [43,44,45].
Treatment of Myeloma
Historically, treatment options for MM with RI were limited, and drug combinations with cytotoxic agents such as melphalan and cyclophosphamide in patients with RI were associated with significant toxicities. Patients presenting with severe AKI were usually excluded from clinical trials of therapy; the evidence for this group of patients is therefore derived from post hoc subgroup analyses or from trials performed in patients with relapsed MM. However, management of patients with MM has evolved considerably over the last two decades, and novel agents are now the backbone of contemporary anti-myeloma therapy (table 2).
Table 2.
Published studies of novel agent-based regimen in myeloma patients with RI
| Study | Year | Number of patients with RI | Requiring dialysis | Disease status | Chemotherapy | Myeloma response | Renal response |
|---|---|---|---|---|---|---|---|
| Bortezomib-based studies | |||||||
| Jagannath et al. [58] | 2005 | 52 (CrCl ≤50 ml/min, with 10 patients with CrCl ≤30 ml/min) | 0 | relapsed or refractory | V or VD | overall response (CR + PR + MR) was seen in 25% | not reported |
| Malani et al. [60] | 2006 | 4 (creatinine >3 mg/dl) | 0 | de novo and relapsed or refractory | V or VD | near CR - 3 (75%) and PR - 1 (25%) | improvement in renal function in all patients, with 3 patients demonstrating creatinine <2 mg/dl |
| Chanan-Khan et al. [61] | 2007 | 24 (creatinine >2 mg/dl) | 23 | relapsed or refractory | V; VD; VTD; VAT, and other combinations | overall response (≥PR) seen in 75%; that included CR - 5 (25%), near CR - 1 (5%), and PR - 9 (45%); MR - 1 (5%) | 4 (16%) patients became dialysis independent |
| Ludwig et al. [62] | 2007 | 8 (CrCl <20 ml/min) | 5 | de novo and relapsed or refractory | V; VD; VAD | CR or near CR - 3 (37.5%), VGPR - 1 (12.5%), and PR - 1 (12.5%) | 5 (62.5%) patients achieved reversal of RI; with creatinine ≤2 mg/dl - 2 (25%) and creatinine <3 mg/dl - 3 (37.5%) patients |
| Blade et al. [63] | 2008 | 193 (CrCl <60 ml/min those with CrCl ≤30 ml/min were excluded) | 0 | relapsed or refractory | PLD + V vs. V | ≥PR - 43 (49%) patients in PLD + V group; ≥PR - 39 (42%) patients in V group | improvement in renal function in both treatment groups, with slightly better response in those on PLD + V |
| San-Miguel et al. [59] | 2008 | 62 (CrCl ≤50 ml/min; included 45 (14%) with CrCl 30–50 ml/min and 17 (5%) with CrCl <30 ml/min) | 0 | relapsed or refractory | V | overall response (≥PR) seen in 23 (40%) patients; that included CR - 4 (7%) and PR - 19 (33%) | not reported |
| San-Miguel et al. [55] | 2008 | 185 (CrCl <60 ml/min) | 0 | de novo | VMP | CR - 28% | not reported |
| Roussou et al. [64] | 2008 | 20 (CrCl ≥2 mg/dl) | 5 | de novo and relapsed or refractory | VD; VTD; | overall response (≥MR) seen in 65%; that included CR - 1 (5%), PR - 11 (55%), and MR - 1 (5%) | reversal of RI (creatinine <1.5 mg/dl) was seen in 8 (40%) patients; 1 of the 5 patients became dialysis independent |
| VAD; | |||||||
| VMTD | |||||||
| Dimopoulos et al. [65] | 2009 | 111 (eGFR ≤50 ml/min; included 92 patients with eGFR 31–50 ml/min and 19 with eGFR ≤30 ml/min) | 0 | de novo | VMP | overall response seen in 68% that included 31% achieving a CR | renal function reversal (eGFR >60 ml/min) seen in 49 (44%) patients that included 42 (37.8%) patients with eGFR 30–50 ml/min and 7 (6.3%) with eGFR ≤30 ml/min at presentation |
| Dimopoulos et al. [37] | 2009 | 46 (eGFR <50 ml/min) | 9 | de novo and relapsed or refractory | VD; VTD; | ≥PR - 29 (63%) | overall renal response seen in 27 (59%) patients that included CRrenal - 14 (30%), PRrenal - 5 (11%), and MRrenal - 8 (17%); 2 of the 9 patients became dialysis independent |
| VAD; | |||||||
| VMTD | |||||||
| Li et al. [66] | 2009 | 18 (creatinine ≥2 mg/dl) | 5 | de novo | VD-T | overall response (≥PR) seen in 83.3%; that included CR - 6 (33.3%), near CR - 3 (16.6%), VGPR - 3 (16.6%), and PR - 3 (16.6%) | overall renal response defined as 50% reduction in serum creatinine level was seen in 7 (38.9%) patients; 1 of the 5 patients became dialysis independent |
| Qayum et al. [67] | 2010 | 6 (CrCl <30 ml/min) | 3 | de novo and relapsed or refractory | V; VD | CR - 2 (33.3%) and PR - 3 (50%) | renal function improved in all patients, with 3 (50%) patients achieving CrCl ≥60 ml/min |
| Morabito et al. [68] | 2010 | 117 (eGFR <80 ml/min; included 12 (10.3%) with eGFR 51–80 ml/min, 23 (19.7%) with eGFR 30–50 ml/min and 82 (70.1%) with eGFR <30 ml/min) | 14 | de novo and relapsed or refractory | VD; V combined with other agents | overall response (≥PR) seen in 83 (73%) patients; that included CR - 23 (19%), near CR - 8 (8%), VGPR - 19 (17%), and PR - 33 (29%) | reversal of RI (normalisation of eGFR) was seen in 41%; 3 of the 14 patients became dialysis independent |
| Roussou et al. [38] | 2010 | 17 (CrCl <50 ml/min) | altogether, 9 patients from the 3 treatment arms were on dialysis | de novo | VD | ≥PR - 82% | ≥PRrenal - 82%; 2 out of the 9 patients recovered renal function, both were on bortezomib |
| Ludwig et al. [40] | 2010 | 68 (CrCl <50 ml/min) | 9 | de novo and relapsed or refractory | VAD | overall response (≥MR) seen in 72%; that included CR or near CR - 38%, VGPR - 15%, PR - 13%, and MR - 6% | overall renal response was seen in 62%, that included CRrenal - 31%, PRrenal - 7%, and MRrenal - 24%; 3 of the 9 patients became dialysis independent |
| Morabito et al. [69] | 2011 | 149 (eGFR ≤50 ml/min; VMPT-VT - 70 patients and VMP - 79 patients) | 0 | de novo | VMPT-VT vs. VMP | overall response (≥PR) was 93.2% in VMPT-VT arm and 83.0% in VMP arm | reversal of RI (eGFR >60 ml/min) was seen in 25.4% of patients in VMPT-VT arm and 40.3% in VMP arm; time to renal function reversal was not significantly different between the 2 arms |
| Costa et al. [70] | 2012 | 14 (CrCl <50 ml/min) | 0 | de novo | VCD | ≥PR - 14 (100%) that included CR in 57.1% | overall renal response seen in 10 (71.4%) of the 14 patients, that included CRrenal - 5 (35.7%), PRrenal - 4 (28.5%), and MRrenal - 1 (7.1%) |
| Dimopoulos et al. [50] | 2013 | 43 (eGFR ≤60 ml/min) | 6 | de novo | VD; VTD; VCD | ≥PR - 81% | ≥PRrenal - 33 (77%) patients, that included 29 (67.4%) patients with CRrenal; 3 of the 6 patients became dialysis independent |
| Yang et al. [72] | 2013 | 30 (creatinine ≥2 mg/dl) | 12 | de novo | VTD | overall response (≥PR) seen in 80%; that included CR - 6 (20%), VGPR - 8 (26.7%), and PR - 10 (33%) | 10 (33%) patients achieved reversal of renal function; 8 of the 12 patients became dialysis independent |
| Dimopoulos et al. [71] | 2013 | 58 (eGFR <50 ml/min) | 0 | relapsed or refractory | VD; VCD; VDR | among those achieving a renal response ≥PR seen in 14 (58.3%) patients | overall renal response seen in 24 patients (41%), that included CRrenal - 13 (22.4%) and MRrenal - 11 (18.9%) |
| Scheid et al. [73] | 2014 | 81 (creatinine ≥2 mg/dl; included 45 patients in VADa-T arm and 36 patients in VAD-V arm) | 0 | de novo | VADa-T VAD-V | overall response (≥PR) was seen in 49% of patients with RI; those on VADa-T arm achieved an overall response in 36% of patients compared to 75% of those on VAD-V arm | overall renal response in VADa-T arm was 63% and in the VAD-V arm was 81% |
| Breitkreutz et al. [74] | 2014 | 13 | 13 | de novo | VAD | overall response (≥PR) prior to ASCT was 83.3%, which improved to 100% after ASCT | after induction with VAD, 38.5% became dialysis independent, with a further 15.4% recovering renal function after ASCT |
| Girnius et al. [56] | 2015 | 27 (eGFR <60 ml/min) | 0 | de novo | VD | not reported separately for patients with RI | 52% had an improvement in eGFR of >20% from baseline |
| Thalidomide-based studies | |||||||
| Fakhouri et al. [135] | 2004 | 7 (CrCl <50 ml/min) | 1 | relapsed or refractory | TD or T alone | CR - 3 (42.8%), PR - 1 (14.2%) and MR - 3 (42.8%) | out of the 5 patients with CrCl <30 ml/min, 2 (40%) patients progressed to RRT and the remaining 3 (60%) patients maintained CrCl <30 |
| Tosi et al. [91] | 2004 | 20 (serum creatinine >130 μmol/l and CrCl <60 ml/min) | 2 | relapsed or refractory | TD or T alone | ≥PR - 9 (45%) | 12 (60%) patients achieved renal function recovery, i.e. serum creatinine <130 μmol/l |
| Tosi et al. [90] | 2010 | 31 (CrCl <50 ml/min) | 7 | de novo | TD | ≥PR - 23 (74%) that included 3 (10%) patients with CR and 5 (16%) with VGPR | 17 (55%) patients achieved CrCl >50 ml/min; 2 out of the 7 patients became dialysis independent |
| Lenalidomide-based studies | |||||||
| Niesvizky et al. [136] | 2007 | 14 (CrCl <40 ml/min) | 0 | de novo | RD | not reported | 3 (21.4%) out of the 14 patients improved renal function with CrCl >40 ml/min |
| Dimopoulos et al. [137] | 2010 | 12 (CrCl <50 ml/min) | 1 | relapsed or refractory | RD | ≥PR - 7 (58%) | 5 (42%) of 12 patients demonstrated improvement in renal function that included CRrenal - 3 (25%) and MRrenal - 2 (17%) |
| Dimopoulos et al. [99] | 2010 | 98 (CrCl <60 ml/min; included 16 patients with CrCl <30 ml/min) | 0 | relapsed or refractory | RD | overall response (≥PR) seen in 54 (55%) patients that included CR - 14 (14.2%), VGPR - 14 (14.2%), and PR - 26 (26.5%) | amongst those with CrCl <60 ml/min, renal function improvement was demonstrated in 72% of patients |
| de la Rubia et al. [100] | 2010 | 15 | 15 (2 patients were on RRT prior to myeloma diagnosis) | relapsed or refractory | RD | overall response (≥PR) seen in 9 (60%) patients; that included CR - 4 (26.6%), VGPR - 1 (6.6%), and PR - 4 (26.6%) | 1 patient became dialysis independent |
| Klein et al. [103] | 2011 | 33 (moderate to severe RI defined as CrCl <50 ml/min) | 5 | relapsed or refractory | RD | overall response (≥PR) seen in 16 (48.5%) patients that included CR - 1 (3%), VGPR - 3 (9%), and PR - 12 (36.3%) | 28.6% demonstrated improvement in renal function |
| Oehrlein et al. [102] | 2012 | 26 (CrCl <60 ml/min; included 11.5% with CrCl <30 ml/min) | 3 | relapsed or refractory | RD | overall response (≥PR) seen in 21 (84%) patients that included CR - 1 (4%), and PR - 20 (80%) | overall renal response seen in 11 (42%) patients; that included CRrenal - 6 (23%), PRrenal - 1 (4%), and MRrenal - 4 (15%); all the 3 patients achieved dialysis independence |
| Hou et al. [138] | 2013 | 62 (CrCl <60 ml/min; included 50 (26.7%) with CrCl 30–59 ml/min and 12 (6.4%) with CrCl <30 ml/min) | 0 | relapsed or refractory | RD | ≥PR - 42% in patients with CrCl 30–59 ml/min | not reported |
| ≥PR - 41.7% in patients with CrCl <30 ml/min | |||||||
| Tosi et al. [101] | 2013 | 20 (CrCl <50 ml/min that included 11 (55%) patients with CrCl <30 ml/min) | 4 | relapsed or refractory | RD | CR - 1 (5%), VGPR - 1 (5%), PR - 6 (30%), and MR - 4 (20%) | CRrenal - 4 (20%) and PRrenal - 3 (15%), 1 patient achieved dialysis independence |
| Dimopoulos et al. [50] | 2013 | 28 (eGFR ≤60 ml/min) | 0 | de novo | RD or MPR | overall response (≥PR) seen in 23 (82%) patients | major renal response (≥PRrenal) seen in 12 (43%) patients that included 10 (36%) patients with CRrenal |
| Ludwig et al. [105] | 2015 | 35 (eGFR <50 ml/min) | 13 | de novo and relapsed or refractory | RD | overall response seen in 24 (68.6%) patients that included CR - 7 (20%), VGPR - 3 (8.6%), and PR - 14 (40%) | renal response seen in 16 (45.7%) patients that included CRrenal - 5 (14,2%), PRrenal - 4 (11.4%), and MRrenal - 7 (20%); 5 patients achieved dialysis independence |
| Yamasaki et al. [139] | 2015 | 15 patients (median eGFR 38.5 ml/min before lenalidomide treatment) | 2 | relapsed or refractory | RD | ≥PR - 15 (100%) that included CR - 2 (14%), VGPR - 1 (7%), and PR - 12 (80%) | post-treatment median eGFR 49.7 ml/min |
PRrenal = Partial renal response; MRrenal = minor renal response; V = bortezomib; VD = bortezomib and dexamethasone; VTD = bortezomib, thalidomide and dexamethasone; VAT = bortezomib, adriamycin and thalidomide; PLD + V = pegylated liposomal doxorubicin and bortezomib; VMP = bortezomib, melphalan and prednisolone; VAD = bortezomib, adriamycin and dexamethasone; VMTD = bortezomib, melphalan, thalidomide and dexamethasone; VMPT-VT = bortezomib, melphalan, prednisolone, thalidomide followed by bortezomib and thalidomide maintenance; VCD = bortezomib, cyclophosphamide and dexamethasone; VDR = bortezomib, dexamethasone and lenalidomide; VADa = vincristine, adriamycin and dexamethasone; T = thalidomide; TD = thalidomide and dexamethasone; RD = lenalidomide and dexamethasone; MPR = melphalan, lenalidomide and prednisolone.
Dexamethasone
Glucocorticoids exert a cytotoxic effect on myeloma cells by inhibiting nuclear factor-κ light chain enhancer of activated B cells (NF-κB) and interleukin (IL)-6 [46,47,48]. Dexamethasone is a potent steroid that has been used both alone and in combination with other agents. In a study that included 112 newly diagnosed MM patients, a 75% reduction in serum M-protein was reported in 43% of patients treated with intermittent courses of dexamethasone. The response rate to dexamethasone used as a single agent was approximately 15% less than that of a vincristine-doxorubicin-dexamethasone (VAD) combination in a previous study, which implied that the majority of the anti-myeloma response by VAD regimen was due to dexamethasone alone [49].
In another study, high-dose dexamethasone with a novel agent was associated with a shorter length of time to renal response compared to high-dose dexamethasone in combination with conventional agents [35]. A similar finding was reported in a further study where treatment with high-dose dexamethasone (≥160 mg/month) was used in combination with thalidomide-based, bortezomib-based or lenalidomide-based regimens [50].
Proteasome Inhibitors
Bortezomib
Bortezomib is a first-in-class selective and reversible 26S proteasome inhibitor with proven efficacy in newly diagnosed MM and in relapsed or refractory MM. It targets the ubiquitin-proteasome pathway and promotes the proliferation and survival of all cells, in particular cancer cells [51]. It decreases myeloma cell binding to bone marrow stromal cells and blocks NF-κB-mediated synthesis of IL-6 by stromal cells [52]. It has anti-angiogenic properties and inhibits vascular endothelial growth factor and IL-6 secretion by the endothelial cells [53]. Among other effects, bortezomib can modulate intracellular signalling mechanisms and gene expression to protect the renal proximal tubular cell from the anti-apoptotic effects of NF-κB [54]. It is metabolised primarily in the liver via cytochrome P450 enzymes, and no dose alteration is necessary in patients with RI. The commonest side effects are peripheral neuropathy and haematological toxicity; electrolyte abnormality, mood disturbances, hypotension and gastrointestinal disturbances can also occur.
A subgroup analysis of the Velcade as Initial Standard Therapy in Multiple Myeloma: Assessment with Melphalan and Prednisolone (VISTA) trial reported no difference in CR rate (28%), time to progression and the OS in the 185 patients with CrCl <60 ml/min compared to the remaining 159 patients who had no RI from the bortezomib arm [55].
A recent phase II study that evaluated the efficacy and tolerability of an alternate dosing regimen for bortezomib in newly diagnosed patients with MM ineligible for stem cell transplantation reported an improvement in renal function in 52% of patients with an eGFR <60 ml/min at diagnosis. The weekly bortezomib regimen was well tolerated by the elderly and frail cohort of patients, with majority of the adverse events reported to be of grade 1/2 severity; only 1 patient in this study developed a grade 3/4 peripheral neuropathy from bortezomib therapy [56].
In another study, subcutaneous bortezomib was an effective alternative in relapsed MM compared to intravenous therapy in a multicentre phase III trial that showed comparable efficacy in the overall response rate, time to progression and 1-year OS in the two treatment groups. RI defined as CrCl ≤60 ml/min at presentation was present in 41% of patients who received subcutaneous and in 32% who had intravenous bortezomib. Patients on subcutaneous bortezomib had lower grade 3 or worse adverse events than those on intravenous treatment (57 vs. 70%, respectively) [57].
A retrospective pooled analysis of the Study of Uncontrolled Multiple Myeloma Managed with Proteasome Inhibition Therapy (SUMMIT) and the Clinical Response and Efficacy Study of Bortezomib in the Treatment of Relapsing Multiple Myeloma (CREST) investigated the response rate and safety of bortezomib in recurrent and/or refractory MM in patients with severe RI as defined by a CrCl of 10–30 ml/min. Ten patients were reported, of whom 6 also received dexamethasone. Overall response was noted in 3 patients (30%); 2 patients achieving partial response (PR) and 1 patient achieving a minimal response (MR). This was one of the earliest studies to report on the clinical benefit of bortezomib in this high-risk group of patients [58].
A subgroup analysis of the Assessment of Proteasome Inhibition for Extending Remissions (APEX) study also studied bortezomib in relapsed MM. Patients treated with bortezomib had a superior disease response (≥PR 38 vs. 18% in CrCl >50 ml/min group; 40 vs. 16% in ≤50 ml/min group) and longer time to progression (6.2 vs. 3.5 months in CrCl >50 ml/min group; 4.9 vs. 2.8 months CrCl ≤50 ml/min group) compared to treatment with high-dose dexamethasone. Adverse event rate was comparable for each group for bortezomib; however, a higher frequency of adverse events and drug discontinuation was seen in the dexamethasone arm [59]. A number of other studies [37,38,40,50,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74] have reported on bortezomib in patients with RI; these are summarised in table 2.
Carfilzomib
Carfilzomib is a new generation proteasome inhibitor approved in the United States for patients with relapsed and/or refractory MM. The PX-171-005 study evaluated the safety and efficacy of carfilzomib in 50 patients with relapsed and/or refractory MM and included 18 (36%) patients with CrCl <50 ml/min and an additional 8 (16%) patients on dialysis. There was no impact of RI on the clearance of carfilzomib. A PR or greater response occurred in 23.5% of patients with a CrCl <50 ml/min and in 37.5% of those on dialysis, 1 of whom achieved renal function recovery and became dialysis independent [75]. An analysis of 4 separate phase II studies that totalled 526 patients, including 23.8% with a CrCl <50 ml/min, demonstrated no worsening in renal function in 86.8% following treatment with carfilzomib; 13.2% had at least one episode of RI and with grade 3/4 adverse events as a consequence occurring in 7.2% [76].
Immunomodulatory Therapy
Thalidomide
Thalidomide was the first immunomodulatory agent to show promise in the treatment of MM. The drug has anti-angiogenic, anti-inflammatory and anti-immunomodulatory properties [77]. Specific effects include blockade or down-regulation of TNF-α, cyclooxygenase-2 and myeloma cell targeted stromal derived factor-1α and its receptor CXCR-4 [78,79]. Thalidomide undergoes biotransformation by non-enzymatic hydrolysis in the liver to form multiple metabolites with <1% excretion of these metabolites in the urine [80,81].
Thalidomide has limited haematological toxicity, and can therefore be used both as a single agent and in combination with other chemotherapeutic agents [82,83,84,85,86]. The pharmacokinetics in patients receiving dialysis has not been extensively studied, and there are reports of hyperkalaemia in patients with RI [77,87]. The most common side effects are peripheral neuropathy that is usually encountered after a prolonged exposure [88], and thromboembolic events. The risk of thromboembolism increases when used in combination with other agents, especially high-dose dexamethasone [89]. Despite over 15 years of clinical use, there is a disappointingly low quantity of useful data on thalidomide in RI.
The efficacy of thalidomide-dexamethasone prior to autologous stem cell transplantation (ASCT) in previously untreated patients presenting with a CrCl of <50 ml/min was reported by Tosi et al. [90]. They noted a ≥PR in 74% of patients that included 26% with a very good partial response (VGPR). An improvement in renal function after induction occurred in 82% of patients with a ≥PR, compared to 37% in those who did not achieve a PR.
In a study of 20 consecutive advanced, refractory or relapsed patients with MM and RI (including 3 patients requiring dialysis) treated with thalidomide alone or in combination with dexamethasone, a PR was noted in 9 patients and an MR in 6 additional patients. Renal function recovery occurred in 12 of 15 patients who responded to treatment; thalidomide alone was associated with a maximal disease response at a median of 6 weeks, and thalidomide plus dexamethasone at a median of 4 weeks. The toxicity of thalidomide as monotherapy or in combination with dexamethasone was similar to that observed in patients with MM and normal renal function [91].
Lenalidomide
Lenalidomide is a second-generation immunomodulatory agent which has direct tumoricidal activity in MM [92,93,94,95]. The kidney is the primary route of elimination, with 84% of the drug secreted in the urine in patients with normal renal function. Therefore, clearance of lenalidomide decreases with a progressive decline in renal function; from 69% in patients with a CrCl 50–80 ml/min, to 38% in patients with CrCl 30–49 ml/min, and 43% in patients with CrCl <30 ml/min. In moderate to severe RI or end-stage kidney disease, the area under the concentration-time curve increases by approximately 185–420%, and the median half-life increases by 6–12 h; furthermore, a single session of 4-hour haemodialysis only removes 31% of the administered dose [96]. As a result, dose recommendations in patients with MM and RI are in place [97]. Lenalidomide therapy is associated with an increased risk of thromboembolic events [91], and neutropenia and thrombocytopenia are also important side effects [98].
Lenalidomide and dexamethasone were assessed in patients with RI in the MM-009 and MM-010 multicentre, phase III clinical trial; 71, 24 and 5% had a CrCl of ≥60, 30–59 and <30 ml/min, respectively. There was no significant difference in the overall response, time to progression and progression-free survival between groups. However, patients with a CrCl <30 ml/min had a shorter survival (18.4 months) than those with a CrCl 30–59 ml/min (29.0 months) or CrCl ≥60 ml/min (38.9 months). A renal response occurred in 72%. Thrombocytopenia and discontinuation of lenalidomide (mainly due to cytopenia) was more common in those with severe RI [99].
de la Rubia et al. [100] reported the use of lenalidomide and dexamethasone in 15 refractory and/relapsed MM patients on dialysis. Thirteen patients received lenalidomide three times a week after dialysis treatment at a dose of 15 mg/day, and 2 patients received lenalidomide at a dose of 5 mg/day and 5 mg on alternate days, respectively. 29% achieved a CR, 7% a VGPR and 29% a PR. One patient who achieved a PR attained independence from dialysis. Haematological toxicity was common; 53% of patients required a reduction in the dose of lenalidomide due to the development of cytopenia. This study showed that with careful monitoring and with appropriate dose adjustments, lenalidomide could be used for relapsing or refractory MM in patients requiring dialysis. Overall, the results of studies to date indicate that lenalidomide therapy is an important treatment option in the management of MM in patients with RI [101,102,103,104,105].
Pomalidomide
Pomalidomide is another new-generation immunomodulatory agent used in combination with dexamethasone for the management of relapsed and/or refractory MM. The superiority of pomalidomide with low-dose dexamethasone compared to pomalidomide alone or high-dose dexamethasone alone was reported in a phase II [106] and a phase III [107] study, respectively; however, individuals with moderate to severe RI at presentation were excluded from both studies. It is metabolised extensively via multiple pathways, with <5% of the administered pomalidomide dose excreted unchanged in the urine. Studies to assess the efficacy and safety of pomalidomide in patients with severe RI are currently underway [108].
Autologous Stem Cell Transplantation
High-dose chemotherapy followed by ASCT is an established treatment option for MM. RI had no impact on stem cell collection and post-transplant engraftment in small single-centre studies [109,110], with some centres reporting cases of recovery from requiring dialysis [110,111].
High-dose melphalan (200 mg/m2) is associated with excessive toxicity; when the dose of melphalan was reduced to 140 mg/m2 in a study where 21 of the 81 patients had advanced RI (creatinine >177 μmol/l with 47% on dialysis), transplant-related mortality was observed in 6 and 13% of patients after single and tandem ASCT, respectively. Dialysis dependence and melphalan dose did not affect event-free survival or OS [112]. In a subsequent study in dialysis patients, a further reduction in melphalan dose (100 mg/m2) produced a similar toxicity profile, transplant-related mortality, disease response and OS compared to patients with no RI [113].
Published data around the disease and renal response in patients with advanced RI are variable. In a single-centre study of 59 patients requiring dialysis, ASCT was associated with independence of dialysis in 13 of 54 patients who survived more than 30 days. A shorter duration of dialysis (≤6 months), achievement of a CR or near CR and CrCl ≥10 ml/min were all associated with recovery of independent kidney function [114.]
More recently, Mayo clinic investigators reported long-term outcomes following ASCT in 30 patients with advanced RI (creatinine >3 mg/dl); 15 patients were receiving dialysis, and only 1 recovered renal function. The non-dialysis patients had a modest improvement in eGFR from 15 to 19 ml/min. CR was noted in 14 patients. Although patients who achieved a CR had a better median eGFR than those who did not, the authors found no association between haematological response and baseline eGFR with renal outcome [115].
Current guidelines support the idea that ASCT is an attractive treatment option in the management of MM; however, careful patient selection is required, especially in patients with advanced RI [116].
Role of Extra-Corporeal Removal of FLC
Therapeutic Plasma Exchange
The largest randomised control trial in patients with MM and severe AKI recruited between 1998 and 2003 failed to show a beneficial effect of plasma exchange (PE) on patient and renal outcomes; the limitations of this study included the lack of a renal biopsy confirming a diagnosis of MCN and the absence of serum or urinary light chain measurement [117].
Subsequently, Leung et al. [118] investigated the efficacy of PE in 40 patients with MM and severe RI that included 9 (22.5%) patients on dialysis. A renal response was noted in 18 (45%) patients, which included 14 patients with MCN who had sFLC levels measured before and after treatment with PE. Of these 14 patients, an sFLC reduction of ≥50% was reported in 64.3%; 7 of these patients had a significant renal response. Two (22%) patients subsequently became dialysis independent. The authors concluded that PE for extra-corporeal FLC removal only had a role in biopsy-proven MCN and in patients who demonstrated a ≥50% reduction in sFLC levels from baseline.
However, PE as an isolated treatment does not provide clinical benefit, and the improvements in clinical outcome associated with a ≥50% reduction in FLC in this study most likely reflect a chemosensitive FLC clone. As FLC have a molecular weight of 25–50 kDa and are distributed in both the intra- and extravascular compartments, a single session of PE removes <10% of extracellular FLC. The current consensus is that FLC removal by PE is unlikely to contribute to improving patient outcome [119,120] and the present focus is on high cut-off (HCO) haemodialysis, which has greater efficacy for FLC removal than PE.
HCO Haemodialysis
Hutchison et al. [121] performed a series of in vitro and in vivo studies to assess the efficacy of several protein-leaking haemodialysers and developed a theoretical model and clinical strategy for extra-corporeal FLC removal in patients with MM presenting with severe RI. From the dialysers tested, the Gambro™ HCO 1100 with a molecular weight cut-off in vivo of around 65 kDa was found to be highly efficient for FLC removal. The model found that extended (8–12 h) daily dialysis would accelerate reduction in sFLC levels provided that the involved FLC was chemo-responsive.
A pilot study reported outcomes in 19 patients with MM who received dialysis for severe AKI, using the HCO 1100 membrane. Thirteen patients who had uninterrupted courses of chemotherapy and extended dialysis demonstrated a sustained FLC reduction, reflecting single-session reductions of 69% in κ-FLC and 71% in λ-FLC levels. Twelve (86%) of the 14 patients who became dialysis independent also had an early and sustained reduction in sFLC levels; median time from presentation to dialysis independence was 24 days. Median survival in patients who had uninterrupted chemotherapy was significantly better compared to those who had treatment suspended due to potential complications [30].
A further report from a multicentre study assessed the relationship between FLC level and renal function in dialysis-dependent patients treated with HCO haemodialysis and chemotherapy. The majority of patients in this study received a novel chemotherapeutic agent. A ≥50% reduction in sFLC level was seen in 68% of patients by day 12 and in 83% of patients by day 21. The majority of patients who demonstrated early reduction in sFLC level also became dialysis independent. Patients who did not achieve independence from dialysis were more likely to have sustained a delay in initiating HCO haemodialysis treatment. In a multivariable model, the factors that were associated with independence from dialysis were FLC reduction at day 12 and day 21 and time to initiating HCO haemodialysis [39].
There are several other small studies which have highlighted the benefit of HCO haemodialysis in renal function recovery in patients with MM and severe RI (table 3) [122,123,124,125,126,127]; however, supplementation of HCO haemodialysis to current standard of care in assisting renal outcome and in doing so long-term patient outcome needs to be carefully investigated, and hence results of the EUropean trial of free LIght chain removal by exTEnded haemodialysis in cast nephropathy (EuLITE) and studies in patients with MM and renal failure due to MCN (MYRE) are awaited [128,129].
Table 3.
Published studies of use of HCO haemodialysis in myeloma patients with severe RI
| Study | Year | Patients on RRT | Disease status | Renal biopsy findings | Chemotherapy | Myeloma response | Renal response |
|---|---|---|---|---|---|---|---|
| Hutchison et al. [121] | 2007 | 5 | de novo | MCN | dexamethasone-based regimen | not reported | 3 (60%) achieved dialysis independence |
| Hutchison et al. [30] | 2009 | 19 | 16 patients – de novo 3 patients – relapsed | MCN | cyclophosphamide - 7; thalidomide - 14; vincristine + doxorubicin - 1; dexamethasone – all patients | not reported; however, sustained FLC reduction noted in 13 (68.4%) patients | 14 (73.6%) patients achieved dialysis independence |
| Peters et al. [140] | 2011 | 5 | de novo | MCN | VD | not reported; however, a mean FLC reduction of 90 ±6% seen after the third session of HCO dialysis, with an FLC reduction of 61 ± 20% per session over 2 weeks | 3 (60%) patients became dialysis independent |
| Sinisalo et al. [125] | 2012 | 7 | de novo | available in 6 (85.7%) patients, all demonstrating MCN | VD - 3; VD + MP - 1; VD + VADa - 1; RVD - 1; VTD - 1 | not reported | 6 (85.7%) patients achieved dialysis independence |
| Hutchison et al. [39] | 2012 | 67 | 50 patients – de novo 17 patients – relapsed | available in 38 (56.7%) patients with 86.7% demonstrating MCN | novel agent used in 56 patients | 42 (61%) patients had a reduction in FLC levels; amongst these, 83% had a ≥50% decline in FLC levels by day 21 | 42 (63%) patients achieved dialysis independence |
| Heyne et al. [127] | 2012 | 19 | 10 patients – de novo 9 patients – relapsed or refractory | available in 6 (31.5%) patients, all demonstrating MCN | novel agent used in 14 patients | ≥PR - 6 (42.8%) | 14 (73.7%) patients achieved dialysis independence |
| Khalahfallah et al. [124] | 2013 | 4 | 3 patients – de novo 1 patient – relapsed | unknown if renal biopsy performed | VAD - 2; VD - 1 TD - 1 (switched to RD) | ≥VGPR - 3 (75%) patients | 3 (75%) patients achieved dialysis independence |
| Tan et al. [141] | 2014 | 6 | de novo | MCN | VCD - 3; CTD - 1; CD - 2 | ≥PR - 4 (66.6%) | 3 (50%) patients achieved dialysis independence |
| Zanetti et al. [122] | 2015 | 21 | de novo | 15 (71.4%) patients demonstrating MCN | VTD - 13 (ASCT eligible) VMP - 8 (ASCT ineligible) | sCR - 4 (19%), VGPR - 10 (48%), and PR - 3 (14%) | 16 (76%) patients achieved dialysis independence |
RRT = Renal replacement therapy; PRrenal = partial renal response; MRrenal = minor renal response; VD = bortezomib and dexamethasone; MP = melphalan and prednisolone; VADa = vincristine, adriamycin and dexamethasone; RVD = lenalidomide, bortezomib and dexamethasone; VTD = bortezomib, thalidomide and dexamethasone; VAD = bortezomib, adriamycin and dexamethasone; TD = thalidomide and dexamethasone; RD = lenalidomide and dexamethasone; CD = cyclophosphamide and dexamethasone; VCD = bortezomib, cyclophosphamide and dexamethasone; CTD = cyclophosphamide, thalidomide and dexamethasone; VMP = bortezomib, melphalan and prednisolone; sCR = stringent complete response.
Future Directions
The outcomes in patients with MM and RI are improving, and newer classes of agents including antibodies (e.g. anti-SLAMF7 - elotuzumab, anti-CD38 - daratumumab) and histone deacetylase inhibitors (e.g. panobinostat and vorinostat) have shown great promise in phase II/III studies, and it would be reasonable to expect the improved myeloma response rates that are being sustained with these agents to lead to better renal outcomes for patients with MM who have RI.
There are several research questions that can stratify the management of patients with MM and RI in the next decade. These include: better defining the relationship between FLC level and renal function at diagnosis; describing the relationship between FLC response and renal function in patients who do not require dialysis at presentation, and understanding the impact of mild-to-moderate RI on long-term patient outcome. Finally, patients with severe RI are usually excluded from major intervention trials; future studies need to address this to improve the evidence base of novel and next-generation therapies.
Conflict of Interest Statement
The authors declare no conflict of interest.
Acknowledgement
Dr. Kassiani Skordilis has very kindly provided the figure for the paper.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK Myeloma Statistics http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/myeloma (accessed October 13, 2015).
- 3.National Cancer Institute SEER Cancer Statistics review, 1975-2008 2011. http://seer.cancer.gov/archive/csr/1975_2008/index.html (accessed October 13, 2015).
- 4.International Myeloma Working Group Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- 5.Eleutherakis-Papaiakovou V, Bamias A, Gika D, Simeonidis A, Pouli A, Anagnostopoulos A, Michali E, Economopoulos T, Zervas K, Dimopoulos MA, et al. Renal failure in multiple myeloma: incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;48:337–341. doi: 10.1080/10428190601126602. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Berg RL, Gassman JJ, Hall PM, Walker WG. Creatinine filtration, secretion and excretion during progressive renal disease. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl. 1989;27:S73–S80. [PubMed] [Google Scholar]
- 7.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E, Richardson P, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 9.Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H. Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol. 2000;54:203–209. [PubMed] [Google Scholar]
- 10.Jovanovic D, Krstivojevic P, Obradovic I, Durdevic V, Dukanovic L. Serum cystatin C and beta2-microglobulin as markers of glomerular filtration rate. Ren Fail. 2003;25:123–133. doi: 10.1081/jdi-120017475. [DOI] [PubMed] [Google Scholar]
- 11.Grubb AO. Cystatin C - properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindstrom V, Grubb A. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40:1921–1926. [PubMed] [Google Scholar]
- 13.Shemesh O, Golbetz H, Kriss JP, Myers BD. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 14.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 15.Horber FF, Scheidegger J, Frey FJ. Overestimation of renal function in glucocorticosteroid treated patients. Eur J Clin Pharmacol. 1985;28:537–541. doi: 10.1007/BF00544064. [DOI] [PubMed] [Google Scholar]
- 16.Nuckel H, Langer C, Herget-Rosenthal S, Wichert M, Assert R, Dohner H, Duhrsen U, Liebisch P. Prognostic significance of serum cystatin C in multiple myeloma. Int J Hematol. 2012;95:545–550. doi: 10.1007/s12185-012-1049-2. [DOI] [PubMed] [Google Scholar]
- 17.Terpos E, Katodritou E, Tsiftsakis E, Kastritis E, Christoulas D, Pouli A, Michalis E, Verrou E, Anargyrou K, Tsionos K, et al. Cystatin-C is an independent prognostic factor for survival in multiple myeloma and is reduced by bortezomib administration. Haematologica. 2009;94:372–379. doi: 10.3324/haematol.2008.000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLennan IC, Cooper EH, Chapman CE, Kelly KA, Crockson RA. Renal failure in myelomatosis. Eur J Haematol Suppl. 1989;51:60–65. doi: 10.1111/j.1600-0609.1989.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 19.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, Behrens J, Smith A, Child JA, Drayson MT. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002 - Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23:9219–9226. doi: 10.1200/JCO.2005.03.2086. [DOI] [PubMed] [Google Scholar]
- 20.Uttervall K, Duru AD, Lund J, Liwing J, Gahrton G, Holmberg E, Aschan J, Alici E, Nahi H. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One. 2014;9:e101819. doi: 10.1371/journal.pone.0101819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonsalves WI, Leung N, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Dingli D, Kapoor P, Go RS, et al. Improvement in renal function and its impact on survival in patients with newly diagnosed multiple myeloma. Blood Cancer J. 2015;5:e296. doi: 10.1038/bcj.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blade J, Fernandez-Llama P, Bosch F, Montoliu J, Lens XM, Montoto S, Cases A, Darnell A, Rozman C, Montserrat E. Renal failure in multiple myeloma: presenting features and predictors of outcome in 94 patients from a single institution. Arch Intern Med. 1998;158:1889–1893. doi: 10.1001/archinte.158.17.1889. [DOI] [PubMed] [Google Scholar]
- 23.Torra R, Blade J, Cases A, Lopez-Pedret J, Montserrat E, Rozman C, Revert L. Patients with multiple myeloma requiring long-term dialysis: presenting features, response to therapy, and outcome in a series of 20 cases. Br J Haematol. 1995;91:854–859. doi: 10.1111/j.1365-2141.1995.tb05400.x. [DOI] [PubMed] [Google Scholar]
- 24.Knudsen LM, Hjorth M, Hippe E. Renal failure in multiple myeloma: reversibility and impact on the prognosis. Nordic Myeloma Study Group. Eur J Haematol. 2000;65:175–181. doi: 10.1034/j.1600-0609.2000.90221.x. [DOI] [PubMed] [Google Scholar]
- 25.Dimopoulos MA, Delimpasi S, Katodritou E, Vassou A, Kyrtsonis MC, Repousis P, Kartasis Z, Parcharidou A, Michael M, Michalis E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25:195–200. doi: 10.1093/annonc/mdt483. [DOI] [PubMed] [Google Scholar]
- 26.Haynes RJ, Read S, Collins GP, Darby SC, Winearls CG. Presentation and survival of patients with severe acute kidney injury and multiple myeloma: a 20-year experience from a single centre. Nephrol Dial Transplant. 2010;25:419–426. doi: 10.1093/ndt/gfp488. [DOI] [PubMed] [Google Scholar]
- 27.Tsakiris DJ, Stel VS, Finne P, Fraser E, Heaf J, de Meester J, Schmaldienst S, Dekker F, Verrina E, Jager KJ. Incidence and outcome of patients starting renal replacement therapy for end-stage renal disease due to multiple myeloma or light-chain deposit disease: an ERA-EDTA Registry study. Nephrol Dial Transplant. 2010;25:1200–1206. doi: 10.1093/ndt/gfp679. [DOI] [PubMed] [Google Scholar]
- 28.Hutchison CA, Cockwell P, Stringer S, Bradwell A, Cook M, Gertz MA, Dispenzieri A, Winters JL, Kumar S, Rajkumar SV, et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J Am Soc Nephrol. 2011;22:1129–1136. doi: 10.1681/ASN.2010080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Basnayake K, Stringer SJ, Hutchison CA, Cockwell P. The biology of immunoglobulin free light chains and kidney injury. Kidney Int. 2011;79:1289–1301. doi: 10.1038/ki.2011.94. [DOI] [PubMed] [Google Scholar]
- 30.Hutchison CA, Bradwell AR, Cook M, Basnayake K, Basu S, Harding S, Hattersley J, Evans ND, Chappel MJ, Sampson P, et al. Treatment of acute renal failure secondary to multiple myeloma with chemotherapy and extended high cut-off hemodialysis. Clin J Am Soc Nephrol. 2009;4:745–754. doi: 10.2215/CJN.04590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutchison CA, Plant T, Drayson M, Cockwell P, Kountouri M, Basnayake K, Harding S, Bradwell AR, Mead G. Serum free light chain measurement aids the diagnosis of myeloma in patients with severe renal failure. BMC Nephrol. 2008;9:11. doi: 10.1186/1471-2369-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying WZ, Allen CE, Curtis LM, Aaron KJ, Sanders PW. Mechanism and prevention of acute kidney injury from cast nephropathy in a rodent model. J Clin Invest. 2012;122:1777–1785. doi: 10.1172/JCI46490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batuman V. The pathogenesis of acute kidney impairment in patients with multiple myeloma. Adv Chronic Kidney Dis. 2012;19:282–286. doi: 10.1053/j.ackd.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Hutchison CA, Batuman V, Behrens J, Bridoux F, Sirac C, Dispenzieri A, Herrera GA, Lachmann H, Sanders PW, et al. The pathogenesis and diagnosis of acute kidney injury in multiple myeloma. Nat Rev Nephrol. 2012;8:43–51. doi: 10.1038/nrneph.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kastritis E, Anagnostopoulos A, Roussou M, Gika D, Matsouka C, Barmparousi D, Grapsa I, Psimenou E, Bamias A, Dimopoulos MA. Reversibility of renal failure in newly diagnosed multiple myeloma patients treated with high dose dexamethasone-containing regimens and the impact of novel agents. Haematologica. 2007;92:546–549. doi: 10.3324/haematol.10759. [DOI] [PubMed] [Google Scholar]
- 36.Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, Niesvizky R, Giralt S, Fermand JP, Blade J, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- 37.Dimopoulos MA, Roussou M, Gavriatopoulou M, Zagouri F, Migkou M, Matsouka C, Barbarousi D, Christoulas D, Primenou E, Grapsa I, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9:302–306. doi: 10.3816/CLM.2009.n.059. [DOI] [PubMed] [Google Scholar]
- 38.Roussou M, Kastritis E, Christoulas D, Migkou M, Gavriatopoulou M, Grapsa I, Psimenou E, Gika D, Terpos E, Dimopoulos MA. Reversibility of renal failure in newly diagnosed patients with multiple myeloma and the role of novel agents. Leuk Res. 2010;34:1395–1397. doi: 10.1016/j.leukres.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Hutchison CA, Heyne N, Airia P, Schindler R, Zickler D, Cook M, Cockwell P, Grima D. Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant. 2012;27:3823–3828. doi: 10.1093/ndt/gfr773. [DOI] [PubMed] [Google Scholar]
- 40.Ludwig H, Adam Z, Hajek R, Greil R, Tothova E, Keil F, Autzinger EM, Thaler J, Gisslinger H, Lang A, et al. Light chain-induced acute renal failure can be reversed by bortezomib-doxorubicin-dexamethasone in multiple myeloma: results of a phase II study. J Clin Oncol. 2010;28:4635–4641. doi: 10.1200/JCO.2010.28.1238. [DOI] [PubMed] [Google Scholar]
- 41.Cockwell P, Hutchison CA. Management options for cast nephropathy in multiple myeloma. Curr Opin Nephrol Hypertens. 2010;19:550–555. doi: 10.1097/MNH.0b013e32833ef72c. [DOI] [PubMed] [Google Scholar]
- 42.Holland MD, Galla JH, Sanders PW, Luke RG. Effect of urinary pH and diatrizoate on Bence Jones protein nephrotoxicity in the rat. Kidney Int. 1985;27:46–50. doi: 10.1038/ki.1985.8. [DOI] [PubMed] [Google Scholar]
- 43.Bataille R. Management of myeloma with bisphosphonates. N Engl J Med. 1996;334:529–530. doi: 10.1056/NEJM199602223340810. [DOI] [PubMed] [Google Scholar]
- 44.Berenson JR, Rosen L, Vescio R, Lau HS, Woo M, Sioufi A, Kowalski MO, Knight RD, Seaman JJ. Pharmacokinetics of pamidronate disodium in patients with cancer with normal or impaired renal function. J Clin Pharmacol. 1997;37:285–290. doi: 10.1002/j.1552-4604.1997.tb04304.x. [DOI] [PubMed] [Google Scholar]
- 45.Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, Navarro-Coy N, Drayson MT, Owen RG, Feyler S, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 47.Ray A, LaForge KS, Sehgal PB. On the mechanism for efficient repression of the interleukin-6 promoter by glucocorticoids: enhancer, TATA box, and RNA start site (Inr motif) occlusion. Mol Cell Biol. 1990;10:5736–5746. doi: 10.1128/mcb.10.11.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrmann F, Andreeff M, Gruss HJ, Brach MA, Lubbert M, Mertelsmann R. Interleukin-4 inhibits growth of multiple myelomas by suppressing interleukin-6 expression. Blood. 1991;78:2070–2074. [PubMed] [Google Scholar]
- 49.Alexanian R, Dimopoulos MA, Delasalle K, Barlogie B. Primary dexamethasone treatment of multiple myeloma. Blood. 1992;80:887–890. [PubMed] [Google Scholar]
- 50.Dimopoulos MA, Roussou M, Gkotzamanidou M, Nikitas N, Psimenou E, Mparmparoussi D, Matsouka C, Spyropoulou-Vlachou M, Terpos E, Kastritis E. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27:423–429. doi: 10.1038/leu.2012.182. [DOI] [PubMed] [Google Scholar]
- 51.Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–421. doi: 10.1016/s1535-6108(04)00120-5. [DOI] [PubMed] [Google Scholar]
- 52.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 53.Roccaro AM, Hideshima T, Raje N, Kumar S, Ishitsuka K, Yasui H, Shiraishi N, Ribatti D, Nico B, Vacca A, et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006;66:184–191. doi: 10.1158/0008-5472.CAN-05-1195. [DOI] [PubMed] [Google Scholar]
- 54.Sarkozi R, Perco P, Hochegger K, Enrich J, Wiesinger M, Pirklbauer M, Eder S, Rudnicki M, Rosenkranz AR, Mayer B, et al. Bortezomib-induced survival signals and genes in human proximal tubular cells. J Pharmacol Exp Ther. 2008;327:645–656. doi: 10.1124/jpet.108.142604. [DOI] [PubMed] [Google Scholar]
- 55.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 56.Girnius SK, Lee S, Kambhampati S, Rose MG, Mohiuddin A, Houranieh A, Zimelman A, Grady T, Mehta P, Behler C, et al. A phase II trial of weekly bortezomib and dexamethasone in veterans with newly diagnosed multiple myeloma not eligible for or who deferred autologous stem cell transplantation. Br J Haematol. 2015;169:36–43. doi: 10.1111/bjh.13243. [DOI] [PubMed] [Google Scholar]
- 57.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 58.Jagannath S, Barlogie B, Berenson JR, Singhal S, Alexanian R, Srkalovic G, Orlowski RZ, Richardson PG, Anderson J, Nix D, et al. Bortezomib in recurrent and/or refractory multiple myeloma. Initial clinical experience in patients with impared renal function. Cancer. 2005;103:1195–1200. doi: 10.1002/cncr.20888. [DOI] [PubMed] [Google Scholar]
- 59.San-Miguel JF, Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, et al. Efficacy and safety of bortezomib in patients with renal impairment: results from the APEX phase 3 study. Leukemia. 2008;22:842–849. doi: 10.1038/sj.leu.2405087. [DOI] [PubMed] [Google Scholar]
- 60.Malani AK, Gupta V, Rangineni R. Bortezomib and dexamethasone in previously untreated multiple myeloma associated with renal failure and reversal of renal failure. Acta Haematol. 2006;116:255–258. doi: 10.1159/000095876. [DOI] [PubMed] [Google Scholar]
- 61.Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, Munshi NC, Schlossman R, Tariman J, Singhal S. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109:2604–2606. doi: 10.1182/blood-2006-09-046409. [DOI] [PubMed] [Google Scholar]
- 62.Ludwig H, Drach J, Graf H, Lang A, Meran JG. Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica. 2007;92:1411–1414. doi: 10.3324/haematol.11463. [DOI] [PubMed] [Google Scholar]
- 63.Blade J, Sonneveld P, San Miguel JF, Sutherland HJ, Hajek R, Nagler A, Spencer A, Robak T, Cibeira MT, Zhuang SH, et al. Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clin Lymphoma Myeloma. 2008;8:352–355. doi: 10.3816/CLM.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 64.Roussou M, Kastritis E, Migkou M, Psimenou E, Grapsa I, Matsouka C, Barmparousi D, Terpos E, Dimopoulos MA. Treatment of patients with multiple myeloma complicated by renal failure with bortezomib-based regimens. Leuk Lymphoma. 2008;49:890–895. doi: 10.1080/10428190801930506. [DOI] [PubMed] [Google Scholar]
- 65.Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, Kropff M, Petrucci MT, Delforge M, Alexeeva J, et al. VMP (bortezomib, melphalan, and prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27:6086–6093. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 66.Li J, Zhou DB, Jiao L, Duan MH, Zhang W, Zhao YQ, Shen T. Bortezomib and dexamethasone therapy for newly diagnosed patients with multiple myeloma complicated by renal impairment. Clin Lymphoma Myeloma. 2009;9:394–398. doi: 10.3816/CLM.2009.n.077. [DOI] [PubMed] [Google Scholar]
- 67.Qayum A, Aleem A, Al Diab AR, Niaz F, Al Momen AK. Rapid improvement in renal function in patients with multiple myeloma and renal failure treated with bortezomib. Saudi J Kidney Dis Transpl. 2010;21:63–68. [PubMed] [Google Scholar]
- 68.Morabito F, Gentile M, Ciolli S, Petrucci MT, Galimberti S, Mele G, Casulli AF, Mannina D, Piro E, Pinotti G, et al. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol. 2010;84:223–228. doi: 10.1111/j.1600-0609.2009.01385.x. [DOI] [PubMed] [Google Scholar]
- 69.Morabito F, Gentile M, Mazzone C, Rossi D, Di Raimondo F, Bringhen S, Ria R, Offidani M, Patriarca F, Nozzoli C, et al. Safety and efficacy of bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in untreated multiple myeloma patients with renal impairment. Blood. 2011;118:5759–5766. doi: 10.1182/blood-2011-05-353995. [DOI] [PubMed] [Google Scholar]
- 70.Costa LJ, Abbas J, Ortiz-Cruz KL, Kang Y, Stuart RK. Outcomes of patients with multiple myeloma and renal impairment treated with bortezomib, cyclophosphamide, and dexamethasone without plasma exchange. Eur J Haematol. 2012;89:432–434. doi: 10.1111/ejh.12008. [DOI] [PubMed] [Google Scholar]
- 71.Dimopoulos MA, Beksac M, Benboubker L, Roddie H, Allietta N, Broer E, Couturier C, Mazier MA, Angermund R, Facon T. Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma. Haematologica. 2013;98:1264–1272. doi: 10.3324/haematol.2013.084376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang G, Chen W, Wu Y. Bortezomib, dexamethasone plus thalidomide for treatment of newly diagnosed multiple myeloma patients with or without renal impairment. Chin J Cancer Res. 2013;25:155–160. doi: 10.3978/j.issn.1000-9604.2013.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scheid C, Sonneveld P, Schmidt-Wolf IG, van der Holt B, el Jarari L, Bertsch U, Salwender H, Zweegman S, Blau IW, Vellenga E, et al. Bortezomib before and after autologous stem cell transplantation overcomes the negative prognostic impact of renal impairment in newly diagnosed multiple myeloma: a subgroup analysis from the HOVON-65/GMMG-HD4 trial. Haematologica. 2014;99:148–154. doi: 10.3324/haematol.2013.087585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breitkreutz I, Heiss C, Perne A, Beimler J, Jager D, Egerer G, Ho AD, Neben K, Zeier M, Goldschmidt H, et al. Bortezomib improves outcome after SCT in multiple myeloma patients with end-stage renal failure. Bone Marrow Transplant. 2014;49:1371–1375. doi: 10.1038/bmt.2014.165. [DOI] [PubMed] [Google Scholar]
- 75.Badros AZ, Vij R, Martin T, Zonder JA, Kunkel L, Wang Z, Lee S, Wong AF, Niesvizky R. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety. Leukemia. 2013;27:1707–1714. doi: 10.1038/leu.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, Badros AZ, Jagannath S, McCulloch L, Rajangam K, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98:1753–1761. doi: 10.3324/haematol.2013.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 78.Fujita J, Mestre JR, Zeldis JB, Subbaramaiah K, Dannenberg AJ. Thalidomide and its analogues inhibit lipopolysaccharide-mediated induction of cyclooxygenase-2. Clin Cancer Res. 2001;7:3349–3355. [PubMed] [Google Scholar]
- 79.Oliveira AM, Maria DA, Metzger M, Linardi C, Giorgi RR, Moura F, Martinez GA, Bydlowski SP, Novak EM. Thalidomide treatment down-regulates SDF-1alpha and CXCR4 expression in multiple myeloma patients. Leuk Res. 2009;33:970–973. doi: 10.1016/j.leukres.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 80.Chen TL, Vogelsang GB, Petty BG, Brundrett RB, Noe DA, Santos GW, Colvin OM. Plasma pharmacokinetics and urinary excretion of thalidomide after oral dosing in healthy male volunteers. Drug Metab Dispos. 1989;17:402–405. [PubMed] [Google Scholar]
- 81.Lepper ER, Smith NF, Cox MC, Scripture CD, Figg WD. Thalidomide metabolism and hydrolysis: mechanisms and implications. Curr Drug Metab. 2006;7:677–685. doi: 10.2174/138920006778017777. [DOI] [PubMed] [Google Scholar]
- 82.Lokhorst HM, van der Holt B, Zweegman S, Vellenga E, Croockewit S, van Oers MH, von dem Borne P, Wijermans P, Schaafsma R, de Weerdt O, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115:1113–1120. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 83.Lokhorst HM, Schmidt-Wolf I, Sonneveld P, van der Holt B, Martin H, Barge R, Bertsch U, Schlenzka J, Bos GM, Croockewit S, et al. Thalidomide in induction treatment increases the very good partial response rate before and after high-dose therapy in previously untreated multiple myeloma. Haematologica. 2008;93:124–127. doi: 10.3324/haematol.11644. [DOI] [PubMed] [Google Scholar]
- 84.Rajkumar SV, Rosinol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, Olesnyckyj M, Yu Z, Knight R, Zeldis JB, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26:2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rajkumar SV, Blood E, Vesole D, Fonseca R, Greipp PR; Eastern Cooperative Oncology Group. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 86.Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Navarro Coy N, Cook G, Feyler S, Johnson PR, Rudin C, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97:442–450. doi: 10.3324/haematol.2011.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Harris E, Behrens J, Samson D, Rahemtulla A, Russell NH, Byrne JL. Use of thalidomide in patients with myeloma and renal failure may be associated with unexplained hyperkalaemia. Br J Haematol. 2003;122:160–161. doi: 10.1046/j.1365-2141.2003.04395_2.x. [DOI] [PubMed] [Google Scholar]
- 88.Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F, Moreau P, Waage A, Spencer A, Ludwig H, et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood. 2008;111:3968–3977. doi: 10.1182/blood-2007-10-117457. [DOI] [PubMed] [Google Scholar]
- 89.Bennett CL, Angelotta C, Yarnold PR, Evens AM, Zonder JA, Raisch DW, Richardson P. Thalidomide- and lenalidomide-associated thromboembolism among patients with cancer. JAMA. 2006;296:2558–2560. doi: 10.1001/jama.296.21.2558-c. [DOI] [PubMed] [Google Scholar]
- 90.Tosi P, Zamagni E, Tacchetti P, Ceccolini M, Perrone G, Brioli A, Pallotti MC, Pantani L, Petrucci A, Baccarani M, et al. Thalidomide-dexamethasone as induction therapy before autologous stem cell transplantation in patients with newly diagnosed multiple myeloma and renal insufficiency. Biol Blood Marrow Transplant. 2010;16:1115–1121. doi: 10.1016/j.bbmt.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 91.Tosi P, Zamagni E, Cellini C, Cangini D, Tacchetti P, Tura S, Baccarani M, Cavo M. Thalidomide alone or in combination with dexamethasone in patients with advanced, relapsed or refractory multiple myeloma and renal failure. Eur J Haematol. 2004;73:98–103. doi: 10.1111/j.1600-0609.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 92.Verhelle D, Corral LG, Wong K, Mueller JH, Moutouh-de Parseval L, Jensen-Pergakes K, Schafer PH, Chen R, Glezer E, Ferguson GD, et al. Lenalidomide and CC-4047 inhibit the proliferation of malignant B cells while expanding normal CD34+ progenitor cells. Cancer Res. 2007;67:746–755. doi: 10.1158/0008-5472.CAN-06-2317. [DOI] [PubMed] [Google Scholar]
- 93.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 94.Gandhi AK, Kang J, Capone L, Parton A, Wu L, Zhang LH, Mendy D, Lopez-Girona A, Tran T, Sapinoso L, et al. Dexamethasone synergizes with lenalidomide to inhibit multiple myeloma tumor growth, but reduces lenalidomide-induced immunomodulation of T and NK cell function. Curr Cancer Drug Targets. 2010;10:155–167. doi: 10.2174/156800910791054239. [DOI] [PubMed] [Google Scholar]
- 95.Tai YT, Li XF, Catley L, Coffey R, Breitkreutz I, Bae J, Song W, Podar K, Hideshima T, Chauhan D, et al. Immunomodulatory drug lenalidomide (CC-5013, IMiD3) augments anti-CD40 SGN-40-induced cytotoxicity in human multiple myeloma: clinical implications. Cancer Res. 2005;65:11712–11720. doi: 10.1158/0008-5472.CAN-05-1657. [DOI] [PubMed] [Google Scholar]
- 96.Chen N, Lau H, Kong L, Kumar G, Zeldis JB, Knight R, Laskin OL. Pharmacokinetics of lenalidomide in subjects with various degrees of renal impairment and in subjects on hemodialysis. J Clin Pharmacol. 2007;47:1466–1475. doi: 10.1177/0091270007309563. [DOI] [PubMed] [Google Scholar]
- 97.Dimopoulos MA, Palumbo A, Attal M, Beksac M, Davies FE, Delforge M, Einsele H, Hajek R, Harousseau JL, da Costa FL, et al. Optimizing the use of lenalidomide in relapsed or refractory multiple myeloma: consensus statement. Leukemia. 2011;25:749–760. doi: 10.1038/leu.2011.3. [DOI] [PubMed] [Google Scholar]
- 98.Reece D, Kouroukis CT, Leblanc R, Sebag M, Song K, Ashkenas J. Practical approaches to the use of lenalidomide in multiple myeloma: a Canadian consensus. Adv Hematol. 2012;2012:621958. doi: 10.1155/2012/621958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dimopoulos M, Alegre A, Stadtmauer EA, Goldschmidt H, Zonder JA, de Castro CM, Masliak Z, Reece D, Olesnyckyj M, Yu Z, et al. The efficacy and safety of lenalidomide plus dexamethasone in relapsed and/or refractory multiple myeloma patients with impaired renal function. Cancer. 2010;116:3807–3814. doi: 10.1002/cncr.25139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de la Rubia J, Roig M, Ibanez A, Garcia I, Vera JA, Aguilar C, del Campo R, Gonzalez N, Martinez R, Palomera L, et al. Activity and safety of lenalidomide and dexamethasone in patients with multiple myeloma requiring dialysis: a Spanish multicenter retrospective study. Eur J Haematol. 2010;85:363–365. doi: 10.1111/j.1600-0609.2010.01500.x. [DOI] [PubMed] [Google Scholar]
- 101.Tosi P, Gamberi B, Castagnari B, Molinari AL, Savini P, Ceccolini M, Tani M, Merli A, Imola M, Mianulli AM, et al. Lenalidomide in combination with dexamethasone in elderly patients with advanced, relapsed or refractory multiple myeloma and renal failure. Mediterr J Hematol Infect Dis. 2013;5:e2013037. doi: 10.4084/MJHID.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oehrlein K, Langer C, Sturm I, Ponisch W, Hahn-Ast C, Kuhn S, Weisel KC. Successful treatment of patients with multiple myeloma and impaired renal function with lenalidomide: results of 4 German centers. Clin Lymphoma Myeloma Leuk. 2012;12:191–196. doi: 10.1016/j.clml.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 103.Klein U, Neben K, Hielscher T, Heiss C, Ho AD, Goldschmidt H. Lenalidomide in combination with dexamethasone: effective regimen in patients with relapsed or refractory multiple myeloma complicated by renal impairment. Ann Hematol. 2011;90:429–439. doi: 10.1007/s00277-010-1080-4. [DOI] [PubMed] [Google Scholar]
- 104.Dimopoulos MA, Swern AS, Li JS, Hussein M, Weiss L, Nagarwala Y, Baz R. Efficacy and safety of long-term treatment with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2014;4:e257. doi: 10.1038/bcj.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ludwig H, Rauch E, Kuehr T, Adam Z, Weissmann A, Kasparu H, Autzinger EM, Heintel D, Greil R, Poenisch W, et al. Lenalidomide and dexamethasone for acute light chain-induced renal failure: a phase II study. Haematologica. 2015;100:385–391. doi: 10.3324/haematol.2014.115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S, Chen C, Lonial S, Jakubowiak A, Bahlis N, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826–1832. doi: 10.1182/blood-2013-11-538835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.San Miguel J, Weisel K, Moreau P, Lacy M, Song K, Delforge M, Karlin L, Goldschmidt H, Banos A, Oriol A, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 108.Dimopoulos MA, Sonneveld P, Siegel D, Palumbo A, San-Miguel J. Carfilzomib and pomalidomide in patients with relapsed and/or refractory multiple myeloma with baseline risk factors. Ann Oncol. 2015;26:2247–2256. doi: 10.1093/annonc/mdv325. [DOI] [PubMed] [Google Scholar]
- 109.Tricot G, Alberts DS, Johnson C, Roe DJ, Dorr RT, Bracy D, Vesole DH, Jagannath S, Meyers R, Barlogie B. Safety of autotransplants with high-dose melphalan in renal failure: a pharmacokinetic and toxicity study. Clin Cancer Res. 1996;2:947–952. [PubMed] [Google Scholar]
- 110.Ballester OF, Tummala R, Janssen WE, Fields KK, Hiemenz JW, Goldstein SC, Perkins JB, Sullivan DM, Rosen R, Sackstein R, et al. High-dose chemotherapy and autologous peripheral blood stem cell transplantation in patients with multiple myeloma and renal insufficiency. Bone Marrow Transplant. 1997;20:653–656. doi: 10.1038/sj.bmt.1700950. [DOI] [PubMed] [Google Scholar]
- 111.Tauro S, Clark FJ, Duncan N, Lipkin G, Richards N, Mahendra P. Recovery of renal function after autologous stem cell transplantation in myeloma patients with end-stage renal failure. Bone Marrow Transplant. 2002;30:471–473. doi: 10.1038/sj.bmt.1703713. [DOI] [PubMed] [Google Scholar]
- 112.Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M, Desikan R, Shaver MJ, Fassas A, McConnell S, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822–829. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 113.Raab MS, Breitkreutz I, Hundemer M, Benner A, Klaus J, Hegenbart U, Moehler T, Ho AD, Zeier M, Goldschmidt H. The outcome of autologous stem cell transplantation in patients with plasma cell disorders and dialysis-dependent renal failure. Haematologica. 2006;91:1555–1558. [PubMed] [Google Scholar]
- 114.Lee CK, Zangari M, Barlogie B, Fassas A, van Rhee F, Thertulien R, Talamo G, Muwalla F, Anaissie E, Hollmig K, et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant. 2004;33:823–828. doi: 10.1038/sj.bmt.1704440. [DOI] [PubMed] [Google Scholar]
- 115.Glavey SV, Gertz MA, Dispenzieri A, Kumar S, Buadi F, Lacy M, Hayman SR, Kapoor P, Dingli D, McCurdy A, et al. Long-term outcome of patients with multiple [corrected] myeloma-related advanced renal failure following auto-SCT. Bone Marrow Transplant. 2013;48:1543–1547. doi: 10.1038/bmt.2013.109. [DOI] [PubMed] [Google Scholar]
- 116.Bird JM, Owen RG, D'Sa S, Snowden JA, Pratt G, Ashcroft J, Yong K, Cook G, Feyler S, Davies F, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 117.Clark WF, Stewart AK, Rock GA, Sternbach M, Sutton DM, Barrett BJ, Heidenheim AP, Garg AX, Churchill DN; Canadian Apheresis Group. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143:777–784. doi: 10.7326/0003-4819-143-11-200512060-00005. [DOI] [PubMed] [Google Scholar]
- 118.Leung N, Gertz MA, Zeldenrust SR, Rajkumar SV, Dispenzieri A, Fervenza FC, Kumar S, Lacy MQ, Lust JA, Greipp PR, et al. Improvement of cast nephropathy with plasma exchange depends on the diagnosis and on reduction of serum free light chains. Kidney Int. 2008;73:1282–1288. doi: 10.1038/ki.2008.108. [DOI] [PubMed] [Google Scholar]
- 119.Cserti C, Haspel R, Stowell C, Dzik W. Light-chain removal by plasmapheresis in myeloma-associated renal failure. Transfusion. 2007;47:511–514. doi: 10.1111/j.1537-2995.2006.01143.x. [DOI] [PubMed] [Google Scholar]
- 120.Winearls CG. Acute myeloma kidney. Kidney Int. 1995;48:1347–1361. doi: 10.1038/ki.1995.421. [DOI] [PubMed] [Google Scholar]
- 121.Hutchison CA, Cockwell P, Reid S, Chandler K, Mead GP, Harrison J, Hattersley J, Evans ND, Chappell MJ, Cook M, et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol. 2007;18:886–895. doi: 10.1681/ASN.2006080821. [DOI] [PubMed] [Google Scholar]
- 122.Zannetti BA, Zamagni E, Santostefano M, De Sanctis LB, Tacchetti P, Mancini E, Pantani L, Brioli A, Rizzo R, Mancuso K, et al. Bortezomib-based therapy combined with high cut-off hemodialysis is highly effective in newly diagnosed multiple myeloma patients with severe renal impairment. Am J Hematol. 2015;90:647–652. doi: 10.1002/ajh.24035. [DOI] [PubMed] [Google Scholar]
- 123.Borrego-Hinojosa J, Perez-del Barrio MP, Biechy-Baldan Mdel M, Merino-Garcia E, Sanchez-Perales MC, Garcia-Cortes MJ, Ocana-Perez E, Gutierrez-Rivas P, Liebana-Canada A. Treatment by long haemodialysis sessions with high cut-off filters in myeloma cast nephropathy: our experience. Nefrologia. 2013;33:515–523. doi: 10.3265/Nefrologia.pre2013.Feb.11932. [DOI] [PubMed] [Google Scholar]
- 124.Khalafallah AA, Loi SW, Love S, Mohamed M, Mace R, Khalil R, Girgs M, Raj R, Mathew M. Early Application of high cut-off haemodialysis for de-novo myeloma nephropathy is associated with long-term dialysis-independency and renal recovery. Mediterr J Hematol Infect Dis. 2013;5:e2013007. doi: 10.4084/MJHID.2013.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sinisalo M, Silvennoinen R, Wirta O. High cut-off hemodialysis and bortezomib-based therapy to rescue kidneys in myeloma-dependent cast nephropathy. Am J Hematol. 2012;87:640. doi: 10.1002/ajh.23189. [DOI] [PubMed] [Google Scholar]
- 126.Martin-Reyes G, Toledo-Rojas R, Torres-de Rueda A, Sola-Moyano E, Blanca-Martos L, Fuentes-Sanchez L, Martinez-Esteban MD, Diez-de los Rios MJ, Bailen-Garcia A, Gonzalez-Molina M, et al. Haemodialysis using high cut-off dialysers for treating acute renal failure in multiple myeloma. Nefrologia. 2012;32:35–43. doi: 10.3265/Nefrologia.pre2011.Nov.11094. [DOI] [PubMed] [Google Scholar]
- 127.Heyne N, Denecke B, Guthoff M, Oehrlein K, Kanz L, Haring HU, Weisel KC. Extracorporeal light chain elimination: high cut-off (HCO) hemodialysis parallel to chemotherapy allows for a high proportion of renal recovery in multiple myeloma patients with dialysis-dependent acute kidney injury. Ann Hematol. 2012;91:729–735. doi: 10.1007/s00277-011-1383-0. [DOI] [PubMed] [Google Scholar]
- 128.Hutchison CA, Cook M, Heyne N, Weisel K, Billingham L, Bradwell A, Cockwell P. European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomised control trial. Trials. 2008;9:55. doi: 10.1186/1745-6215-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bridoux F, Fermand JP. Optimizing treatment strategies in myeloma cast nephropathy: rationale for a randomized prospective trial. Adv Chronic Kidney Dis. 2012;19:333–341. doi: 10.1053/j.ackd.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Dawson AA, Ogston D. Factors influencing the prognosis in myelomatosis. Postgrad Med J. 1971;47:635–638. doi: 10.1136/pgmj.47.552.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kyle RA. Multiple myeloma: review of 869 cases. Mayo Clin Proc Mayo Clin. 1975;50:29–40. [PubMed] [Google Scholar]
- 132.Rayner HC, Haynes AP, Thompson JR, Russell N, Fletcher J. Perspectives in multiple myeloma: survival, prognostic factors and disease complications in a single centre between 1975 and 1988. Q J Med. 1991;79:517–525. [PubMed] [Google Scholar]
- 133.Knudsen LM, Hippe E, Hjorth M, Holmberg E, Westin J. Renal function in newly diagnosed multiple myeloma - a demographic study of 1353 patients. The Nordic Myeloma Study Group. Eur J Haematol. 1994;53:207–212. doi: 10.1111/j.1600-0609.1994.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 134.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc Mayo Clin. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 135.Fakhouri F, Guerraoui H, Presne C, Peltier J, Delarue R, Muret P, Knebelmann B. Thalidomide in patients with multiple myeloma and renal failure. Br J Haematol. 2004;125:96–97. doi: 10.1111/j.1365-2141.2004.04875.x. [DOI] [PubMed] [Google Scholar]
- 136.Niesvizky R, Naib T, Christos PJ, Jayabalan D, Furst JR, Jalbrzikowski J, Zafar F, Mark T, Lent R, Pearse RN, et al. Lenalidomide-induced myelosuppression is associated with renal dysfunction: adverse events evaluation of treatment-naive patients undergoing front-line lenalidomide and dexamethasone therapy. Br J Haematol. 2007;138:640–643. doi: 10.1111/j.1365-2141.2007.06698.x. [DOI] [PubMed] [Google Scholar]
- 137.Dimopoulos MA, Christoulas D, Roussou M, Kastritis E, Migkou M, Gavriatopoulou M, Matsouka C, Mparmparoussi D, Psimenou E, Grapsa I, et al. Lenalidomide and dexamethasone for the treatment of refractory/relapsed multiple myeloma: dosing of lenalidomide according to renal function and effect on renal impairment. Eur J Haematol. 2010;85:1–5. doi: 10.1111/j.1600-0609.2010.01432.x. [DOI] [PubMed] [Google Scholar]
- 138.Hou J, Du X, Jin J, Cai Z, Chen F, Zhou DB, Yu L, Ke X, Li X, Wu D, et al. A multicenter, open-label, phase 2 study of lenalidomide plus low-dose dexamethasone in Chinese patients with relapsed/refractory multiple myeloma: the MM-021 trial. J Hematol Oncol. 2013;6:41. doi: 10.1186/1756-8722-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yamasaki S, Kohno K, Kadowaki M, Takase K, Okamura S. Dose-adjusted lenalidomide combined with low-dose dexamethasone rescues older patients with bortezomib-resistant multiple myeloma. Intern Med. 2015;54:1711–1715. doi: 10.2169/internalmedicine.54.4075. [DOI] [PubMed] [Google Scholar]
- 140.Peters NO, Laurain E, Cridlig J, Hulin C, Cao-Huu T, Frimat L. Impact of free light chain hemodialysis in myeloma cast nephropathy: a case-control study. Hemodial Int Int Symp Home Hemodial. 2011;15:538–545. doi: 10.1111/j.1542-4758.2011.00587.x. [DOI] [PubMed] [Google Scholar]
- 141.Tan J, Lam-Po-Tang M, Hutchison CA, de Zoysa JR. Extended high cut-off haemodialysis for myeloma cast nephropathy in Auckland, 2008-2012. Nephrology (Carlton) 2014;9:432–435. doi: 10.1111/nep.12267. [DOI] [PubMed] [Google Scholar]