Abstract
Background
Asia is the largest, most populous and most heterogeneous continent in the world. The number of patients with end-stage renal disease is growing rapidly in Asia.
Summary
A fully informed report on the status of dialysis therapies including hemodialysis (HD) is limited by the lack of systematic registries. Available data suggest remarkable heterogeneities, with some countries like Taiwan, Japan and Korea exhibiting well-established HD systems, high prevalence and universal access to all patients, while low- and low-middle income countries are unable to provide HD to eligible patients because of high cost and poor healthcare systems. Many Asian countries have unregulated dialysis units, with poor standards of delivery, quality control and outcome reporting. This leads to high mortality due to preventable complications like infections. Modeling data suggest that at least 2.9 million people need dialysis in Asia, which represents a gap in availability of dialysis to the tune of −66%. The population is projected to grow rapidly in the coming years. Several countries are expanding access to HD. Innovative modifications in dialysis practice are being made to optimize outcomes. It is important to develop robust systems of documentation and outcome reporting to evaluate the effects of such changes. HD needs to develop in conjunction with effective preventive programs and improvement of health systems.
Key Messages
The practice of HD in Asia is growing and evolving. Rapid expansion will improve the currently dismal access to care for large sections of the population. Quality issues need to be addressed if the full benefit of this therapy is to reach the population. Developed countries of Asia can provide substantial messages to developing economies. HD programs must develop in conjunction with prevention efforts.
Facts from East and West
(1) While developed Western and Asian countries provide end-stage renal disease patients full access to HD, healthcare systems from South and South-East Asia can offer access to HD only to a limited fraction of the patients in need. Even though the annual costs of HD are much lower in less developed countries (for instance 30 times lower in India compared to the US), patients often cannot afford costs not covered by health insurance. (2) The recommended dialysis pattern in the West is at least three sessions weekly with high-flux dialyzers. Studies from Shanghai and Taiwan might however indicate a benefit of twice versus thrice weekly sessions. In less developed Asian countries, a twice weekly pattern is common, sometimes with dialyzer reuse and inadequate water treatment. A majority of patients decrease session frequency or discontinue the program due to financial constraint. (3) As convective therapies are gaining popularity in Europe, penetration in Asia is low and limited by costs. (4) In Asian countries, in particular in the South and South-East, hepatitis and tuberculosis infections in HD patients are higher than in the West and substantially increase mortality. (5) Progress has recently been made in countries like Thailand and Brunei to provide universal HD access to all patients in need. Nevertheless, well-trained personnel, reliable registries and better patient follow-up would improve outcomes in low-income Asian countries.
Key Words: Asia, Cost of care, Economics, End-stage renal disease, Hemodialysis
Introduction
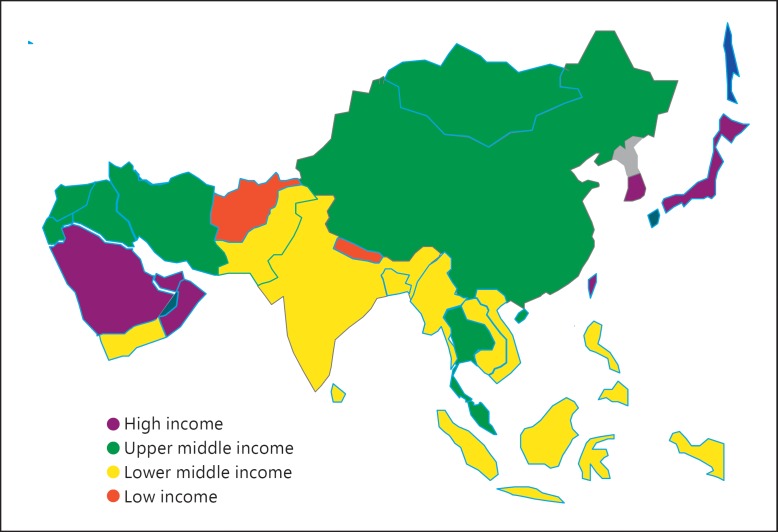
Largest and most populous of Earth's continents, Asia comprises about 30% of the world's land area with 60% of the world population (3.6 billion people) [1]. According to the World Bank, most Asian countries fall in the low or lower middle income bracket (fig. 1). Even within these broad categories, these countries vary in their level of economic development and healthcare indicators. Within country variations are also seen in large countries like India and China (table 1). Demographic, nutritional and epidemiological transitions have changed disease presentation in Asia. Chronic non-communicable diseases, such as diabetes, hypertension and metabolic syndrome, have been gaining in prominence. In parallel with the overall disease burden, the leading causes of chronic kidney disease (CKD) have shifted from infection- and public health-related diseases, such as glomerulonephritis and interstitial nephritis, to diabetic nephropathy and hypertensive nephrosclerosis [2,3]. In recent reports coming from Asia, these conditions now account for about 40–50% of all cases with end-stage renal disease (ESRD), the best-known consequence of CKD [2,4,5]. This different is important, because individuals on hemodialysis (HD) secondary to ESRD due to diabetes and hypertension carry a significant burden of other comorbidities, which impact outcomes.
Fig. 1.
World Bank economic classification of Asian countries.
Table 1.
Regional variations in health status in Asia
| Life expectancy, years | Asian countries | Chinese provinces | Indian states |
|---|---|---|---|
| >80 | Japan, Korea, Singapore, Australia | ||
| 75.0–79.9 | Shanghai, Beijing | Kerala | |
| 70.0–74.9 | Malaysia, Vietnam, Sri Lanka | Zhejiang, 20 others | Punjab |
| 65.0–69.9 | Philippines, Thailand, Indonesia, Bangladesh | Inner Mongolia, 6 others | Maharashtra, 5 others |
| 60.0–64.9 | Laos, Myanmar, Pakistan | Tibet | Andhra Pradesh, 7 others |
Burden of Disease
An important indicator of the state of health in a community is accurate enumeration of the causes of death. One in three deaths worldwide occur just in India and China. It would therefore be ideal to know the proportion of all deaths due to ESRD. Unfortunately, systematic causes of death reporting systems are lacking in most Asian countries. Recently, the Institute of Health Metrics produced estimates of the most important causes of death and disability in the periodic Global Burden of Disease (GBD) study report. According to the recent GBD report [6], CKD showed an almost 100% increase in the number of deaths over 20 years from 1990.
The Asian continent has the countries with the highest ESRD incidence and prevalence in the world (Taiwan, Japan). Data on the accurate incidence and prevalence of various stages of CKD in most Asian countries are hard to come by because of the absence of national or regional registries. Publications based on individual experiences and hospital-based data have generally underestimated the true figures [7,8,9].
The population of ESRD patients requiring dialysis in Asia is expanding at a rate higher than elsewhere in the world. In many Asian countries, including China, the Philippines and Malaysia, the annual growth is in excess of 10% [10]. This pace is likely to accelerate further because of the progressive aging and increase in numbers of people with diabetes and hypertension [11]. The demand for dialysis in India is said to be growing at the rate of 31% per annum, and the value of the dialysis market has increased from USD 100 million in 2007 to USD 150 million in 2012 [12].
The demography of the ESRD population is Asia is also remarkable for two features: the relatively younger age - at least two decades younger than that seen in the developed countries - and a high prevalence (17–20%) of CKD of uncertain etiology [5,13,14].
Healthcare Systems and Financing
The healthcare systems in the countries of Asia range from extremely well-developed, as in Singapore, Hong Kong, Japan and Korea, to very poorly developed, as in Nepal, Bangladesh, Vietnam and Cambodia. Most countries are between the two extremes and in various stages of development. Broadly, the base of the public sector healthcare pyramid is formed by primary health centers which provide basic preventive care. Above them are district and regional hospitals, but the referral link between these two tiers of facilities is poor [8,15]. The latter are often underfunded and ill-equipped for managing complex problems like ESRD. Public sector tertiary care hospitals are located in large cities, overcrowded and understaffed and thus limited in their ability to meet with the ever-increasing demand. In many countries, specialist referral care is provided largely by private sector hospitals which are expensive and out of reach of the average citizens [16,17].
Although dialysis patients constitute only a small proportion of the total population, the funding needed for treating these patients is considerable, out of proportion to the overall healthcare spending [18]. The growing number of ESRD patients and the high costs of therapy impose a large financial burden, more so in countries with low incomes. Emerging economies lack the technical, human and financial resources needed to establish appropriate policies for ESRD treatment, making it a political and economic challenge.
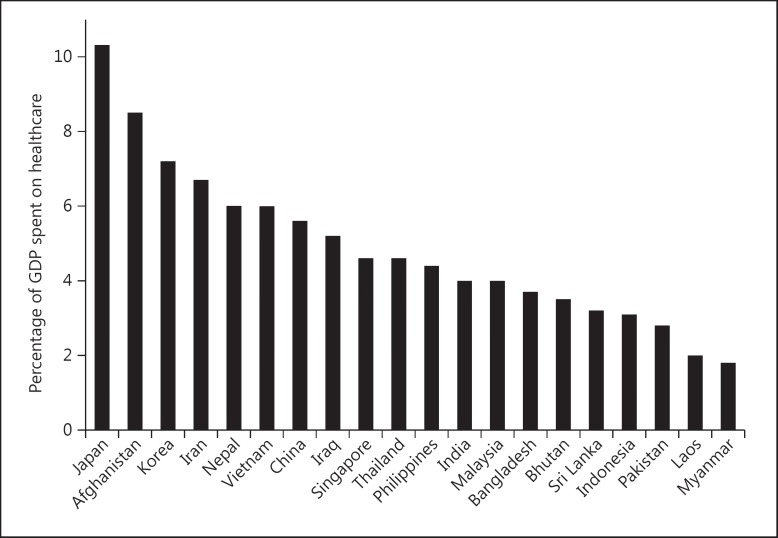
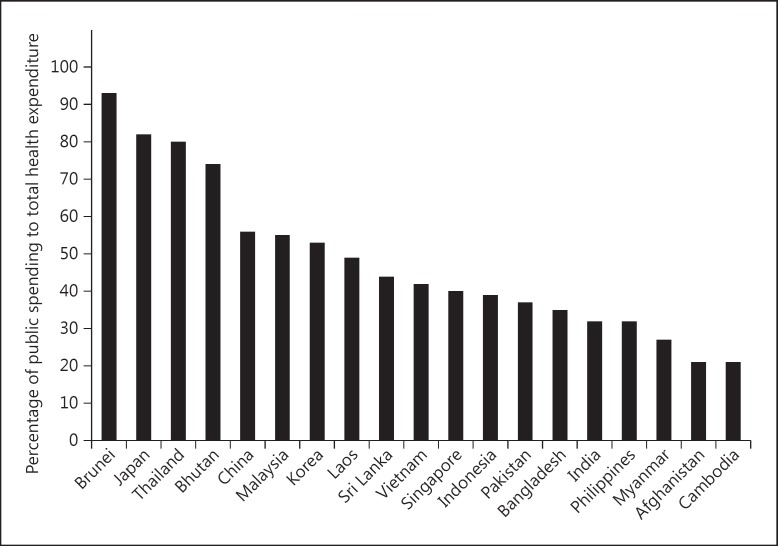
Health spending accounts for only 2–5% of the GDP in various developing Asian countries, far less than the OECD average of 9.3% (fig. 2). In countries with low spending, the major expenditure goes towards preventive programs, family welfare and nutrition, staff salaries and maintenance of basic hospital infrastructure. Attention to specialist-, technology- and resource-intensive treatments like dialysis does not occupy high priority. In several countries, the share of public spending on healthcare is low, which raises the cost of care (fig. 3).
Fig. 2.
Bar chart showing the percentage of GDP spent on healthcare in Asian countries (data from http://data.worldbank.org/).
Fig. 3.
Bar chart showing the percentage of public expenditure as part of overall healthcare spending in Asian countries (data from http://data.worldbank.org/).
Policies for Care of Patients with ESRD
Government policies for the care of ESRD patients vary from country to country in Asia. Citizens of Japan, Taiwan, Singapore and Korea have long enjoyed universal access to dialysis. More recently, Malaysia, Thailand, Brunei as well as parts of China and India have also introduced universal dialysis coverage to their citizens. In some instances, beneficiaries need to make a co-payment. In large countries, there are significant within-country heterogeneities in healthcare delivery systems, leading to lack of uniformity in the delivery of care.
In regions without universal access, payment for dialysis is often met by out of pocket expenditure, leading to catastrophic impoverishment. In one study from India [19], about 60% of patients reported severe catastrophic expenditure on pre-transplant dialysis (fig. 4). As a result, large sections of the society remain effectively excluded from this potentially transformative treatment.
Fig. 4.
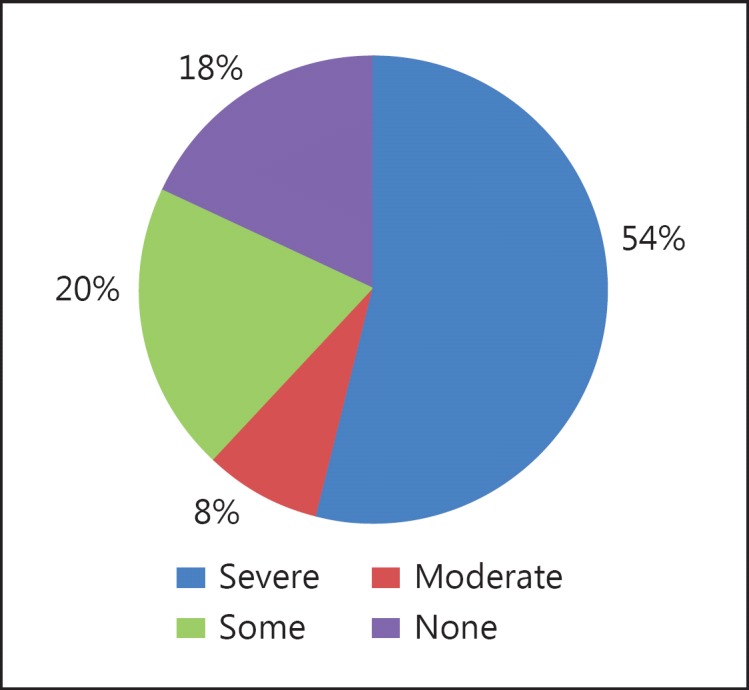
Catastrophic healthcare spending on dialysis (reproduced from [19]).
The cost of care in public sector hospitals is lower because of the subsidy provided by governments. The subsidy can vary depending on the size and location of the hospital, the number of dialysis machines in the unit, and university or local affiliation. However, public hospitals are overrun by a large load of patients with acute kidney injury and able to accept only a limited number of patients for maintenance dialysis. In order to ensure optimal utilization of the limited resources, these hospitals limit access to HD for kidney transplant candidates or those with reversible acute insults. This limitation has created a demand for dialysis services in private hospitals. The charges in these hospitals are substantially higher and depend upon hospital size, type (single or multiple specialties), location, reputation and additional facilities [20].
Incidence, Prevalence and Gaps in Dialysis Treatment
Estimating the exact incidence and prevalence of dialysis populations in Asian countries is a major challenge. The job is made difficult by the lack of a systematic data collection facility and the absence of a referral chain. Moreover, an unspecified but potentially large number of patients in remote rural locations never come to the attention of nephrologists and remain unaccounted for in incidence/prevalence estimates. It is reasonable to expect that a variable proportion of individuals who are eligible to receive dialysis are unable to get it.
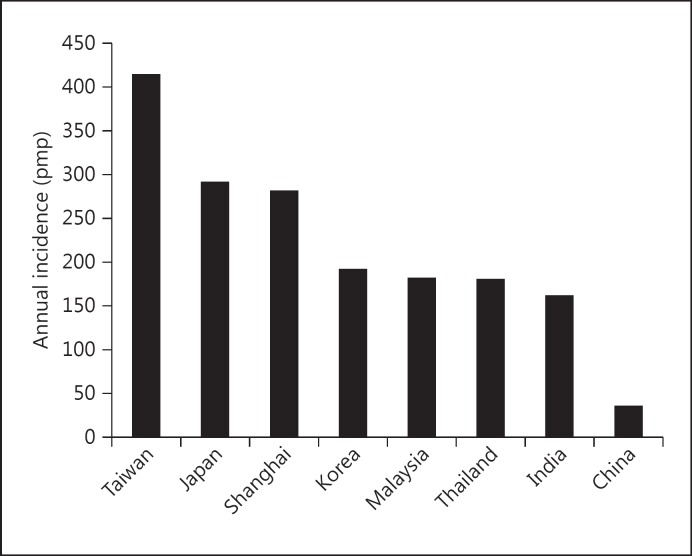
Recently, the establishment of registries has allowed a more accurate estimate in several countries/regions. According to a recent review [21], there were seven registries in Asia, with variable levels of accessibility, data quality and outcome reporting (table 2). Figures 5 and 6 show the current data on the incidence and prevalence of ESRD in Asian countries.
Table 2.
Features of dialysis registries in Asia (modified from [21])
| Registry name (common abbreviation), year of establishment | Accessibility | Patient-level data availability | Treatments | Outcomes |
|---|---|---|---|---|
| Hong Kong Renal Registry (HKRR), 1995 | + | + | +++ | +++ |
| Korean Renal Registry, 1985 | ++ | ++ | +++ | +++ |
| Malaysian National Renal Registry (NRR), 1993 | ++ | +++ | +++ | +++ |
| Shanghai Dialysis Registry, 1996 | + | + | ++ | +++ |
| Singapore Renal Registry, 2001 | +++ | ++ | +++ | +++ |
| Taiwan Renal Registry Data System (TWRDS), 1987 | + | + | +++ | + |
| Thailand Renal Replacement Therapy Registry (TRT), 1997 | ++ | + | +++ | + |
Accessibility: +++ (good): information including annual reports, publications and aggregate data accessible via website, publicly available records, or with assistance from registry staff. ++ (moderate): information in local language only or limited publicly available information including on website; with additional searches, basic information may be available in reports or in published research; more information may be accessible via third-party collaborators (e.g. registry researchers or local academics). + (limited/unclear): very limited information available publicly or unclear.
Patient-level data availability: +++: available to external researchers directly or through application and review; may include usage fee. ++: conditional access, e.g. via third-party collaborators. +: not available to external researchers or access process unclear.
Treatments: +++: submodality available. ++: modality available but not submodality. +: modality not available or availability unclear.
Outcomes: +++: mortality/survival and/or hospitalization/complication data available. ++: mortality/survival or hospitalization/complication data not reported; surrogates such as laboratory result data reported. +: no reported outcome or surrogate data or availability unclear.
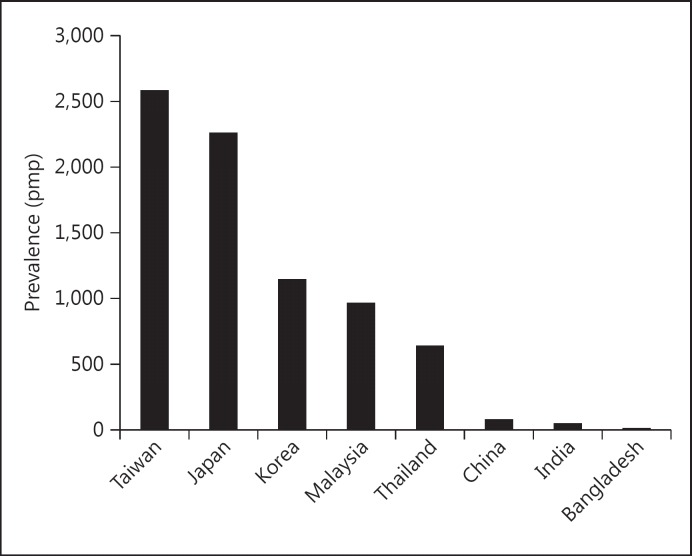
Fig. 5.
Annual incidence of ESRD in Asian countries (data from [14], with permission).
Fig. 6.
Prevalence of ESRD in Asian countries (data from [14], with permission).
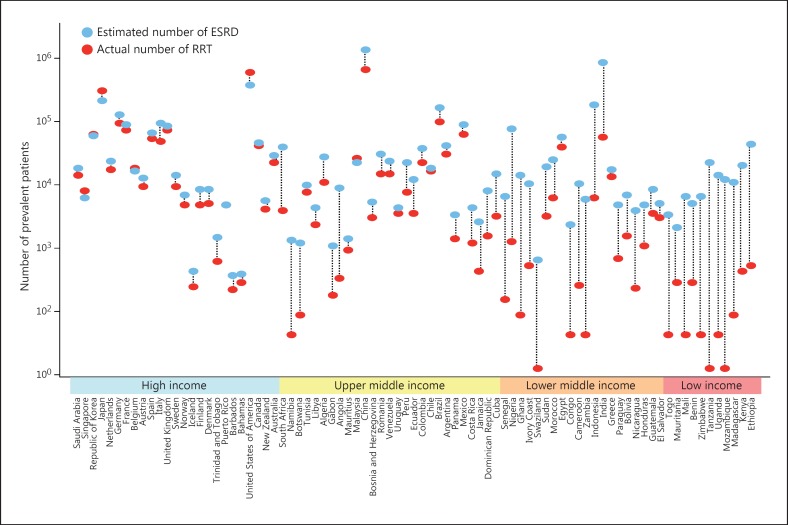
A recent study [22] explored the magnitude of burden of untreated ESRD around the world. Age-specific ESRD incidence and prevalence data were collected from 123 countries. The authors correlated the prevalence with various disease- and population-related factors. Only life expectancy and per capita GNP of a country were able to predict prevalence. This allowed the development of a model to estimate prevalence for countries from where data were not available. Data on actual numbers on renal replacement therapy (RRT) obtained from existing sources and on the basis of modeling showed that globally, 2.618 million people received RRT in 2010. About 968,000 were on RRT in Asia (232 pmp), with 909,000 (218 pmp) on dialysis. Next, estimates were developed to get the number of people who would need dialysis. Two estimates were developed - a high estimate based on data from Japan, Taiwan, Singapore and the US, where almost all patients with ESRD are eligible to get RRT, and a conservative model based on data from 16 high-income countries where access to dialysis is slightly less liberal. According to these projections, the number of individuals who need RRT in Asia ranges between 2.9 and 5.6 million (690–1,352 pmp, conservative and high estimates). This indicates a gap in availability of dialysis to the tune of −66% (95% CI −70 to −63, conservative model) to −83% (95% CI −95 to −81, high estimate). Figure 7 shows the gaps in access to RRT in individual countries. Finally, projected data for GDP and life expectancy from the World Bank and the World Health Organization was used to estimate the numbers that would need RRT in coming years. Calculations suggest 2.16 million people needing RRT in Asia in 2030, an increase of 223%. These figures present a daunting challenge for the nephrology community and health policy makers in the region.
Fig. 7.
Gap between the actual number of patients undergoing RRT and the estimated number of patients with ESRD for each country.
With economic prosperity and expansion of dialysis services, the dialysis population in most developing countries is expanding at a high rate. The annual growth in China, India, the Philippines, Thailand and Malaysia now exceeds 10% [10]. In developed Asian countries such as Japan, Taiwan and Korea, the proportion of patients receiving dialysis has reached 0.1–0.2% of the population.
Financing of Dialysis
The cost of RRT remains a major problem in ensuring provision of RRT to all ESRD patients. Data on ESRD treatment costs are available from some Asian countries. Expenses on dialysis accounted for 4.1% of the 1996 healthcare budget in Japan and for 3.24% of the national health expenditure in South Korea in 2004. Thailand started universal coverage for dialysis in 2008. For that year, the total spending on dialysis was THB 160 million (0.2% of the total budget). By 2012, this figure had grown to THB 3.9 billion (3.4% of total budget) [23].
Although the cost of dialysis in many Asian countries is relatively modest, compared to incomes these figures become unaffordable. By way of example, a recent analysis estimated that a single HD session costs around USD 30 in an Indian public hospital, so that dialysis twice a week for a year would cost around USD 3,000 [24]. This is 10- to 20-fold less than the cost of dialysis treatment in many wealthy countries. However, the unadjusted gross national income per capita in India is USD 1,040, and two-thirds of the population of 1.2 billion live on less than USD 2 a day [25]. Therefore, without financial support dialysis will remain beyond the financial reach of the majority of patients with ESRD in the developing countries of Asia. Frequent hospitalizations add to the financial burden [19].
The source of funding for dialysis therapy - determined by the local healthcare financing system - varies between countries and regions of some large countries. Examples of variations in funding within a country include a variety of insurance schemes in China and India. In China, people living in urban areas who work for the government get generous insurance coverage, whereas farmers living in rural areas have to make substantial co-payments. In India, some state governments have started insurance schemes to cover dialysis therapy for people with incomes below the poverty line. New models of dialysis delivery have been started in which units are set up by private players within a public delivery system that provides dialysis services at an agreed cost subsidized by the government. However, these models are yet to be pragmatically evaluated.
There are substantial variations in the components of coverage, and often there is a cap on the total expenditure that limits its implementation. People working for the government and in organized corporate employment sectors have access to dialysis as part of their health benefits, but often the extra cost incurred on dialysis in a private sector hospital is not reimbursed, even when there are no vacancies in public hospitals [8]. In recent years, certain organizations have started negotiating reimbursement rates for their employees with private hospitals. Charitable organizations and government-administered relief funds provide limited assistance to poor patients getting treatment in a government hospital. Generally, such assistance covers a few weeks of treatment. One study estimated that approximately two-thirds of patients took help from employers or accepted charity, one-third sold property or family valuables such as jewelry, and one-fourth took loans to cover the cost of RRT [26]. In view of the high cost of HD, many countries offer peritoneal dialysis (PD) as the preferred form of dialysis.
As the economies grow, countries in Asia are increasingly adopting universal coverage for dialysis. Government revenue is the main source of funding for dialysis in Hong Kong and Japan. In Taiwan, healthcare insurance covers the expense of dialysis for most ESRD patients. Malaysia has adopted a mixed private and public model for financing dialysis therapy [27]. Recently, Thailand has started providing dialysis to all citizens. In order to limit the expenditure incurred on dialysis, Hong Kong and Thailand have adopted a ‘PD first’ policy, limiting HD only for those who are either medically unsuitable or fail PD [23].
Patterns of Dialysis and Impact on Outcomes
The practice pattern of dialysis is diverse throughout Asia. The practice patterns in developed nations like Japan, Korea, Singapore, Hong Kong and Taiwan are similar to those in the developed economies, with the default modality being three times per week 4-hour sessions of high-flux HD and no dialyzer reuse. In contrast, countries with immature HD programs exhibit a substantial degree of variability in terms of frequency, duration and technology used to provide HD [20]. Quite often, the most frequent prescription is twice a week dialysis in 4-hour sessions using a low-flux dialyzer. The dialyzers and/or tubings are reused several times to save costs.
The main driver behind such variations is financial. Reports from South and South-East Asia suggest that many patients receive dialysis only when uremia becomes overwhelming and/or life-threatening complications like hyperkalemia, fluid overload, encephalopathy or metabolic acidosis force presentation. Data from the Indian CKD registry showed that over 50% of patients saw a nephrologist for the first time only with stage 5 CKD [5]. In a study from a large public sector hospital in India, more than 75% of those presenting needed emergency dialysis within 48 h of presentation [28].
After the initial emergency is tided over, patients cut down on dialysis frequency for financial reasons. This has an inevitable impact on outcomes. In a cohort of more than 1,200 consecutive ESRD patients referred to a public sector hospital, the mean HD duration was <1 month [29]. About 10% of patients died in the hospital and another 60% left the program and were lost to follow-up. Only 2% were on HD for >6 months. It is not uncommon for patients to reduce the dialysis frequency as financial resources dwindle, leading ultimately to discontinuation of dialysis or death [30].
In recent years, there has been a resurgence of interest in regular twice a week dialysis. In an observational study from Taiwan [31], patients dialyzed twice (n = 23) versus thrice (n = 51) weekly had a slower decline in residual kidney function, as indicated by higher urine output and better creatinine clearance over an 18-month follow-up period. Patients dialyzed twice weekly also had lower levels of serum β2 microglobulin, fewer intradialytic hypotensive episodes and fewer hospitalizations. In another study from Shanghai, patients on twice a week dialysis (n = 58) showed greater time to decline in residual renal function as measured by urine output compared to those on thrice a week HD (n = 110). The odds ratio of residual kidney function loss for each additional HD treatment per week was 7.2 [32]. This longer time could however have resulted because the twice a week dialysis patients started with a higher urine output. Based on these observations and some physiological parameters, criteria for starting patients on twice a week dialysis with the possibility of increasing the frequency have been proposed [33]. These patients need close monitoring to ensure they continue to receive adequate dialysis. The Indian Society of Nephrology has made similar recommendations [34]. Appropriately designed and adequately powered studies, including randomized clinical trials, are needed to establish the efficacy and cost-effectiveness of this approach.
Dialyzer Reuse and Water Treatment
To save costs, dialyzer reuse is practically universal in all the developing countries of Asia [35]. The reuse practice, however, is not standardized. Untrained personnel perform dialyzer cleaning and reprocessing manually and the efficiency of reuse is not appropriately evaluated by standard measurements, with resultant effects on dialysis adequacy and outcomes.
Similarly, the quality of water treatment systems is uneven in many geographies. The quality of feed water is variable and the treatment systems are not optimized to ensure generation of a consistent supply of water that meets with acceptable quality standards. Testing frequencies are low and documentation poor. This issue is of particular relevance in countries where periodic rains and flooding lead to erosion of soil and variable contamination of surface water, which is the usual source for dialysis water.
Use of Convective Therapies
The use of convective therapies, in particular online hemodiafiltration, has been shown to be associated with improved outcomes and is gaining popularity, especially in Europe. At present, the use of convective HD is low in Asia. A survey of South-East Asian countries showed varying numbers of patients on HD, online HD and continuous ambulatory PD in different countries within the region. The number of facilities offering online hemodiafiltration ranges from 3 in Vietnam to 500 in Korea. Data from Taiwan and Japan are not available [36]. Some centers in India have started electively offering hemodiafiltration to patients who do not do well on HD. Important considerations for setting up online hemodiafiltration include ensuring availability of ultrapure water and high cost of the dialysis equipment. In view of these limitations, it is out of the reach of common ESRD patients and unlikely to gain wide acceptance in low to middle income countries.
Comorbidity Burden and Management
Patients on dialysis have multiple comorbidities which are the main determinants of survival and quality of life of these patients. Robust data on the burden of comorbidities, their management and impact on outcomes amongst dialysis patients in Asia are lacking. Emerging data from the UK [37,38] suggest that despite living in the same environment, immigrant Asian patients with chronic conditions had a poorer understanding of their illness compared to white patients. Increasing understanding of kidney disease by Asian patients and their families may be one way of improving the attitude towards and the understanding of this chronic disease. It has also been observed that elderly Asians have a higher incidence of mental illness compared to Caucasians in the UK.
Infections
Infections are important causes of morbidity, hospitalization and mortality in HD patients in Asia [39]. The annual mortality rate secondary to sepsis in these patients is 100- to 300-fold higher than that of the general population [40]. Advanced age, diabetes, malnutrition, frailty and comorbidities further increase the infection risk [41]. Because of delayed presentation, a large proportion of patients in the developing countries of Asia are started on dialysis with temporary vascular access with femoral and uncuffed internal jugular catheters, thereby incurring an increased risk of catheter-related sepsis, with significant morbidity and mortality [42].
Hepatitis Virus Infection
Despite the widespread availability of effective immunization and reduction in transfusion needs secondary to use of erythropoiesis-stimulating agents (ESAs), hepatitis virus infection remains a problem amongst the dialysis population in Asia. Suboptimal infection control practices are responsible for horizontal transmission of infection in HD units. In a study from Vietnam, the seroprevalence of HBV and HCV was 7 and 6%, respectively, and co-infection was noted in 1%. The major risk factors include multiple blood transfusions, multiple visits to different HD units and frequency of HD [43]. Many dialysis units refuse to serve patients infected with any of the hepatitis viruses.
In a recent review of data from over 200,000 dialysis patients (HD 173,788, PD 27,802) in seven Asian-Pacific countries [44], HBsAg positivity rates varied between 1.3 and 14.6%. The positivity rates amongst HD and PD patients were no different in China, Malaysia, Hong Kong and Thailand. Japan and Taiwan showed a higher prevalence of PD patients whereas the situation was reversed in Korea. HCV infection is even more prevalent in Asia, with several HCV-hyperendemic areas in Japan, the Middle East, Vietnam and Taiwan [45]. China alone has more subjects with HCV infection than all of Europe or the Americas. The prevalence of HCV infection in dialysis patients ranges between 0.7 and 18.1% [44]. Unlike HBV, the prevalence was consistently higher in HD patients (7.9%) compared to those on PD (3%). Moreover, HD patients also showed higher seroconversion rates in comparison with PD patients (0.1 vs. 0.03 per 100 patient-years at risk). The annual incidence of HCV infections ranged from 0% in Thai PD patients to 18.1% in Indian HD patients. Despite global recommendations to the contrary, some Indian experts have suggested the need to dialyze hepatitis C-infected patients in a separate area, as for hepatitis B [46]. The prevalence figures for both HBV and HCV are likely underreported because most studies have reported positivity on the basis of antibody positivity rather than nucleic acid testing. Isolated studies have shown the presence of occult infection, leading to the recommendation that nucleic acid testing rather than serologic testing be used in endemic areas [47]. The emergence of new orally active drugs has the potential to revolutionize the treatment of HCV infection. However, these drugs are either contraindicated or have nor been tested in ESRD patients. Despite this caution, sporadic off-label use of these drugs has started [48].
Tuberculosis
South and South-East Asia as well as the Western Pacific Regions reported the largest number of new tuberculosis (TB) cases in the world in 2013, accounting for 56% of new cases globally. The prevalence is particularly high in India, China and Indonesia [49,50]. The incidence of TB in CKD is 10–15 times higher than that in the general population, which increases to >50-fold amongst those on HD [51,52]. About 7–10% of patients on maintenance dialysis develop clinically overt TB. In old studies from the region, the overall mortality rate amongst ESRD patients with TB was 20–30% [51,52]. Issues of concern related to the diagnosis and management of TB in HD patients include difficulties in making a timely diagnosis of active and occult TB because of overlap in the clinical features with uremia and lack of agreement on the optimal method of detection of latent TB [53], and in managing interaction between anti-tubercular agents and several drugs that HD patients need to take. Worth mentioning are the interaction between the calcium channel blocker amlodipine and rifampicin. The latter can increase amlodipine metabolism, leading to worsening of hypertension. The reduction of adverse outcomes secondary to TB depends on a high index of suspicion, employing aggressive invasive investigations to establish the diagnosis and instituting therapy in a timely manner. Neither Mantoux testing nor the newer IFN-γ release assays have been found to be useful for identifying latent TB amongst HD patients in endemic regions [54].
Anemia Management
The prevalence of anemia amongst HD patients in many Asian countries is high because of inadequate dialysis and inadequate ESA dosing. An important issue is the lack of quality control over the generic versions of ESAs, used extensively throughout Asia because of economic reasons. The complexity of the manufacturing process for these recombinant proteins can result in changes that may affect safety. In a study from Thailand [55], out of 30 patients exhibiting a sudden loss of efficacy after treatment with biosimilar erythropoietin, 23 showed erythropoietin-neutralizing antibodies, and bone marrow biopsies showed absence of erythroblasts. Other factors contributing to worsening anemia include iron, folate and vitamin B12 deficiency in the vegetarian population. Inadequate dialysis and poor appetite further complicates the situation. A recent study from China [56] showed that daily dialysis increased hemoglobin levels, decreased erythropoietin requirements and improved physical and mental health scores.
Outcome Reporting and Survival
The Dialysis Outcomes and Practice Patterns Study (DOPPS) has documented variations in dialysis practice, HD facilities and patient outcomes across many countries. Japan is the only Asian country to feature in the DOPPS reports [57]. According to a recent review [21], five out of the seven registries in Asia were collecting mortality, survival and/or hospitalization and complication data. Table 2 shows the features of dialysis registries in Asia.
The survival of maintenance HD patients is not uniform across Asia. A study using data from the Korean registry reported a 10-year survival of >60%. In a propensity score-matched cohort, their survival was significantly better compared to that of PD patients [58]. According to the 2003 DOPPS report, Japanese patients on dialysis enjoyed the longest survival compared to patients in other parts of the world, with 1-year crude mortality rates of 6.6% [59]. About 91% of patients commencing dialysis under the age of 70 were still alive after 12 months of treatment [60]. Data from the Taiwan registry showed a first-year mortality of 15% and a 5-year survival of 54% in those who were started on dialysis between 1990 and 2001. Interestingly, the 5-year survival amongst those started on dialysis in the 2000–2009 period declined to 44% [61]. The reasons for this reduction in survival were unclear. According to the most recent report of the Malaysian Dialysis and Transplant Registry [62], the annual death rates amongst patients on HD were between 11 and 13% in a 10-year period starting 2004. During the same period, the annual mortality rate was 14–18% amongst PD patients. As seen in other parts of the world, cardiovascular disease was the commonest causes of death, and mortality was higher amongst diabetics.
The obesity paradox on survival of maintenance HD patients stands true in Asia as well [63]. In a cohort of 20,818 Korean patients on HD as compared to 10,000 American and European patients each, higher BMI was associated with greater survival.
Outcome reporting systems remain disappointing, however, throughout South Asia. Single-center reports suggest high mortality and dropout rates, to the tune of 66% within 3 months. In a study from India comparing the outcomes of 558 dialysis patients belonging to different socioeconomic groups, the mortality rate was 38.1% in those with low as compared to 4.2% in those with high socioeconomic status [64]. More recently, efforts are being made in the region to develop reporting systems through the use of modern information technology systems [65]. The reported 1-year survival rates are 70% in Myanmar and about 90% in the Philippines [36].
Future Directions
The HD landscape in Asia offers evolving, interesting and contrasting challenges. On the one hand, countries with very large numbers of patients on HD are investigating mechanisms that will reduce or reverse the growth of those in need for dialysis. Preventive programs to slow the progression of CKD in order to reduce the burden on healthcare systems have been instituted in Taiwan, Japan and Korea, with varying degrees of success. On the other hand, with economic prosperity and easier access to information, in particular from online resources, an increasing proportion of the aspirational citizens in the developing countries of Asia expect access to RRT. This change in healthcare seeking behavior has led to a dramatic increase in demand for dialysis. Of particular concern are the quality of dialysis and outcomes in new standalone dialysis centers. Market forces have fueled the growth in the number of dialysis units, especially in small towns, where the healthcare regulatory mechanisms are relatively lax [15]. Several of these centers operate in a laissez-faire manner, without access to a trained nephrologist and with suboptimal essential services like water treatment system, infection control measures or ancillary services. Patient scheduling, dialysis prescription and treatment times are decided arbitrarily. Sessions are cut short to accommodate extra patients. Such practices have a large-scale adverse impact on outcomes. Inevitably, once the initial aspiration of gaining access to therapy is met, there will be demand for providing good-quality HD. Innovative models of operation of dialysis center are being investigated. As in the Western countries, chains of dialysis centers are being set up. Such a consolidation has several potential advantages: business and financial advantages like volume-based and technical efficiency, improved information and tracking systems, access to affordable capital, vertical service-product integration, professional management, improved compliance with processes and protocols, and structured accountability as well as clinical advantages such as standardization of care, robust quality improvement and integrated information reporting systems.
The other major challenge is the shortage of trained manpower, nephrologists, dialysis technicians, nurses, vascular access surgeons and support staff. Emigration of trained personnel from poor countries to Europe, North America and Australia further worsens the shortage. Innovative schemes are required to fund treatment costs. Microfinancing, which depends upon regular small contributions, has been started in parts of India.
The lack of access to HD leading to premature deaths needs an urgent and multipronged approach, starting with documentation of magnitude, advocacy, healthcare system reforms and research. Effective approaches are needed for the prevention of ESRD using innovative models of care. The success of such strategies is becoming evident in Taiwan. Finally, cost-effective dialysis techniques should be developed. In partnership with the International Society of Nephrology and the Asia-Pacific Society of Nephrology, the George Institute for Global Health has announced a competition for an affordable HD machine, with a USD 100,000 prize for the winner (http://www.georgeinstitute.org/projects/dialysis-competition).
In conclusion, the study of HD in Asia presents a picture of contrasts, with high prevalence and universal access in some countries, but limitation because of high cost and poor healthcare systems in others. The population of patients needing HD is projected to grow rapidly in the coming years. Most countries are developing models to expand access of their populations to HD. Innovative modifications in dialysis practice are being made to optimize outcomes relative to cost. It is important to develop robust systems of documentation and outcome reporting to evaluate the effects of such changes. HD needs to develop in conjunction with effective preventive programs and improvement of health systems.
Disclosure Statement
The authors state no conflicts of interest.
References
- 1.Asia Population 2015 - World Population Review. Available at http://worldpopulationreview.com/continents/asia-population/ (accessed October 2, 2015).
- 2.Jones CA, Krolewski AS, Rogus J, et al. Epidemic of end-stage renal disease in people with diabetes in the United States population: do we know the cause? Kidney Int. 2005;67:1684–1691. doi: 10.1111/j.1523-1755.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 3.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–340. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 4.Gooneratne IK, Ranaweera AK, Liyanarachchi NP, et al. Epidemiology of chronic kidney disease in a Sri Lankan population. Int J Diabetes Dev Ctries. 2008;28:60–64. doi: 10.4103/0973-3930.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajapurkar MM, John GT, Kirpalani AL, et al. What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrol. 2012;13:10. doi: 10.1186/1471-2369-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chugh KS, Jha V, Chugh S. Economics of dialysis and renal transplantation in the developing world. Transplant Proc. 1999;31:3275–3277. doi: 10.1016/s0041-1345(99)00722-8. [DOI] [PubMed] [Google Scholar]
- 8.Jha V. End-stage renal care in developing countries: the India experience. Ren Fail. 2004;26:201–208. doi: 10.1081/jdi-120039516. [DOI] [PubMed] [Google Scholar]
- 9.Jha V. Current status of chronic kidney disease care in southeast Asia. Semin Nephrol. 2009;29:487–496. doi: 10.1016/j.semnephrol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Lee G. End-stage renal disease in the Asian-Pacific region. Semin Nephrol. 2003;23:107–114. doi: 10.1053/snep.2003.50009. [DOI] [PubMed] [Google Scholar]
- 11.Wu AY, Kong NC, de Leon FA, et al. An alarmingly high prevalence of diabetic nephropathy in Asian type 2 diabetic patients: the MicroAlbuminuria Prevalence (MAP) Study. Diabetologia. 2005;48:17–26. doi: 10.1007/s00125-004-1599-9. [DOI] [PubMed] [Google Scholar]
- 12.Gross A.India's dialysis market Published on July 10, 2013 in Medical Device Daily by Pacific Bridge Medical. Available at http://www.pacificbridgemedical.com/publications/high-rates-of-chronic-kidney-disease-lead-to-medtech-opportunities-in-india/ (accessed October 2, 2015).
- 13.Agarwal SK, Dash SC, Irshad M, et al. Prevalence of chronic renal failure in adults in Delhi, India. Nephrol Dial Transplant. 2005;20:1638–1642. doi: 10.1093/ndt/gfh855. [DOI] [PubMed] [Google Scholar]
- 14.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 15.Jha V. Current status of end-stage renal disease care in India and Pakistan. Kidney Int Suppl (2011) 2013;3:157–160. [Google Scholar]
- 16.Singh A, Prakash G. Public-private partnerships in health services delivery. Public Manage Rev. 2010;12:829–856. [Google Scholar]
- 17.Chakraborty D.The private sector's role in achieving universal health coverage in India Available at http://www.emhn.org/sites/default/files/Chakraborty_PPPs_EMHN.pdf
- 18.De Vecchi AF, Dratwa M, Wiedemann ME. Healthcare systems and end-stage renal disease (ESRD) therapies - an international review: costs and reimbursement/funding of ESRD therapies. Nephrol Dial Transplant. 1999;14(suppl 6):31–41. doi: 10.1093/ndt/14.suppl_6.31. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran R, Jha V. Kidney transplantation is associated with catastrophic out of pocket expenditure in India. PLoS One. 2013;8:e67812. doi: 10.1371/journal.pone.0067812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jha V, Chugh KS. The practice of dialysis in the developing countries. Hemodial Int. 2003;7:239–249. doi: 10.1046/j.1492-7535.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 21.Liu FX, Rutherford P, Smoyer-Tomic K, et al. A global overview of renal registries: a systematic review. BMC Nephrol. 2015;16:31. doi: 10.1186/s12882-015-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 23.Tantivess S, Werayingyong P, Chuengsaman P, et al. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ. 2013;346:f462. doi: 10.1136/bmj.f462. [DOI] [PubMed] [Google Scholar]
- 24.Khanna U. The economics of dialysis in India. Indian J Nephrol. 2009;19:1–4. doi: 10.4103/0971-4065.50671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Central Intelligence Agency The World Factbook. South Asia: India Available at https://www.cia.gov/library/publications/the-world-factbook/geos/in.html (accessed October 2, 2015).
- 26.Mani MK. The management of end-stage renal disease in India. Artif Organs. 1998;22:182–186. doi: 10.1046/j.1525-1594.1998.06070.x. [DOI] [PubMed] [Google Scholar]
- 27.Morad Z, Lee DG, Lim YN, et al. Peritoneal dialysis in Malaysia. Perit Dial Int. 2005;25:426–431. [PubMed] [Google Scholar]
- 28.Parameswaran S, Geda SB, Rathi M, et al. Referral pattern of patients with end-stage renal disease at a public sector hospital and its impact on outcome. Natl Med J India. 2011;24:208–213. [PubMed] [Google Scholar]
- 29.Rao M, Juneja R, Shirly RB, et al. Haemodialysis for end-stage renal disease in Southern India - a perspective from a tertiary referral care centre. Nephrol Dial Transplant. 1998;13:2494–2500. doi: 10.1093/ndt/13.10.2494. [DOI] [PubMed] [Google Scholar]
- 30.Chugh KS, Jha V. Differences in the care of ESRD patients worldwide: required resources and future outlook. Kidney Int Suppl. 1995;50:S7–S13. [PubMed] [Google Scholar]
- 31.Lin YF, Huang JW, Wu MS, et al. Comparison of residual renal function in patients undergoing twice-weekly versus three-times-weekly haemodialysis. Nephrology. 2009;14:59–64. doi: 10.1111/j.1440-1797.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Wang M, Li H, et al. Association of initial twice-weekly hemodialysis treatment with preservation of residual kidney function in ESRD patients. Am J Nephrol. 2014;40:140–150. doi: 10.1159/000365819. [DOI] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Unruh M, Zager PG, et al. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64:181–186. doi: 10.1053/j.ajkd.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jha V.The Indian Society of Nephrology Guidelines for hemodialysis units Available at http://www.theisn.org/education/education-topics/hemodialysis/item/948-a-commentary-on-the-indian-society-of-nephrology-guidelines-for-hemodialysis-units
- 35.Dhrolia MF, Nasir K, Imtiaz S, et al. Dialyzer reuse: justified cost saving for South Asian region. J Coll Physicians Surg Pak. 2014;24:591–596. [PubMed] [Google Scholar]
- 36.Naramura T, Hyodo T, Kokubo K, et al. Dialysis and quality of dialysate in Southeast Asian developing countries. Nephron Extra. 2014;4:64–69. doi: 10.1159/000362454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson E, Randhawa G. An examination of concordance and cultural competency in the diabetes care pathway: South Asians living in the United Kingdom. Indian J Nephrol. 2012;22:424–430. doi: 10.4103/0971-4065.106033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson E, Randhawa G, Farrington K, et al. Lack of awareness of kidney complications despite familiarity with diabetes: a multi-ethnic qualitative study. J Ren Care. 2011;37:2–11. doi: 10.1111/j.1755-6686.2011.00199.x. [DOI] [PubMed] [Google Scholar]
- 39.Jha V, Chugh S, Chugh KS. Infections in dialysis and transplant patients in tropical countries. Kidney Int. 2000;57:85–93. [Google Scholar]
- 40.Gomez AT, Kiberd BA, Royston JP, et al. Comorbidity burden at dialysis initiation and mortality: a cohort study. Can J Kidney Health Dis. 2015;2:34. doi: 10.1186/s40697-015-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Laupland KB, Gregson DB, Zygun DA, et al. Severe bloodstream infections: a population-based assessment. Crit Care Med. 2004;32:992–997. doi: 10.1097/01.ccm.0000119424.31648.1e. [DOI] [PubMed] [Google Scholar]
- 42.Kosa SD, Lok CE. The economics of hemodialysis catheter-related infection prophylaxis. Semin Dial. 2013;26:482–493. doi: 10.1111/sdi.12115. [DOI] [PubMed] [Google Scholar]
- 43.Duong CM, Olszyna DP, McLaws ML. Hepatitis B and C virus infections among patients with end stage renal disease in a low-resourced hemodialysis center in Vietnam: a cross-sectional study. BMC Public Health. 2015;15:192. doi: 10.1186/s12889-015-1532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson DW, Dent H, Yao Q, et al. Frequencies of hepatitis B and C infections among haemodialysis and peritoneal dialysis patients in Asia-Pacific countries: analysis of registry data. Nephrol Dial Transplant. 2009;24:1598–1603. doi: 10.1093/ndt/gfn684. [DOI] [PubMed] [Google Scholar]
- 45.Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol. 2009;24:336–345. doi: 10.1111/j.1440-1746.2009.05789.x. [DOI] [PubMed] [Google Scholar]
- 46.Agarwal SK. Hemodialysis of patients with HCV infection: isolation has a definite role. Nephron Clin Pract. 2011;117:c328–c332. doi: 10.1159/000319984. [DOI] [PubMed] [Google Scholar]
- 47.Potsangbam G, Yadav A, Chandel N, et al. Challenges in containing the burden of hepatitis B infection in dialysis and transplant patients in India. Nephrology. 2011;16:383–388. doi: 10.1111/j.1440-1797.2010.01429.x. [DOI] [PubMed] [Google Scholar]
- 48.Fabrizi F, Messa P. Therapy of hepatitis C by direct-acting anti-virals: the end of HCV in dialysis population? Expert Rev Clin Pharmacol. 2015;8:785–793. doi: 10.1586/17512433.2015.1086266. [DOI] [PubMed] [Google Scholar]
- 49.Goodchild M, Sahu S, Wares F, et al. A cost-benefit analysis of scaling up tuberculosis control in India. Int J Tuberc Lung Dis. 2011;15:358–362. [PubMed] [Google Scholar]
- 50.World Health Organization . Global tuberculosis report 2014. Geneva: WHO; 2014. [Google Scholar]
- 51.Costa JM, Meyers AM, Botha JR, et al. Mycobacterial infections in recipients of kidney allografts. A seventeen-year experience. Acta Med Port. 1988;1:51–57. [PubMed] [Google Scholar]
- 52.Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis. 1998;27:1266–1277. doi: 10.1086/514993. [DOI] [PubMed] [Google Scholar]
- 53.Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shankar MS, Aravindan AN, Sohal PM, et al. The prevalence of tuberculin sensitivity and anergy in chronic renal failure in an endemic area: tuberculin test and the risk of post-transplant tuberculosis. Nephrol Dial Transplant. 2005;20:2720–2724. doi: 10.1093/ndt/gfi141. [DOI] [PubMed] [Google Scholar]
- 55.Praditpornsilpa K, Tiranathanagul K, Kupatawintu P, et al. Biosimilar recombinant human erythropoietin induces the production of neutralizing antibodies. Kidney Int. 2011;80:88–92. doi: 10.1038/ki.2011.68. [DOI] [PubMed] [Google Scholar]
- 56.Jiang JL, Ren W, Song J, et al. The impact of short daily hemodialysis on anemia and the quality of life in Chinese patients. Braz J Med Biol Res. 2013;46:629–633. doi: 10.1590/1414-431X20132832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisoni RL, Greenwood RN. Selected lessons learned from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Contrib Nephrol. 2005;149:58–68. doi: 10.1159/000085458. [DOI] [PubMed] [Google Scholar]
- 58.Kim H, Kim KH, Park K, et al. A population-based approach indicates an overall higher patient mortality with peritoneal dialysis compared to hemodialysis in Korea. Kidney Int. 2014;86:991–1000. doi: 10.1038/ki.2014.163. [DOI] [PubMed] [Google Scholar]
- 59.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 60.Masakane I. High-quality dialysis: a lesson from the Japanese experience. Effects of membrane material on nutritional status and dialysis-related symptoms. Clin Kidney J. 2010;3:i28–i35. doi: 10.1093/ndtplus/sfq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu MS, Wu IW, Hsu KH. Survival analysis of Taiwan Renal Registry Data System (TWRDS) 2000-2009. Acta Nephrologica. 2012;26:104–108. [Google Scholar]
- 62.Malaysian Society of Nephrology 21th Report of the Malaysian Dialysis and Transplant Registry 2013 Available at http://www.msn.org.my/fwbPagePublic.jsp?fwbPageId=pMdtr2013 (accessed October 2, 2015).
- 63.Park J, Jin DC, Molnar MZ, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clin Proc. 2013;88:479–486. doi: 10.1016/j.mayocp.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abraham G, Jayaseelan T, Matthew M, et al. Resource settings have a major influence on the outcome of maintenance hemodialysis patients in South India. Hemodial Int. 2010;14:211–217. doi: 10.1111/j.1542-4758.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- 65.Jha V, John O, Joshi R, et al. Dialysis outcomes in India: a pilot study. Nephrology. 2015;20:329–334. doi: 10.1111/nep.12404. [DOI] [PubMed] [Google Scholar]