Abstract
Background
Antineutrophil cytoplasmic autoantibodies (ANCA) are associated with a spectrum of necrotizing vasculitis including granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, and renal-limited necrotizing and crescentic glomerulonephritis. Clinical observations and in vitro and in vivo experimental evidence strongly indicate that ANCA are pathogenic.
Summary
The etiology and pathogenesis of ANCA-associated vasculitis (AAV) are multifactorial, with contributions from genetic factors, environmental exposures, infections, characteristics of the innate and adaptive immune system, and the intensity and duration of the injury. Acute vascular inflammation is induced when resting neutrophils that have ANCA autoantigens sequestered in cytoplasmic granules are exposed to priming factors - for example, cytokines induced by infection or phlogogenic factors released by complement activation - that cause the release of ANCA antigens on the surface of neutrophils and in the microenvironment around the neutrophils. ANCA bind to these ANCA antigens, which activates neutrophils by Fcγ receptor engagement and F(ab′)2 binding at the neutrophil cell surface. ANCA-activated neutrophils release factors that activate the alternative complement pathway, which generates C5a, a chemoattractant for neutrophils; C5a also primes the arriving neutrophils for activation by ANCA. Activated neutrophils adhere to and penetrate vessel walls, and they release toxic oxygen radicals and destructive enzymes that cause apoptosis and necrosis of the neutrophils as well as of the adjacent vessel wall cells and matrix.
Key Messages
Patients with active AAV have ongoing asynchronous onsets of countless acute lesions, with each lesion evolving through stereotypical phases within 1 or 2 weeks. Induction of remission results in termination of new waves of acute lesions and allows all lesions to progress to scarring or resolution.
Key Words: ANCA, Antineutrophil cytoplasmic autoantibodies, Autoantibodies, Polyangiitis, Vasculitis
Introduction
Antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (AAV) is a category of vasculitis and predominantly involves small vessels (arteries, arterioles, venules, and veins) in any organ of the body [1,2]. AAV pathogenic mechanisms should explain the pathologic and clinical manifestations of AAV. Pathologically, in the acute phase AAV is characterized by necrotizing vasculitis with infiltrating neutrophils and monocytes (fig. 1) [3]. Within days, the neutrophils undergo leukocytoclasis and disappear, to be replaced by mononuclear leukocytes including macrophages, monocytes, and T lymphocytes. Immunopathologically, AAV is characterized by the absence or a paucity of immunoglobulin deposition in vessel walls, which indicates that the pathogenesis of AAV is different from the pathogenesis of vasculitis, which has extensive vessel wall deposition of immunoglobulin, indicative of immune complex vasculitis or antiglomerular basement membrane disease [1]. AAV has different clinicopathologic phenotypes, including granulomatosis with polyangiitis (GPA; formerly known as Wegener's granulomatosis), microscopic polyangiitis (MPA), eosinophilic GPA (EGPA; formerly known as Churg-Strauss syndrome), and renal-limited necrotizing and crescentic glomerulonephritis (NCGN) [1]. The approximately 10% of AAV patients who are ANCA negative have clinical and pathologic manifestations similar to those of ANCA-positive patients [4], and thus are likely to have a similar if not identical final pathogenic pathway leading to vasculitis.
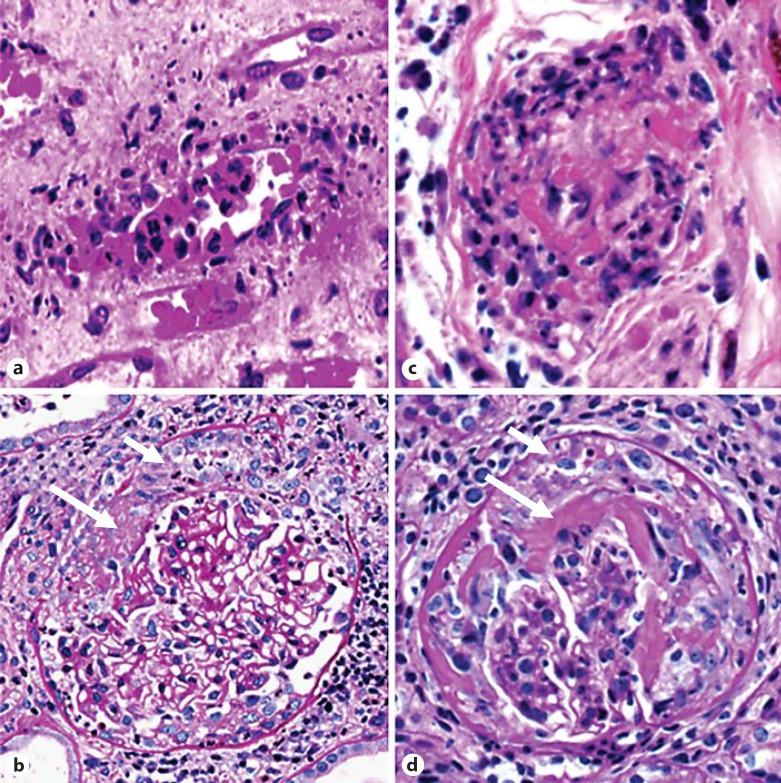
Fig. 1.
Pathology of AAV. a, c Acute leukocytoclastic angiitis in a patient with AAV (a) and a mouse with a model of AAV induced by the injection of anti-MPO IgG (c). In both a and c, a small vessel has mural and perivascular influx of leukocytes, including numerous neutrophils, some of which are undergoing leukocytoclasis. b, d NCGN in a patient with AAV (b) and in a mouse with a model of AAV induced by the injection of anti-MPO IgG (d). Note the fibrin in Bowman's space (long arrows) and the cellular crescent (short arrows).
The two major target antigens for ANCA in patients with vasculitis are myeloperoxidase (MPO) [5] and proteinase 3 (PR3) [6] in the granules of neutrophils and lysosomes of monocytes. Another autoantigen target that has been reported is lysosomal-associated membrane protein 2 (LAMP2) [7], but the frequency of this autoantibody in AAV is not fully understood [8]. The specificity of ANCA for MPO versus PR3 correlates with the spectrum of pathologic and clinical features that is most likely to be found in a given patient [9], with MPO-ANCA patients having more MPA features and PR3-ANCA patients having more GPA features. A genome-wide association study (GWAS) showed that genetic characteristics, including major histocompatibility complex traits, correlated better with ANCA autoantigen specificity than with different kinds of clinicopathologic expression of AAV (such as MPA vs. GPA) [10], which is not surprising given the link between the peptide specificity of human leukocyte antigen (HLA) molecules that present antigens and the resulting antigen specificity of T-cell receptors and immunoglobulin antigen-binding domains.
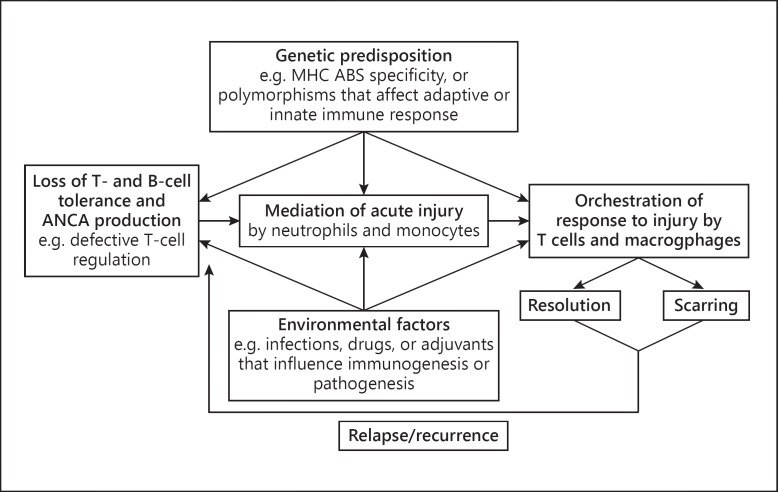
As with most autoimmune diseases, the etiology and pathogenesis of AAV are undoubtedly multifactorial, with contributions from genetic factors, environmental exposures, infections, characteristics of the innate and adaptive immune system, and the intensity and duration of the injury [11]. Among different patients, differing etiologic and synergistic factors may lead to AAV, which may influence the differences in clinicopathologic phenotypes among patients. The pathogenesis of AAV follows the general pattern of other autoimmune inflammatory diseases in that there is a beginning, in which the aberrant pathogenic autoimmune response is generated; an active injury phase, mediated by effector leukocytes that, in the case of AAV, are predominantly neutrophils, and a response-to-injury phase, which, in AAV, predominantly involves monocytes, macrophages, and T lymphocytes (fig. 2). If the acute injury is mild enough, it will resolve completely (e.g. complete disappearance of a purpuric rash); however, if full resolution is not possible, the outcome is often scarring, which may result in residual dysfunction (e.g. necrotizing glomerular injury leading to glomerular scarring with residual renal insufficiency and proteinuria). It is important to recognize that, based on observations in patients and animal models, patients with active AAV have ongoing asynchronous onsets of countless acute lesions occurring in multiple organs, with each lesion evolving through the stereotypical phases from acute to chronic, probably over a span of only 1 or 2 weeks. Therapeutic induction of remission results in termination of new waves of acute lesions and allows all lesions to progress to scarring or resolution. Relapse entails the initiation of new waves of acute lesions.
Fig. 2.
Diagram depicting the multifactorial influences on the pathogenesis of AAV. Reproduced with slight changes from Jennette and Falk [11] with permission. MCH = Major histocompatibility complex; ABS = antigen-binding site.
The pathogenic mechanism that will be reviewed in this article describes a sequence of events that does not simply happen once during the course of AAV, but rather continues to occur at multiple sites as long as there is active AAV. Thus, if a patient continues to have active disease, new lesions will appear in the initial acute phase at all times until remission is induced.
Clinical Evidence Supporting the Pathogenicity of ANCA
A number of clinical observations support the pathogenicity of ANCA. Bansal and Tobin [12] reported a case of transplacental passage of MPO-ANCA from a mother with MPA to a neonate that resulted in neonatal pulmonary hemorrhage and renal disease within days after birth. This report provides direct clinical evidence for the pathogenic role of MPO-ANCA in humans; however, no additional examples of this phenomenon have been reported, and the delivery of a healthy newborn despite transplacental transfer of MPO-ANCA from a mother with MPA was described [13].
ANCA small vessel vasculitis is characterized by vascular inflammation that is highly correlated with the presence of ANCA in the circulation of patients. Either MPO-ANCA or PR3-ANCA are present in 90% of patients with GPA, MPA, EGPA, and renal-limited NCGN [4]. In general, high levels of ANCA are present in patients with active disease, and the titers fall during therapy; however, this is not an absolute correlation using current clinical assays for ANCA. Epitope-specific assays for ANCA that can identify autoantibodies which are particularly pathogenic may show a better correlation between ANCA titers and disease activity [14].
Another observation in humans that supports a pathogenic role of ANCA is the close association of development of ANCA disease with specific drug treatment, including propylthiouracil, minocycline, pimagedine, hydralazine, and levamisole-adulterated cocaine [15,16]. Renal remission typically occurs in these patients after drug withdrawal, immunosuppressive treatment, and decline in ANCA [15,16,17].
Targeted therapies that reduce autoantibodies and deplete B cells are effective treatments in AAV, supporting a pathogenic role for ANCA. For example, patients who had undergone removal of circulating ANCA by plasma exchange had a lower risk for progression to end-stage renal disease at 1 year than patients who had not received a plasma exchange [18]. Further, depleting B cells - for example, with anti-CD20 antibodies - is effective in the induction of remission and maintenance of remission [19]. This beneficial effect of targeted antibody and B-cell treatments in AAV patients supports a pathogenic role for circulating ANCA.
In vitro Evidence Supporting and Elucidating the Pathogenicity of ANCA
Numerous in vitro studies have demonstrated that ANCA activate both neutrophils and monocytes. Incubation of MPO-ANCA or PR3-ANCA with normal human neutrophils that have been primed by proinflammatory stimuli such as tumor necrosis factor (TNF)-α, bacterial lipopolysaccharide (LPS), or the complement anaphylatoxin C5a induces a respiratory burst that generates toxic oxygen free radicals and degranulation, which in turn release numerous destructive enzymes to cause injury to and death of cultured endothelial cells [20,21,22,23,24,25,26,27,28,29,30].
ANCA are predominantly IgG class autoantibodies that can engage Fcγ receptors (FcγR) [23] and can bind to ANCA antigens (such as MPO and PR3) at the surface of primed neutrophils [26], resulting in neutrophil activation [20]. Engagement of FcγR appears to be the major driver of activation. The priming of neutrophils causes autoantigen translocation to the surface of cells, so that F(ab′)2 of ANCA can interact with the autoantigens [26,28]. Phosphorylation of p38 mitogen-activated protein kinase and the extracellular signal-regulated kinase are involved in neutrophil priming and activation, and blockade of mitogen-activated protein kinase prevents this priming and activation [27,31,32]. Simvastatin, a 3-hydroxy-3-methylglutaryl-coenzyme A inhibitor, is capable of inhibiting the ANCA-induced degranulation of neutrophils [33]. These in vitro finding support a pathogenic role for ANCA and also suggest potential novel therapeutic options for ANCA disease.
In addition to neutrophils, monocytes contain the ANCA antigens MPO and PR3, and can be activated in vitro by ANCA similarly to neutrophils, with a resultant production of proinflammatory cytokines including monocyte chemoattractant protein 1 (MCP-1, also known as CCL2) and IL-8 [34,35,36]. IL-8 can attract and activate neutrophils and thus could amplify neutrophil-mediated injury. MCP-1 attracts monocytes and macrophages and could participate in the transition of ANCA-induced vascular injury from predominantly neutrophil-rich inflammation to predominantly monocyte- and/or macrophage-rich inflammation, including granulomatous inflammation [36]. Monocytes are conspicuous at sites of ANCA vascular inflammation as early as the first 1 or 2 days, and there is evidence for systemic activation of monocytes in patients with active ANCA-associated disease [37,38,39].
Animal Model Evidence Supporting and Elucidating the Pathogenicity of ANCA
The most convincing evidence that autoantibodies with specificity for recognized ANCA autoantigens are pathogenic comes from animal models of AAV. Several animal models of disease caused by ANCA have been described [40]. The first described, and most extensively studied, is a mouse model developed by Xiao et al. [41]. MPO knockout mice were immunized with murine MPO to produce high titers of anti-murine MPO antibodies. Intravenous injection of the purified anti-MPO IgG into wild-type or immunodeficient Rag2-/- mice (lacking immune-competent B and T cells) induced pauci-immune NCGN and systemic small vessel vasculitis which closely mimic human ANCA disease (fig. 1) [41]. Depending on modulating factors such as infection or modification of innate immune capabilities, mice can be induced to have more or less pulmonary alveolar hemorrhagic capillaritis or necrotizing granulomatosis [unpubl. observations]. Thus, anti-MPO antibodies alone, in the absence of functional T cells, are sufficient to cause acute lesions virtually identical to human AAV. As will be discussed later, T cells are important in the initial induction and maturation of the autoimmune ANCA response, in the amplification of acute injury, and in the chronic response to acute injury.
Another mouse model was created by transplanting wild-type bone marrow cells with MPO into irradiated MPO knockout mice that had been immunized with murine MPO and had high titers of anti-MPO antibodies. Once the transplanted MPO-positive neutrophils appeared in the circulation, the mice developed glomerulonephritis, whereas control mice that received bone marrow cells from MPO knockout mice did not, demonstrating that induction of glomerulonephritis required both anti-MPO antibodies and MPO-positive neutrophils in the circulation [42].
A rat model has also been reported that supports a pathogenic role for ANCA [43,44]. Rats immunized with human MPO develop anti-MPO antibodies that cross-react with rat MPO. These rats developed NCGN and pulmonary vasculitis.
No convincing models of PR3-AAV have been widely accepted, although several models have been proposed [45,46,47,48]. Because these models use mice, one possible explanation for the failure to produce a model of PR3-ANCA disease is that the low level of PR3 in circulating mouse neutrophils is not sufficient to mediate activation by anti-PR3 antibodies.
Neutrophils as Effector Cells in AAV Pathogenesis
Clinical evidence and in vitro studies suggest that neutrophils are important effector cells in the pathogenesis of human AAV. In renal biopsies from patients, activated neutrophils are present in affected glomeruli, and the number of activated intraglomerular neutrophils correlates with the severity of renal injury as reflected in serum creatinine levels [49]. In vitro, ANCA can activate cytokine-primed neutrophils, causing an oxidative burst, degranulation, release of inflammatory cytokines, and damage to endothelial cells [20,21,22,23,24,25,26,27,28,29].
To support the hypothesis that neutrophils are key effector cells in the pathogenesis of MPO-ANCA-mediated NCGN in the experimental model, wild-type mice were depleted of circulating neutrophils by injection of anti-neutrophil monoclonal antibody (NIMP-R14) and then injected with pathogenic anti-MPO IgG. These mice were completely protected from anti-MPO IgG-induced NCGN, while all control mice developed NCGN [50]. These experiments provide direct evidence that neutrophils play a major role in the pathogenesis of anti-MPO-induced NCGN in this animal model and implicate neutrophils in the induction of human ANCA disease. This raises the possibility that therapeutic strategies for reducing circulating neutrophils could be beneficial to patients with ANCA-induced NCGN. This finding does not exclude the possibility that monocytes contribute to lesion induction or progression, but, at least in this model, monocytes are not sufficient to cause the acute necrotizing lesions.
Cytokines and Chemokines in AAV Pathogenesis
Clinical and experimental evidence indicates that proinflammatory stimuli, for example induced by infections, can be a synergistic factor in the onset, recurrence, and exacerbation of AAV. AAV occurs and relapses more often in winter and spring, when there is a higher incidence of infections [51]. In vitro, anti-MPO IgG induces a respiratory burst of murine neutrophils more effectively after priming with proinflammatory stimuli such as TNF-α, LPS, or C5a [20,30,32,52]. To test the synergistic effect of proinflammatory factors in the anti-MPO IgG-induced NCGN mouse model, wild-type mice were injected with bacterial LPS as a proinflammatory stimulus along with anti-MPO antibodies [52]. Compared to control mice that received anti-MPO without LPS, mice that receive anti-MPO and LPS developed more severe anti-MPO-induced NCGN and had increased levels of circulating TNF. Anti-TNF treatment attenuated the LPS-mediated aggravation of anti-MPO IgG-induced glomerulonephritis.
Complement in ANCA Pathogenesis
Until animal model experiments demonstrated a major role for complement in the pathogenesis of AAV, complement activation had not been suspected as a major pathogenic factor because of the relative paucity of complement component deposition at sites of vascular inflammation and glomerulonephritis in AAV compared to the more extensive localization of complement at sites of inflammation induced by recognized forms of immune complex-mediated inflammation that were known to involve extensive complement activation. The mouse model of anti-MPO IgG-induced NCGN identified an unsuspected role of complement activation [53] that has subsequently been supported by observations in patients [54,55,56,57,58,59]. The NCGN induced by transfer of anti-MPO IgG into wild-type mice or anti-MPO splenocytes into immunodeficient (Rag2-/-) mice could be completely blocked by complement depletion using cobra venom factor [53]. This finding was confirmed by the failure of C5 knockout mice to develop NCGN after injection of anti-MPO antibodies. The role of specific complement activation pathways was investigated using mice with knockout of classic and lectin-binding pathway component C4 and alternative pathway component factor B. After injection of anti-MPO IgG, C4 knockout mice developed disease comparable to that in wild-type mice; however, factor B knockout mice developed no disease. These observations demonstrated that the alternative complement activation pathway plays a critical pathogenic role in ANCA-induced NCGN, and that the classic and lectin pathways are not required [53].
A number of subsequent observations in AAV patients support a role for complement activation in pathogenesis, including evidence for complement activation at tissue sites of vascular inflammation and detection of biomarkers for complement activation in plasma and urine [54,55,56,57,58,59]. For example, the complement membrane attack complex C3d and factor B are detected in the lesion sites of patients with AAV [54]. Clinical studies have reported that plasma levels of C3a, C5a, soluble C5b-9, and factor Bb were higher in active AAV than in AAV in remission [56]; that levels of plasma complement factor H, a regulator of the alternative complement activation pathway, were significantly lower in active AAV patients compared with AAV patients in remission and normal controls, and also that plasma factor H levels were inversely correlated with circulating levels of C3a, C5a, and Sc5b-9, and positively correlated with circulating levels of C3 [59].
Based on animal model studies [30,53], the activation of C5 and engagement of C5a receptor (C5aR) on neutrophils should be critical events in the induction of ANCA disease. To test this hypothesis, mice deficient in C5aR were injected with anti-MPO IgG. Protection against anti-MPO-induced NCGN was observed in these mice [60]. Moreover, transgenic mice with mouse C5aR replaced by human C5aR (C5aR/CD88) that were treated with a small-molecule antagonist of human C5aR/CD88 (CCX168) showed a significant amelioration of anti-MPO-induced NCGN [60]. These observations demonstrate that C5a engagement of C5aR has a critical role in the pathogenesis, and that blockade of C5aR/CD88 might have a therapeutic benefit for patients with AAV and glomerulonephritis [60]. A clinical trial is currently underway to assess the safety and efficacy of CCX168 in patients with AAV.
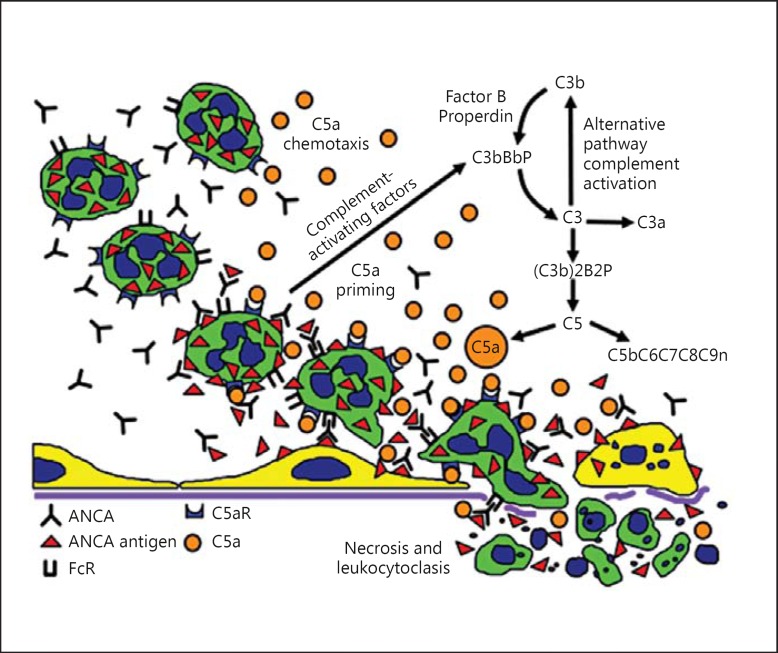
The in vitro and in vivo studies summarized thus far in this review support the pathogenic sequence for the acute vascular lesion of AAV that is illustrated in figure 2[61]. Beginning in the upper left of the illustration, resting neutrophils have the target ANCA autoantigen sequestered in cytoplasmic granules. Exposure to priming factors, for example cytokines induced by a synergistic infection or phlogogenic factors released by complement activation, causes priming of neutrophils with release of ANCA antigens (e.g. MPO and PR3) on the surface of the neutrophils and in the microenvironment around the neutrophils [20,22,52]. If ANCA are present in the plasma, they bind to ANCA antigens and activate neutrophils by FcγR engagement and F(ab′)2 binding at the neutrophil cell surface [23,25,26]. ANCA-activated neutrophils release factors that activate the alternative complement pathway [53,60], which generates C5a, a chemoattractant for the accumulation of more neutrophils at the site of activation. C5a also primes the arriving neutrophils for activation by ANCA [30,60]. The activated neutrophils adhere to and penetrate vessel walls, and they release toxic oxygen radicals and destructive enzymes that cause apoptosis and necrosis of the neutrophils as well as of the adjacent vessel wall cells and matrix (depicted at the right of fig. 3) [21,22,24,29].
Fig. 3.
Putative pathogenic sequence for acute vascular injury in AAV. Beginning in the upper left, resting neutrophils have ANCA autoantigens sequestered in the cytoplasmic granules. Exposure to priming factors, for example cytokines induced by infection or phlogogenic factors released by complement activation, causes priming of neutrophils with release of ANCA antigens (e.g. MPO and PR3) on the surface of neutrophils and in the microenvironment around the neutrophils. ANCA bind to ANCA antigens and activate neutrophils by FcγR engagement and F(ab′)2 binding at the neutrophil cell surface. ANCA-activated neutrophils release factors that activate the alternative complement pathway, which generates C5a, a chemoattractant for neutrophils. C5a also primes the arriving neutrophils for activation by ANCA. Activated neutrophils adhere to and penetrate vessel walls, and they release toxic oxygen radicals and destructive enzymes that cause apoptosis and necrosis of the neutrophils as well as of the adjacent vessel wall cells and matrix. Modified from Jennette et al. [61] with permission.
The same general pathogenic process could cause the initial lesion of the extravascular necrotizing granulomatosis of GPA and EGPA [11]. A synergistic proinflammatory condition, such as an infection or allergy, could attract primed neutrophils to the extravascular tissue. If ANCA are present in the extravascular interstitial fluid, the primed neutrophils would be activated by ANCA, which would initiate an inflammatory amplification loop causing a destructive necrotizing lesion. The acute injury would initiate a response to the injury by monocytes that transform into macrophages (including giant cells) and recruit T cells, which are the constituent cells of granulomatous inflammation.
T Cells in ANCA Pathogenesis
T cells are undoubtedly critically involved in the genesis of the ANCA autoimmune response, both through active B-cell help by T cells in order to induce a pathogenic autoantibody response with the production of IgG subclass antibodies and through ineffective suppression of the autoimmune ANCA response by regulatory T cells [62]. Free et al. [63] have observed that AAV patients have both disruption of the suppressive regulatory T cell network as well as an increased frequency of a distinct proinflammatory effector T cell subset.
The research group of Kitchen and Holdsworth [64,65,66,67] have used a mouse model to study potential pathogenic roles for T cells in AAV. They induced glomerulonephritis by immunizing mice with human MPO, which induced B- and T-cell autoimmunity to mouse MPO. When these mice were given a dose of anti-glomerular basement membrane antibody that was not pathogenic in unimmunized control mice, neutrophils, CD4-positive cells, and macrophages accumulated in glomeruli and induced glomerulonephritis with crescents. They concluded that ANCA induce glomerular neutrophil infiltration and MPO deposition. Anti-MPO CD4-positive cells subsequently recognize the MPO as a planted antigen in glomeruli and amplified glomerular injury [64]. More recently, the same researchers investigated the role of autoreactive MPO-specific CD4-positive T cells in another mouse model of glomerulonephritis. They reported that transfer of epitope-specific MPO-specific CD4-positive T cells into immunodeficient Rag1 knockout mice induced focal necrotizing glomerulonephritis when glomerular MPO deposition was induced either by passive transfer of MPO-ANCA and LPS or by planting the MPO epitope conjugated to a murine anti-glomerular basement membrane monoclonal antibody. They concluded that ANCA-activated neutrophils not only induce injury but also deposit MPO in glomeruli, allowing autoreactive anti-MPO CD4-positive T cells to participate in the induction of glomerular lesions [65]. The homology between the pathogenic human MPO B-cell epitope recognized by MPO-ANCA with the nephritogenic murine T-cell MPO epitope supports the relevance of this model to human disease.
The same research group also showed the importance of T cells in the pathogenesis of their model of AAV by blocking the induction of MPO autoimmunity by impairing central thymic T cells or peripheral regulatory T cells [66], or by inducing tolerance to the nephritogenic MPO peptide by nasal insufflation of the peptide [67].
Genetic Association in AAV
Patients with ANCA have a wide spectrum of NCGN severity and distribution of systemic vasculitis. For example, at the time of renal biopsy, 90% of patients have crescents, ranging from crescents in 100% to <5% of glomeruli with an average of 50% [68]. Most patients have systemic vasculitis, but a minority has renal-limited disease. Patients with systemic disease may have an MPA, GPA, or EGPA phenotype [2]. This marked disease heterogeneity may be influenced at least in part by genetic factors, which is in accord with GWAS data showing an association between AAV and genetic factors [10]. A genetic influence also has been suggested by familial occurrences [69] and a greater frequency in first-degree relatives [70]. A US cohort study showed that GPA was more prevalent in Caucasians than Blacks [71]. In New Zealand cohorts in 2003, the 5-year GPA incidence among New Zealand Maori or Asians was approximately half that among New Zealand Europeans, whereas it was twice that among New Zealand Pacific Islanders [71]. The overall prevalence was 2 times higher among subjects of European ancestry than among non-Europeans in a French urban multiethnic population in 2000 [72]. These studies support a role for genetic factors in AAV pathogenesis.
The HLA genetic region has a ‘susceptibilizing’ and modulatory role in many autoimmune diseases, including AAV. A strong association of the HLA-DRB1*15 allele was found in PR3-ANCA disease, with a 73.3-fold higher risk in African-American patients in a small cohort of 32 African-American and 96 Caucasian patients, suggesting HLA-DRB1*15 alleles contribute to the pathogenesis of PR3-ANCA disease [73]. In a study genotyping the same area from 152 Chinese AAV patients, HLA-DRB1*1101 and DRB1*1202 were significantly more frequent in patients with MPA and GPA, respectively [74]. The strong association of AAV with a single nucleotide polymorphism (SNP) in the HLA-DPB1 area was identified in two GWAS conducted by the European Vasculitis Genetic Consortium (EVGC) [10] and the Vasculitis Clinical Research Consortium (VCRC) [75]. The EVGC GWAS also showed the striking finding that the SNPs in the loci of HLA-DP, SERPINA1 (the gene encoding α1-antitrypsin, a major inhibitor of PR3), and PRTN3 (the gene encoding PR3) were strongly associated with PR3-ANCA, and that a SNP in HLA-DQ was more significantly associated with MPO-ANCA. These genetic associations were stronger for ANCA specificity than for AAV clinical syndromes, suggesting that PR3-AAV and MPO-AAV are genetically distinct autoimmune diseases [10]. The strong associations of PR3-ANCA and MPO-ANCA disease with distinct HLA molecules suggest that HLA-determined immune responses against PR3 and MPO have a central role in the pathogenesis of ANCA disease.
A mouse model of AAV also has demonstrated a marked influence of the genetic background on the susceptibility to and severity of NCGN induced by anti-MPO [76]. Intravenous injections of anti-MPO IgG induced glomerular crescents in >60% of glomeruli in 129S6/SvEv and CAST/EiJ mice, but in <1% of glomeruli in A/J, DBA/1J, DBA/2J, NOD/LtJ, and PWK/PhJ mice. C57BL6J, 129S1/SvImJ, LP/J, WSB/EiJ, NZO/HILtJ, and C3H mice showed an intermediate severity. Experiments using bone marrow chimeric mice and in vitro studies of neutrophil activation by anti-MPO IgG indicated that the severity of NCGN is mediated by genetically determined differences in the ability of neutrophils to be activated by anti-MPO. The absence of a dominant quantitative trait locus suggested that the observed differences in severity are the result of multiple gene interactions rather than a single gene effect [76].
Immunogenesis of the ANCA Autoimmune Response
As already reviewed, substantial evidence supports a pathogenic role for ANCA in AAV; however, the origin of the ANCA autoimmune response is less well understood. Several hypotheses have been proposed for the nature and origin of the autoantigens that induce the pathogenic ANCA response, including exposure to exogenous antigens such as infectious pathogens and drugs, endogenous autoantigens such as antisense peptides and peptides derived from alternatively spliced transcripts, or display of self-antigens along with adjuvant effects from apoptotic cells or neutrophil extracellular traps that are presenting the antigens. In fact, there may be multiple different mechanisms that can induce a pathogenic autoimmune response.
Several microbial agents such as Staphylococcus aureus[77] and Ross River virus[78] have been implicated in the pathogenesis of AAV. The molecular mimicry between bacterial and self-antigens has been proposed as a mechanism for the induction of pathogenic ANCA. Kain et al. [7] reported the presence of antibodies to LAMP2 in the circulation of AAV patients. The authors were able to induce glomerulonephritis in rabbits by injection of rabbit anti-human LAMP2 that cross-reacted with rat LAMP2. Human LAMP2 is located in neutrophils, endothelial cells, and other cell types, and the epitope that is recognized by ANCA has complete homology with FimH, a bacterial adhesion molecule. Rats immunized with FimH produced antibodies to rat and human LAMP2 and developed pauci-immune NCGN. These observations suggest that infection with fimbriated bacteria bearing FimH may trigger cross-reactive autoimmune responses to LAMP2 and cause AAV. These observations could not be reproduced by another research group [8]. Most recently, another observation by Kim et al. [79] revealed that when C57BL/6 mice were immunized with a recombinant serine protease of Saccharomonospora viridis, which has >30% homology with human PR3, all mice produced high levels of autoantibodies to mouse PR3, which showed a cytoplasmic ANCA staining pattern on mouse neutrophils. More interestingly, some of those mice (40%) developed lung granulomas.
Another mechanism proposed for the genesis of pathogenic ANCA is the initiation of the autoimmune response by peptides that are complementary to the autoantigens [80]. In this study, patients with AAV due to PR3-ANCA were found to have not only circulating antibodies against PR3 peptides (anti-PR3) but also antibodies against antisense peptides. Specific memory T cells against a PR3 antisense peptide have also been detected in patients with PR3-ANCA [81]. The PR3 sense peptides and antisense peptides [complementary PR3 (c-PR3)] are encoded by the sense strand and the antisense strand of the PR3 gene, respectively. The idiotypic antibodies against c-PR3 can induce anti-idiotypic antibodies that react with the original PR3. The researchers also found that immunization of mice with c-PR3 triggered the production of anti-PR3 antibodies [80]. The initiation of anti-c-PR3 immune responses could be triggered by endogenous c-PR3 peptides or exogenous peptides that mimic the c-PR3 peptides, which could be derived from a microbe. Interestingly, c-PR3 has homology with multiple microbial peptides including peptides from Ross River virus and S. aureus that are associated with AAV [80,82], raising the possibility that infections with microbes may cause the ANCA autoimmune response and AAV via the idiotypic mechanism and complementary molecular mimicry. This theory of an immune response against a peptide that is antisense or complementary to the autoantigen is supported by the observation that pathogenic antiglomerular basement membrane antibodies can be induced by injecting rats with a peptide that is complementary to the autoantigen glomerular basement membrane peptide [83].
Disclosure Statement
The authors have no conflicts to report that are relevant to this review.
References
- 1.Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CGM, McCluskey RT, Sinico RA, Rees AJ, van Es LA, Waldherr R, Wiik A. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC, Falk RJ. Small vessel vasculitis. N Engl J Med. 1997;337:1512–1523. doi: 10.1056/NEJM199711203372106. [DOI] [PubMed] [Google Scholar]
- 3.Jennette JC, Falk RJ. The role of pathology in the diagnosis of systemic vasculitis. Clin Exp Rheumatol. 2007;25(suppl 44):52–56. [PubMed] [Google Scholar]
- 4.Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, Lesavre P, Noël LH. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. 2005;20:1392–1399. doi: 10.1093/ndt/gfh830. [DOI] [PubMed] [Google Scholar]
- 5.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 6.Jennette JC, Hoidal JH, Falk RJ. Specificity of anti-neutrophil cytoplasmic autoantibodies for proteinase 3. Blood. 1990;75:2263–2264. [PubMed] [Google Scholar]
- 7.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roth AJ, Brown MC, Smith RN, Badhwar AK, Parente O, Chung HC, Bunch DO, McGregor JG, Hogan SL, Hu Y, Yang JJ, Berg EA, Niles J, Jennette JC, Preston GA, Falk RJ. Anti-LAMP-2 antibodies are not prevalent in patients with antineutrophil cytoplasmic autoantibody glomerulonephritis. J Am Soc Nephrol. 2012;23:545–555. doi: 10.1681/ASN.2011030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lionaki S, Blyth ER, Hogan SL, Hu Y, Senior JBA, Jennette CE, Nachman PH, Jennette JC, Falk RJ. Classification of antineutrophil cytoplasmic autoantibody vasculitides: the role of antineutrophil cytoplasmic autoantibody specificity for myeloperoxidase or proteinase 3 in disease recognition and prognosis. Arthritis Rheum. 2012;64:3452–3462. doi: 10.1002/art.34562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, Jayne DR, Baslund B, Brenchley P, Bruchfeld A, Chaudhry AN, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367:214–223. doi: 10.1056/NEJMoa1108735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jennette JC, Falk RJ. Pathogenesis of ANCA-associated vasculitis: observations, theories and speculations. Presse Med. 2013;42(pt 2):493–498. doi: 10.1016/j.lpm.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Bansal PJ, Tobin MC. Neonatal microscopic polyangiitis secondary to transfer of maternal myeloperoxidase-antineutrophil cytoplasmic antibody resulting in neonatal pulmonary hemorrhage and renal involvement. Ann Allergy Asthma Immunol. 2004;93:398–401. doi: 10.1016/S1081-1206(10)61400-7. [DOI] [PubMed] [Google Scholar]
- 13.Silva F, Specks U, Sethi S, Irazabal MV, Fervenza FC. Successful pregnancy and delivery of a healthy newborn despite transplacental transfer of antimyeloperoxidase antibodies from a mother with microscopic polyangiitis. Am J Kidney Dis. 2009;54:542–545. doi: 10.1053/j.ajkd.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Roth AJ, Ooi J, Hess JJ, van Timmeren MM, Berg EA, Jennette CE, McGregor JA, Burkart M, Hogan SL, Hu Y, Winnik W, Nachman PH, Stegeman CA, Niles J, Heeringa P, Kitching AR, Holdsworth S, Jennette JC, Preston GA, Falk RJ. ANCA epitope specificity determines pathogenicity, detectability and clinical predictive value. J Clin Invest. 2013;123:1773–1783. doi: 10.1172/JCI65292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug-induced vasculitis. Ann Pharmacother. 2002;36:130–147. doi: 10.1345/aph.1A124. [DOI] [PubMed] [Google Scholar]
- 16.Pendergraft WF, 3rd, Niles JL. Trojan horses: drug culprits associated with antineutrophil cytoplasmic autoantibody (ANCA) vasculitis. Curr Opin Rheumatol. 2014;26:42–49. doi: 10.1097/BOR.0000000000000014. [DOI] [PubMed] [Google Scholar]
- 17.Visavachaipan N, Ong-Ajyooth L, Chanchairujuira T, Parichatikanond P, Choensuchon B. Clinical features and outcomes in patient with antineutrophil cytoplasmic autoantibody-positive glomerulonephritis associated with propylthiouracil treatment in Siriraj Hospital. J Med Assoc Thai. 2010;93(suppl 1):S139–S146. [PubMed] [Google Scholar]
- 18.Jayne DR, Gaskin G, Rasmussen N, Abramowicz D, Ferrario F, Guillevin L, Mirapeix E, Savage CO, Sinico RA, Stegeman CA, Westman KW, van der Woude FJ, de Lind van Wijngaarden RA, Pusey CD; European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vasculitis. J Am Soc Nephrol. 2007;18:2180–2188. doi: 10.1681/ASN.2007010090. [DOI] [PubMed] [Google Scholar]
- 19.Jones RB. Rituximab in the treatment of anti-neutrophil cytoplasm antibody-associated vasculitis. Nephron Clin Pract. 2014;128:243–249. doi: 10.1159/000368580. [DOI] [PubMed] [Google Scholar]
- 20.Falk RJ, Terrell RS, Charles LA, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci USA. 1990;87:4115–4119. doi: 10.1073/pnas.87.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewert BH, Jennette JC, Falk RJ. Anti-myeloperoxidase antibodies stimulate neutrophils to damage human endothelial cells. Kidney Int. 1992;41:375–383. doi: 10.1038/ki.1992.52. [DOI] [PubMed] [Google Scholar]
- 22.Savage CO, Gaskin G, Pusey CD, Pearson JD. Myeloperoxidase binds to vascular endothelial cells, is recognized by ANCA and can enhance complement dependent cytotoxicity. Adv Exp Med Biol. 1993;336:121–123. doi: 10.1007/978-1-4757-9182-2_20. [DOI] [PubMed] [Google Scholar]
- 23.Porges AJ, Redecha PB, Kimberly WT, Csernok E, Gross WL, Kimberly RP. Anti-neutrophil cytoplasmic antibodies engage and activate human neutrophils via Fcγ RIIa. J Immunol. 1994;153:1271–1280. [PubMed] [Google Scholar]
- 24.Ewert BH, Becker ME, Jennette JC, Falk RJ. Antimyeloperoxidase antibodies induce neutrophil adherence to cultured human endothelial cells. Ren Fail. 1995;17:125–133. doi: 10.3109/08860229509026249. [DOI] [PubMed] [Google Scholar]
- 25.Kocher M, Siegel ME, Edberg JC, Kimberly RP. Cross-linking of Fcγ receptor IIa and Fcγ receptor IIIb induces different proadhesive phenotypes on human neutrophils. J Immunol. 1997;159:3940–3948. [PubMed] [Google Scholar]
- 26.Kettritz R, Jennette JC, Falk RJ. Cross-linking of ANCA-antigens stimulates superoxide release by human neutrophils. J Am Soc Nephrol. 1997;8:386–394. doi: 10.1681/ASN.V83386. [DOI] [PubMed] [Google Scholar]
- 27.Kettritz R, Schreiber A, Luft FC, Haller H. Role of mitogen-activated protein kinases in activation of human neutrophils by antineutrophil cytoplasmic antibodies. J Am Soc Nephrol. 2001;12:37–46. doi: 10.1681/ASN.V12137. [DOI] [PubMed] [Google Scholar]
- 28.Williams JM, Ben-Smith A, Hewins P, Dove SK, Hughes P, McEwan R, Wakelam MJO, Savage COS. Activation of the G heterotrimeric G protein by ANCA IgG F(ab′)2 fragments is necessary but not sufficient to stimulate the recruitment of those downstream mediators used by intact ANCA IgG. J Am Soc Nephrol. 2003;14:661–669. doi: 10.1097/01.asn.0000050223.34749.f4. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Garfield A, Rainger GE, Savage CO, Nash GB. Mediation of endothelial cell damage by serine proteases, but not superoxide released from antineutrophil cytoplasmic antibody-stimulated neutrophils. Arthritis Rheum. 2006;54:1619–1628. doi: 10.1002/art.21773. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber A, Xiao H, Jennette JC, Schneider W, Luft FC, Kettritz R. C5a receptor mediates neutrophil activation and ANCA-induced glomerulonephritis. J Am Soc Nephrol. 2009;20:289–298. doi: 10.1681/ASN.2008050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Veen BS, Chen M, Müller R, van Timmeren MM, Petersen AH, Lee PA, Satchell SC, Mathieson PW, Saleem MA, Stegeman CA, Zwerina J, Molema G, Heeringa P. Effects of p38 mitogen-activated protein kinase inhibition on anti-neutrophil cytoplasmic autoantibody pathogenicity in vitro and in vivo. Ann Rheum Dis. 2011;70:356–365. doi: 10.1136/ard.2010.129106. [DOI] [PubMed] [Google Scholar]
- 32.Hao J, Meng LQ, Xu PC, Chen M, Zhao MH. p38MAPK, ERK and PI3K signaling pathways are involved in C5a-primed neutrophils for ANCA-mediated activation. PLoS One. 2012;7:e38317. doi: 10.1371/journal.pone.0038317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Ani B. Simvastatin inhibits neutrophil degranulation induced by antineutrophil cytoplasm auto-antibodies and N-formyl-methionine-leucine phenylalanine (fMLP) peptide. Saudi Med J. 2013;34:477–483. [PubMed] [Google Scholar]
- 34.Casselman BL, Kilgore KS, Miller BF, Warren JS. Antibodies to neutrophil cytoplasmic antigens induce monocyte chemoattractant protein-1 secretion from human monocytes. J Lab Clin Med. 1995;126:495–502. [PubMed] [Google Scholar]
- 35.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, α1-antitrypsin, and Fcγ receptors. J Clin Invest. 1997;100:1416–1424. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jennette JC, Falk RJ. ANCAs are also anti-monocyte cytoplasmic autoantibodies. Clin J Am Soc Nephrol. 2015;10:4–6. doi: 10.2215/CJN.11501114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidner S, Carl M, Riess R, Rupprecht HD. Histologic analysis of renal leukocyte infiltration in antineutrophil cytoplasmic antibody-associated vasculitis: importance of monocyte and neutrophil infiltration in tissue damage. Arthritis Rheum. 2004;50:3651–3657. doi: 10.1002/art.20607. [DOI] [PubMed] [Google Scholar]
- 38.Zhao L, David MZ, Hyjek E, Chang A, Meehan SM. M2 macrophage infiltrates in the early stages of ANCA-associated pauci-immune necrotizing GN. Clin J Am Soc Nephrol. 2015;10:54–62. doi: 10.2215/CJN.03230314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller Kobold AC, Kallenberg CG, Tervaert JW. Monocyte activation in patients with Wegener's granulomatosis. Ann Rheum Dis. 1999;58:237–245. doi: 10.1136/ard.58.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jennette JC, Xiao H, Falk R, Gasim AM. Experimental models of vasculitis and glomerulonephritis induced by antineutrophil cytoplasmic autoantibodies. Contrib Nephrol. 2011;169:211–220. doi: 10.1159/000314776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y, Maeda N, Falk RJ, Jennette JC. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest. 2002;110:955–963. doi: 10.1172/JCI15918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schreiber A, Xiao H, Falk RJ, Jennette JC. Bone marrow-derived cells are sufficient and necessary targets to mediate glomerulonephritis and vasculitis induced by anti-myeloperoxidase antibodies. J Am Soc Nephrol. 2006;17:3355–3364. doi: 10.1681/ASN.2006070718. [DOI] [PubMed] [Google Scholar]
- 43.Little MA, Smyth CL, Yadav R, Ambrose L, Cook HT, Nourshargh S, Pusey CD. Antineutrophil cytoplasm antibodies directed against myeloperoxidase augment leukocyte-microvascular interactions in vivo. Blood. 2005;106:2050–2058. doi: 10.1182/blood-2005-03-0921. [DOI] [PubMed] [Google Scholar]
- 44.Little MA, Smyth L, Salama AD, Mukherjee S, Smith J, Haskard D, Nourshargh S, Cook HT, Pusey CD. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol. 2009;174:1212–1220. doi: 10.2353/ajpath.2009.080458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfister H, Ollert M, Fröhlich LF, Quintanilla-Martinez L, Colby TV, Specks U, Jenne DE. Antineutrophil cytoplasmic autoantibodies against the murine homolog of proteinase 3 (Wegener autoantigen) are pathogenic in vivo. Blood. 2004;104:1411–1418. doi: 10.1182/blood-2004-01-0267. [DOI] [PubMed] [Google Scholar]
- 46.van der Geld YM, Hellmark T, Seiga D, Heeringa P, Huitema MG, Limburg P, Kallenberg CGM. Rats and mice immunized with chimeric human/mouse proteinase 3 produce autoantibodies to mouse Pr3 and rat granulocytes. Ann Rheum Dis. 2007;66:1679–1682. doi: 10.1136/ard.2006.064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Primo VC, Marusic S, Franklin CC, Goldmann WH, Achaval CG, Smith RN, Arnaout MA, Nikolic B. Anti-PR3 immune responses induce segmental and necrotizing glomerulonephritis. Clin Exp Immunol. 2010;159:327–337. doi: 10.1111/j.1365-2249.2009.04072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Little MA, Al-Ani B, Ren S, Al-Nuaimi H, Leite M Jr, Alpers CE, Savage CO, Duffield JS. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One. 2012;7:e28626. doi: 10.1371/journal.pone.0028626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brouwer E, Huitema MG, Mulder AH, Heeringa P, van Goor H, Tervaert JW, Weening JJ, Kallenberg CG. Neutrophil activation in vitro and in vivo in Wegener's granulomatosis. Kidney Int. 1994;45:1120–1131. doi: 10.1038/ki.1994.149. [DOI] [PubMed] [Google Scholar]
- 50.Xiao H, Heeringa P, Liu Z, Huugen D, Hu PQ, Maeda N, Falk RJ, Jennette JC. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol. 2005;167:39–45. doi: 10.1016/S0002-9440(10)62951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tidman M, Olander R, Svalander C, Danielsson D. Patients hospitalised because of small vessel vasculitis with renal involvement in the period 1975-1995: organ involvement, anti-neutrophil cytoplasmic antibodies patterns, seasonal attack rates and fluctuation of annual frequencies. J Intern Med. 1998;244:133–141. doi: 10.1046/j.1365-2796.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- 52.Huugen D, Xiao H, van Esch A, Falk RJ, Peutz-Kootstra CJ, Buurman WA, Tervaert JW, Jennette JC, Heeringa P. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-α. Am J Pathol. 2005;167:47–58. doi: 10.1016/s0002-9440(10)62952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170:52–64. doi: 10.2353/ajpath.2007.060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M, Xing GQ, Yu F, Liu G, Zhao MH. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol Dial Transplant. 2009;24:1247–1252. doi: 10.1093/ndt/gfn586. [DOI] [PubMed] [Google Scholar]
- 55.Xing GQ, Chen M, Liu G, Heeringa P, Zhang JJ, Zheng X, et al. Complement activation is involved in renal damage in human antineutrophil cytoplasmic autoantibody associated pauci-immune vasculitis. J Clin Immunol. 2009;29:282–291. doi: 10.1007/s10875-008-9268-2. [DOI] [PubMed] [Google Scholar]
- 56.Gou SJ, Yuan J, Chen M, Yu F, Zhao MH. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2013;83:129–137. doi: 10.1038/ki.2012.313. [DOI] [PubMed] [Google Scholar]
- 57.Gou SJ, Yuan J, Wang C, Zhao MH, Chen M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clin J Am Soc Nephrol. 2013;8:1884–1891. doi: 10.2215/CJN.02790313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molad Y, Tovar A, Ofer-Shiber S. Association of low serum complement C3 with reduced patient and renal survival in antimyeloperoxidase-associated small-vessel vasculitis. Nephron Clin Pract. 2014;126:67–74. doi: 10.1159/000357154. [DOI] [PubMed] [Google Scholar]
- 59.Chen SF, Wang FM, Li ZY, Yu F, Zhao MH, Chen M. Plasma complement factor H is associated with disease activity of patients with ANCA-associated vasculitis. Arthritis Res Ther. 2015;17:129. doi: 10.1186/s13075-015-0656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao H, Dairaghi DJ, Powers JP, Ertl LS, Baumgart T, Wang Y, Seitz LC, Penfold ME, Gan L, Hu P, Lu B, Gerard NP, Gerard C, Schall TJ, Jaen JC, Falk RJ, Jennette JC. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol. 2014;25:225–231. doi: 10.1681/ASN.2013020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jennette JC, Xiao H, Hu P. Complement in ANCA-associated vasculitis. Semin Nephrol. 2013;33:557–564. doi: 10.1016/j.semnephrol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lepse N, Abdulahad WH, Kallenberg CG, Heeringa P. Immune regulatory mechanisms in ANCA-associated vasculitides. Autoimmun Rev. 2011;11:77–83. doi: 10.1016/j.autrev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Free ME, Bunch DO, McGregor JA, Jones BE, Berg EA, Hogan SL, Hu Y, Preston GA, Jennette JC, Falk RJ, Su MA. Patients with antineutrophil cytoplasmic antibody-associated vasculitis have defective Treg cell function exacerbated by the presence of a suppression-resistant effector cell population. Arthritis Rheum. 2013;65:1922–1933. doi: 10.1002/art.37959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ruth AJ, Kitching AR, Kwan RY, Odobasic D, Ooi JD, Timoshanko JR, Hickey MJ, Holdsworth SR. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1940–1949. doi: 10.1681/ASN.2006020108. [DOI] [PubMed] [Google Scholar]
- 65.Ooi JD, Chang J, Hickey MJ, Borza DB, Fugger L, Holdsworth SR, Kitching AR. The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci USA. 2012;109:E2615–E2624. doi: 10.1073/pnas.1210147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan DS, Gan PY, O'Sullivan KM, Hammett MV, Summers SA, Ooi JD, Lundgren BA, Boyd RL, Scott HS, Kitching AR, Chidgey AP, Holdsworth SR. Thymic deletion and regulatory T cells prevent antimyeloperoxidase GN. J Am Soc Nephrol. 2013;24:573–585. doi: 10.1681/ASN.2012090898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gan PY, Tan DS, Ooi JD, Alikhan MA, Kitching AR, Holdsworth SR. Myeloperoxidase peptide-based nasal tolerance in experimental ANCA-associated GN. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015010089. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jennette JC. Rapidly progressive and crescentic glomerulonephritis. Kidney Int. 2003;63:1164–1177. doi: 10.1046/j.1523-1755.2003.00843.x. [DOI] [PubMed] [Google Scholar]
- 69.Knight A, Sandin S, Askling J. Risks and relative risks of Wegener's granulomatosis among close relatives of patients with the disease. Arthritis Rheum. 2008;58:302–307. doi: 10.1002/art.23157. [DOI] [PubMed] [Google Scholar]
- 70.Cotch MF, Rao JK. New insights into the epidemiology of systemic vasculitis. Curr Opin Rheumatol. 1996;8:19–25. doi: 10.1097/00002281-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 71.O'Donnell JL, Stevanovic VR, Frampton C, Stamp LK, Chapman PT. Wegener's granulomatosis in New Zealand: evidence for a latitude dependent incidence gradient. Intern Med J. 2007;37:242–246. doi: 10.1111/j.1445-5994.2006.01297.x. [DOI] [PubMed] [Google Scholar]
- 72.Mahr A, Guillevin L, Poissonnet M, Aymé S. Prevalences of polyarteritis nodosa, microscopic polyangiitis, Wegener's granulomatosis, and Churg-Strauss syndrome in a French urban multiethnic population in 2000: a capture-recapture estimate. Arthritis Rheum. 2004;51:92–99. doi: 10.1002/art.20077. [DOI] [PubMed] [Google Scholar]
- 73.Cao Y, Schmitz JL, Yang JJ, Hogan SL, Bunch DO, Hu Y, Jennette CE, Berg EA, Arnett FC Jr, Jennette JC, Falk RJ, Preston GA. DRB1*15 allele is a risk factor for PR3-ANCA disease in African Americans. J Am Soc Nephrol. 2011;22:1161–1167. doi: 10.1681/ASN.2010101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luo H, Chen M, Yang R, Xu PC, Zhao MH. The association of HLA-DRB1 alleles with antineutrophil cytoplasmic antibody-associated systemic vasculitis in Chinese patients. Hum Immunol. 2011;72:422–425. doi: 10.1016/j.humimm.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 75.Xie G, Roshandel D, Sherva R, Monarch PA, Lu EY, Kung T, Carrington K, Zhang SS, Pulit SL, Ripke S, et al. Association of granulomatosis with polyangiitis (Wegener's) with HLA-DPB1*04 and SEMA6A gene variants: evidence from genome-wide analysis. Arthritis Rheum. 2013;65:2457–2468. doi: 10.1002/art.38036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao H, Ciavatta D, Aylor DL, Hu P, de Villena FP, Falk RJ, Jennette JC. Genetically determined severity of anti-myeloperoxidase glomerulonephritis. Am J Pathol. 2013;182:1219–1226. doi: 10.1016/j.ajpath.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Popa ER, Stegeman CA, Kallenberg CG, Tervaert JW. Staphylococcus aureus and Wegener's granulomatosis. Arthritis Res. 2002;4:77–79. doi: 10.1186/ar392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotizing glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed) 1982;285:606. doi: 10.1136/bmj.285.6342.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YC, Choi YS, Alam J, Kim Y, Baek KJ, Koh J, Song YW, Chung DH, Choi Y. Induction of proteinase 3-anti-neutrophil cytoplasmic autoantibodies by proteinase 3-homologous bacterial protease in mice. Immunol Res. 2015 doi: 10.1007/s12026-015-8687-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Pendergraft WF 3rd, Preston GA, Shah RR, Tropsha A, Carter CW Jr, Jennette JC, Falk RJ. Autoimmunity is triggered by cPR-3(105-201), a protein complementary to the autoantigen proteinase 3. Nat Med. 2004;10:72–79. doi: 10.1038/nm968. [DOI] [PubMed] [Google Scholar]
- 81.Yang JJ, Bautz DJ, Lionaki S, Hogan SL, Chin H, Tisch RM, Schmitz JL, Pressler BM, Jennette JC, Falk RJ, Preston GA. ANCA patients have T cells responsive to complementary PR 3 antigen. Kidney Int. 2008;74:1159–1169. doi: 10.1038/ki.2008.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Preston GA, Pendergraft WF 3rd, Falk RJ. New insights that link microbes with the generation of antineutrophil cytoplasmic autoantibodies: the theory of autoantigen complementarity. Curr Opin Nephrol Hypertens. 2005;14:217–222. doi: 10.1097/01.mnh.0000165886.93427.b1. [DOI] [PubMed] [Google Scholar]
- 83.Reynolds J, Preston GA, Pressler BM, Hewins P, Brown M, Roth A, Alderman E, Bunch D, Jennette JC, Cook HT, Falk RJ, Pusey CD. Autoimmunity to the alpha 3 chain of type IV collagen in glomerulonephritis is triggered by ‘autoantigen complementarity'. J Autoimmun. 2015;59:8–18. doi: 10.1016/j.jaut.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]