Abstract
Background
Chronic hemodialysis (HD) in the 1960s encompassed a wide variety of prescriptions from twice weekly to five times per week HD. Over time, HD prescriptions in the West became standardized at three times per week, 2.5–4 h per session, with occasional additional treatments for volume overload.
Summary
When clinical trials of dialysis dose failed to show significant benefit of extending time compared with the traditional dialysis prescription, interest in more frequent HD was renewed. Consequently, there has been growth in home HD therapies as well as alternative dialysis prescriptions. Data from recent randomized clinical trials have demonstrated the benefits and risks of these more frequent therapies, with surprising differences in outcomes between short daily HD and long nocturnal HD. More frequent therapies improve control of both hypertension and hyperphosphatemia, but at the expense of increased vascular access complications and, at least for nocturnal HD, a faster loss of residual renal function.
Key Messages
In the West, the standard HD prescription is three treatments per week with a minimal time of 3.0 h and dialysis is performed in an outpatient dialysis center. A minority of patients will have a fourth treatment per week for volume issues. Alternative HD prescriptions, although rare, are more available compared to the recent past.
Facts from East and West
(1) While developed Western and Asian countries provide end-stage renal disease patients full access to HD, healthcare systems from South and South-East Asia can offer access to HD only to a limited fraction of the patients in need. Even though the annual costs of HD are much lower in less developed countries (for instance 30 times lower in India compared to the US), patients often cannot afford costs not covered by health insurance. (2) The recommended dialysis pattern in the West is at least three sessions weekly with high-flux dialyzers. Studies from Shanghai and Taiwan might however indicate a benefit of twice versus thrice weekly sessions. In less developed Asian countries, a twice weekly pattern is common, sometimes with dialyzer reuse and inadequate water treatment. A majority of patients decrease session frequency or discontinue the program due to financial constraint. (3) As convective therapies are gaining popularity in Europe, penetration in Asia is low and limited by costs. (4) In Asian countries, in particular in the South and South-East, hepatitis and tuberculosis infections in HD patients are higher than in the West and substantially increase mortality. (5) Progress has recently been made in countries like Thailand and Brunei to provide universal HD access to all patients in need. Nevertheless, well-trained personnel, reliable registries and better patient follow-up would improve outcomes in low-income Asian countries.
Key Words: Adequacy of dialysis, Hemodialysis, Mortality, Residual renal function, Vascular access
The History of Chronic Hemodialysis
Since the initial development of chronic hemodialysis (HD) therapy at the Northwest Kidney Center in 1960, there have been numerous developments that have improved the treatment of end-stage renal disease (ESRD) using HD. Prior to 1960, HD had been reserved for the treatment of acute renal failure, due to the need to perform a surgical cutdown to access the vasculature required to perform HD. The development of the Scribner shunt in 1960 [1] allowed for the development of chronic HD programs. These programs, very limited in scope initially, became more feasible after the development of a more durable arteriovenous fistula by Dr. Cimino and colleagues in 1966 [2]. These developments, in part, led to legislation in the US Congress which provided Medicare coverage of ESRD as part of Social Security Amendments [3]. When enrollment for this program commenced in July 1973, only 11,000 patients were being dialyzed [4]. By 1990, almost 200,000 Americans were being treated for ESRD [5], with an annual rate of increase in the numbers of in-center HD patients of 5% for the years 1984–1986 and of 10% for the years 1987–1989 [6,7]. The number of patients under ESRD treatment was 378,862, including 275,053 dialysis patients and 103,809 patients with a functioning transplant. Subsequent data from 2010 indicated that the number of ESRD patients had increased to 615,899, including 430,273 dialysis patients and 185,626 patients with a functioning transplant [8]. It is estimated that more than 533,000 people in the US will be on dialysis by 2020, according to United States Renal Data System estimates based on a Markov model [9]. Similar increases have been seen throughout the Western world [10,11,12,13].
Costs of Chronic Hemodialysis
The cost of providing care to ESRD patients has continued to rise. In 2000, the Centers for Medicare & Medicaid Services spent USD 14 billion on the ESRD program; this amount has risen to USD 34.3 billion in 2010, with an average cost of USD 87,945 per person per year for HD patients [8]. Inpatient services accounted for 38% of these costs, with 35% for outpatient care, 19% for physician/supplier costs and 8% for outpatient medications as paid by Medicare Part D. Therefore, much of the cost of ESRD care is due to morbidity, perhaps a result of inadequacies in dialysis therapy, hence the importance of improving the ‘adequacy’ of dialysis so that the morbidity, mortality and costs could be reduced.
Adequacy of Hemodialysis - Early Data
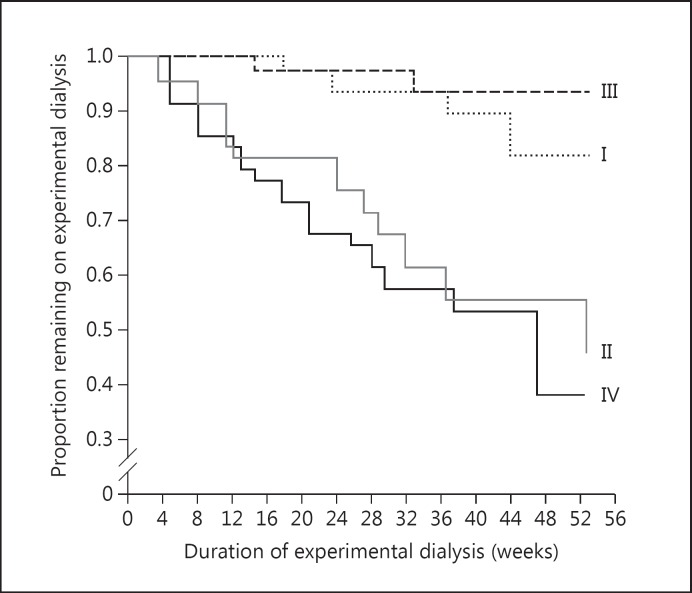
As HD matured, there was a great interest in determining what an adequate dose of HD was. A variety of methods were proposed to measure adequacy of dialysis, including measurement of blood urea nitrogen (BUN) and the square meter hypothesis. The first large clinical trial on the dose of dialysis, the National Cooperative Dialysis Study (NCDS) [14], was conducted in the late 1970s and early 1980s. In the one-year period immediately following the study conclusion, significantly more deaths occurred in the high time-averaged concentration of urea groups than the low time-averaged concentration of urea groups (fig. 1). A re-analysis of the study data, using a formal mechanistic analysis approach [15], introduced the concept of Kt/V to measure the dose of dialysis, where K is the urea clearance, t is the duration of the dialysis session in minutes on dialysis and V is the volume of distribution of urea. It was found that Kt/V varied inversely with morbidity, defined as ‘dropout’ or ‘percent failure’, and the relationship between Kt/V and morbidity was characterized as a step function rather than an exponentially decreasing function. Using this approach, the percent failure for Kt/V values ≤0.8 was expressed at a high constant value (55%) while that for Kt/V values of ≥0.9 was expressed at a low constant value (10%) [15], which led to the conclusion that adequate dialysis for patients receiving HD three times per week was defined by a delivered Kt/V of 1.0 per treatment and that a Kt/V of >1.0 per treatment was of no apparent benefit. A reappraisal of the NCDS database by Keshaviah [16], however, edited the mistakenly recorded data and fit them more appropriately to an exponential function. This re-analysis therefore suggested that delivered Kt/V values >1.0 per treatment resulted in a concomitant improvement in clinical outcomes.
Fig. 1.
Patient survival in the NCDS. Group I: Long duration and low BUN. Group II: long duration and high BUN. Group III: short duration and low BUN. Group IV: short duration and high BUN. Long duration was about 4.5 h while short duration was about 3.25 h. High BUN was about 105–110 mg/dl while low BUN was about 70–75 mg/dl. From [14].
The provision of HD to a larger numbers of patients in a number of first-world countries inevitably led to comparison of mortality rates in chronic HD patients by country. A study by Held et al. [17] reported 5-year mortality estimates for ESRD patients for the years 1983–1988, controlled for age differences between countries as well as the rates of diabetes mellitus. The mortality rate for the US ESRD population was 15% higher than those in the countries of the European Dialysis and Transplant Association (EDTA) and 30% higher than in Japan. One of the many possible reasons for this higher mortality rate in US patients was that the dialysis treatment times were decreased in the US in response to a decrease in the level of Medicare reimbursements for HD that was implemented in 1983. This change in reimbursement level also led to a decrease in the dialysis unit staffing per patient. Thus, the mean treatment time and Kt/V for the US ESRD population was 9.8 h/week and 1.0, respectively, compared to 12.1 h/week and 1.3 in the EDTA population.
The data from Held et al. [17], several large observational studies on the relationship between dialysis dose and mortality [18,19,20,21] as well as the conclusions from a professional consensus panel led three national organizations, including the National Institutes of Health [22], the Renal Physicians Association [23] and the Hemodialysis Adequacy Work Group (NKF-DOQI) [24], to advocate a urea reduction ratio of 65% as the threshold for ‘adequate’ HD and to profile providers accordingly.
Adequacy of Dialysis and Dialysis Flux - More Recent Studies
At about the same time, the National Institutes of Health funded a randomized clinical trial to test the impact of dialysis dose and dialysis flux on mortality in chronic HD patients. The HEMO study [25] was the first randomized clinical trial that examined the effects of both the dose of dialysis and the level of flux of the dialyzer membrane on mortality and morbidity among patients on maintenance HD. In this 2 × 2 factorial design, 1,846 patients were randomly assigned to a standard dose (equilibrated Kt/Vurea [eKt/Vurea] of 1.0, approximately equivalent to a urea reduction ratio of 65% or a single-pool Kt/Vurea [spKt/Vurea] of 1.20) [26] or a high dose (eKt/Vurea of 1.4, approximately equivalent to a urea reduction ratio of 75% or a spKt/Vurea of 1.60) and to a low-flux or high-flux dialyzer. Flux was defined by the clearance of β2 microglobulin, with low-flux dialyzers defined as having a β2 microglobulin clearance of <10 ml/min and high-flux dialyzers defined as having a β2 microglobulin clearance of ≥20 ml/min. The study had an 84% power to detect a 25% reduction in the primary endpoint of all-cause mortality for each intervention.
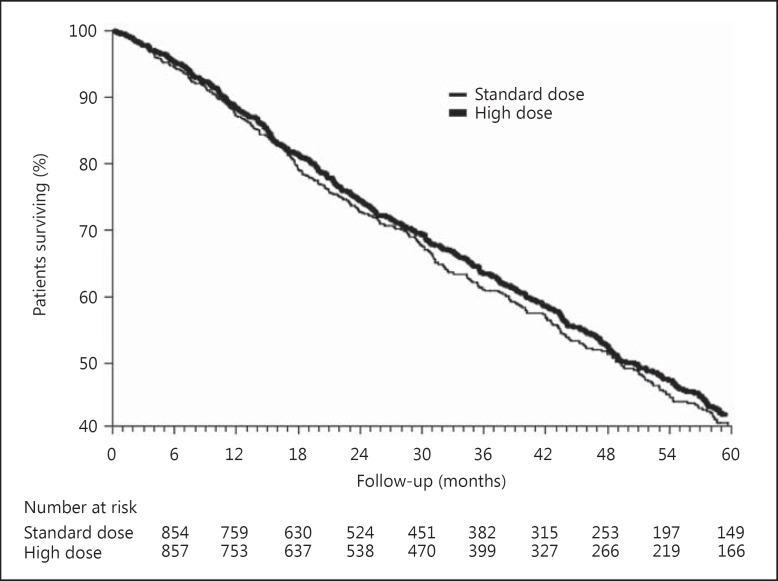
During the course of the study, concerns were raised that the standard-dose arm of the trial was unethical as it provided a substandard dose of dialysis. These concerns led to a modification of the protocol such that the low standard and high eKt/Vurea doses were raised to 1.05 and 1.45, respectively. Despite these initial concerns regarding study design, the relative risk (RR) of death in the high-dose group (mean eKt/Vurea of 1.53 ± 0.09, mean spKt/Vurea of 1.71 ± 0.11) versus the standard-dose group (mean eKt/Vurea of 1.16 ± 0.08, mean spKt/Vurea of 1.32 ± 0.09) was not statistically different, with a RR of 0.96 (95% confidence interval [CI] 0.84–1.10, p = 0.53; fig. 2). Subgroup analysis suggested that females had a statistically significantly lower mortality rate with the higher dialysis dose while males had a higher mortality rate that was not statistically significant. The results for the flux intervention were also negative, as those patients randomized to the high-flux arm had a mortality rate that was no different from that of patients randomized to the low-flux arm (RR 0.92, 95% CI 0.81–1.05, p = 0.23).
Fig. 2.
Survival curves for the HEMO study dose intervention. From [25].
The flux arm of the study was complicated by the observation, unknown at the time that the study was designed, that the dialyzer flux varied by both the specific dialyzer membrane used as well as the method of reuse [27]. Thus, cellulose triacetate membranes that were reused using Renalin© (peracetic acid mixture) had a marked decline in β2 microglobulin clearance with each reuse, while all membranes had an increase in β2 microglobulin clearance if bleach was used for the reuse method. These observations led to a restriction on the number of times that cellulose triacetate dialyzers could be reused in order to maintain separation between the two flux arms of the study. The separation of the flux arms was thus lower than initially planned, with a mean β2 microglobulin clearance of 33.8 ± 11.4 ml/min in the high-flux group versus 3.4 ± 7.2 ml/min in the low-flux group [28]. Thus it is not known whether a higher β2 microglobulin clearance would have positively impacted overall mortality rates. Post hoc analysis revealed that in the high-flux group, there were significant risk reductions in the risk of death from cardiac causes and in the combined outcome of first hospitalization for cardiac causes or death from cardiac causes, neither of which were designated as the main secondary outcomes for the HEMO study. In addition, those participants who had been receiving dialysis for >3.7 years had a lower risk of death if they were randomized to the high-flux arm of the trial.
Two other randomized trials have addressed the issue of dialysis flux. The Membrane Permeability Outcome trial was a prospective clinical trial where 738 incident HD patients were randomized to low- or high-flux dialyzers, with stratification based on a serum albumin level >4 g/dl or ≤4 g/dl [29]. There was no significant difference in mortality between the high- and low-flux groups; however, in the low serum albumin subgroup, there was a lower mortality in the high-flux versus the low-flux group (RR 0.49, 95% CI 0.28–0.87). The EGE study was a 2 × 2 factorial randomized controlled trial that compared the effects of high- versus low-flux dialyzers on a combined outcome of fatal and nonfatal cardiovascular events [30]. In this study of 704 participants, there was no statistically significant difference in the primary outcome between high- and low-flux dialyzers (hazard ratio [HR] 0.73, 95% CI 0.49–1.08, p = 0.1). A post hoc analysis revealed that among those participants with either an arteriovenous fistula or with diabetes mellitus, the use of high-flux dialyzers improved cardiovascular event-free survival. Finally, a meta-analysis of these three trials demonstrated that the use of high-flux dialyzers was associated with a decrease in cardiovascular mortality compared to participants using low-flux membranes (HR = 0.82, 95% CI 0.70–0.96) [30].
Current Hemodialysis Prescriptions
Despite the negative results of the HEMO study regarding the dose of dialysis, the dose of dialysis in the US, as measured by spKt/Vurea, climbed throughout the late 1990s and early 2000s, perhaps in part due to the wide publicity that the HEMO study received in order to enhance recruitment among the 72 participating clinics [31]; based on data collected by the Centers for Medicare & Medicaid Services, the spKt/Vurea per HD session rose from 1.39 in 1997 to 1.52 in 2003 [32]. Cross-sectional data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) data from 2011 provide information on the dose of dialysis in Western countries [33]. Less than 5% of patients in Western countries dialyze less than three times per week. The mean dialysis session length is usually in the range of 220–245 min per session. Blood flow rate is quite variable, with the highest rate in North America (413 ± 68 ml/min), followed by Europe, Australia and New Zealand (317 ± 57 ml/min) and the lowest value in Japan at (202 ± 29 ml/min). The dose of dialysis, as expressed by the standardized Kt/V, was fairly similar in the 2.1–2.3 range, with numbers in the lower range more likely to be seen in Japan. The percentage of patients with a standardized Kt/V <2.0 ranged from a low of 13.8% in North America to 25.3% in Japan. The percentage of patients dialyzing more than three times per week ranged from 14% in Sweden, 8% in Spain, 5% in Canada, 4% in Italy and 2% in Australia and New Zealand to levels <1% in the US, Germany, Japan, France and the United Kingdom.
More Frequent Hemodialysis
The negative results of the HEMO study also led to a critical reappraisal of whether three times per week HD could provide a high enough dose of dialysis to impact mortality rate. It was noted that patients in the high-dose arm of the trial received only approximately 90 more minutes of dialysis per week (15% increase in total time per week) compared to patients in the standard-dose arm. This observation led to the concept that improvements in mortality would require the delivery of much higher doses of dialysis that could not be achieved with the traditional three times per week prescription. It is interesting to note that both short daily and nocturnal HD have their origins in the early days of chronic HD therapy. In the late 1960s and early 1970s, daily in-center HD was conducted both in the US and in Europe [34,35], as was home HD [36,37,38,39]. An early study by Bonomini et al. [35] in 1972 noted that in six patients who had uremic-type symptoms despite increasing the time on dialysis from 22 to 30 h per week, changing patients to short daily dialysis (3–4 h for 5 days per week) led to resolution of signs and symptoms of advanced uremia such as anorexia, amenorrhea, severe anemia, impotence, insomnia, polyneuropathy, pruritus and restless leg syndrome. Improvements in these symptoms of uremia, as well as in blood pressure control and left ventricular hypertrophy, were noted by other investigators in both Europe and North America [40,41,42,43].
Thus, the negative HEMO study results, coupled with prior knowledge of outcomes, on a small scale, from observational and cross-over studies with more frequent HD, led to the development of the Frequent Hemodialysis Network (FHN) Daily and Nocturnal studies. A number of different HD prescriptions were proposed for these studies in the early design phases of protocol development; however, it was eventually decided that providing a maximal dose of the effects of these more frequent dialysis modalities on outcomes would be more likely to result in a positive outcome. As both the FHN Daily Trial and the FHN Nocturnal Trial were designed to enroll only a small number of patients, the primary outcomes, of necessity, needed to be ‘soft’ outcomes and not hard outcomes such as hospitalizations or mortality [44]. Thus, for both studies, participants were followed for 12 months and the two co-primary composite outcomes were death or change in left ventricular mass by cardiac magnetic resonance imaging and death or change in the RAND physical health composite from the SF-36. There were a number of secondary outcomes, including measures of cognitive performance, self-reported depression, laboratory markers of nutrition, mineral metabolism and anemia, blood pressure as well as rates of hospitalization and vascular access interventions. The inclusion and exclusion criteria for the two trials were similar, with the exception of residual renal function, in which the exclusion criteria was higher in the Nocturnal Trial (>10 ml/min/1.73 m2 as calculated as the average of the urea and creatinine clearances) than in the Daily Trial (>3 ml/min/ 1.73 m2 of urea clearance).
Short Daily Hemodialysis
The FHN Daily Trial was a multicenter, prospective, randomized trial of frequent HD performed six times per week, in-center, compared to conventional HD performed three times per week in-center [45]. The 120 participants assigned to the standard arm continued on their pre-study dialysis prescription as long as the minimum target eKt/Vurea was at least 1.1 and the session length was between 2.5 and 4.0 h. The 125 participants in the frequent arm had dialysis prescriptions factored by V2/3 instead of V to both minimize the effect of patients' body mass on the prescription and also to avoid very long dialysis sessions for patients with higher body mass. Thus, the target eKt/Vn in the frequent arm was 0.9, where Vn = 3.271 × V2/3, with a session length that was between 1.5 and 2.75 h. Compliance with the dialysis prescription was acceptable in the frequent arm with participants averaging 5.2 sessions per week. There was excellent separation between groups in regards to dialysis dose, with a weekly standard Kt/Vurea of 3.54 ± 0.56 in the frequent arm compared to 2.49 ± 0.27 in the standard arm. The two co-primary outcomes were significantly improved in the frequent arm, with a reduction in the left ventricular mass/death HR (HR = 0.61, 95% CI 0.46–0.82) and an improvement in the physical health composite/death HR (HR = 0.70, 95% CI 0.53–0.92). Participants in the frequent HD arm also demonstrated improvement in the control of hypertension and hyperphosphatemia [46]. There were no significant improvements in the other secondary outcomes, however, including anemia [47], measures of cognitive performance [48], depressive symptoms and self-reported mental health [49] and nutrition and body composition [50]. Adverse effects in the frequent arm included an increased risk for a first-access event (HR 1.76, 95% CI 1.11–2.79) as well as an increase in total arteriovenous repairs (p = 0.011), likely secondary to the increased rate of cannulation per week in the frequent arm [51].
A long-term follow-up of the FHN Daily Trial has recently been published whereby the vital status of these participants was followed for a median of 3.6 years after randomization [52]. Most of the participants (81%) who were in the frequent arm returned within 2 months after the completion of the trial to a dialysis prescription <4.5 treatments per week. Despite this change to a more conventional dialysis prescription, participants in the frequent arm showed a significantly lower death rate during extended follow-up, with a relative mortality HR of 0.54 (95% CI 0.31–0.93). If participants were censored after receiving a kidney transplant, the relative HR was 0.56 (95% CI 0.32–0.99). The overall mortality rate was substantially lower in the frequent arm compared to the standard arm (0.043 versus 0.082 deaths per patient-year). These findings are analogous to the NCDS, in which survival benefits were seen several years after the conclusion on the randomized portion of that trial [14].
Long Nocturnal Hemodialysis
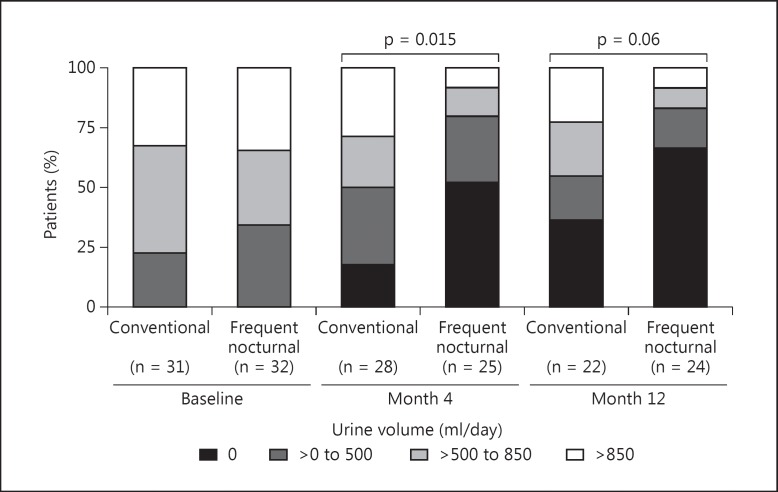
These findings are in marked contradistinction to the FHN Nocturnal Trial. The Nocturnal Trial was a multicenter, prospective, randomized trial in which participants were randomized to either three times per week conventional HD lasting 2.5–5 h per session or nocturnal HD six times per week with a duration of 6.0–8.0 h per session [53]. Due to difficulties with recruitment, the sample size was reduced from 250 to 90 patients. As in the FHN Daily Trial, there was excellent separation between groups with a mean ± standard deviation weekly standard Kt/Vurea of 2.59 ± 0.69 in the standard arm compared to 4.72 ± 1.08 in the frequent arm. Dialysis sessions were performed 2.91 ± 0.21 times per week in the standard arm compared to 5.06 ± 0.8 times per week in the frequent arm. The mean weekly duration of dialysis was 12.6 ± 3.9 h in the standard arm and 30.8 ± 9.1 h in the intensive arm. There was no benefit shown in the frequent arm for either of the two co-primary outcomes, with a HR for the left ventricular mass/death outcome of 0.68 (95% CI 0.44–1.07, p = 0.095) and a HR for the physical health component/death outcome of 0.91 (95% CI 0.58–1.43, p = 0.68). There was improvement, however, in the control of both hypertension and hyperphosphatemia. In those participants in the frequent arm who dialyzed >35 h per week, 60% required the addition of phosphorus to the dialysate to help prevent hypophosphatemia. There was no difference, however, between groups for the other secondary outcomes, including anemia, depression, cognitive function or nutrition (as measured by serum albumin levels) [47,48,49,50]. Finally, in the more frequent arm, there was not only a trend for increased vascular events [51] (p = 0.076), there was also a more rapid decline in residual renal function over the 12-month intervention period (fig. 3) [54].
Fig. 3.
Change in urine volume during the FHN Nocturnal Trial. From [54].
The Alberta Kidney Disease Network Study was also a randomized trial of more frequent nocturnal HD compared to conventional HD [55]. In this trial, 52 participants were followed for 6 months after randomization; the primary outcome was change in left ventricular mass, while secondary outcomes were focused on quality of life, mineral metabolism, control of hypertension and anemia. There was a significant decline in left ventricular mass in the frequent arm (-15.3 g, 95% CI −29.6 to −1.0) that was of greater magnitude than that seen in the FHN Nocturnal Trial (-10.8 g, 95% CI −23.7 to +1.8). This difference in the left ventricular mass finding may be secondary to several issues, including technical differences in how left ventricular mass was measured, the higher baseline left ventricular mass in the Alberta Trial compared to the FHN Trial, and the longer vintage of ESRD in the Alberta Trial (median of 3.5 years) versus the FHN Nocturnal Trial (median of 1.08 years, 10th and 90th percentiles 0.1–10.7 years), which likely also resulted in differences in residual renal function at baseline. A higher residual renal function would result in lower interdialytic weight gains, which in turn may mitigate any positive effects on left ventricular mass due to the nocturnal intervention. There was improvement in control of both hypertension [55] and hyperphosphatemia [56], but no improvements in anemia [55] or quality of life [57].
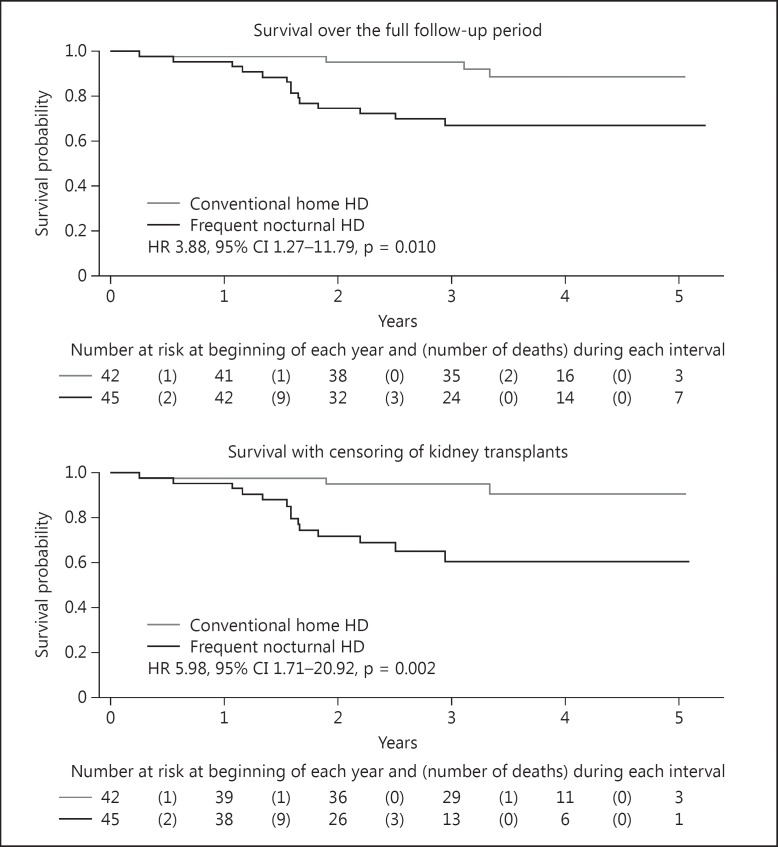
Similar to the FHN Daily Trial, a long-term extension study was conducted in participants in the FHN Nocturnal Trial [58]. Upon conclusion of the 1-year FHN Trial, these participants were free to change their HD prescription. Participants were followed for an average of 3.7 years. The overall mortality HR for those receiving nocturnal HD was 3.88 (95% CI 1.27–11.79, p = 0.010). If participants who received a renal transplant are censored at the time of renal transplantation, the HR is 5.98 (95% CI 1.71–20.92, p = 0.002; fig. 4). If one accounts for changes in modality by using an as treated analysis by dialysis prescription, with a 12-month running treatment average, the HR for mortality was still elevated at 3.06 (95% CI 1.11–8.43, p = 0.03). A Bayesian analysis was performed due to the small numbers of participants in order to provide a complementary approach to interpreting data when small sample sizes are present. These Bayesian analyses revealed that the probability of a clinically significant benefit were very small, suggesting that it was very unlikely that the utilization of frequent nocturnal HD would result in an improvement in the mortality rate compared to conventional three times per week HD.
Fig. 4.
Survival in the FHN Nocturnal Trial during the randomized trial and the post randomization follow-up period. From [53].
The Future of Hemodialysis
What does the future of HD hold? In the short term, there are several new home HD machines that are in various stages of clinical development that may make home HD less complicated and thus help decrease caregiver burden [59]. Longer-term, continued improvement in miniaturization and nanotechnology may lead to the development of viable wearable HD devices [60,61] and bioengineered kidneys, including de novo organogenesis, cell transplantation, growing a kidney in vivo, reseeding a kidney and implantable artificial kidneys [61,62,63,64]. These latter therapies may ultimately provide a definitive treatment for ESRD and replace HD as a treatment for ESRD.
Disclosure Statement
The author has no competing financial interests to declare.
References
- 1.Quinton W, Dillard D, Scribner BH. Cannulation of blood vessels for prolonged hemodialysis. Trans Am Soc Artif Intern Organs. 1960;6:104–113. [PubMed] [Google Scholar]
- 2.Brescia MJ, Cimino JE, Appel K, Hurwich BJ. Chronic hemodialysis using venipuncture and a surgically created arteriovenous fistula. N Engl J Med. 1966;275:1089–1092. doi: 10.1056/NEJM196611172752002. [DOI] [PubMed] [Google Scholar]
- 3.Rettig RA, Marks EL. The federal government and social planning for end-stage renal disease: past, present, and future. Santa Monica: RAND Corporation; 1983. http://www.rand.org/pubs/notes/N1922. [Google Scholar]
- 4.Evans RW, Blagg CR, Bryan FA Jr. Implications for health care policy. A social and demographic profile of hemodialysis patients in the United States. JAMA. 1981;245:487–491. doi: 10.1001/jama.245.5.487. [DOI] [PubMed] [Google Scholar]
- 5.USRDS Annual Data Report. Bethesda, MD: The National Institute of Health NIDDK; 1991. [Google Scholar]
- 6.Sicad GA. How will hemodialysis change over the next decade? Semin Dial. 1991;4:233–234. [Google Scholar]
- 7.United States Renal Data System . 2002 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2002. pp. 151–164. [Google Scholar]
- 8.United States Renal Data System . 2013 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Google Scholar]
- 9.Collins AJ, Foley RN, Gilbertson DT, Chen SC. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(suppl 1):S5–S11. doi: 10.2215/CJN.05980809. [DOI] [PubMed] [Google Scholar]
- 10.ERA-EDTA Registry ERA-EDTA Registry 2002 Annual Report Amsterdam, The Netherlands, Academic Medical Center, 5-1-2004.
- 11.ERA-EDTA Registry . ERA-EDTA Registry Annual Report 2013. Amsterdam, The Netherlands: Academic Medical Center, Department of Medical Informatics; 2015. [Google Scholar]
- 12.ANZDATA Registry. 37th Report, Preliminary Report 2014 . Summary of Dialysis and Transplantation in Australia and New Zealand. Adelaide, Australia: Australia and New Zealand Dialysis and Transplant Registry; 2015. [Google Scholar]
- 13.ANZDATA Registry Report 2002. Adelaide Australia: Australia and New Zealand Dialysis and Transplant Registry; 2015. [Google Scholar]
- 14.Parker TF, Laird NM, Lowrie EG. Comparison of the study groups in the National Cooperative Dialysis Study and a description of morbidity, mortality, and patient withdrawal. Kidney Int Suppl. 1983;13:S42–S49. [PubMed] [Google Scholar]
- 15.Gotch FA, Sargent JA. A mechanistic analysis of the National Cooperative Dialysis Study (NCDS) Kidney Int. 1985;28:526–534. doi: 10.1038/ki.1985.160. [DOI] [PubMed] [Google Scholar]
- 16.Keshaviah P. Urea kinetic and middle molecule approaches to assessing adequacy of emodialysis and CAPD. Kidney Int. 1993;43(suppl 40):S28–S38. [PubMed] [Google Scholar]
- 17.Held PJ, Brunner F, Odaka M, Garcia JR, Port FK, Gaylin DS. Five-year survival for end-stage renal disease patients in the United States, Europe, and Japan, 1982 to 1987. Am J Kidney Dis. 1990;15:451–457. doi: 10.1016/s0272-6386(12)70363-3. [DOI] [PubMed] [Google Scholar]
- 18.Owen WF Jr, Lew NL, Liu Y, Lowrie EG, Lazarus JM. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med. 1993;329:1001–1006. doi: 10.1056/NEJM199309303291404. [DOI] [PubMed] [Google Scholar]
- 19.Collins AJ, Ma JZ, Umen A, Keshaviah P. Urea index and other predictors of hemodialysis patient survival. Am J Kidney Dis. 1994;23:272–282. doi: 10.1016/s0272-6386(12)80984-x. [DOI] [PubMed] [Google Scholar]
- 20.Hakim RM, Breyer J, Ismail N, Schulman G. Effects of dose of dialysis on morbidity and mortality. Am J Kidney Dis. 1994;23:661–669. doi: 10.1016/s0272-6386(12)70276-7. [DOI] [PubMed] [Google Scholar]
- 21.Parker TF III, Husni L, Huang W, Lew N, Lowrie EG. Survival of hemodialysis patients in the United States is improved with a greater quantity of dialysis. Am J Kidney Dis. 1994;23:670–680. doi: 10.1016/s0272-6386(12)70277-9. [DOI] [PubMed] [Google Scholar]
- 22.Morbidity and mortality of renal dialysis an NIH consensus conference statement. Consensus Development Conference Panel. Ann Intern Med. 1994;121:62–70. doi: 10.7326/0003-4819-121-1-199407010-00013. [DOI] [PubMed] [Google Scholar]
- 23.Renal Physicians Association Working Committee on clinical practice guidelines Clinical Practice Guidelines on adequacy of hemodialysis. Clinical Practice Guideline. 1993;(1) Washington. [Google Scholar]
- 24.NKF-DOQI clinical practice guidelines for hemodialysis adequacy. National Kidney Foundation. Am J Kidney Dis. 1997;30(3 suppl 2):S15–S66. doi: 10.1016/s0272-6386(97)70027-1. [DOI] [PubMed] [Google Scholar]
- 25.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–2019. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 26.Daugirdas JT, Greene T, Depner TA, Leypoldt J, Gotch F, Schulman G, et al. Factors that affect postdialysis rebound in serum urea concentration, including the rate of dialysis: results from the HEMO Study. J Am Soc Nephrol. 2004;15:194–203. doi: 10.1097/01.asn.0000103871.20736.0c. [DOI] [PubMed] [Google Scholar]
- 27.Cheung AK, Agodoa LY, Daugirdas JT, Depner TA, Gotch FA, Greene T, et al. Effects of hemodialyzer reuse on clearances of urea and beta2-microglobulin. The Hemodialysis (HEMO) Study Group. J Am Soc Nephrol. 1999;10:117–127. doi: 10.1681/ASN.V101117. [DOI] [PubMed] [Google Scholar]
- 28.Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–555. doi: 10.1681/ASN.2005020132. [DOI] [PubMed] [Google Scholar]
- 29.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20:645–654. doi: 10.1681/ASN.2008060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer SC, Rabindranath KS, Craig JC, Roderick PJ, Locatelli F, Strippoli GF. High-flux versus low-flux membranes for end-stage kidney disease. Cochrane Database Syst Rev. 2012;9:CD005016. doi: 10.1002/14651858.CD005016.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eknoyan G, Levey GJ, Agodoa LY, Daugirdas JT, Kusek JW, Levin NW, et al. The hemodialysis (HEMO) study: rationale for selection of interventions. Semin Dial. 1996;9:24–33. [Google Scholar]
- 32.Centers for Medicare & Medicaid Services 2003 Annual Report: End Stage Renal Disease Clinical Performance Measures Project. Am J Kidney Dis. 2004;44(suppl 1):S1–S92. doi: 10.1053/s0272-6386(04)00719-x. [DOI] [PubMed] [Google Scholar]
- 33.Bieber B, Qian J, Anand S, Yan Y, Chen N, Wang M, et al. Two-times weekly hemodialysis in China: frequency, associated patient and treatment characteristics and Quality of Life in the China Dialysis Outcomes and Practice Patterns study. Nephrol Dial Transplant. 2014;29:1770–1777. doi: 10.1093/ndt/gft472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Palma JR, Pecker EA, Maxwell MH. A new automatic coil dialyzer system for ‘daily’ dialysis. Proc Eur Dial Transplant Assoc. 1969;6:26–34. [Google Scholar]
- 35.Bonomini L, Mioli V, Albertazzi A, Scolari P. Daily-dialysis programme: indications and results. Proc Eur Dial Transplant Assoc. 1972;9:44–52. [PubMed] [Google Scholar]
- 36.Eschbach JW Jr, Wilson WE Jr, Peoples RW, Wakefield AW, Babb AL, Scribner BH. Unattended overnight home hemodialysis. Trans Am Soc Artif Intern Organs. 1966;12:346–356. [PubMed] [Google Scholar]
- 37.Eschbach JW Jr, Barnett BM, Cole JJ, Daly S, Scribner BH. Hemodialysis in the home. A new approach to the treatment of chronic uremia. Ann Intern Med. 1967;67:1149–1162. doi: 10.7326/0003-4819-67-6-1149. [DOI] [PubMed] [Google Scholar]
- 38.Fenton SS, Blagg CR, Eschbach JW Jr, Scribner BH. Treatment of end-stage renal disease by home hemodialysis. J Am Med Womens Assoc. 1968;23:1096–1103. [PubMed] [Google Scholar]
- 39.Blagg CR, Hickman RO, Eschbach JW, Scribner BH. Home hemodialysis: six years' experience. N Engl J Med. 1970;283:1126–1131. doi: 10.1056/NEJM197011192832102. [DOI] [PubMed] [Google Scholar]
- 40.Kooistra MP, Vos J, Koomans HA, Vos PF. Daily home haemodialysis in the Netherlands: effects on metabolic control, haemodynamics, and quality of life. Nephrol Dial Transplant. 1998;13:2853–2860. doi: 10.1093/ndt/13.11.2853. [DOI] [PubMed] [Google Scholar]
- 41.Buoncristiani U. Fifteen years of clinical experience with daily haemodialysis. Nephrol Dial Transplant. 1998;13(suppl 6):148–151. doi: 10.1093/ndt/13.suppl_6.148. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds JT, Homel P, Cantey L, Evans E, Harding P, Gotch F, et al. A one-year trial of in-center daily hemodialysis with an emphasis on quality of life. Blood Purif. 2004;22:320–328. doi: 10.1159/000079186. [DOI] [PubMed] [Google Scholar]
- 43.Lindsay RM, Leitch R, Heidenheim AP, Kortas C. The London Daily/Nocturnal Hemodialysis Study - study design, morbidity, and mortality results. Am J Kidney Dis. 2003;42(1 suppl):S5–S12. doi: 10.1016/s0272-6386(03)00531-6. [DOI] [PubMed] [Google Scholar]
- 44.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71:349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 45.FHN Trial Group In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daugirdas JT, Chertow GM, Larive B, Pierratos A, Greene T, Ayus JC, et al. Effects of frequent hemodialysis on measures of CKD mineral and bone disorder. J Am Soc Nephrol. 2012;23:727–738. doi: 10.1681/ASN.2011070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ornt DB, Larive B, Rastogi A, Rashid M, Daugirdas JT, Hernandez A, et al. Impact of frequent hemodialysis on anemia management: results from the Frequent Hemodialysis Network (FHN) Trials. Nephrol Dial Transplant. 2013;28:1888–1898. doi: 10.1093/ndt/gfs593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, Mehta RL, Kliger AS, et al. Effect of more frequent hemodialysis on cognitive function in the Frequent Hemodialysis Network trials. Am J Kidney Dis. 2013;61:228–237. doi: 10.1053/j.ajkd.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unruh ML, Larive B, Chertow GM, Eggers PW, Garg AX, Gassman J, et al. Effects of 6-times-weekly versus 3-times-weekly hemodialysis on depressive symptoms and self-reported mental health: Frequent Hemodialysis Network (FHN) Trials. Am J Kidney Dis. 2013;61:748–758. doi: 10.1053/j.ajkd.2012.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaysen GA, Greene T, Larive B, Mehta RL, Lindsay RM, Depner TA, et al. The effect of frequent hemodialysis on nutrition and body composition: Frequent Hemodialysis Network Trial. Kidney Int. 2012;82:90–99. doi: 10.1038/ki.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suri RS, Larive B, Sherer S, Eggers P, Gassman J, James SH, et al. Risk of vascular access complications with frequent hemodialysis. J Am Soc Nephrol. 2013;24:498–505. doi: 10.1681/ASN.2012060595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chertow GM, Levin NW, Beck GJ, Daugirdas JT, Eggers PW, Kliger AS, Larive B, et al. Long-term effects of frequent in-center hemodialysis. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015040426. Epub ahead of print. DOI: 10.1681/ASN. 2015040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rocco MV, Lockridge RS Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–1091. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daugirdas JT, Greene T, Rocco MV, Kaysen GA, Depner TA, Levin NW, et al. Effect of frequent hemodialysis on residual kidney function. Kidney Int. 2013;83:949–958. doi: 10.1038/ki.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 56.Walsh M, Manns BJ, Klarenbach S, Tonelli M, Hemmelgarn B, Culleton B. The effects of nocturnal compared with conventional hemodialysis on mineral metabolism: a randomized-controlled trial. Hemodial Int. 2010;14:174–181. doi: 10.1111/j.1542-4758.2009.00418.x. [DOI] [PubMed] [Google Scholar]
- 57.Manns BJ, Walsh MW, Culleton BF, Hemmelgarn B, Tonelli M, Schorr M, et al. Nocturnal hemodialysis does not improve overall measures of quality of life compared to conventional hemodialysis. Kidney Int. 2009;75:542–549. doi: 10.1038/ki.2008.639. [DOI] [PubMed] [Google Scholar]
- 58.Rocco MV, Daugirdas JT, Greene T, Lockridge RS, Chan C, Pierratos A, et al. Long-term effects of frequent nocturnal hemodialysis on mortality: the Frequent Hemodialysis Network (FHN) Nocturnal Trial. Am J Kidney Dis. 2015;66:459–468. doi: 10.1053/j.ajkd.2015.02.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agar JW, Perkins A, Heaf JG. Home hemodialysis: infrastructure, water, and machines in the home. Hemodial Int. 2015;19(suppl 1):S93–S111. doi: 10.1111/hdi.12290. [DOI] [PubMed] [Google Scholar]
- 60.Davenport A, Ronco C, Gura V. From wearable ultrafiltration device to wearable artificial kidney. Contrib Nephrol. 2011;171:237–242. doi: 10.1159/000327172. [DOI] [PubMed] [Google Scholar]
- 61.Fissell WH, Roy S, Davenport A. Achieving more frequent and longer dialysis for the majority: wearable dialysis and implantable artificial kidney devices. Kidney Int. 2013;84:256–264. doi: 10.1038/ki.2012.466. [DOI] [PubMed] [Google Scholar]
- 62.Kim S, Fissell WH, Humes DH, Roy S. Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed) 2015;7:215–228. doi: 10.2741/e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peloso A, Katari R, Murphy SV, Zambon JP, DeFrancesco A, Farney AC, et al. Prospect for kidney bioengineering: shortcomings of the status quo. Expert Opin Biol Ther. 2015;15:547–558. doi: 10.1517/14712598.2015.993376. [DOI] [PubMed] [Google Scholar]
- 64.Jansen J, Fedecostante M, Wilmer MJ, van den Heuvel LP, Hoenderop JG, Masereeuw R. Biotechnological challenges of bioartificial kidney engineering. Biotechnol Adv. 2014;32:1317–1327. doi: 10.1016/j.biotechadv.2014.08.001. [DOI] [PubMed] [Google Scholar]