Abstract
The clinical consequences of untreated, severe anemia in patients with nondialysis CKD can be significant, but disparities exist in the anemia treatment guidelines and position papers issued from working groups and associations across the world. These differ in hemoglobin target and iron levels and their emphasis on various iron markers and other clinical outcomes. Not surprisingly, disparities are observed in anemia treatment strategies among patients with nondialysis CKD across different areas of the world. Over the past decade, the prescription and dosage of both iron therapies and erythropoiesis-stimulating agents have shifted, with notable regional differences observed. Moreover, there is ongoing debate regarding oral versus intravenous administration of iron. Compared with oral iron therapy, which often leads to gastrointestinal adverse events, low patient adherence, and low efficacy, intravenous iron administration has been associated with potential serious adverse events, such as anaphylaxis. New iron–based compounds and drugs currently under development are reviewed to describe their potential benefits in the treatment of anemia in patients with CKD. New oral compounds, including iron–based phosphate binders, heme iron polypeptide, and liposomal iron, show different rates of absorption with possibly different efficacy and improved tolerability. These new potential therapies offer health care providers additional anemia treatment options for their patients with CKD; however, the management of anemia in the CKD population continues to present challenges that require prospective studies to identify the optimal iron therapy for patients.
Keywords: chronic kidney disease, anemia, intravenous, anemia, Erythropoiesis, Ferric Compounds, Humans, Iron, Patient Compliance, Prospective Studies, Renal Insufficiency, Chronic
Introduction
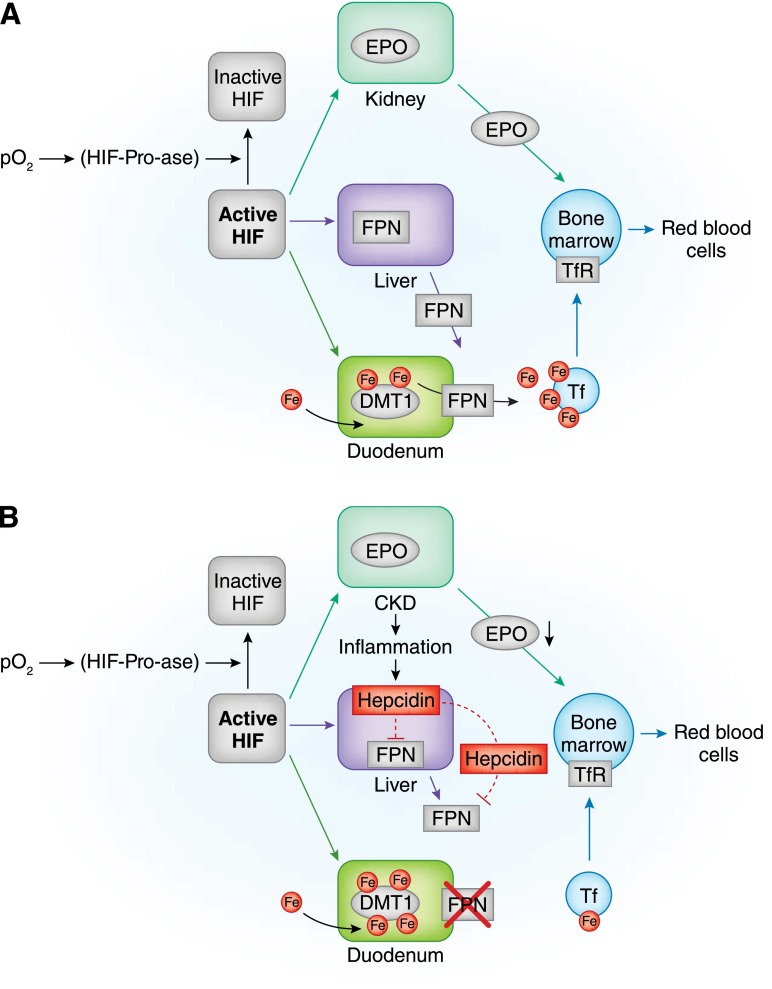
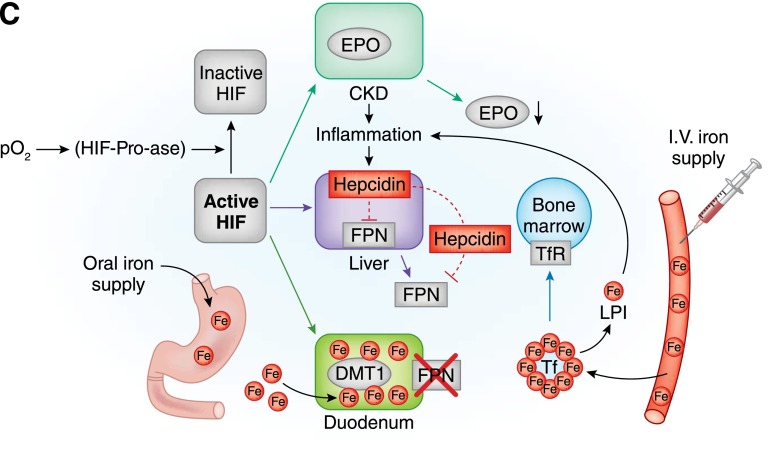
Guidelines for treating anemia in patients with CKD, particularly patients with nondialysis (ND) -CKD, vary, especially across different areas of the world (1–8). In addition, the growing number of patients with CKD and anemia and the increasingly insufficient number of nephrologists have led primary care physicians, particularly those in the United States, to take on the burden of care for many of these patients (9). There are clinical and logistical concerns in treating to correct anemia in this patient population because of the scarcity of evidence regarding treatment of patients with ND-CKD compared with the dialysis CKD population. There are several causes, including the lack of access to facilities for intravenous (iv) administration, conflicting reports regarding safety of treatments, and poor tolerability and efficacy of oral preparations, particularly in patients with CKD stage 5. These concerns have caused confusion as to how to optimize anemia therapy for patients with ND-CKD and led some health care providers to choose not to treat anemia in these patients at all. However, untreated moderate to severe anemia in patients with CKD has been associated with left ventricular hypertrophy, congestive heart failure, and coronary heart disease (9). Additionally, untreated anemia can have consequences regarding the patient’s overall health–related quality of life (HRQoL). Moreover, recent experimental (10) and clinical (11) evidence points to iron status as a modulator of fibroblast growth factor 23 (FGF23) and phosphate metabolism, thus broadening iron’s potential clinical implications. Conversely, the consequence of excess iron can promote oxidative stress, leading to protein oxidation and lipid peroxidation. These processes may accelerate cellular apoptosis as shown in nonclinical studies; however, the association with advancing cardiovascular disease is proposed but not yet confirmed (12). A consensus is required to aid health care providers in choosing the best therapeutic plan to treat iron deficiency anemia in patients with ND-CKD. An overview of the current understanding of anemia in CKD, underlying mechanisms of erythropoiesis, and iron absorption is provided in Figure 1.
Figure 1.
Anemia, erythropoiesis, and iron absorption in normal subjects and patients with CKD. (A) In normal subjects, by inhibiting hypoxia–inducible factor–prolyl–hydroxylases (HIF-Pro-ase), hypoxia stabilizes hypoxia-inducible factor (HIF), thus increasing its activity. In time, the HIF signaling cascade increases renal synthesis of erythropoietin (EPO), liver synthesis of ferroportin (FPN), and duodenal synthesis of divalent metal transporter 1 (DMT1). By targeting duodenal cells, FPN favors iron transfer into blood. In blood, iron is bound to transferrin (Tf) and transported to transferrin receptor (TfR) –expressing cells. Bone marrow cells supplied with iron and stimulated by EPO can efficiently increase red blood cell production. (B) In chronic inflammatory states, like chronic renal failure , increments in HIF do not efficiently increase renal synthesis of EPO or liver synthesis of FPN. This last effect also results from increased liver synthesis of epcidin, an inflammatory cytokine capable of inhibiting both FPN liver synthesis and its binding to duodenal cells. Intestinal absorption of iron is blunted with possibly increased intracellular iron concentrations. With lower iron availability and inadequate EPO stimulation, bone marrow cannot efficiently increase red blood cell production. (C) Iron supply in chronic renal failure can be obtained by either oral or intravenous administration. The oral route can be regarded as physiologic but is hampered by reduced efficiency of intestinal absorption and gastrointestinal side effects; the unphysiologic intravenous administration is hindered by possible toxic effects through production of labile plasma iron (LPI). Lines indicate activation, and dashed lines indicate inhibition. Fe, iron; I.V., intravenous; pO2, partial pressure of oxygen.
Figure 1.
Continued.
Current Treatment Guidelines
Taking into account these various anemia therapy approaches, treatment guidelines and position papers by a number of societies and working groups across the world have been developed. Guidance regarding patients with ND-CKD include the Kidney Disease Improving Global Outcomes (KDIGO) (1) and the Kidney Disease Outcomes Quality Initiative (2) guidelines and position papers from the Canadian Society of Nephrology (3–6), the National Institute for Health and Care Excellence (NICE) (7), and European Renal Best Practice (8) (Table 1). Although there are some exceptions, these guidelines and position papers are generally consistent regarding the definition of hemoglobin (Hb) thresholds of anemia. However, they are less consistent in recommendations regarding iron supplementation and erythropoiesis–stimulating agent (ESA) use and defining target Hb ranges and maximum ferritin levels. Regional differences in treatment preferences are also evident, because the position papers from outside of the United States, including those from the NICE and the Canadian Society of Nephrology, generally consider HRQoL effects, whereas the United States–based guidance does not emphasize HRQoL parameters. However, all of the guidelines and position papers agree that any iron deficiency should be addressed before initiating ESA therapy and suggest that the decision to prescribe ESAs should be on the basis of Hb levels and clinical factors. These guidelines and position papers are updated periodically to reflect new research results; however, because of differences in how clinical trial results are evaluated and the lack of strong evidence supporting particular approaches, the interpretation of these results can be conflicting and lead to widely different treatment recommendations. In particular, studies of iron therapy have not provided clear results as opposed to the evidence supporting ESA use.
Table 1.
Summary of current clinical guidelines to treat anemia in adult patients with nondialysis-dependent CKD
| Guideline | Diagnosis of Anemia | ESA Use | Iron Use | Hb Target | TSAT/Ferritin Target | Phosphorus Target | Adjuvants |
| KDIGO (1,83) | Hb<130 g/L in men >15 yr; Hb<120 g/L in women >15 yr | Initiate ESA therapy in those with Hb<100 g/L after all other correctable causes of anemia have been addressed and symptoms of anemia are present and for avoiding transfusions; therapy should be individualized for each patienta | 1- to 3-mo trial of oral iron or iv ironb if TSAT is <30% and ferritin is <500 μg/L or ESA therapy is to be avoided or decreased (if already on ESA therapy) | Hb≤115 g/L in adults on ESA therapy but should be on the basis of individual patients | TSAT>30%/ferritin >500 μg/L | CKD stages 3–5: Maintain serum phosphorus within the normal range | Not recommended: androgens, vitamin C, vitamin D, vitamin E, folic acid, l-carnitine, and pentoxifylline |
| KDOQI (2,84) | Hb<135 g/L in adult men; Hb<120 g/L in adult women | Initial ESA dose and ESA dose adjustments determined by the patient’s Hb level, target Hb level, rate of increase in Hb level, and clinical circumstancesc | Oral or iv iron administered to maintain ferritin >100 μg/L and TSAT>20% during ESA treatmentd | Hb>110 g/L; ESA-treated patients should not be routinely maintained at Hb≥130 g/L | TSAT≥20%/ferritin >100 and <500 μg/L | Stages 3 and 4 CKD: ≥2.76 and <4.61 mg/dl; stage 5 CKD: >3.50 and <5.51 mg/dl | Not routinely recommended: l-carnitine and ascorbate; not recommended: androgens |
| Canadian Society of Nephrology (3–6,85) | Hb<135 g/L in men ≥18 yr; Hb<120 g/L in women ≥18 yr | Initiate ESA therapy when iron stores have been corrected, other reversible causes of anemia have been treated, and Hb level is sustained at <100 g/L in patients not receiving ESA therapye; for patients receiving ESA therapy, target Hb is 110 g/L | Oral or iv iron to maintain target concentrations of TSAT and ferritinf; iron is not recommended for patients without evidence of classic iron deficiency whose Hb is >110 g/L | Hb<110 g/L (acceptable Hb range =100–120 g/L) | TSAT>20%/ferritin >100 μg/L; iron should be considered if patient is below target Hb or requires high ESA doses and has ferritin >800 μg/L and TSAT<25% | Does not recommend monitoring phosphorus for stage 3 CKD; stages 4 and 5 CKD: Maintain within normal range | Not recommended: androgens; insufficient evidence to recommend: l-carnitine, vitamin C, ultrapure dialysis, pentoxifylline, statins, vitamin B6, and quotidian dialysis |
| NICE (7) | Hb<110 g/L or development of symptoms attributable to anemia (tiredness, shortness of breath, lethargy, and palpitations) | ESA therapy should not be initiated in the presence of absolute iron deficiency without also managing the iron deficiency; ESAs not recommended in the presence of comorbidities or a prognosis that is likely to negate the benefits of correcting the anemiag | Oral or iv iron to maintain hypochromic RBC, reticulocyte, or TSAT and ferritin targets | 100–120 g/L; rate of Hb increase should be between 10 and 20 g/L per mo in patients on ESA therapyh | Recommend using hypochromic RBC >6% (unless ferritin is >800 μg/L) or reticulocyte count >29 pg (unless ferritin is >800 μg/L); if tests are not available, TSAT>20%/ferritin >100 μg/L; maintain ferritin <800 μg/L in patients on iron therapy | Recommend following British, American, and European treatment guidelines | Not recommended: androgens, vitamin C, folic acid, and carnitine |
| ERBP (8,86) | Hb<135 g/L in adult men ≤70 yr; Hb<132 g/L in adult men >70 yr; Hb<120 g/L in adult women | In low-risk patients or those in whom a clear benefit in quality of life can be foreseen, ESA therapy can be considered at Hb<120 g/L; in high-risk patients, ESA therapy should be initiated at Hb values between 90 and 100 g/Li | Oral or iv iron if TSAT is <20% and ferritin is <100 μg/L or if Hb increases without ESA therapy, TSAT is <25%, and ferritin is <200 μg/L; in patients on ESA therapy in whom increase in Hb or decrease in ESA dose is desired, oral or iv iron may be given when TSAT is <30% and ferritin is <300 μg/L | 100–120 g/L; not >130 g/L for patients receiving ESA therapy; approximately 100 g/L in high-risk patients | TSAT≥20%/ferritin =100 μg/L; should not exceed TSAT>30%/ferritin >500 μg/L | For CKD stages 3–5, maintain within normal range | Not recommended |
ESA, erythropoiesis-stimulating agent; Hb, hemoglobin; TSAT, saturated transferrin; KDIGO, Kidney Disease Improving Global Outcomes; iv, intravenous; KDOQI, Kidney Disease Outcomes Quality Initiative; NICE, National Institute for Health and Care Excellence; RBC, red blood cell; ERBP, European Renal Best Practice.
ESA dosing should be on the basis of the individual patient Hb, body weight, and clinical circumstances and should be adjusted on the basis of Hb, rate of change in Hb, current ESA dose, and clinical circumstances. The choice of ESA should be on the basis of the balance of pharmacodynamics, safety information, clinical outcome data, cost, and availability. Subcutaneous dosing is recommended for administration of ESA therapy to patients with nondialysis CKD. Initiation of ESA therapy should be with great caution, if at all, in patients with active malignancy or a history of stroke.
The route of iron administration should be on the basis of the severity of iron deficiency, the availability of venous access, response to prior oral iron therapy, side effects with prior iron therapy, patient compliance, and cost.
The route of ESA administration should be determined by the CKD stage, treatment setting, efficacy, safety, and class of ESA used in adults. The anticipated frequency and pain of administration and the potential effects on the child and family should also be considered in pediatric patients. Convenience favors subcutaneous administration and less frequent ESA administration in adult patients with nondialysis CKD.
Clinicians should judge the individual patient’s clinical status and ESA responsiveness and base iron treatment decisions on this assessment.
ESAs should be administered subcutaneously on the basis of improved efficacy and convenience.
Oral iron is the preferred first–line therapy in patients with nondialysis CKD. In patients who do not meet serum ferritin or TSAT targets on oral iron or in whom oral iron is not tolerated, iv iron may be used. The medical decision regarding the use of iron therapy should be guided by results of iron status tests together with Hb levels, ESA dose, and patient status.
When ESA has been initiated, the effectiveness of the ESA should be assessed after an agreed on interval. Where appropriate, a mutual decision between the clinician, the patient with anemia and CKD, and the family/caregivers should be made on whether to continue ESA therapy. The patient and clinician should agree to the route of ESA administration taking into account the individual patient’s clinical course, pain of injection, frequency of administration, lifestyle and preferences, efficacy, and cost.
When determining individual Hb targets for patients with anemia and CKD, take into account patient preferences, symptoms, comorbidities, and the required treatment.
The decision on whether and when to begin ESA therapy in patients with nondialysis CKD should be individualized and take into account the rate of fall of Hb, prior response to iron therapy, risk of transfusion, risks related to ESA therapy, and the presence of symptoms attributable to anemia. Hb values should not routinely be allowed to fall to <100 g/L in patients with nondialysis CKD. Therapy with ESAs should not be started if there is a temporary and obvious cause of anemia that is potentially reversible. Risk factors for stroke, a history of malignancy, or the presence of active malignancy should be considered when weighing the risk-to-benefit ratio of ESA therapy.
As reflected in the various criteria used in the guidelines and position papers described above, the evaluation of therapies for anemia presents a challenge to health care providers, because so many measures of effectiveness need to be considered, including efficacy targets, safety evaluations, and HRQoL. The most frequently used efficacy measurements to assess anemia therapies are Hb, ferritin (as a marker of iron stores), and transferrin saturation (TSAT; as a marker of available iron). Apoferritin is a hollow protein sphere that surrounds a core of hydrous ferric oxide, in which up to 4500 iron atoms can be stored bound to oxygen in the ferric state (13). Although all ferritins contain the same core structure, there are variations in the ferritin molecule depending on cell type and function (13,14). Interestingly, serum ferritin is derived from the same gene sequence as intracellular ferritin; however, serum ferritin is iron poor, because it consists predominantly of l-chain subunits, and there may be a combination of ferritin and apoferritin (not combined with iron) (15). In addition, there is current controversy regarding whether serum ferritin contains iron. Ferritin serves to sequester iron for storage; thus, when intracellular iron increases, ferritin synthesis increases, and expression of cell surface transferrin receptors decreases (16). The iron storage disorder called hereditary hemochromatosis, an autosomal recessive genetic disorder that usually results from defects in the HFE gene, although rare, should also be taken into consideration when evaluating patients with CKD for anemia (17). However, the specificity and sensitivity of ferritin and TSAT for the evaluation of iron status are not ideal, especially when evaluating iron repletion and excess iron, because they can be affected by other causes, such as inflammation (ferritin) and malnutrition (TSAT), providing misleading results (18).
Consequently, investigation into other biomarkers of iron deficiency has begun. Reticulocyte Hb content has been shown to be a more stable and sensitive marker of iron deficiency than ferritin or TSAT in a clinical trial of erythropoietin (EPO) -treated patients on dialysis but is not routinely tested in patients with ND-CKD (19). Other measures include the percentage of labile plasma iron (20), soluble transferrin receptor 1 (21), and hepcidin levels (22,23), but these assays must be further refined before general use (24,25).
In addition to efficacy measurements, the safety of treatments to correct iron deficiency anemia has become a growing concern, because severity of associated adverse events (AEs) needs to be carefully monitored. Also, although often neglected (26), the patient’s overall HRQoL, including physical functioning, pain, general health and vitality, social functioning, and mental and emotional health (27), is another outcome to consider when choosing a treatment plan and biomarker targets. For example, HRQoL should be considered when choosing an upper Hb target, particularly in younger patients without comorbidities, because physical activity would be important to most of these patients.
ESA Therapy
Since the approval of recombinant human EPO by the Food and Drug Administration (FDA) in 1989, ESAs have become a key component of anemia treatment plans for patients with CKD. Therapy with ESAs is intended to stimulate erythropoiesis and consequently, increase Hb levels; however, as described above, target Hb levels are debated as well as the correlation between high Hb levels and HRQoL. Some studies have shown that higher Hb levels have a significant association with improved HRQoL scores, including both physical and mental summary scores and general health scores (28,29). In the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta Trial, significantly better HRQoL measures were observed in the group treated with epoetin to maintain high Hb levels (130–150 g/L) over the group with a lower Hb target (105–115 g/L) (30). In the Correction of Hemoglobin and Outcomes in Renal insufficiency (CHOIR) Study, primary analysis of the composite events of death, myocardial infarction, hospitalization caused by congestive heart failure, and stroke showed a higher rate in patients with ND-CKD treated with weekly epoetin targeting a higher Hb level compared with those targeting a lower Hb level (135 versus 113 g/L; 17% versus 13.5%; P=0.03) (31). Attempting to attain the higher Hb concentrations required higher doses of epoetin, and these doses were associated with more AEs. The CHOIR Study also indicated no significant increase in overall HRQoL outcomes at higher Hb values. The placebo–controlled Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT), which evaluated patients with CKD and type 2 diabetes, found no differences in the composite primary end point of mortality and cardiovascular comorbidities, whereas there was increased risk of stroke and neoplasia in ESA-treated patients; however, a secondary analysis showed that patients in the ESA group reported a significant improvement in fatigue compared with those in the placebo group as well as numerically, but not significantly, higher improvements in other HRQoL measures in the ESA group (32).
In the United States, EPO dosages decreased by about 20% from 2005 to 2009 among patients with ND-CKD (33). Additionally, an analysis of ESA use among the ND-CKD population in the United States before and after the release of results from the TREAT showed that, in the 2 years after the release of the study results, ESA use declined 38% and 22% among patients with ND-CKD stage 3 or 4, respectively (34). Also, a 15% decrease was observed in Hb levels >120 g/L among patients with ND-CKD (33,35). In 2011, the FDA changed ESA prescribing to remove the target Hb range of 10–12 g/dl and replaced it with the recommendation to consider initiating ESA treatment when Hb is <10 g/dl and symptoms of anemia are present (36).
In contrast to the trends observed in the United States, between 2008 and 2013 in Sweden, ESA doses slightly increased among patients with ND-CKD (37). In Taiwan, restrictive criteria for ESA reimbursement were introduced by the National Health Insurance Administration in 1996, resulting in much lower ESA dosing and use (38). These guidelines provide strict criteria for when ESA therapy can be initiated in patients with ND-CKD (serum creatinine >6 mg/dl and a hematocrit <28% to maintain a hematocrit level not exceeding 30%), limiting its use in this patient population (38).
Iron Therapy
The treatment guidelines universally recommend iron therapy to correct iron deficiency in patients with ND-CKD (Table 1); however; the type of iron therapy and route of administration have been topics of much controversy, with differences observed regionally. In patients with ND-CKD in Sweden, oral iron therapy decreased from 15.1% in 2008 to 11.0% in 2013, whereas iv iron administration increased from 3.8% in 2008 to 5.5% in 2013 (37). Although changes in iron therapy administration among the ND-CKD population have not been collected in the United States or many other regions of the world, trends in recent years in the United States among the dialysis CKD population have shown an increase in iv iron administration (39). The reason for these regional differences in iron use could be because of differences in guidelines, but there are not enough data regarding the ND-CKD population at this time to draw such conclusions. The CKD Outcomes and Practice Patterns Study team is a collaborative research relationship among experts in France, Germany, Brazil, and the United States that uses its international scope to facilitate comparisons of nephrology practices in different countries. The overall goal is to develop an evidence base for the effective treatment of advanced CKD, thereby improving care and outcomes for future patients. Numerous topics are currently being explored, including anemia; thus, these studies may provide insight into these trends in the future.
Changes in iron therapy trends have been observed regionally and over the past decade, and ferritin levels in the dialysis population have shown increasing variation among countries, with the United States presenting with the highest national average of ferritin and Japan showing the lowest (40). These country-specific differences are not as clearly identified in the ND-CKD population. In the latest study from Sweden of patients with ND-CKD, average serum ferritin levels did not change significantly over the period from 2008 to 2013, despite increases in iv iron use over that same period (37). It would be interesting to identify whether the increase in ferritin levels within the United States CKD population occurs earlier during disease management or if the increase in ferritin coincides with the incidence of dialysis. Despite the recognition that ferritin is a poor marker of iron stores, it remains to be identified if elevated levels of ferritin contribute to poorer outcomes, especially in patients with ND-CKD. For example, a prospective study in patients with ND-CKD showed a dose dependency between iv iron doses and liver iron concentration; however, no dose dependency was observed in changes in serum ferritin or TSAT concentrations (18,41–43).
Iron supplementation can be administered by iv and oral routes (1,2,7,8). The KDIGO guidelines recommend that, for patients with ND-CKD who require iron supplementation, the route of iron administration should be selected on the basis of severity of iron deficiency, availability of venous access, response to prior oral iron therapy, side effects with prior oral or iv iron therapy, patient adherence, and cost (1). Health care providers have begun reconsidering oral iron therapy over iv iron therapy, particularly for the ND-CKD population, for multiple reasons, including safety concerns regarding the regulation of iv iron, ease of administration, vein preservation for future vascular access, and cost. Typical AEs associated with oral iron administration are nausea, vomiting, constipation, and a metallic taste, which are usually reported as relatively mild in intensity (44).
iv Iron Therapies
iv Iron was evaluated for treating anemia as early as 1932 (45), but the 10% ferric ammonium citrate formulation produced severe AEs. Updated iv iron formulations were introduced for treating anemia beginning in 1964. Early iv iron formulations, such as high molecular weight dextran, raised concerns of severe anaphylactoid reactions (46). Newer formulations have sought to reduce immunogenicity by reducing the size and branch characteristics of the polysaccharide, which may reduce some of the safety issues associated with earlier formulations (47).
Moreover, treatment with iv iron has also raised more serious safety concerns, such as for renal injury (48), cardiovascular injury (49), calciphylaxis (50), and labile iron toxicity (51). The administration of iv iron has been shown to cause oxidative stress from large amounts of iron being introduced in the blood during a single infusion (48). In an in vitro study comparing iv iron dextran, iron sucrose, iron gluconate, and iron oligosaccharide, all formulations produced oxidative stress, which was shown by increases in lipid peroxidation (52). However, differences in cell death were observed in this study where ferric sucrose caused the highest rate of cell death, followed by ferric glucose, ferric dextrose, and ferric oligosaccharide, indicating significantly different toxicity profiles (52). These in vitro data have been supported by animal studies (53) as well as studies in patients on hemodialysis (54,55) and patients with ND-CKD (56). In a study of patients with ND-CKD treated with iv ferric carboxymaltose, levels of both oxidative stress and inflammatory markers increased after iron treatment, regardless of response to the iron therapy (56). Oxidative stress markers analyzed included oxidized low–density lipid protein, protein carbonyl groups, erythrocyte superoxide dismutase, and glutathione peroxidase. Glutathione peroxidase activity was lower in nonresponders compared with responders; however, levels of all other oxidative stress markers did not show significant differences between the two groups. The long-term effects of such iron–induced oxidative stress have not been determined.
Recent clinical trial results have continued to present conflicting efficacy and safety conclusions regarding the use of iv iron to treat anemia in the ND-CKD population. In the randomized, clinical trial the Randomized Trial to Evaluate Intravenous and Oral Iron in Chronic Kidney Disease (REVOKE) that investigated iv iron sucrose (200 mg once every 2 weeks for a total of 1 g) versus oral ferrous sulfate (325 mg three times daily for 8 weeks) in patients with ND-CKD and moderate to severe anemia, efficacy measurements were similar between the two treatment groups. However, the trial was terminated early because of an increased risk of serious AEs, particularly cardiovascular AEs and infections, in the iv iron treatment group (57). In contrast, another randomized clinical trial known as the Ferinject Assessment in Patients with Iron Deficiency Anemia and Non-Dialysis-Dependent Chronic Kidney Disease Study evaluated the difference between iv ferric carboxymaltose (high [1000 mg followed by 500–1000 mg every 4 weeks depending on ferritin level] and low [200 mg followed by 200 mg every 4 weeks] doses) and oral iron therapy (100 mg ferrous sulfate twice daily) in the same population and found that iv iron therapy was more effective in reaching and maintaining Hb levels and reduced the need for additional anemia management (58). The investigators did not observe the same increase in serious AEs as those reported in the REVOKE. Similarly, in an open-label clinical trial comparing iv ferric carboxymaltose (1000 mg up to three times over 8 weeks) with oral ferrous sulfate (325 mg three times daily), statistically significant increases in Hb, ferritin, and TSAT were observed in the iv versus oral group, with significantly fewer treatment–related AEs in the iv iron group (59). A recent phase 3, open-label clinical study compared the use of iv iron isomaltoside 1000 (500–1000 mg weekly) with oral ferrous sulfate (100 mg twice daily) in ESA-naïve patients with ND-CKD over 8 weeks and found that the iv iron group had statistically significant improvement of iron deficiency markers, including Hb, serum ferritin, and TSAT concentrations, over the oral iron group (60). A similar number of AEs was reported in each treatment group; however, a larger percentage of patients in the oral ferrous sulfate group (4.3% versus 0.9%) discontinued the trial because of AEs. Thus, safety results in regards to iv iron administration are different among studies, and the long-term consequences of continued iv iron are yet to be determined.
Other considerations for iv versus oral iron administration include ease of administration and cost. Oral iron is inexpensive and administered at home. Patients receiving iv iron are required to visit a health care facility for treatment by a skilled health care professional. Although considered rare, AEs (especially with first treatment or initiation of a new formulation), including acute chest and back tightness, allergic reactions, or anaphylactic shock (61), can occur, and, thus, the iv iron therapies should only be administered in facilities where resuscitation and treatments for anaphylaxis are readily available (62). Although rare, the chance of allergic reactions with iron treatment prompted the European Medicines Agency to release new recommendations to manage the risk of allergic reactions with iron-containing medicines in 2013 (63). In addition, recurrent infusions of iron can lead to problems with venous anatomy and quality, thereby compromising future vascular access construction (1), which needs to be preserved for potential future hemodialysis treatments.
Oral Iron Therapies
The use of oral iron in medicine has a more colorful history (64). Although oral ferrous sulfate was empirically prescribed as early as the 1700s to treat “chlorosis” in adolescent girls, which was characterized by “pale color, palpitation of the heart,” and “confusion of the spirits,” it was only in the 1830s that any rationale for this treatment for anemia was provided (64). Still, it remained contested into the late 1890s whether inorganic iron could be incorporated into Hb, and the relationship between oral iron and anemia was finally accepted with the advent of radioactive tracer experiments in the late 1930s that showed gut iron absorption (64).
The bioavailability of oral iron sources has been a major concern for the treatment of anemia. Iron is absorbed in the intestine in a highly regulated metabolic cycle. Hepcidin has been shown to be an important regulator of iron metabolism by inhibiting release of intestinal iron being absorbed into the blood (65). Hepcidin inhibition increases during states of inflammation, such as inflammation experienced by patients with CKD, and during iron overload, resulting in decreased iron absorption (Figure 1) (65,66). Thus, new oral iron therapies are required to provide efficacy with better patient compliance. The most promising of the new therapies along with conventional options for oral iron supplementation are reviewed in Table 2, and a summary of oral iron therapies is also provided in Table 2.
Table 2.
Oral iron supplementation therapies in patients with nondialysis-dependent CKD
| Therapy | Chemical Formula | Therapy Duration | Efficacy Measuresa | Adverse Events | Studies in Nondialysis CKD | |||
| Hb | Fer | TSAT | Frequency, %b | Most Common Adverse Eventsc | ||||
| Ferrous sulfate | FeSO4 | 8 wk | X | X | X | 68.1 | Stool discoloration, URI, diarrhea, traumatic injury, fall, constipation, dyspnea, chest pain, dizziness, UTI, hyperkalemia, hypertension | Agarwal et al. (57) |
| Ferrous sulfate | FeSO4 | 52 wk | X | X | X | 81.7 | Diarrhea, constipation, hypertension, peripheral edema, dyspepsia, UTI, nasopharyngitis | Macdougall et al. (58) |
| Ferrous sulfate | FeSO4 | 56 d | X | — | X | 59.2 | Constipation | Qunibi et al. (59) |
| Ferrous sulfate | FeSO4 | 12 mo | — | — | — | NR | NR | McMahon et al. (87) |
| Ferrous sulfate | FeSO4 | 6 wk | — | X | X | 20d | Constipation, stool discoloration, nausea, diarrhea | Agarwal et al. (88) |
| Ferrous sulfate | FeSO4 | 29 d | X | — | — | NR | NR | Charytan et al. (89) |
| Ferrous sulfate | FeSO4 | 56 d | X | X | X | 19 | Constipation, diarrhea, nausea, vomiting | Van Wyck et al. (90) |
| Ferrous sulfate | FeSO4 | 3 mo | X | — | — | NR | NR | Aggarwal et al. (91) |
| Ferrous sulfate | FeSO4 | 5.2 mo | X | X | NR | NR | NR | Stoves et al. (92) |
| Ferrous fumarate | C4H2FeO4 | 21 d | X | X | X | 52 | Constipation, diarrhea | Spinowitz et al. (93) |
| Ferric citrate | FeC3H5O (COO)3 | 12 wk | X | X | X | 68.3 | Diarrhea, constipation, abdominal discomfort, abdominal distensione | Yokoyama et al. (94) |
| Ferric citrate | FeC3H5O (COO)3 | 12 wk | X | X | X | 69.3 | Stool discoloration, diarrhea, constipation, nausea, vomiting, URI | Block et al. (74) |
| Heme iron | 6 mo | X | X | X | NR | Constipation, abdominal cramps, muscle cramps, bloating, diarrhea, nausea | Nagaraju et al. (80) | |
| Liposomal iron | Fe4(P2O7)3 | 3 mo | X | — | — | 3.1d | — | Pisani et al. (81) |
Hb, hemoglobin; Fer, ferritin; TSAT, transferrin saturation, X, evidence of therapeutic efficacy for the given measure; URI, upper respiratory tract infection; UTI, urinary tract infection; —, lack of efficacy evidence; NR, not reported.
Assessed at the conclusion of treatment.
Unless otherwise noted, percentage of subjects experiencing at least one adverse event.
Unless otherwise noted, most common treatment–emergent adverse events occurring in ≥5% of participants.
Percentage of subjects experiencing at least one treatment–related adverse event.
Most common treatment–emergent and –related adverse events occurring in ≥5% of participants.
Iron Salts
Iron salts have historically been the most common form of oral iron supplementation used in patients with ND-CKD. Iron salts exist as the ferrous (Fe2+) and ferric (Fe3+) forms. For gut absorption, iron must be in the ferrous form; thus, dietary ferric iron is reduced to the ferrous form to be absorbed (67). Prescribed oral iron salt preparations are in the ferrous form, including ferrous sulfate, ferrous fumarate, ferrous gluconate, and polysaccharide iron complex (68). Among these, ferrous sulfate is by far the most frequently prescribed for treating anemia in patients with ND-CKD (44). These oral iron salts have been shown to increase Hb, ferritin, and TSAT in this population (69,70). Administration of these iron salts can produce a rapid release of iron ions in the gastrointestinal tract, leading to oxidative stress and resulting AEs, particularly those affecting the gastrointestinal tract (67,71). Mild to moderate AEs of constipation, nausea, vomiting, and diarrhea are often reported in trials of oral iron salts, such as ferrous sulfate, but serious AEs related to treatment are rare. Recent evidence has also suggested that unabsorbed oral iron can lead to changes in the intestinal microbiome by stimulating the growth of bacterial pathogens, which threaten the natural microbiota of the gut and lead to additional AEs (72). Because of frequent gastrointestinal side effects, patients with ND-CKD often have reduced adherence to oral iron therapy (44).
Iron–Based Phosphate Binders
Ferric citrate is an oral compound comprising trivalent iron with citrate. Originally developed and approved for use as a phosphate binder, it is water soluble and dissociates into ferric iron and citrate in the intestinal lumen (73). In a phase 2 study in patients with ND-CKD to evaluate the change in serum phosphate and TSAT, ferric citrate increased measures of iron repletion, including Hb, ferritin, and TSAT (74). As with other iron preparations, the most frequently observed AEs with ferric citrate were gastrointestinal (74). A phase 3 trial (NCT02268994) is currently underway in the United States to evaluate the effect of ferric citrate on changes in Hb compared with placebo in patients with ND-CKD. Sucroferric oxhydroxide is another iron–based phosphate binder recently approved for treatment of patients with CKD; however, the clinical trials showed no clinical or statistical increase in iron parameters when used as a phosphate binder in patients with CKD on dialysis (75).
Recent studies of the molecular mechanism of phosphate homeostasis have suggested a link between hyperphosphatemia and iron deficiency through the key regulatory hormone FGF23 (11). In addition, a historical population–based study found that higher phosphorus levels in patients with early CKD resulted in a greater chance of having anemia (76). Interestingly, ferric citrate, being administered with meals as a phosphate binder, has been shown to reduce FGF23 and increase iron parameters in ND-CKD (74).
Heme Iron Polypeptide
Heme iron polypeptide is an oral iron therapy that uses the heme porphyrin ring to supply iron to sites of absorption in the intestinal lumen (77). Because heme iron is absorbed via a different receptor than in nonheme (ionic) iron, the bioavailability of heme iron polypeptide in healthy individuals is significantly greater than that for nonheme iron (78). However, this increase has not uniformly translated into markedly improved outcomes in clinical studies (79). Nevertheless, heme iron polypeptide increases levels of Hb, ferritin, and TSAT in patients with ND-CKD (80).
As with other forms of oral iron supplementation, AEs associated with heme iron polypeptide therapy are primarily related to the gastrointestinal system in patients with ND-CKD. These AEs occurred at a frequency similar to that observed with iv iron therapy, including constipation, abdominal cramps, muscle cramps, bloating, diarrhea, and nausea (80).
Liposomal Iron
Liposomal iron is an oral iron preparation in which iron, as ferric pyrophosphate, is carried within a phospholipid membrane. Liposomal iron does not directly contact the intestinal mucosa before its release by liver enzymes. Consequently, this method of iron supplementation is associated with high gastrointestinal absorption, high bioavailability, and a low incidence of side effects (77).
Liposomal iron is an investigational drug currently under study. AEs associated with liposomal iron therapy in patients with ND-CKD occur at a lower frequency than typically observed for other oral or iv iron supplementation strategies (81). These AEs are generally mild and primarily related to the gastrointestinal system, with the most common being constipation, diarrhea, nausea, and headache. This therapy increases iron stores and Hb levels in patients with ND-CKD, but early data suggest that it does so less effectively than iv iron therapy (81).
Other Approaches to Treat Iron Deficiency Anemia in Patients with CKD
Although ESAs and iron therapy continue to be the main therapies used in the treatment of anemia in patients with CKD, other approaches to anemia treatment are currently being investigated in clinical studies. These include hypoxia–inducible factor stabilizers and hepcidin modulators (82). In addition to ESA and iron therapies, these could offer alternative or complementary therapies for the treatment of anemia in patients with CKD.
Conclusions
Despite disparities regarding target iron levels and the absence of reliable markers of iron deficiency, the importance of correcting iron deficiency in patients with CKD is indisputable. Oral iron therapies offer safe and effective options to treat iron deficiency anemia in patients with CKD in a physiologic way. In contrast to iv iron therapies, oral iron treatments require few resources for administration and are not associated with potential serious AEs. However, traditional oral iron treatments are not optimal because of increased gastrointestinal AEs, lack of patient adherence, and very often, lack of efficacy in patients with stage 5 CKD. Overall, treatments currently under development for iron deficiency anemia may represent an improvement in therapeutic options to treat iron deficiency anemia.
Disclosures
F.L. was a member of an advisory board and/or speaker at meetings supported by Astellas, Akebia, Amgen, Inc. (Thousand Oaks, CA), GlaxoSmithKline (Brentford, United Kingdom), Janssen Biotech (Horsham, PA), Keryx Biopharmaceuticals, Inc., Pharmacosmos, Roche (Basel, Switzerland), and Takeda Chemical Industries (Osaka, Japan). F.L. is a council member of the International Society of Nephrology, country coordinator for the Dialysis Outcomes and Practice Patterns Study, and on the editorial boards of many journals, including the Journal of the American Society of Nephrology, Nephrology Dialysis Transplantation, Blood Purification, and the Journal of Nephrology. S.M. was an advisory board member and speaker at meetings supported by Amgen, Inc., AbbVie, Keryx Biopharmaceuticals, Inc., and Shire. S.M. is on the editorial board of Nephrology Dialysis Transplantation and the Journal of Nephrology. J.Y. was an advisory board member for Keryx Biopharmaceuticals, Inc., Fresenius Medical Care-North America, Corvidia, AbbVie, ZS Pharma (Coppell, TX), and Relypsa. J.Y. is on the editorial staff of the Nephrology Self-Assessment Program, Advances in Chronic Kidney Disease, and on the Editorial Board of the Clinical Journal of the American Society of Nephrology.
Acknowledgments
We acknowledge Lisa Ambrosini Vadola, medical writer and consultant (Whitsell Innovations, Inc., Chapel Hill, NC) for her assistance in the preparation of this manuscript with financial support from Keryx Biopharmaceuticals, Inc.
The preparation of this manuscript has been financially supported by Keryx Biopharmaceuticals, Inc.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.KDIGO: KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 2.Levin A, Rocco M; KDOQI; National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47[Suppl 3]: S11–S145, 2006 [DOI] [PubMed] [Google Scholar]
- 3.White CT, Barrett BJ, Madore F, Moist LM, Klarenbach SW, Foley RN, Culleton BF, Tonelli M, Manns BJ; Canadian Society of Nephrology: Clinical practice guidelines for evaluation of anemia. Kidney Int Suppl 74: S4–S6, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Madore F, White CT, Foley RN, Barrett BJ, Moist LM, Klarenbach SW, Culleton BF, Tonelli M, Manns BJ; Canadian Society of Nephrology: Clinical practice guidelines for assessment and management of iron deficiency. Kidney Int Suppl 74: S7–S11, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Moist LM, Foley RN, Barrett BJ, Madore F, White CT, Klarenbach SW, Culleton BF, Tonelli M, Manns BJ; Canadian Society of Nephrology: Clinical practice guidelines for evidence-based use of erythropoietic-stimulating agents. Kidney Int Suppl 74: S12–S18, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Klarenbach SW, Moist LM, Foley RN, Barrett BJ, Madore F, White CT, Culleton BF, Tonelli M, Manns BJ; Canadian Society of Nephrology: Clinical practice guidelines for supplemental therapies and issues. Kidney Int Suppl 74: S19–S24, 2008 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence: Anaemia Management in Chronic Kidney Disease–Partial Update, London, National Clinical Guideline Center, Royal College of Physicians, 2015 [Google Scholar]
- 8.Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, Cameron S; European Best Practice Guidelines Working Group: Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 19[Suppl 2]: ii1–ii47, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt RJ, Dalton CL: . Treating anemia of chronic kidney disease in the primary care setting: Cardiovascular outcomes and management recommendations. Osteopath Med Prim Care 1: 14, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phan O, Maillard M, Malluche HH, Stehle JC, Funk F, Burnier M: . Effects of sucroferric oxyhydroxide compared to lanthanum carbonate and sevelamer carbonate on phosphate homeostasis and vascular calcifications in a rat model of chronic kidney failure. BioMed Res Int 2015: 515606, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf M, White KE: . Coupling fibroblast growth factor 23 production and cleavage: Iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 23: 411–419, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdougall IC, Bircher AJ, Eckardt KU, Obrador GT, Pollock CA, Stenvinkel P, Swinkels DW, Wanner C, Weiss G, Chertow GM; Conference Participants: Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 89: 28–39, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Theil EC: . Ferritin: Structure, gene regulation, and cellular function in animals, plants, and microorganisms. Annu Rev Biochem 56: 289–315, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Jacobs A, Miller F, Worwood M, Beamish MR, Wardrop CA: . Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. BMJ 4: 206–208, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen LA, Gutierrez L, Weiss A, Leichtmann-Bardoogo Y, Zhang DL, Crooks DR, Sougrat R, Morgenstern A, Galy B, Hentze MW, Lazaro FJ, Rouault TA, Meyron-Holtz EG: . Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 116: 1574–1584, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Besarab A, Frinak S, Yee J: . An indistinct balance: The safety and efficacy of parenteral iron therapy. J Am Soc Nephrol 10: 2029–2043, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Vujić M: Molecular basis of HFE-hemochromatosis. Front Pharmacol 5: 42, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrari P, Kulkarni H, Dheda S, Betti S, Harrison C, St Pierre TG, Olynyk JK: . Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol 6: 77–83, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fishbane S, Shapiro W, Dutka P, Valenzuela OF, Faubert J: . A randomized trial of iron deficiency testing strategies in hemodialysis patients. Kidney Int 60: 2406–2411, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Pootrakul P, Breuer W, Sametband M, Sirankapracha P, Hershko C, Cabantchik ZI: . Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded beta-thalassemia/HbE patients treated with an oral chelator. Blood 104: 1504–1510, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Skikne BS, Flowers CH, Cook JD: . Serum transferrin receptor: A quantitative measure of tissue iron deficiency. Blood 75: 1870–1876, 1990 [PubMed] [Google Scholar]
- 22.Babitt JL, Lin HY: . Molecular mechanisms of hepcidin regulation: Implications for the anemia of CKD. Am J Kidney Dis 55: 726–741, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M: . Immunoassay for human serum hepcidin. Blood 112: 4292–4297, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Gaweda AE, Ginzburg YZ, Chait Y, Germain MJ, Aronoff GR, Rachmilewitz E: . Iron dosing in kidney disease: Inconsistency of evidence and clinical practice. Nephrol Dial Transplant 30: 187–196, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung M, Moorthy D, Hadar N, Salvi P, Iovin RC, Lau J: Biomarkers for Assessing and Managing Iron Deficiency Anemia in Late-Stage Chronic Kidney Disease. Comparative Effectiveness Review No. 83. (Prepared by the Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I.) AHRQ Publication No. 12(13)-EHC140-EF, 2012. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. Accessed August 25, 2015 [PubMed]
- 26.Locatelli F, Del Vecchio L: . Haemoglobin levels and health-related quality of life: A neglected hard end point. Nephrol Dial Transplant 29: 1272–1274, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Cruz MC, Andrade C, Urrutia M, Draibe S, Nogueira-Martins LA, Sesso RC: . Quality of life in patients with chronic kidney disease. Clinics (Sao Paulo) 66: 991–995, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein FO, Story K, Firanek C, Mendelssohn D, Barre P, Takano T, Soroka S, Mujais S: . Health-related quality of life and hemoglobin levels in chronic kidney disease patients. Clin J Am Soc Nephrol 4: 33–38, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Goeij MCM, Meuleman Y, van Dijk S, Grootendorst DC, Dekker FW, Halbesma N; PREPARE-2 Study Group: Haemoglobin levels and health-related quality of life in young and elderly patients on specialized predialysis care. Nephrol Dial Transplant 29: 1391–1398, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Drüeke TB, Locatelli F, Clyne N, Eckardt K-U, Macdougall IC, Tsakiris D, Burger H-U, Scherhag A; CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer MA, Burdmann EA, Chen C-Y, Cooper ME, de Zeeuw D, Eckardt K-U, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Regidor D, McClellan WM, Kewalramani R, Sharma A, Bradbury BD: . Changes in erythropoiesis-stimulating agent (ESA) dosing and haemoglobin levels in US non-dialysis chronic kidney disease patients between 2005 and 2009. Nephrol Dial Transplant 26: 1583–1591, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Thamer M, Zhang Y, Kshirsagar O, Cotter DJ, Kaufman JS: . Erythropoiesis-stimulating agent use among non-dialysis-dependent CKD patients before and after the trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) using a large US health plan database. Am J Kidney Dis 64: 706–713, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miskulin DC, Zhou J, Tangri N, Bandeen-Roche K, Cook C, Ephraim PL, Crews DC, Scialla JJ, Sozio SM, Shafi T, Jaar BG, Boulware LE; DEcIDE Network Patient Outcomes in End Stage Renal Disease Study Investigators: Trends in anemia management in US hemodialysis patients 2004-2010. BMC Nephrol 14: 264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.FDA Drug Safety Communication: Modified Dosing Recommendations to Improve the Safe Use of Erythropoiesis-Stimulating Agents (ESAs) in Chronic Kidney Disease. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm#hcp Accessed December 14, 2015
- 37.Evans M, Suttorp MM, Bellocco R, Hoekstra T, Qureshi AR, Dekker FW, Carrero JJ: Trends in haemoglobin, erthyropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013 [published online ahead of August 4, 2015]. Nephrol Dial Transplant. [DOI] [PubMed]
- 38.Hung SC, Kuo KL, Tarng DC, Hsu CC, Wu MS, Huang TP: . Anaemia management in patients with chronic kidney disease: Taiwan practice guidelines. Nephrology (Carlton) 19: 735–739, 2014 [DOI] [PubMed] [Google Scholar]
- 39.US DOPPS Practice Pattern Monitor: August 2015. Available at: http://www.dopps.org/DPM. Accessed October 26, 2015
- 40.Fuller DS, Bieber BA, Pisoni RL, Li Y, Morgenstern H, Akizawa T, Jacobson SH, Locatelli F, Port FK, Robinson BM: . International comparisons to assess effects of payment and regulatory changes in the United States on Anemia practice in patients on hemodialysis: The Dialysis Outcomes and Practice Patterns Study [published online ahead of print November 18, 2015]. J Am Soc Nephrol doi:ASN.2015060673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fishbane S, Pollack S, Feldman HI, Joffe MM: . Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988-2004. Clin J Am Soc Nephrol 4: 57–61, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Besarab A: . Anemia and iron management. Semin Dial 24: 498–503, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Kalantar-Zadeh K, Kalantar-Zadeh K, Lee GH: . The fascinating but deceptive ferritin: To measure it or not to measure it in chronic kidney disease? Clin J Am Soc Nephrol 1[Suppl 1]: S9–S18, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Camaschella C: . Iron-deficiency anemia. N Engl J Med 372: 1832–1843, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Heath CW, Strauss MB, Castle WB: . Quantitative aspects of iron deficiency in hypochromic anemia: (The parenteral administration of iron). J Clin Invest 11: 1293–1312, 1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamstra RD, Block MH, Schocket AL: . Intravenous iron dextran in clinical medicine. JAMA 243: 1726–1731, 1980 [PubMed] [Google Scholar]
- 47.Balakrishnan VS, Rao M, Kausz AT, Brenner L, Pereira BJ, Frigo TB, Lewis JM: . Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest 39: 489–496, 2009 [DOI] [PubMed] [Google Scholar]
- 48.Agarwal R, Vasavada N, Sachs NG, Chase S: . Oxidative stress and renal injury with intravenous iron in patients with chronic kidney disease. Kidney Int 65: 2279–2289, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Hayat A: . Safety issues with intravenous iron products in the management of anemia in chronic kidney disease. Clin Med Res 6: 93–102, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farah M, Crawford RI, Levin A, Chan Yan C: . Calciphylaxis in the current era: Emerging ‘ironic’ features? Nephrol Dial Transplant 26: 191–195, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Kletzmayr J, Sunder-Plassmann G, Hörl WH: . High dose intravenous iron: A note of caution. Nephrol Dial Transplant 17: 962–965, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Zager RA, Johnson AC, Hanson SY, Wasse H: . Parenteral iron formulations: A comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis 40: 90–103, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Lim CS, Vaziri ND: . Iron and oxidative stress in renal insufficiency. Am J Nephrol 24: 569–575, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Tovbin D, Mazor D, Vorobiov M, Chaimovitz C, Meyerstein N: . Induction of protein oxidation by intravenous iron in hemodialysis patients: Role of inflammation. Am J Kidney Dis 40: 1005–1012, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Pai AB, Conner T, McQuade CR, Olp J, Hicks P: . Non-transferrin bound iron, cytokine activation and intracellular reactive oxygen species generation in hemodialysis patients receiving intravenous iron dextran or iron sucrose. Biometals 24: 603–613, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Prats M, Font R, García C, Muñoz-Cortés M, Cabré C, Jariod M, Romeu M, Giralt M, Martinez-Vea A: . Oxidative stress markers in predicting response to treatment with ferric carboxymaltose in nondialysis chronic kidney disease patients. Clin Nephrol 81: 419–426, 2014 [DOI] [PubMed] [Google Scholar]
- 57.Agarwal R, Kusek JW, Pappas MK: . A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int 88: 905–914, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macdougall IC, Bock AH, Carrera F, Eckardt K-U, Gaillard C, Van Wyck D, Roubert B, Nolen JG, Roger SD; FIND-CKD Study Investigators: FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 29: 2075–2084, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qunibi WY, Martinez C, Smith M, Benjamin J, Mangione A, Roger SD: . A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant 26: 1599–1607, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalra PA, Bhandari S, Saxena S, Agarwal D, Wirtz G, Kletzmayr J, Thomsen LL, Coyne DW: . A randomized trial of iron isomaltoside 1000 versus oral iron in non-dialysis-dependent chronic kidney disease patients with anaemia [published online ahead of print August 6, 2015]. Nephrol Dial Transplant [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Auerbach M, Ballard H: . Clinical use of intravenous iron: Administration, efficacy, and safety. Hematology (Am Soc Hematol Educ Program) 2010: 338–347, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Rampton D, Folkersen J, Fishbane S, Hedenus M, Howaldt S, Locatelli F, Patni S, Szebeni J, Weiss G: . Hypersensitivity reactions to intravenous iron: Guidance for risk minimization and management. Haematologica 99: 1671–1676, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.European Medicines Agency: New Recommendations to Manage Risk of Allergic Reactions with Intravenous Iron-Containing Medicines. EMA/377372/2013, 2013
- 64.Sheftel AD, Mason AB, Ponka P: . The long history of iron in the Universe and in health and disease. Biochim Biophys Acta 1820: 161–187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganz T: . Hepcidin and its role in regulating systemic iron metabolism. Hematology (Am Soc Hematol Educ Program) 1: 29–35, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Nakanishi T, Hasuike Y, Nanami M, Yahiro M, Kuragano T: . Novel iron-containing phosphate binders and anemia treatment in CKD: Oral iron intake revisited [published online ahead of print July 3, 2015]. Nephrol Dial Transplant [DOI] [PubMed] [Google Scholar]
- 67.Geisser P, Burckhardt S: . The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics 3: 12–33, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hudson JQ, Comstock TJ: . Considerations for optimal iron use for anemia due to chronic kidney disease. Clin Ther 23: 1637–1671, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Rozen-Zvi B, Gafter-Gvili A, Paul M, Leibovici L, Shpilberg O, Gafter U: . Intravenous versus oral iron supplementation for the treatment of anemia in CKD: Systematic review and meta-analysis. Am J Kidney Dis 52: 897–906, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Liles AM: . Intravenous versus oral iron for treatment of iron deficiency in non-hemodialysis-dependent patients with chronic kidney disease. Am J Health Syst Pharm 69: 1206–1211, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE: . Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94: 329–354, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kortman GAM, Raffatellu M, Swinkels DW, Tjalsma H: . Nutritional iron turned inside out: Intestinal stress from a gut microbial perspective. FEMS Microbiol Rev 38: 1202–1234, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Umanath K, Niecestro R, Lewis JB, Dwyer JP: . Ferric citrate: A novel phosphate-binding agent. Expert Rev Endocrinol Metab 8: 13–19, 2013 [DOI] [PubMed] [Google Scholar]
- 74.Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, Wolf M, Chertow GM: . A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3-5. Am J Kidney Dis 65: 728–736, 2015 [DOI] [PubMed] [Google Scholar]
- 75.Floege J, Covic AC, Ketteler M, Mann JF, Rastogi A, Spinowitz B, Chong EM, Gaillard S, Lisk LJ, Sprague SM; Sucroferric Oxyhydroxide Study Group: Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 30: 1037–1046, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tran L, Batech M, Rhee CM, Streja E, Kalantar-Zadeh K, Jacobsen SJ, Sim JJ: Serum phosphorus and association with anemia among a large diverse population with and without chronic kidney disease [published online ahead of print August 8, 2015]. Nephrol Dial Transplant. [DOI] [PMC free article] [PubMed]
- 77.Del Vecchio L, Locatelli F: . Anemia in chronic kidney disease patients: Treatment recommendations and emerging therapies. Expert Rev Hematol 7: 495–506, 2014 [DOI] [PubMed] [Google Scholar]
- 78.Seligman PA, Moore GM, Schleicher RB: . Clinical studies of HIP: An oral heme-iron product. Nutr Res 20: 1279–1286, 2000 [Google Scholar]
- 79.Dull RB, Davis E: . Heme iron polypeptide for the management of anaemia of chronic kidney disease. J Clin Pharm Ther 40: 386–390, 2015 [DOI] [PubMed] [Google Scholar]
- 80.Nagaraju SP, Cohn A, Akbari A, Davis JL, Zimmerman DL: . Heme iron polypeptide for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients: A randomized controlled trial. BMC Nephrol 14: 64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pisani A, Riccio E, Sabbatini M, Andreucci M, Del Rio A, Visciano B: . Effect of oral liposomal iron versus intravenous iron for treatment of iron deficiency anaemia in CKD patients: A randomized trial. Nephrol Dial Transplant 30: 645–652, 2015 [DOI] [PubMed] [Google Scholar]
- 82.Macdougall IC: . New anemia therapies: Translating novel strategies from bench to bedside. Am J Kidney Dis 59: 444–451, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group: KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed]
- 84.NKF KDOQI: NKF KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Available at: www2.kidney.org/professionals/KDOQI/guidelines_bone/Guide3.htm. Accessed August 25, 2015
- 85.Manns BJ, Hodsman A, Zimmerman DL, Mendelssohn DC, Soroka SD, Chan C, Jindal K, Klarenbach S: . Canadian Society of Nephrology commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD). Am J Kidney Dis 55: 800–812, 2010 [DOI] [PubMed] [Google Scholar]
- 86.Goldsmith DJ, Covic A, Fouque D, Locatelli F, Olgaard K, Rodriguez M, Spasovski G, Urena P, Zoccali C, London GM, Vanholder R: . Endorsement of the Kidney Disease Improving Global Outcomes (KDIGO) Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Guidelines: A European Renal Best Practice (ERBP) commentary statement. Nephrol Dial Transplant 25: 3823–3831, 2010 [DOI] [PubMed] [Google Scholar]
- 87.McMahon LP, Kent AB, Kerr PG, Healy H, Irish AB, Cooper B, Kark A, Roger SD: . Maintenance of elevated versus physiological iron indices in non-anaemic patients with chronic kidney disease: A randomized controlled trial. Nephrol Dial Transplant 25: 920–926, 2010 [DOI] [PubMed] [Google Scholar]
- 88.Agarwal R, Rizkala AR, Bastani B, Kaskas MO, Leehey DJ, Besarab A: . A randomized controlled trial of oral versus intravenous iron in chronic kidney disease. Am J Nephrol 26: 445–454, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Charytan C, Qunibi W, Bailie GR; Venofer Clinical Studies Group: Comparison of intravenous iron sucrose to oral iron in the treatment of anemic patients with chronic kidney disease not on dialysis. Nephron Clin Pract 100: c55–c62, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Van Wyck DB, Roppolo M, Martinez CO, Mazey RM, McMurray S; United States Iron Sucrose (Venofer) Clinical Trials Group: A randomized, controlled trial comparing IV iron sucrose to oral iron in anemic patients with nondialysis-dependent CKD. Kidney Int 68: 2846–2856, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Aggarwal HK, Nand N, Singh S, Singh M, Hemant, Kaushik G: . Comparison of oral versus intravenous iron therapy in predialysis patients of chronic renal failure receiving recombinant human erythropoietin. J Assoc Physicians India 51: 170–174, 2003 [PubMed] [Google Scholar]
- 92.Stoves J, Inglis H, Newstead CG: . A randomized study of oral vs intravenous iron supplementation in patients with progressive renal insufficiency treated with erythropoietin. Nephrol Dial Transplant 16: 967–974, 2001 [DOI] [PubMed] [Google Scholar]
- 93.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJG: . Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol 19: 1599–1605, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, Kumagai Y, Block GA: . Ferric citrate hydrate for the treatment of hyperphosphatemia in nondialysis-dependent CKD. Clin J Am Soc Nephrol 9: 543–552, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]