Abstract
Background and objectives
Colonic microbial metabolism substantially contributes to uremic retention solutes in CKD. p-Cresyl sulfate is the main representative of this group of solutes, relating to adverse outcomes. Other than sulfate conjugation, p-cresol is subjected to endogenous glucuronide conjugation. Whether the balance between sulfate and glucuronide conjugation is relevant in CKD is unexplored.
Design, setting, participants, & measurements
We prospectively followed 488 patients with CKD stages 1–5 (enrollment between November of 2005 and September of 2006; follow-up until December of 2010). Serum and urine levels of p-cresyl sulfate and p-cresyl glucuronide were measured using liquid chromatography-mass spectrometry. Total amount of microbial p-cresol was calculated by the sum of serum p–cresyl sulfate and p-cresyl glucuronide. Outcome analysis was performed for mortality and cardiovascular disease.
Results
Serum p–cresyl sulfate was a median of 193.0-fold (interquartile range, 121.1–296.6) higher than serum p–cresyl glucuronide, with a significant correlation between eGFR and proportion of serum p–cresyl sulfate to glucuronide (rho=0.23; P=0.001). There was also a significant correlation between eGFR and proportion of 24-hour urinary excretion of p-cresyl sulfate to glucuronide (rho=0.32; P<0.001). Higher serum p–cresol and lower proportion of serum p–cresyl sulfate to glucuronide were jointly and significantly associated with mortality (hazard ratio per SD higher, 1.58; 95% confidence interval, 1.10 to 2.29; P=0.01 and hazard ratio, 0.65; 95% confidence interval, 0.47 to 0.89; P<0.01, respectively) and cardiovascular disease (hazard ratio, 1.68; 95% confidence interval, 1.27 to 2.22; P<0.001 and hazard ratio, 0.55; 95% confidence interval, 0.42 to 0.72; P<0.001, respectively) after adjustment for eGFR, Framingham risk factors, mineral bone metabolism markers, C-reactive protein, and albumin.
Conclusions
p-Cresol shows a preponderance of sulfate conjugation, although a relatively diminished sulfotransferase activity can be suggested in patients with advanced CKD. Along with total p–cresol burden, a relative shift from sulfate to glucuronide conjugation is independently associated with mortality and cardiovascular disease, warranting increased focus to the dynamic interplay between microbial and endogenous metabolism.
Keywords: chronic kidney disease; intestine; Cresols; Follow-Up Studies; Humans; Microbiota; Protein Binding; Renal Insufficiency, Chronic; risk factors; Sulfotransferases
Introduction
There has been mounting evidence that the colonic microbial metabolism contributes substantially to uremic retention solutes accumulating in patients with CKD (1,2). p-Cresyl sulfate (PCS) can be considered representative of this group of solutes and has been associated with overall mortality, cardiovascular disease, and progression of CKD (3–7). In addition, mechanistic studies relate PCS to oxidative stress, endothelial dysfunction, proximal tubular injury, and insulin resistance (8–10).
Other than sulfate conjugation, microbial p-cresol is subjected to endogenous glucuronidation, with serum levels of p-cresyl glucuronide (PCG) being substantially lower than those of PCS (11,12). Although it can be hypothesized that this imbalance is caused by differences in phase 2 metabolism, possibly influenced by renal dysfunction, this has not been studied to date. Furthermore, renal handling of PCG is unknown but may be different compared with PCS, which mainly depends on tubular secretion (13–16). In addition, although it is well established that serum PCS is highly protein bound (17,18), protein binding characteristics of serum PCG are less clear, possibly contributing to differential renal clearance of PCS and PCG. A better understanding of the determinants of serum PCG is needed, because PCG has also been related to adverse outcomes in patients with CKD (19). Whether a relative shift of p-cresol from sulfation to glucuronidation or vice versa is relevant in CKD remains, however, unknown.
Therefore, we explored the behavior of PCS and PCG in patients with CKD not yet on dialysis focusing on potential differences in phase 2 metabolism: protein binding and renal clearance between both p–cresol derivatives. In addition, the relative contribution of sulfation and glucuronidation of p-cresol to adverse outcomes was examined.
Materials and Methods
Study Population
This is an ancillary analysis of the Leuven Mild-to-Moderate CKD Study (NCT00441623) (5). Prevalent patients with CKD followed at the nephrology outpatient clinic of University Hospitals Leuven who were 18 years of age or older and able to provide consent were eligible for inclusion. Patients were screened between November of 2005 and September of 2006. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of University Hospitals Leuven. Informed consent was obtained from all patients.
Biochemical Measurements
Serum levels of PCS and PCG were quantified using a dedicated ultraperformance liquid chromatography–tandem mass spectrometry method (Supplemental Material). To gain additional insights in the behavior of PCS and PCG, we also measured free solute levels, 24-hour urinary excretion, and renal clearance (total and free) in a subgroup of patients with availability of 24-hour urinary collection. Completeness was assessed using 24-hour urinary creatinine excretion, and collections were considered complete when creatinine excretion was within 2 SDs of the mean creatinine excretion for the geographic region of this study (the International Study of Salt and Blood Pressure Study) (20). Free solute levels were measured after ultrafiltration of serum using the Centrifree UF Device (molecular mass cutoff of 30 kD; EMD Millipore, Billerica, MA). Free solute fraction was defined as a ratio of free to total solute level. Assuming steady-state conditions and negligible nonrenal clearance, 24-hour urinary excretions of PCS and PCG can be considered estimates of endogenous sulfate and glucuronide conjugation of p-cresol, respectively. In addition, combined 24-hour urinary excretion of PCS and PCG equals total intestinal uptake of precursor p-cresol.
Equilibrium Dialysis
To explore protein binding characteristics of PCG, we performed equilibrium dialysis using the HTDialysis 96b System (molecular mass cutoff of 12–14 kD; cell volume of 150 μl; HTDialysis, Gales Ferry, CT). Experimental solutions were prepared by spiking PCG at different concentrations (2, 5, 10, 25, 50, 75, 100, 200, 400, 800, and 1200 μM) in an albumin solution (target of 40 g/L dissolved in PBS), healthy serum, uremic serum, or healthy serum with addition of PCS (target 200 μM). PCG, fatty acid free human serum albumin, and PBS were purchased from Sigma-Aldrich (St. Louis, MO). Pooled healthy serum was obtained from eight healthy study participants, whereas pooled uremic serum was derived from eight patients on hemodialysis taken immediately before the midweek hemodialysis session. PCS was synthesized according to the work by Feigenbaum and Neuberg (21). To determine binding equilibrium, experimental solutions were dialyzed against an equal volume of PBS with temperature kept constant at 37°C for a duration of 4 hours. All experiments were performed in octet, with pooling of four chamber volumes of each experimental condition for quantification. Free fractions of PCG were defined as the ratios between solute concentrations in PBS solution and experimental solution.
Outcome Analyses
After inclusion, patients were prospectively followed at the nephrology outpatient clinic at 3- to 6-month intervals. In the original study, follow-up was available until December 1, 2008. For this study, follow-up was extended until December 31, 2010. End point evaluation has been described previously (5) and consisted of overall mortality and first cardiovascular event (Supplemental Material).
Statistical Analyses
Data are expressed as means (SDs) for normally distributed variables or medians (interquartile ranges [IQRs]) for non–normally distributed variables. Correlations were calculated by Spearman rank correlation coefficients. Differences were tested using Wilcoxon rank sum, Kruskal–Wallis, or chi-squared test as indicated. Linear regression analysis was performed for renal clearance (total and free) of PCS and PCG, free fraction of PCS and PCG, and proportion of 24-hour urinary excretion of PCS to PCG. Time to first event analysis was performed for both serum total p–cresol (sum of serum total PCS and PCG) and proportion of serum total PCS to PCG using Cox proportional hazards analysis. For multivariate analysis, we used a double–backward elimination approach, with inclusion of all variables at P<0.20 for secondary backward elimination at P<0.05. To test the proportionality assumption, each model was tested against log(time). For overall mortality, data were censored at start of RRT, loss to follow-up, or the end of study observation period. With respect to cardiovascular disease, additional censoring was performed for noncardiovascular death. All statistical analyses were performed using SAS (version 9.3; SAS Institute Inc., Cary, NC).
Results
Study Population
Study population consisted of 499 patients with CKD stages 1–5 and has been described previously (5). Of these, total serum levels of PCS and PCG were measured in 488 patients for outcome analysis. In addition, 24-hour urinary collection was available in 203 patients, in whom we also measured free serum levels, 24-hour urinary excretion, and renal clearance (total and free) of PCS and PCG. Other than a small age difference, we observed no significant differences in baseline characteristics between both groups (Table 1).
Table 1.
Study population
| Variable | Subgroup, n=203 | Full Group, n=488 | P Value |
|---|---|---|---|
| Age, yr | 60 (47–72) | 64 (50–74) | 0.02 |
| Sex, men/women (%) | 120/83 (59.1/40.9) | 270/218 (55.3/44.7) | 0.37 |
| Albumin, g/dl | 4.51 (4.20–4.68) | 4.48 (4.24–4.69) | 0.81 |
| Creatinine, mg/dl | 1.81 (1.29–2.50) | 1.78 (1.26–2.43) | 0.45 |
| eGFR, ml/min per 1.73 m2 | 34 (23–56) | 35 (23–56) | 0.87 |
| Creatinine clearance, ml/min | 40 (27–61) | NA | NA |
| 24-h Proteinuria, g | 0.31 (0.11–1.13) | NA | NA |
| Serum total PCS, μM | 49.7 (21.0–104.1) | 53.2 (21.6–106.5) | 0.24 |
| Serum free PCS, μM | 1.8 (0.6–4.1) | NA | NA |
| Serum total PCG, μM | 0.22 (0.08–0.60) | 0.23 (0.08–0.59) | 0.85 |
| Serum free PCG, μM | 0.13 (0.05–0.50) | NA | NA |
Data are expressed as medians (interquartile ranges) or proportions. NA, not available; PCS, p-cresyl sulfate; PCG, p-cresyl glucuronide.
Serum Level of PCS Versus PCG
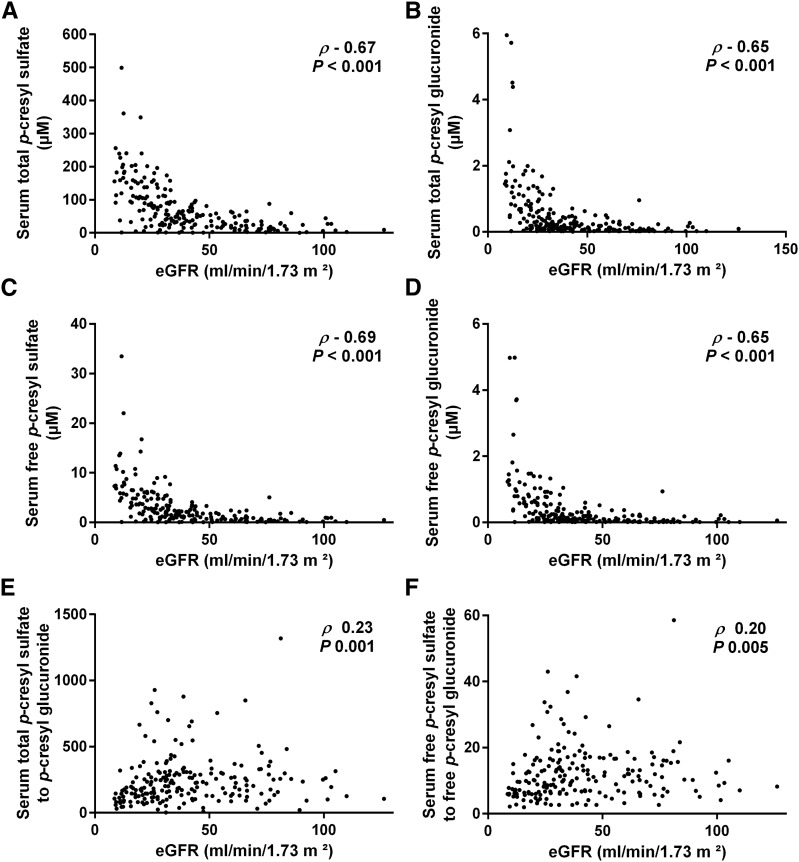
Total serum levels of PCS amounted to a median of 49.7 μM (IQR, 21.0–104.1), being approximately 193.0-fold (IQR, 121.1–296.6) higher than those of PCG. There was a statistically significant correlation between serum total PCS and PCG (rho=0.88; P<0.001), and both serum total PCS and PCG were significantly correlated with eGFR (rho=−0.67; P<0.001 for PCS and rho=−0.65; P<0.001 for PCG) (Figure 1, A and B). In addition, we observed a significant relationship between proportion of serum total PCS to PCG and eGFR (rho=0.23; P=0.001) (Figure 1E), with relatively more serum total PCG in patients with advanced CKD, especially when eGFR is <30 ml/min per 1.73 m2 (Supplemental Figure 1A).
Figure 1.
Serum levels of p-cresyl sulfate and p-cresyl glucuronide versus eGFR. Correlation between eGFR and (A) serum total p–cresyl sulfate, (B) serum total p–cresyl glucuronide, (C) serum free p–cresyl sulfate, (D) serum free p–cresyl glucuronide, (E) proportion of serum total p–cresyl sulfate to p-cresyl glucuronide, and (F) proportion of serum free p–cresyl sulfate to p-cresyl glucuronide.
Free serum levels of PCS (median =1.8 μM; IQR, 0.6–4.1) were also 10.1-fold (IQR, 6.4–15.5) higher than those of PCG. In addition, free serum levels of PCS and PCG were significantly correlated with each other (rho=0.93; P<0.001), their corresponding total serum levels (rho=0.97; P<0.001 for PCS and rho=0.97; P<0.001 for PCG, respectively), and eGFR (rho=−0.69; P<0.001 for PCS and rho=−0.65; P<0.001 for PCG, respectively) (Figure 1, C and D). Also, the proportion of serum free PCS to PCG was significantly lower in patients with lower eGFR (rho=0.20; P<0.01) (Figure 1F, Supplemental Figure 1B).
24-Hour Urinary Excretion of PCS Versus PCG
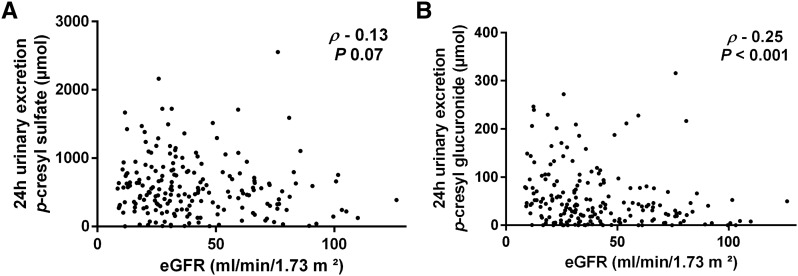
To estimate the degree of sulfate versus glucuronide conjugation, we calculated 24-hour urinary excretions of PCS and PCG; 24-hour urinary excretion of PCS amounted to a median of 50.7 μM (IQR, 271.0–77.5), being 12.7-fold (IQR, 8.1–18.6) higher than 24-hour urinary excretion of PCG (both correlating with each other; rho=0.78; P<0.001). A significant correlation was noted between 24-hour urinary excretion and total serum levels of both compounds (rho=0.68; P<0.001 for PCS and rho=0.69; P<0.001 for PCG). There was also a significant correlation between eGFR and 24-hour urinary excretion of PCG (rho=−0.25; P<0.001) and a borderline significant correlation between eGFR and 24-hour urinary excretion of PCS (rho=−0.13; P=0.07) (Figure 2). In addition, we observed a significant relationship between proportion of 24-hour urinary excretion of PCS to PCG and eGFR (rho=0.32; P<0.001), with relatively more 24-hour urinary excretion of PCG in patients with advanced CKD. To estimate total intestinal uptake of precursor p-cresol, 24-hour urinary excretions of PCS and PCG were combined, showing a significant correlation with eGFR (rho=−0.15; P=0.04). In linear regression analysis, eGFR (β=0.43 per ml/min per 1.73 m2 [natural logarithmic transformation]; P=0.03) but not 24-hour urinary excretion of p-cresol (β=0.03 per μmol [natural logarithmic transformation]; P=0.74) was associated with proportion of 24-hour urinary excretion of PCS to PCG. There was a significant correlation between 24-hour urinary excretion of urea and 24-hour urinary excretions of PCS (rho=0.32; P<0.001), PCG (rho=0.20; P<0.01), and p-cresol (rho=0.31; P<0.001), whereas there was no correlation between 24-hour urinary excretion of urea and proportion of 24-hour urinary excretion of PCS to PCG (rho=0.11; P=0.13).
Figure 2.
The 24-hour urinary excretions of p-cresyl sulfate and p-cresyl glucuronide versus eGFR. Correlation between eGFR and 24-hour urinary excretion of (A) p-cresyl sulfate and (B) p-cresyl glucuronide.
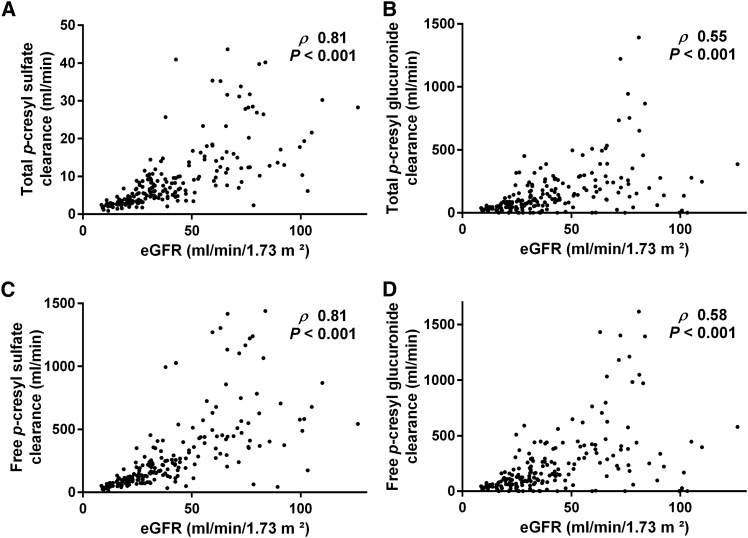
Renal Clearance of PCS Versus PCG
Total renal clearance of PCS amounted to a median of 6.6 ml/min (IQR, 3.6–12.0), which was correlated with (rho=0.70; P<0.001) but substantially lower than total renal clearance of PCG (median =98.9 ml/min; IQR, 40.6–212.4). Correlation between eGFR and total clearance was nominally higher for PCS (rho=0.81; P<0.001) than for PCG (rho=0.55; P<0.001) (Figure 3, A and B). Furthermore, there was a correlation between eGFR and fractional excretion, albeit more pronounced for total PCS (median =16.6%; IQR, 13.2 to 22.3; rho=0.29; P<0.001) than for total PCG (median =259.5%; IQR, 161.1–385.1; rho=0.16; P=0.02). However, we observed no relationship between proportion of total clearance of PCS to PCG and eGFR (rho=−0.01; P=0.90).
Figure 3.
Renal clearances of p-cresyl sulfate and p-cresyl glucuronide versus eGFR. Correlation between eGFR and renal clearance of (A) total p–cresyl sulfate, (B) total p–cresyl glucuronide, (C) free p–cresyl sulfate, and (D) free p–cresyl glucuronide.
When focusing on free solute renal clearance, clearance of PCS (median =190.0 ml/min; IQR, 94.2–374.6) was correlated with (rho=0.72; P<0.001) but higher than clearance of PCG (median =136.5 ml/min; IQR, 57.5–295.9) (Figure 3, C and D). Again, correlation between eGFR and free clearance was nominally higher for PCS (rho=0.81; P<0.001) than for PCG (rho=0.58; P<0.001), and correlation between eGFR and fractional excretion was stronger for free PCS (median =463.2%; IQR, 353.3–625.8; rho=0.52; P<0.001) than for free PCG (median =371.5%; IQR, 232.3–567.3; rho=0.25; P<0.001). There was no relationship between eGFR and proportion of free clearance of PCS to PCG (rho=0.08; P=0.27).
In linear regression analysis, eGFR and serum albumin were significantly associated with total and free renal clearances of PCS, with higher serum albumin relating to lower total clearance but also, higher free clearance. For PCG, eGFR but not serum albumin was a determinant of both total and free clearance (Table 2).
Table 2.
Linear regression analysis of renal clearance of p-cresyl sulfate and p-cresyl glucuronide
| Variable | β | P Value |
|---|---|---|
| p-Cresyl sulfate | ||
| Total renal clearance, ml/min (Ln) | ||
| eGFR, ml/min per 1.73 m2 (Ln) | 1.07 | <0.001 |
| Serum albumin, g/dl | −0.18 | 0.03 |
| Model R2 | 0.65 | |
| Free renal clearance, ml/min (Ln) | ||
| eGFR, ml/min per 1.73 m2 (Ln) | 1.22 | <0.001 |
| Serum albumin, g/dl | 0.19 | 0.05 |
| Model R2 | 0.65 | |
| p-Cresyl glucuronide | ||
| Total renal clearance, ml/min (Ln) | ||
| eGFR, ml/min per 1.73 m2 (Ln) | 0.91 | <0.001 |
| Serum albumin, g/dl | −0.07 | 0.81 |
| Model R2 | 0.09 | |
| Free renal clearance, ml/min (Ln) | ||
| eGFR, ml/min per 1.73 m2 (Ln) | 1.01 | <0.001 |
| Serum albumin, g/dl | 0.05 | 0.85 |
| Model R2 | 0.12 |
Ln, natural logarithmic transformation.
Protein Binding of PCS Versus PCG
Median free fraction of PCS was 3.5% (IQR, 2.9–4.2), whereas median free fraction of PCG was 72.8% (IQR, 62.2–80.0). Free fractions of PCS and PCG correlated with each other (rho=0.50; P<0.001) and with eGFR (rho=−0.35; P<0.001 for PCS and rho=−0.33; P<0.001 for PCG) and albumin (rho=−0.45; P<0.001 for PCS and rho=−0.26; P<0.001 for PCG). In linear regression analysis, both lower eGFR and lower serum albumin were associated with higher free fraction of PCS and PCG (Table 3).
Table 3.
Linear regression analysis of free fractions of p-cresyl sulfate and p-cresyl glucuronide
| Variable | β | P Value |
|---|---|---|
| Free fraction of p-cresyl sulfate, % | ||
| eGFR, ml/min per 1.73 m2 (Ln) | −0.15 | <0.001 |
| Serum albumin, g/dl | −0.36 | <0.001 |
| Model R2 | 0.25 | |
| Free fraction of p-cresyl glucuronide, % | ||
| eGFR, ml/min per 1.73 m2 (Ln) | −6.78 | <0.001 |
| Serum albumin, g/dl | −7.31 | <0.01 |
| Model R2 | 0.11 |
Ln, natural logarithmic transformation.
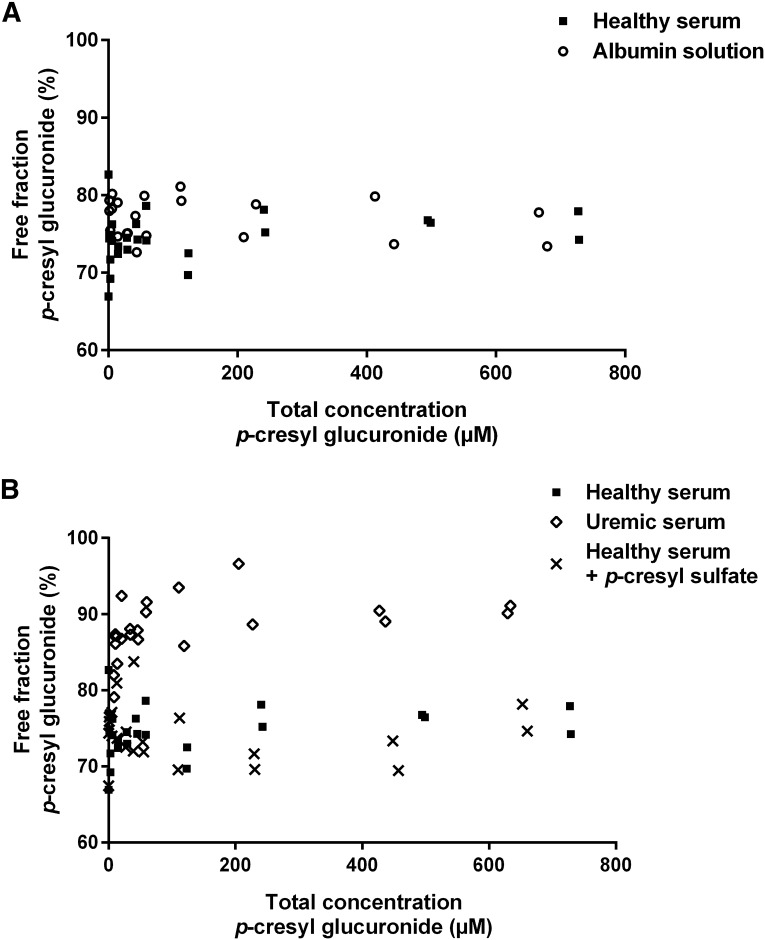
To extend clinical data, we performed ex vivo equilibrium dialysis (Figure 4). Mean free fraction of PCG was 76.9% (SD=2.6) in albumin in PBS solution, being slightly higher than mean free fraction in healthy serum (74.5%; SD=3.3; P<0.01). Comparing healthy versus uremic serum, a significantly higher mean free fraction of PCG was noted in uremic serum (88.1%; SD=3.7; P<0.001). There was no difference in free fraction of PCG between healthy serum and healthy serum with addition of PCS (74.3%; SD=3.7; P=0.88). In addition, there was no correlation between total spiked concentrations of PCG and free fraction of PCS in both healthy (rho=−0.02; P=0.92) and uremic serum (rho=−0.23; P=0.28), with mean free fraction of PCS being higher in uremic than in healthy serum (7.2%; SD=0.71 versus 3.7%; SD=0.71; P<0.001).
Figure 4.
Protein binding characteristics of p-cresyl glucuronide. Free fraction of p-cresyl glucuronide in (A) albumin in PBS solution versus healthy serum and (B) healthy serum versus uremic serum and healthy serum with addition of p-cresyl sulfate.
Outcome Analyses
We investigated the relationship between serum total p–cresol (sum of serum total PCS and PCG), proportion of serum total PCS to PCG, and adverse outcomes (Table 4). During follow-up, we noted 51 deaths and 75 cardiovascular events. Higher serum total p–cresol and lower proportion of serum total PCS to PCG were jointly associated with mortality, even after adjustment for eGFR, Framingham risk factors, mineral bone metabolism markers, albumin, and C-reactive protein (hazard ratio [HR] per SD higher, 1.58; 95% confidence interval [95% CI], 1.10 to 2.29; P=0.01 for p-cresol and HR, 0.65; 95% CI, 0.47 to 0.89; P<0.01 for proportion of PCS to PCG). In addition, higher serum total p–cresol and lower proportion of serum total PCS to PCG were independent predictors for cardiovascular events (HR, 1.68; 95% CI, 1.27 to 2.22; P<0.001 for p-cresol and HR, 0.55; 95% CI, 0.42 to 0.72; P<0.001 for proportion of PCS to PCG). Additional analyses also showed a significant independent relationship between higher proportion of serum total PCG to p-cresol along with higher serum total p–cresol and both overall mortality and cardiovascular disease (Supplemental Table 1). Finally, higher serum total PCG itself was a significant and independent predictor of adverse outcomes (Supplemental Table 2).
Table 4.
Cox proportional hazards analysis of overall mortality and cardiovascular disease for serum total p–cresol and proportion of serum total p–cresyl sulfate to p-cresyl glucuronide
| Variable | Hazard Ratio Per 1 SD Higher (95% Confidence Interval) | P Value |
|---|---|---|
| Mortality | ||
| Model 1 | ||
| p-Cresol (Ln) | 1.90 (1.37 to 2.61) | <0.001 |
| p-Cresyl sulfate to glucuronide (Ln) | 0.59 (0.45 to 0.78) | <0.001 |
| Model 2 | ||
| p-Cresol (Ln) | 1.58 (1.10 to 2.29) | 0.01 |
| p-Cresyl sulfate to glucuronide (Ln) | 0.65 (0.47 to 0.89) | <0.01 |
| Cardiovascular disease | ||
| Model 1 | ||
| p-Cresol (Ln) | 2.10 (1.61 to 2.75) | <0.001 |
| p-Cresyl sulfate to glucuronide (Ln) | 0.53 (0.41 to 0.69) | <0.001 |
| Model 2 | ||
| p-Cresol (Ln) | 1.68 (1.27 to 2.22) | <0.001 |
| p-Cresyl sulfate to glucuronide (Ln) | 0.55 (0.42 to 0.72) | <0.001 |
Model 1 included serum total p–cresol and proportion of serum total p–cresyl sulfate to p-cresyl glucuronide. Model 2 included additional adjustment for eGFR (Ln), age, sex, systolic BP, current smoker, diabetes mellitus, cholesterol, calcium, phosphate, parathyroid hormone (Ln), C-reactive protein (Ln), and albumin. Serum total p–cresol is the sum of serum total p–cresyl sulfate and p-cresyl glucuronide. Ln, natural logarithmic transformation.
Discussion
In this study, we explored the differential behavior of PCS and PCG in patients with CKD not yet on dialysis. The key findings are as follows: (1) total and free serum levels of PCS are substantially higher than those of PCG; (2) p-cresol is predominantly subjected to sulfation, although the contribution of glucuronidation was greater among patients with lower eGFR; (3) renal clearances of PCS and PCG depend on tubular secretion, with serum levels of albumin also contributing to renal clearance of PCS; (4) protein binding of serum PCS is substantially higher compared with PCG, and protein binding of both p–cresol derivatives is diminished in patients with advanced CKD and lower serum albumin; and (5) a relative shift from sulfation to glucuronidation along with higher serum total p–cresol associates with mortality and cardiovascular disease.
PCS originates from colonic microbial fermentation of tyrosine to p-cresol with subsequent endogenous sulfate conjugation (2,22). PCG is another p–cresol derivative, albeit with substantially lower total serum levels compared with PCS (11,12). Because the differential behavior of PCS and PCG has been largely unexplored, we measured serum levels and 24-hour urinary excretion of both p–cresol derivatives in our Leuven Mild-to-Moderate CKD Study cohort.
In agreement with previous studies (11,12), total serum levels of PCG were considerably lower than those of PCS in patients across the whole range of eGFRs. In addition, when focusing on free serum solute levels, the balance between PCS and PCG, albeit less pronounced, was still in favor of PCS. Because these findings can be explained by differences in both phase 2 metabolism and renal clearance, we compared 24-hour urinary excretion and renal clearance between both solutes. Assuming steady-state conditions and negligible nonrenal clearance, 24-hour urinary excretion of PCS and PCG can be considered an estimate of endogenous sulfate and glucuronide conjugation of p-cresol. Because we noted a substantially higher 24-hour urinary excretion of PCS, it can be suggested that phase 2 metabolism of p-cresol shows preponderance of sulfate conjugation. Furthermore, we observed a lower proportion of both serum and 24-hour urinary excretion of PCS to PCG in patients with lower eGFR, which is indicative of a relatively diminished sulfotransferase activity in patients with advanced CKD. These findings confirm and extend a previous observation of a decreased serum total PCS-to-PCG ratio in patients on maintenance hemodialysis, in whom a different dialytic clearance should be taken into account (23). In agreement, glucuronide conjugation of paracetamol was also more pronounced compared with sulfate conjugation when administered to patients with CKD, again pointing to a decrease in sulfotransferase function, especially for sulfotransferase 1A1 (24). A relative shift from sulfate to glucuronide conjugation may, however, be detrimental, because we showed that lower proportion of serum total PCS to PCG along with higher serum total p–cresol burden were independent predictors of mortality and cardiovascular disease. Serum PCG has already been related to worse survival in CKD, although direct comparison with PCS was not performed (19). Mechanistic studies of PCG are also rather scarce compared with those of PCS. In this regard, it has been shown that PCG per se has no effect on leukocyte oxidative burst activity, whereas it may induce a synergistic activating effect in the presence of PCS (12). In addition, the effect of PCG on proximal tubular cells is equivocal (25,26). Additional research is required to elucidate the relevance of a decrease in sulfotransferase activity in patients with advanced CKD with respect to phase 2 metabolism of p-cresol and possibly, also endogenous and drug metabolism in general.
Furthermore, we studied renal clearance of both p–cresol derivatives. Total clearance of PCS was lower compared with that of PCG, whereas free clearance of PCS exceeded free clearance of PCG. Because free renal clearances of PCS and PCG were higher than eGFR or creatinine clearances, active tubular secretion can be expected for both solutes. Furthermore, fractional excretion was lower in patients with advanced CKD, thus also pointing to saturation of tubular transport for PCS and PCG. Although mechanisms underlying tubular secretion of PCS are increasingly being unraveled (13,14,27), less is known about renal handling of PCG. Recent data point to potential involvement of breast cancer resistance protein and multidrug resistance–associated protein 4 for tubular transport of PCG, whereas secretion of PCS may depend on multidrug resistance–associated protein 4 but not on breast cancer resistance protein (23). Differences in free renal clearance of PCS and PCG may also be derived from their binding characteristics, because it has been suggested that protein binding enhances clearance by providing a readily accessible reservoir for efficient removal of the solute throughout its passage within the native kidney (15). Interestingly, even in the range present in our cohort, lower serum albumin was a significant determinant of lower free renal clearance of PCS, while also being associated with higher total renal clearance.
Finally, we investigated protein binding characteristics of PCG, showing rather low protein binding compared with that of PCS. Although serum albumin was the major protein responsible for PCG binding, which was also observed for PCS (17), there was no competitive binding between PCG and PCS, thus possibly pointing to another albumin binding site for PCG than for PCS (Sudlow site 2) (28). In addition, protein binding of PCG was significantly diminished in patients with advanced CKD as well as in experimental uremic conditions. Diminished protein binding along with renal function decline have already been noted for PCS (17) but also, various relevant drugs (29) and may relate to hypoalbuminemia, chemical modifications or conformational changes of albumin, and competitive binding with increasing levels of uremic retention solutes (30).
There are limitations to our study. First, the study design precludes causal inferences. Second, because measurements of proteinuria were only available in a subgroup of patients, adjustment for proteinuria was not possible in outcome analysis. Third, phase 2 metabolism of p-cresol was estimated by 24-hour urinary excretions of PCS and PCG, assuming negligible nonrenal clearance. Preliminary data in healthy volunteers show only minor biliary excretion of both PCS and PCG.
In conclusion, p-cresol shows preponderance of sulfate conjugation, although relatively diminished sulfotransferase activity can be observed in patients with advanced CKD. Along with total p–cresol burden, a relative shift from sulfate to glucuronide conjugation is independently associated with mortality and cardiovascular disease. The pathophysiologic relevance of these findings requires additional investigation.
Disclosures
None.
Supplementary Material
Acknowledgments
Technical assistance by T. Coopmans and M. Dekens is highly appreciated.
R.P. is the recipient of a fellowship of Research Foundation–Flanders (FWO) grant 11E9813N. Part of the research has been funded by FWO grant G077514N.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00160116/-/DCSupplemental.
References
- 1.Aronov PA, Luo FJ, Plummer NS, Quan Z, Holmes S, Hostetter TH, Meyer TW: Colonic contribution to uremic solutes. J Am Soc Nephrol 22: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer TW, Hostetter TH: Uremic solutes from colon microbes. Kidney Int 81: 949–954, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Bammens B, Evenepoel P, Keuleers H, Verbeke K, Vanrenterghem Y: Free serum concentrations of the protein-bound retention solute p-cresol predict mortality in hemodialysis patients. Kidney Int 69: 1081–1087, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Liabeuf S, Barreto DV, Barreto FC, Meert N, Glorieux G, Schepers E, Temmar M, Choukroun G, Vanholder R, Massy ZA; European Uraemic Toxin Work Group (EUTox) : Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol Dial Transplant 25: 1183–1191, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Meijers BK, Claes K, Bammens B, de Loor H, Viaene L, Verbeke K, Kuypers D, Vanrenterghem Y, Evenepoel P: p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin J Am Soc Nephrol 5: 1182–1189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meijers BK, Bammens B, De Moor B, Verbeke K, Vanrenterghem Y, Evenepoel P: Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int 73: 1174–1180, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Wu IW, Hsu KH, Lee CC, Sun CY, Hsu HJ, Tsai CJ, Tzen CY, Wang YC, Lin CY, Wu MS: p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant 26: 938–947, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meijers BK, Van Kerckhoven S, Verbeke K, Dehaen W, Vanrenterghem Y, Hoylaerts MF, Evenepoel P: The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am J Kidney Dis 54: 891–901, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S, Nakajima M, Kataoka K, Kim-Mitsuyama S, Tanaka M, Fukagawa M, Otagiri M, Maruyama T: p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int 83: 582–592, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Koppe L, Pillon NJ, Vella RE, Croze ML, Pelletier CC, Chambert S, Massy Z, Glorieux G, Vanholder R, Dugenet Y, Soula HA, Fouque D, Soulage CO: p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol 24: 88–99, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Loor H, Bammens B, Evenepoel P, De Preter V, Verbeke K: Gas chromatographic-mass spectrometric analysis for measurement of p-cresol and its conjugated metabolites in uremic and normal serum. Clin Chem 51: 1535–1538, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Meert N, Schepers E, Glorieux G, Van Landschoot M, Goeman JL, Waterloos MA, Dhondt A, Van der Eycken J, Vanholder R: Novel method for simultaneous determination of p-cresylsulphate and p-cresylglucuronide: Clinical data and pathophysiological implications. Nephrol Dial Transplant 27: 2388–2396, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto Y, Watanabe H, Noguchi T, Kotani S, Nakajima M, Kadowaki D, Otagiri M, Maruyama T: Organic anion transporters play an important role in the uptake of p-cresyl sulfate, a uremic toxin, in the kidney. Nephrol Dial Transplant 26: 2498–2502, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, Sakaguchi Y, Sugimoto R, Kaneko K, Iwata H, Kotani S, Nakajima M, Ishima Y, Otagiri M, Maruyama T: Human organic anion transporters function as a high-capacity transporter for p-cresyl sulfate, a uremic toxin. Clin Exp Nephrol 18: 814–820, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Sirich TL, Aronov PA, Plummer NS, Hostetter TH, Meyer TW: Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 84: 585–590, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poesen R, Viaene L, Verbeke K, Claes K, Bammens B, Sprangers B, Naesens M, Vanrenterghem Y, Kuypers D, Evenepoel P, Meijers B: Renal clearance and intestinal generation of p-cresyl sulfate and indoxyl sulfate in CKD. Clin J Am Soc Nephrol 8: 1508–1514, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B: Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm Drug Dispos 34: 165–175, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Sirich TL, Meyer TW, Gondouin B, Brunet P, Niwa T: Protein-bound molecules: A large family with a bad character. Semin Nephrol 34: 106–117, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Liabeuf S, Glorieux G, Lenglet A, Diouf M, Schepers E, Desjardins L, Choukroun G, Vanholder R, Massy ZA; European Uremic Toxin (EUTox) Work Group : Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS One 8: e67168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intersalt Cooperative Research Group : Intersalt: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feigenbaum J, Neuberg CA: Simplified method for the preparation of aromatic sulfuric acid esters. J Am Chem Soc 63: 3529–3530, 1941 [Google Scholar]
- 22.Evenepoel P, Meijers BK, Bammens BR, Verbeke K: Uremic toxins originating from colonic microbial metabolism. Kidney Int Suppl 114: S12–S19, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Mutsaers HA, Caetano-Pinto P, Seegers AE, Dankers AC, van den Broek PH, Wetzels JF, van den Brand JA, van den Heuvel LP, Hoenderop JG, Wilmer MJ, Masereeuw R: Proximal tubular efflux transporters involved in renal excretion of p-cresyl sulfate and p-cresyl glucuronide: Implications for chronic kidney disease pathophysiology. Toxicol In Vitro 29: 1868–1877, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Martin U, Temple RM, Winney RJ, Prescott LF: The disposition of paracetamol and the accumulation of its glucuronide and sulphate conjugates during multiple dosing in patients with chronic renal failure. Eur J Clin Pharmacol 41: 43–46, 1991 [DOI] [PubMed] [Google Scholar]
- 25.Mutsaers HA, van den Heuvel LP, Ringens LH, Dankers AC, Russel FG, Wetzels JF, Hoenderop JG, Masereeuw R: Uremic toxins inhibit transport by breast cancer resistance protein and multidrug resistance protein 4 at clinically relevant concentrations. PLoS One 6: e18438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poveda J, Sanchez-Niño MD, Glorieux G, Sanz AB, Egido J, Vanholder R, Ortiz A: p-cresyl sulphate has pro-inflammatory and cytotoxic actions on human proximal tubular epithelial cells. Nephrol Dial Transplant 29: 56–64, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Mutsaers HA, Wilmer MJ, van den Heuvel LP, Hoenderop JG, Masereeuw R: Basolateral transport of the uraemic toxin p-cresyl sulfate: Role for organic anion transporters? Nephrol Dial Transplant 26: 4149, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Watanabe H, Noguchi T, Miyamoto Y, Kadowaki D, Kotani S, Nakajima M, Miyamura S, Ishima Y, Otagiri M, Maruyama T: Interaction between two sulfate-conjugated uremic toxins, p-cresyl sulfate and indoxyl sulfate, during binding with human serum albumin. Drug Metab Dispos 40: 1423–1428, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Vanholder R, Van Landschoot N, De Smet R, Schoots A, Ringoir S: Drug protein binding in chronic renal failure: Evaluation of nine drugs. Kidney Int 33: 996–1004, 1988 [DOI] [PubMed] [Google Scholar]
- 30.Meijers BK, Bammens B, Verbeke K, Evenepoel P: A review of albumin binding in CKD. Am J Kidney Dis 51: 839–850, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.