Abstract
The Nephrology Quiz and Questionnaire remains an extremely popular session for attendees of the Annual Kidney Week Meeting of the American Society of Nephrology. During the 2015 meeting, the conference hall was once again overflowing with eager quiz participants. Topics covered by the experts included electrolyte and acid-base disorders, glomerular disease, ESRD and dialysis, and kidney transplantation. Complex cases representing each of these categories together with single best answer questions were prepared and submitted by the panel of experts. Before the meeting, training program directors of nephrology fellowship programs and nephrology fellows in the United States answered the questions through an internet-based questionnaire. During the live session, members of the audience tested their knowledge and judgment on the same series of case-oriented questions in a quiz. The audience compared their answers in real time using a cellphone application containing the answers of the nephrology fellows and training program directors. The results of the online questionnaire were displayed, and then, the quiz answers were discussed. As always, the audience, lecturers, and moderators enjoyed this highly educational session. This article recapitulates the session and reproduces selected content of educational value for the readers of the Clinical Journal of the American Society of Nephrology. Enjoy the clinical cases and expert discussions.
Keywords: peritoneal dialysis, hemodialysis access, hemodialysis adequacy, innovation, Ethnic Groups, Humans, Kidney Failure, Chronic, nephrology
Introduction: Mark A. Perazella and Michael J. Choi (Comoderators)
For most American Society of Nephrology (ASN) Kidney Week attendees, case–based clinical nephrology talks are one of the most exciting venues. The Nephrology Quiz and Questionnaire (NQ&Q) is the essence of clinical nephrology and represents what drew all of us into the field of nephrology. This year’s NQ&Q in beautiful San Diego, with full-house attendance, was no exception. The expert discussants prepared vignettes of puzzling cases, which illustrated some topical, challenging, or controversial aspect of the diagnosis or management of key clinical areas of nephrology. These eight interesting cases were presented and eloquently discussed by our four expert ASN faculty. Subsequently, each discussant prepared a manuscript summarizing his or her case discussions, which serves as the main text of this article.
In this NQ&Q, Dr. Charmaine E. Lok challenges the reader with two interesting dialysis cases. The audience responses are reviewed along with the training program director and nephrology fellow responses obtained before the meeting. Dr. Lok thoughtfully synthesizes the essential clinical, laboratory, and specific dialysis–related data available in the cases. She then walks the reader through the diagnosis and management of two cases that highlights challenging issues related to dialysis modality choice in some patients with complex medical problems as well as the appropriate choice of initial vascular access and the complications associated with dialysis catheters. Overall, an educational experience was had for all who attended the session. We hope that this distillate from San Diego will serve the subscribers of the Clinical Journal of the American Society of Nephrology well and provide some fresh insights into the complexity and vibrancy of clinical nephrology for those who were unable to attend the meeting.
At first glance, the cases presented at this year’s ASN NQ&Q for RRT may appear disparate; however, underlying these cases is the core message of the critical need to think outside the box when necessary to problem solve and respond to each patient’s differing needs to achieve the best quality dialytic care.
Case 1
A 61-year-old man with autosomal dominant polycystic kidney disease (PCKD) has been followed by you in a multidisciplinary CKD clinic for 23 months. His eGFR is now 18 ml/min per 1.73 m2 and has been stable for the last 15 months. He manages his own restaurant and works long hours. Because of the stability of his renal function and his busy lifestyle, he is reluctant to attend educational sessions on RRT modalities. He is convinced that he will find a living kidney donor and receive a preemptive transplant, although no donors have been identified yet.
He is married, lives in a two-story home, and claims he is adamantly “needle-phobic.” He has hypertension, mitral valve prolapse, and a body mass index (BMI) of 32. He is on an angiotensin converting enzyme inhibitor (ACEI) and a diuretic.
He sees you urgently in your clinic, because he is having signs and symptoms consistent with an infected cyst. You take advantage of this visit and discuss options of RRT: in–center conventional hemodialysis (HD; three times per week), peritoneal dialysis (PD), and home HD. Your facility is able to support all of these options.
Question 1A
Which of the following statements is true regarding RRT?
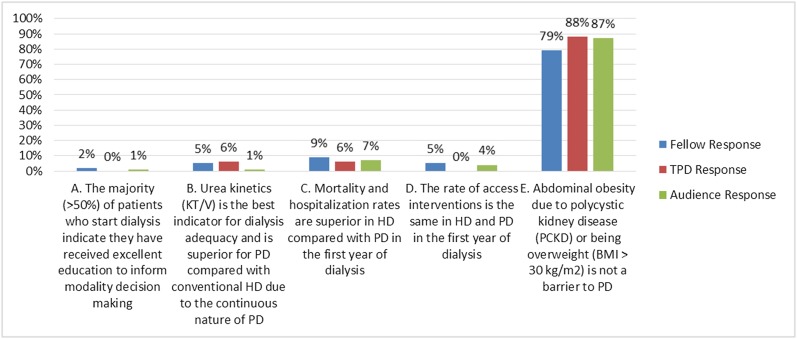
(A) The majority (>50%) of patients who start dialysis indicate that they have received excellent education to inform modality decision making
(B) Urea kinetics (Kt/V) are the best indicator for dialysis adequacy and superior for PD compared with conventional HD because of the continuous nature of PD
(C) Mortality and hospitalization rates are superior in HD compared with PD in the first year of dialysis
(D) The rate of access interventions is the same in HD and PD in the first year of dialysis
(E) A large abdomen related to PCKD or being overweight (BMI>30) is not a barrier to PD
Answer: E.
Discussion of Question 1A
Although this section of the NQ&Q is not on CKD and its management, the effect of predialysis care sets the stage for each patient’s ESRD life plan. For this patient, in selecting a modality for chronic dialysis along his ESRD life plan, his large abdomen related to his PCKD and being overweight is not a barrier to PD (choice E is correct).
When patients have stage 4 or 5 CKD, they are often in denial and unable to make decisions to plan appropriately and/or implement management strategies needed for initiating RRT. A common scenario is when dialysis finally becomes inevitable, and a single decision is made to initiate a dialysis modality for the immediate need. Often, an ESRD life plan is not discussed and/or conceived; the lack of such an ESRD life plan can have later downstream negative consequences.
In this case, the patient has established CKD and has been appropriately followed in a multidisciplinary CKD clinic. The benefits of a multidisciplinary team approach to CKD care are well established, including delayed progression to ESRD and improved survival (1–3), improved patient psychologic profile (better moods and less anxiety), less physical functional disability (4,5), fewer HD catheter starts (2), and increased choice and likelihood of home–based dialysis modalities (2). However, we do not typically provide “excellent education to inform modality decision making.” In a large survey of >2000 patients, <25% of patients indicated that heath care providers gave “excellent” information when choosing HD or PD (6). Other large studies also found that a significant proportion of patients (approximately 40%–65%) felt that they did not have a choice in dialysis modality or that they were not informed of their choices (7,8) (choice A is incorrect). However, patient involvement in modality decision making is associated with greater patient satisfaction once on dialysis (8). Indeed, the component of multidisciplinary team care that may be most impactful with respect to modality choice is patient education (9). The importance of patient and modality education is recognized by the 2008 Medicare Improvements for Patients and Providers Act (United States), where reimbursement is provided for six sessions of comprehensive CKD education. Recently, a large study showed that such education can be provided in four phases using Patient Decision Aids aimed at two objectives: “to progressively provide information and to guide patients to identify their personal values and important lifestyle aspects and preferences, facilitating the deliberation and ultimately the choice”; of patients who used Patient Decision Aids, 45% choose PD (10).
PD is steadily increasing in the United States with the change in financial reimbursement and recognition of PD’s equivalence and/or potential benefits in terms of patient survival, access interventions required in the first year of initiating dialysis, hospitalizations, cost savings, and patient lifestyle flexibility compared with HD (11,12) (choices C and D are incorrect). Dialysis adequacy, as determined by Kt/V urea, is measured differently for HD and PD and cannot be directly compared (choice B is incorrect). Furthermore, using urea clearance and values derived from urea kinetic models, such as Kt/V, as the sole gold standard for assessing dialysis adequacy continues to be vigorously debated (13,14). Although Kt/V can be easily obtained as a performance measure and relevant for policymakers, administrators, and insurers, more patient–relevant variables, such as a patient’s overall wellbeing and how they are feeling—although more challenging to objectively measure—are arguably more clinically important indicators of optimal and not just adequate dialysis.
Contraindications to PD should be individually assessed. Absolute contraindications include (1) conditions that would prevent effective PD (i.e., leading to significantly reduced peritoneal membrane surface area or poor dialysate flow) or increase the risk of infections (e.g., abdominal surgeries resulting in multiple adhesions, or uncontrolled active inflammatory bowel conditions) and (2) an absence of needed assistance in a patient who is mentally or physically incapable of performing PD. Indeed, a majority (>75%) of patients with ESRD are eligible for PD (15). In this patient, a BMI>30 and having PCKD are not contraindications to PD (16,17) (Figure 1).
Figure 1.
Answer for Case 1, Question 1A: Which of the following statements is true regarding RRT? The correct answer is E. BMI, body mass index; HD, hemodialysis; PD, peritoneal dialysis; TPD, training program directors.
The patient finally decides that he will perform PD when he reaches ESRD. He makes significant lifestyle changes and loses weight (BMI reduced from 32 to 29) over 9 months. Later, he is admitted into hospital with nausea, vomiting, and diarrhea from food poisoning (not from his restaurant). Hospitalization is complicated by a serious pneumonia associated with hypotension and need for intravenous antibiotics. He suffers from severe AKI without recovery of his kidney function. He becomes oliguric and volume overloaded (uncomfortable extremity and scrotal swelling along with mild shortness of breath but O2 saturation ≥92%) and develops hyperkalemia (K=5.6). A trial of discontinuing ACEI and diuretic was attempted.
Question 1B
Which is the next best step in the management of this patient?
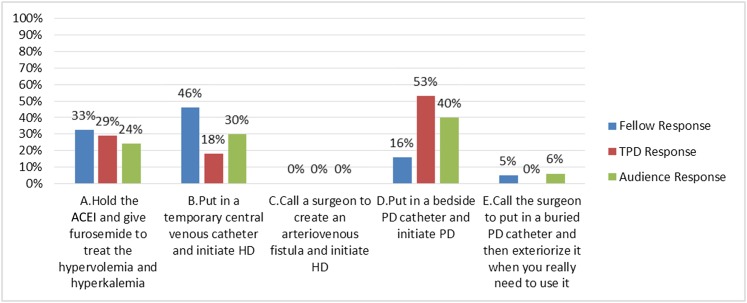
(A) Give more furosemide to treat the hypervolemia and hyperkalemia
(B) Put in a temporary central venous catheter (CVC) and initiate HD
(C) Call a surgeon to create an arteriovenous fistula and initiate HD
(D) Put in a bedside PD catheter and initiate PD
(E) Call a surgeon to put in a buried PD catheter and then exteriorize it when you really need to use it
Answer: D.
Discussion of Question 1B
This patient has already decided on PD and needs to initiate dialysis; thus, putting in a bedside PD catheter to initiate PD is most efficient (choice D is correct). Additional attempts at medical management of his kidney failure are unlikely to be successful. Ultimately, this patient requires dialysis to manage his acute on chronic kidney failure (choice A is incorrect). In facilities where PD is unavailable, insertion of a temporary CVC to initiate HD is reasonable, because the short timeframe would prohibit the creation and proper maturation of an arteriovenous fistula for functional use in this situation. However, in this patient, PD resources are available, and PD was the patient’s modality of choice (choices B and C are incorrect).
Urgent start PD is a relatively new term for an old procedure that has gained considerable exposure over the last decade. Although traditional PD requires a 2- to 4-week healing period after PD catheter insertion before training is started, urgent start PD refers to PD initiation earlier than 2 weeks after PD catheter insertion. Typically, urgent start PD is performed with low-fill volumes with the patient in the supine position with a cycler to reduce intra-abdominal pressure and avoid pericatheter or incisional leaks. It is an excellent HD CVC sparing strategy with the additional benefits of PD (18). However, proper training and logistic support is required throughout all phases of its implementation. Although it is conceptually appealing, in many urgent dialysis start situations, patients have had little or no education about CKD or ESRD, let alone dialysis modality. They are often overwhelmed with the need to start dialysis in addition to the burden of their underlying medical circumstances and may not be emotionally or cognitively able to understand the processes and implications of PD catheter insertion and urgent start PD. Thus, the first phase of the process of urgent start PD is to ensure that the hospital or facility has the support of experienced and dedicated dialysis educators for patients and their families to ensure that the right decision is made for urgent PD start with a view of continued long–term PD. The next phases, including PD catheter insertion and the practical implementation of an urgent start PD program, are beyond the scope of this discussion but are reviewed elsewhere (19–21).
In this case, our patient was a well informed patient with CKD who had an acute decline in his kidney function. Here, a PD catheter insertion to initiate PD for AKI (with continued long–term PD) is appropriate. Such acute or urgent start PD was first described in the 1950s and was popular in the 1970s (22). Later, its use waned in favor of HD-based continuous replacement renal therapy, especially in acute care units. In these situations of acute initiations of PD, there may also be reduced efficiency in large-sized and/or hypercatabolic patients if the fill volume cannot be adequately adjusted. However, it has the advantages of practical simplicity with limited equipment and training required, gentle dialysis and ultrafiltration in hemodynamically unstable patients, and it does not require anticoagulation. Early studies showed a lower mortality rate and a higher rate of renal recovery compared with HD (23). Perhaps the greatest practical challenge in treating a patient with AKI using PD is timely PD catheter insertion (within 12–36 hours). Traditionally, surgeons placed PD catheters using open surgical and then, laprascopic techniques; next, interventional radiologists and nephrologists gained familiarity with radiologic and bedside techniques. Regardless of the operator’s discipline, the key to successful PD catheter placement is appropriate training and experience with a given technique (24,25). Because of lack of timely access to PD catheter insertions for acute dialysis starts by surgery or radiology in our institution, our nephrology division supported a bedside PD catheter program initiated by a sole staff nephrologist. Preliminary results (n=53) from our center have shown a success rate of 93% (7% primary failure rate), with 100% used for dialysis (18). Long–term committed use of PD after insertion by nephrologists compared with surgeons or radiologists has been reported in a study of >3000 patients on PD (26). Thus, a key to facilitating PD in a patient needing urgent start is to have timely and safe PD catheter insertion by trained and committed operators who have shown successful PD use after catheter insertion—be it by surgical, percutaneous, or various other bedside techniques (Figure 2).
Figure 2.
Answer for Case 1, Question 1B: Which is the next best step in the management of this patient? The correct answer is D. ACEI, angiotension converting enzyme inhibitor; HD, hemodialysis; PD, peritoneal dialysis; TPD, training program directors.
Case 2
A 26-year-old previously active woman is referred to your multidisciplinary CKD clinic by a colleague. She has established primary FSGS, and her kidneys are failing rapidly, despite appropriate immunosuppressive therapy. Her other significant active medical issues are migraine headaches and ongoing active ulcerative colitis. She works as a sales person in a high–end clothing store and is fiercely independent, with a very active social life. When she comes to your clinic, she complains of nausea and poor appetite but denies persistent vomiting, and she has tolerable pruritus.
On examination, her BP is 145/90 (bilaterally), heart rate is 72 bpm, and respiratory rate is 18. She has significant anasarca but no cardiac gallop or rub. She does not have asterixis. Her Allen test is positive.
Her trend in eGFR decline is rapid and now <11 ml/min; K=4.8 mmol/L, and bicarbonate=18 mmol/L.
Question 2A
At this point, what dialysis access is the best choice for this patient?
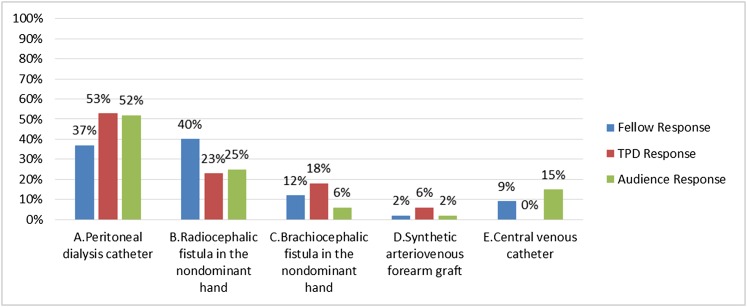
(A) PD catheter
(B) Radiocephalic fistula in the nondominant hand
(C) Brachiocephalic fistula in the nondominant hand
(D) Synthetic arteriovenous forearm graft
(E) CVC
Answer: D.
Discussion of Question 2A
This young patient needs to urgently (not emergently) start dialysis and has a long ESRD lifetime ahead of her. The principle of a distal to proximal approach to vascular access in this patient is most appropriate; given the lack of time for fistula maturation, a forearm arteriovenous graft is appropriate in this patient (choice D is correct). Indeed, this patient is volume overloaded and has a rapid and progressive decline in her kidney function. Although she has no signs or symptoms of uremia requiring emergency dialysis (e.g., life-threatening hyperkalemia or pulmonary edema/volume overload refractory to medical management, pericarditis, altered consciousness/seizures, uremic bleeding, or intractable nausea/vomiting/pruritus), she is anticipated to start dialysis within 2–3 weeks. This case is typical of a patient requiring urgent start dialysis. In this type of dialysis start, the patient may have had timely and/or excellent pre–ESRD care, but nevertheless, the patient’s underlying kidney condition has had a natural rapid decline in function, or unforeseen circumstances occurred that expedited the patient’s need for dialysis. An urgent start may also be viewed differently than an emergent start, where dialysis is required imminently to correct life-threatening conditions (above). A patient needing urgent start dialysis may have signs and symptoms of uremia or volume overload but nonlife-threatening conditions, where dialysis is anticipated to be required in approximately 2 weeks (±1 week). It is the timeframe in which it is difficult to have a fistula planned, created, and matured enough to be successfully used for functional dialysis (27) (choice B is incorrect; details below). In such circumstances where the patient is independent and active, home PD would have been an excellent option; however, this patient cannot have PD, because she has ongoing active ulcerative colitis (choice A is incorrect).
In all patients in whom HD is the most appropriate dialysis modality, guidelines and national quality improvement projects, such as the Fistula First, Catheter Last Initiative, greatly emphasize fistula first … if possible. Is a fistula possible in this patient? Undoubtedly. She is young, with renal limited disease and no prior central venous disruptions, such as a pacemaker; thus, a fistula is likely possible. However, it is sometimes necessary to think outside the box and beyond expected norms to really respond to each of your patient’s differing needs to achieve the best quality dialytic care. Is a fistula really appropriate for this patient at this time? No. Depending on geography and local institutional practices, it may take longer than 1–2 weeks for surgical referral and creation (28,29). Even if it could be created within 72 hours, it would still require approximately 4 weeks minimum to mature enough to support dialysis with the high blood flow rates prescribed in North America. However, average maturation times range from 4 to 9 months (30,31). In the interim, our patient would require a temporary CVC for dialysis. This would put her at risk of complications, including bacteremia, central stenosis, and shortened ipsilateral arteriovenous access cumulative patency (32,33). Approximately 20%–60% of fistulas fail to mature (34,35) and require up to two to three interventions to facilitate maturation (36,37) (choices B and E are incorrect).
Upper arm fistulas have been reported to have better maturation success compared with forearm fistulas because of larger vessel sizes in the upper arm (38). In an elderly patient with limited life expectancy, a brachiocephalic fistula may have been an acceptable choice, even if the patient needed a CVC on a short-term basis. However, this patient is young and vibrant and has a long life expectancy. The median cumulative patency of a brachiocephalic fistula ranges from 1.6 to 3.6 years (39,40). By bypassing options at the wrist and forearm, her life expectancy has been shortened by the duration of the brachiocephalic fistula’s patency (choice C is incorrect). For this reason, national vascular access guidelines recommend a distal to proximal approach for access placement to enable expanded options for vascular access placement. At this time, her ESRD life plan needs to be considered. One would expect this patient to initiate HD and hopefully get a kidney transplant, and depending on what happens to her inflammatory bowel disease, she may return back to PD or HD when her transplant fails. At that time, she would only be in her 40s or early 50s—when she still has a significant lifetime ahead of her and would need vascular access.
Thus, in this case of a young woman with an urgent need for dialysis start, a forearm graft would be the best option. Grafts have been shown to have equivalent or even superior patency to arteriovenous fistulas (41–43). Early cannulation grafts can be used within 72 hours, if needed, and avoid CVC placement (44). The blood flows through the forearm graft would enhance the cephalic vein so that if it became problematic, it could be converted to a fistula, which would then be anticipated to have a short maturation time (because of prior development of the cephalic vein) (45). Hopefully, she would get a transplant by the time the graft becomes problematic (Figure 3).
Figure 3.
Answer for Case 2, Question 2A: At this point, what dialysis access is the best choice for this patient? The correct answer is D. TPD, training program directors.
One week later, she hosts a party with lots of salty foods and presents to the emergency department with volume overload—short of breath and impending intubation. A left internal jugular CVC is placed, and she receives acute HD and adequate ultrafiltration.
Question 2B
Which of the following statements is true regarding central venous stenosis from catheters?
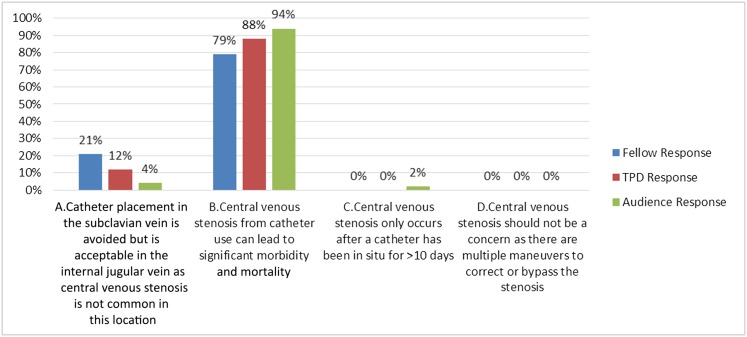
(A) Catheter placement in the subclavian vein is avoided but acceptable in the internal jugular vein, because central venous stenosis is not common in this location
(B) Central venous stenosis from catheter use can lead to significant morbidity and mortality
(C) Central venous stenosis only occurs after a catheter has been in situ for >10 days
(D) Central venous stenosis should not be a concern, because there are multiple simple conventional maneuvers to correct or bypass the stenosis
Answer: B.
Discussion of Question 2B
Central venous stenosis from catheter use is often associated with significant morbidity and mortality (choice B is the correct answer). Central venous stenosis is defined as a >50% lesion in the internal jugular, subclavian, or axillary veins that affects the HD circuit by interfering with a return of blood to the superior vena cava and leading to venous hypertension (46). It is unclear at what time point central venous stenosis occurs (choice C is incorrect). Some patients can have multiple CVCs without issue, and some may have central stenosis even without ever having a CVC in situ. However, it is associated with greater number of CVCs, longer duration of CVC use, and dialysis vintage (47,48). It is often unrecognized and may only become apparent after an arteriovenous access is placed, leading to increased venous return and clinical signs and symptoms (46,48). Central venous stenosis has been reported in about 50% of patients with vascular access problems who undergo venography (47,48). Although subclavian vein catheterization has largely been abandoned because of problems with central vein stenosis, the incidence of central vein stenosis with internal jugular catheters has been reported to be 36% (48) (choice A is incorrect). The greatest clinically relevant sequelae of central venous stenosis for this patient and many other patients is the inability to create an arteriovenous access—fistula or graft—because of restricted venous inflow. This leaves the patient CVC dependent if the other limb cannot be used for arteriovenous access creation (already used or has complications making the side unsuitable for arteriovenous access). Sometimes, the temporary CVC in situ causes the central stenosis but ends up being the object keeping the vein open. After that CVC is removed, it may be difficult to recannulate the vein. Indeed, there are maneuvers to re-establish patency, such as balloon angioplasty, stents, and complicated recanalization procedures; if these fail, the patient would be left with transhepatic, translumbar, femoral, iliac, or direct atrial access. To gain access to the right atrium, highly unconventional maneuvers, such as the inside-out procedure (video in ref. 49), have been attempted with success (50) (choice D is incorrect). If the stenosis is successfully bypassed, unique devices, such as the hemodialysis reliable outflow graft (a hybrid between a prosthetic graft and a tunneled catheter), may be an option. The distal graft portion is subcutaneous and used for cannulation, whereas the proximal catheter portion ends at the right atrium, maintaining patency of the central venous outflow (after it is accessed). As with a graft or a CVC, it too has its own complications. It is best, if possible, to avoid a CVC, the risk of central stenosis, and its complications (Figure 4).
Figure 4.
Answer for Case 2, Question 2B: Which of the following statements is true regarding central venous stenosis from catheters? The correct answer is B. TPD, training program directors.
This patient’s surgeon elected to place a left brachiocephalic fistula, which failed to mature over 6 months.
Question 2C
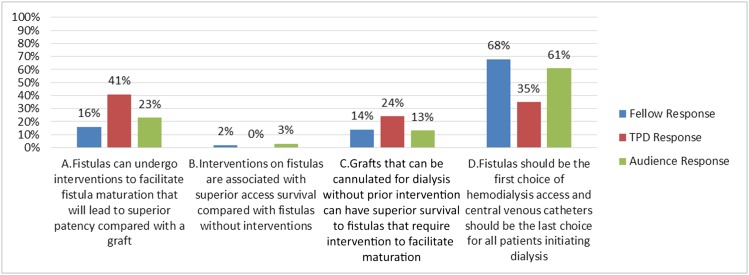
Which of the following statements is true?
(A) Fistulas can undergo interventions to facilitate fistula maturation that will lead to superior patency compared with a graft
(B) Interventions on fistulas are associated with superior access survival compared with fistulas without interventions
(C) Grafts that can be cannulated for dialysis without prior intervention can have superior survival to fistulas that require intervention to facilitate maturation
(D) Fistulas should be the first choice of HD access, and CVCs should be the last choice for all patients initiating dialysis
Answer: C.
Discussion of Question 2C
Although Fistula First, Catheter Last has been highly promoted, one or even two sizes do not fit all. This is particularly relevant with new data reporting that grafts that are cannulated without prior intervention can have superior survival compared with fistulas that require intervention to mature (51) (choice C is correct). Indeed, fistulas that require interventions to facilitate maturation have inferior survival to those that mature without intervention (52) (choice B is incorrect). Also, cumulative patencies of fistulas and grafts over the past decade have been shown to be equivalent (41–43). These data differ from earlier data on which the superiority of fistulas was first established (53). It is very important to re-evaluate data and adjust our practices accordingly to provide optimal and current patient care (choices A and D are incorrect) (Figure 5).
Figure 5.
Answer for Case 2, Question 2C: Which of the following statements is true? The correct answer is C. TPD, training program directors.
When clinicians, researchers, and administrators stop thinking outside the box and settle for the expected norm for a patient in whom this norm does not apply, we blind ourselves to potential innovation and progress to improve care for individual patients. Who would have thought that one could access a patient’s central system from the inside out to fix the problem of central stenosis (49,50) or that fistulas could be created without surgery (using percutaneous magnet–based technology) to potentially improve the problem of fistula nonmaturation (54)? Although these are extreme examples that are not yet mainstream, we can still do our part as clinicians when in front of an individual patient. It starts by taking a little time up front to get to know your patient but saves a lot of time in their ESRD lifetime—that the clinician has presumably committed to overseeing—to prevent dissatisfaction and complications later on. Be it dialysis adequacy, dialysis access, or something else, we should think of how best to manage that specific patient’s care, perhaps not even by thinking outside the box but by thinking as if there were no box! Lastly, when challenged by the discordance between policy and new evidence as it applies to an individual patient, the decision about the optimal care for a truly informed patient can often be elucidated by asking the patient about their preference and doing what’s right for them.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Goldstein M, Yassa T, Dacouris N, McFarlane P: Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis 44: 706–714, 2004 [PubMed] [Google Scholar]
- 2.Lacson E Jr., Wang W, DeVries C, Leste K, Hakim RM, Lazarus M, Pulliam J: Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis 58: 235–242, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Devins GM, Mendelssohn DC, Barré PE, Binik YM: Predialysis psychoeducational intervention and coping styles influence time to dialysis in chronic kidney disease. Am J Kidney Dis 42: 693–703, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Klang B, Björvell H, Berglund J, Sundstedt C, Clyne N: Predialysis patient education: Effects on functioning and well-being in uraemic patients. J Adv Nurs 28: 36–44, 1998 [DOI] [PubMed] [Google Scholar]
- 5.White CA, Pilkey RM, Lam M, Holland DC: Pre-dialysis clinic attendance improves quality of life among hemodialysis patients. BMC Nephrol 3: 3, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer SC, de Berardis G, Craig JC, Tong A, Tonelli M, Pellegrini F, Ruospo M, Hegbrant J, Wollheim C, Celia E, Gelfman R, Ferrari JN, Törok M, Murgo M, Leal M, Bednarek-Skublewska A, Dulawa J, Strippoli GF: Patient satisfaction with in-centre haemodialysis care: An international survey. BMJ Open 4: e005020, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song MK, Lin FC, Gilet CA, Arnold RM, Bridgman JC, Ward SE: Patient perspectives on informed decision-making surrounding dialysis initiation. Nephrol Dial Transplant 28: 2815–2823, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Biesen W, van der Veer SN, Murphey M, Loblova O, Davies S: Patients’ perceptions of information and education for renal replacement therapy: An independent survey by the European Kidney Patients’ Federation on information and support on renal replacement therapy. PLoS One 9: e103914, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manns BJ, Taub K, Vanderstraeten C, Jones H, Mills C, Visser M, McLaughlin K: The impact of education on chronic kidney disease patients’ plans to initiate dialysis with self-care dialysis: A randomized trial. Kidney Int 68: 1777–1783, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Prieto-Velasco M, Quiros P, Remon C; Spanish Group for the Implementation of a Shared Decision Making Process for RRT Choice with Patient Decision Aid Tools: The concordance between patients’ renal replacement therapy choice and definitive modality: Is it a utopia? PLoS One 10: e0138811, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Oliver MJ, Verrelli M, Zacharias JM, Blake PG, Garg AX, Johnson JF, Pandeya S, Perl J, Kiss AJ, Quinn RR: Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 27: 810–816, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Vanholder R, Glorieux G, Eloot S: Once upon a time in dialysis: The last days of Kt/V? Kidney Int 88: 460–465, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Daugirdas JT: Kt/V (and especially its modifications) remains a useful measure of hemodialysis dose. Kidney Int 88: 466–473, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Jager KJ, Korevaar JC, Dekker FW, Krediet RT, Boeschoten EW; Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Study Group: The effect of contraindications and patient preference on dialysis modality selection in ESRD patients in The Netherlands. Am J Kidney Dis 43: 891–899, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Johnson DW, Herzig KA, Purdie DM, Chang W, Brown AM, Rigby RJ, Campbell SB, Nicol DL, Hawley CM: Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int 20: 715–721, 2000 [PubMed]
- 17.Hadimeri H, Johansson AC, Haraldsson B, Nyberg G: CAPD in patients with autosomal dominant polycystic kidney disease. Perit Dial Int 18: 429–432, 1998 [PubMed]
- 18.Lok CE: Urgent peritoneal dialysis or hemodialysis catheter dialysis. J Vasc Access 17[Suppl 1]: 56–59, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Arramreddy R, Zheng S, Saxena AB, Liebman SE, Wong L: Urgent-start peritoneal dialysis: A chance for a new beginning. Am J Kidney Dis 63: 390–395, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghaffari A, Kumar V, Guest S: Infrastructure requirements for an urgent-start peritoneal dialysis program. Perit Dial Int 33: 611–617, 2013 [DOI] [PMC free article] [PubMed]
- 21.Casaretto A, Rosario R, Kotzker WR, Pagan-Rosario Y, Groenhoff C, Guest S: Urgent-start peritoneal dialysis: Report from a U.S. private nephrology practice. Adv Perit Dial 28: 102–105, 2012 [PubMed] [Google Scholar]
- 22.Maxwell MH, Rockney RE, Kleeman CR, Twiss MR: Peritoneal dialysis. 1. Technique and applications. J Am Med Assoc 170: 917–924, 1959 [DOI] [PubMed] [Google Scholar]
- 23.Passadakis PS, Oreopoulos DG: Peritoneal dialysis in patients with acute renal failure. Adv Perit Dial 23: 7–16, 2007 [PubMed] [Google Scholar]
- 24.Wong LP, Liebman SE, Wakefield KA, Messing S: Training of surgeons in peritoneal dialysis catheter placement in the United States: A national survey. Clin J Am Soc Nephrol 5: 1439–1446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo YI, Shin SK, Lee JH, Song JO, Park JH: Immediate initiation of CAPD following percutaneous catheter placement without break-in procedure. Perit Dial Int 27: 179–183, 2007 [PubMed]
- 26.Perl J, Pierratos A, Kandasamy G, McCormick BB, Quinn RR, Jain AK, Huang A, Paterson JM, Oliver MJ: Peritoneal dialysis catheter implantation by nephrologists is associated with higher rates of peritoneal dialysis utilization: A population-based study. Nephrol Dial Transplant 30: 301–309, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Rayner HC, Pisoni RL, Gillespie BW, Goodkin DA, Akiba T, Akizawa T, Saito A, Young EW, Port FK; Dialysis Outcomes and Practice Patterns Study: Creation, cannulation and survival of arteriovenous fistulae: Data from the Dialysis Outcomes and Practice Patterns Study. Kidney Int 63: 323–330, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 21: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Goldfarb-Rumyantzev AS, Syed W, Patibandla BK, Narra A, Desilva R, Chawla V, Hod T, Vin Y: Geographic disparities in arteriovenous fistula placement in patients approaching hemodialysis in the United States. Hemodial Int 18: 686–694, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Kimball TA, Barz K, Dimond KR, Edwards JM, Nehler MR: Efficiency of the kidney disease outcomes quality initiative guidelines for preemptive vascular access in an academic setting. J Vasc Surg 54: 760–765, 2011 [DOI] [PubMed]
- 31.Biuckians A, Scott EC, Meier GH, Panneton JM, Glickman MH: The natural history of autologous fistulas as first-time dialysis access in the KDOQI era. J Vasc Surg 47: 415–421, 2008 [DOI] [PubMed]
- 32.Rehman R, Schmidt RJ, Moss AH: Ethical and legal obligation to avoid long-term tunneled catheter access. Clin J Am Soc Nephrol 4: 456–460, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Shingarev R, Barker-Finkel J, Allon M: Association of hemodialysis central venous catheter use with ipsilateral arteriovenous vascular access survival. Am J Kidney Dis 60: 983–989, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CE, Zhang JC, Garg AX, Kosa SD, Quinn RR, Moist LM: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kidney Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Dember LM, Beck GJ, Allon M, Delmez JA, Dixon BS, Greenberg A, Himmelfarb J, Vazquez MA, Gassman JJ, Greene T, Radeva MK, Braden GL, Ikizler TA, Rocco MV, Davidson IJ, Kaufman JS, Meyers CM, Kusek JW, Feldman HI; Dialysis Access Consortium Study Group: Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: A randomized controlled trial. JAMA 299: 2164–2171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller GA, Hwang W, Preddie D, Khariton A, Savransky Y: Percutaneous salvage of thrombosed immature arteriovenous fistulas. Semin Dial 24: 107–114, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Falk A: Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol 17: 807–813, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Dageforde LA, Harms KA, Feurer ID, Shaffer D: Increased minimum vein diameter on preoperative mapping with duplex ultrasound is associated with arteriovenous fistula maturation and secondary patency. J Vasc Surg 61: 170–176, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Oliver MJ, McCann RL, Indridason OS, Butterly DW, Schwab SJ: Comparison of transposed brachiobasilic fistulas to upper arm grafts and brachiocephalic fistulas. Kidney Int 60: 1532–1539, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Lok CEOM, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV: Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int 67: 2462–2469, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allemang MT, Schmotzer B, Wong VL, Lakin RO, Woodside KJ, Schulak JA, Wang J, Kashyap VS: Arteriovenous grafts have higher secondary patency in the short term compared with autologous fistulae. Am J Surg 208: 800–805, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Cui J, Steele D, Wenger J, Kawai T, Liu F, Elias N, Watkins MT, Irani Z: Hemodialysis arteriovenous fistula as first option not necessary in elderly patients [published online ahead of print January 6, 2016]. J Vasc Surg [DOI] [PubMed]
- 44.Al Shakarchi J, Houston G, Inston N: Early cannulation grafts for haemodialysis: A systematic review. J Vasc Access 16: 493–497, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Slayden GC, Spergel L, Jennings WC: Secondary arteriovenous fistulas: Converting prosthetic AV grafts to autogenous dialysis access. Semin Dial 21: 474–482, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Collin G, Jones RG, Willis AP: Central venous obstruction in the thorax. Clin Radiol 70: 654–660, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Hernández D, Díaz F, Rufino M, Lorenzo V, Pérez T, Rodríguez A, De Bonis E, Losada M, González-Posada JM, Torres A: Subclavian vascular access stenosis in dialysis patients: Natural history and risk factors. J Am Soc Nephrol 9: 1507–1510, 1998 [DOI] [PubMed] [Google Scholar]
- 48.MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M: Central vein stenosis: A common problem in patients on hemodialysis. ASAIO J 51: 77–81, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Gurley J: Surfacer, 2016. Available at: http://bluegrassvascular.com/company/. Accessed January 28, 2016
- 50.Elayi CS, Allen CL, Leung S, Lusher S, Morales GX, Wiisanen M, Aikat S, Kakavand B, Shah JS, Moliterno DJ, Gurley JC: Inside-out access: A new method of lead placement for patients with central venous occlusions. Heart Rhythm 8: 851–857, 2011 [DOI] [PubMed]
- 51.Harms JC, Rangarajan S, Young CJ, Barker-Finker J, Allon M: Outcomes of arteriovenous fistulas and grafts with or without intervention prior to successful use. J Vasc Surg 2016, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee T, Ullah A, Allon M, Succop P, El-Khatib M, Munda R, Roy-Chaudhury P: Decreased cumulative access survival in arteriovenous fistulas requiring interventions to promote maturation. Clin J Am Soc Nephrol 6: 575–581, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Kidney Foundation-Dialysis Outcomes Quality Initiative: NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis 30[Suppl 3]: S150–S191, 1997 [PubMed] [Google Scholar]
- 54.Rajan DK, Lok CE: Promises for the future: Minimally invasive fistula creation. J Vasc Access 16[Suppl 9]: S40–S41, 2015 [DOI] [PubMed] [Google Scholar]