Abstract
Background and Objectives
Protein energy wasting and systemic inflammation are prevalent in maintenance hemodialysis (MHD) patients. Omega-3 (ω-3) fatty acids have anti-inflammatory properties and have been shown to improve protein homeostasis. We hypothesized that administration of high-dose (2.9 g/d) ω-3 would be associated with decreased muscle protein breakdown in MHD patients with systemic inflammation.
Design, setting, participants & measurements
This is a substudy from a randomized, placebo-controlled study (NCT00655525). Patients were recruited between September 2008 and June 2011. Primary inclusion criteria included signs of chronic inflammation (average C-reactive protein of ≥5 mg/L over three consecutive measurements), lack of active infectious or inflammatory disease, no hospitalization within 1 month prior to the study, and not receiving steroids (>5 mg/d) and/or immunosuppressive agents. The primary outcomes were forearm muscle and whole body protein breakdown and synthesis before and after the intervention. The patients received ω-3 (n=11) versus placebo (n=9) for 12 weeks. Analysis of covariance was used to compare outcome variables at 12 weeks. Models were adjusted for a propensity score that was derived from age, sex, race, baseline high sensitivity C-reactive protein, diabetes mellitus, and fat mass because the groups were not balanced for several characteristics.
Results
Compared with placebo, ω-3 supplementation was significantly associated with decreased muscle protein breakdown at 12 weeks (−31, [interquartile range, −98–−13] versus 26 [interquartile range, 13–87] µg/100 ml per min; P=0.01), which remained significant after multivariate adjustment (−46, [95% confidence interval, −102 to −1] µg/100 ml per min). ω-3 Supplementation resulted in decreased forearm muscle protein synthesis while the rate in the placebo group increased; however, there is no longer a statistically significant difference in skeletal muscle protein synthesis or in net protein balance after multivariate adjustment. There was no statistically significant effect of ω-3 supplementation on whole body protein synthesis or breakdown.
Conclusions
High-dose ω-3 supplementation over 12 weeks in MHD patients with systemic inflammation was associated with attenuation of forearm muscle protein breakdown but did not influence skeletal muscle protein synthesis, skeletal muscle net protein balance or any component of the whole-body protein balance. These results should be interpreted cautiously given the imbalance in the two groups and the short duration of the intervention.
Keywords: hemodialysis, protein energy wasting, muscle protein turnover, omega-3 fatty acids, diabetes mellitus, Forearm, Homeostasis, Humans, Inflammation, Muscle Proteins
Introduction
Maintenance hemodialysis (MHD) patients are prone to have multiple catabolic processes that lead to a unique form of metabolic and nutritional derangement termed as protein energy wasting (PEW) of kidney disease (1,2). PEW is associated with major adverse clinical outcomes and is considered to be a significant comorbid condition leading to increased rates of hospitalization and death in patients undergoing MHD.
Systemic inflammation is highly prevalent in MHD patients. Multiple studies in several chronic disease states have shown that markers of systemic inflammation, such as high sensitivity C-reactive protein (hs-CRP) and IL-6, are associated with worse nutrition status, and systemic inflammation is known to be involved in different forms of cachexia such as those seen in cancer. Accordingly, it is likely that systemic inflammation plays a central role in development and worsening of PEW in MHD patients. However, there are limited data of anti-inflammatory interventions in MHD patients showing improvements in nutrition markers.
ω-3 Fatty acids have multiple metabolic effects including anti-inflammatory properties (3). Among those, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) also have direct muscle anabolic actions independent of their anti-inflammatory actions (4–6) suggesting that this might be a valuable therapeutic approach for the treatment of PEW in MHD patients (4,7). In this study, we aimed to estimate the association between high-dose ω-3 fatty acid supplementation on the rates of muscle protein synthesis and breakdown in MHD patients with underlying systemic inflammation. We hypothesized that administration of 2.9 g/d ω-3 for 12 weeks would effectively decrease muscle protein breakdown based on the data indicating that inflammation primarily increases protein catabolism (8).
Materials and Methods
Participants
Patients were recruited from the Vanderbilt University Medical Center (VUMC) dialysis units and the Veteran Affairs Tennessee Valley Healthcare System (VATHS) between September 2008 and June 2011. Inclusion criteria included being on MHD for >6 months, well-functioning hemodialysis vascular access or permanent dialysis catheter, signs of chronic inflammation (average C-reactive protein [CRP] of ≥5 mg/L over three consecutive measurements), and acceptable dialysis adequacy (kt/V>1.2). Exclusion criteria included active infectious or inflammatory disease, hospitalization within 1 month prior to study, and receiving steroids (>5 mg/d) and/or immunosuppressive agents. The study was approved by the institutional review boards from both VUMC and VATHS, and signed informed consent was obtained from all study patients.
Experimental Protocol
Intervention.
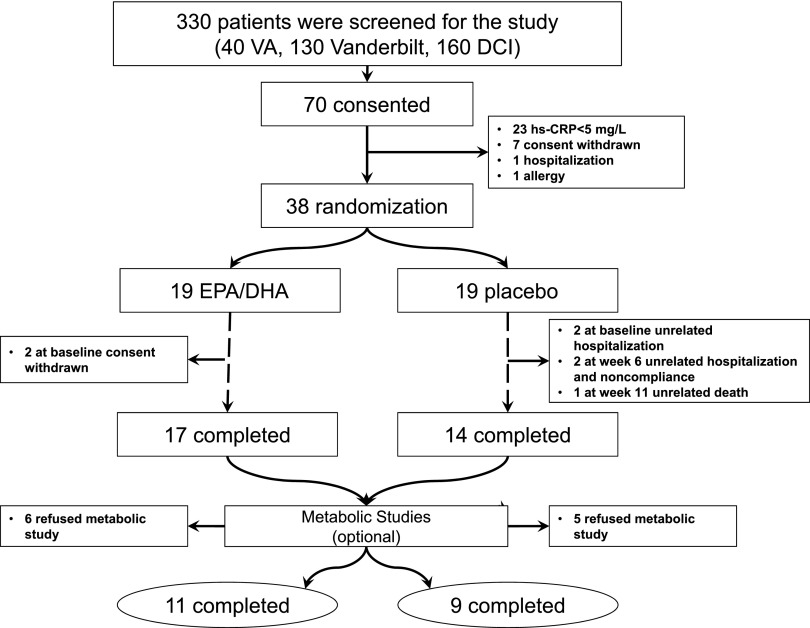
This was a substudy of a placebo-controlled, double-blind randomized trial aimed at examining anti-inflammatory properties of the effects of ω-3 fatty acids in MHD patients. Both aims of the protocol were registered at clinicaltrials.gov prior to start of the intervention (NCT00655525) (9). Of 330 screened patients, 38 prevalent MHD patients were randomized in a 1:1 ratio to receive 2.9 g of ω-3 (2:1 ratio of EPA/DHA) or placebo daily for 12 weeks (Ocean Nutrition via Yasoo Health, Inc.) (Figure 1). From the 38 randomized patients, 20 opted to participate in the protein metabolism substudy, which happened simultaneously during the trial period. Accordingly, the randomization procedure was only done for the parent study and was not applicable to this particular substudy. The rationale for the dose of EPA/DHA is based on multiple studies showing the effectiveness on cardiovascular risk factors at this dose (10). The investigational pharmacy dispensed the monthly pill supply to participants. Review of compliance (pill counting) and adverse events were discussed with the participant monthly.
Figure 1.
Patient recruitment and randomization flow diagram. DCI, Dialysis Clinics, Inc.; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; hs-CRP, high sensitivity C-reactive protein; VA, Veterans Affairs.
Measurements and Outcomes.
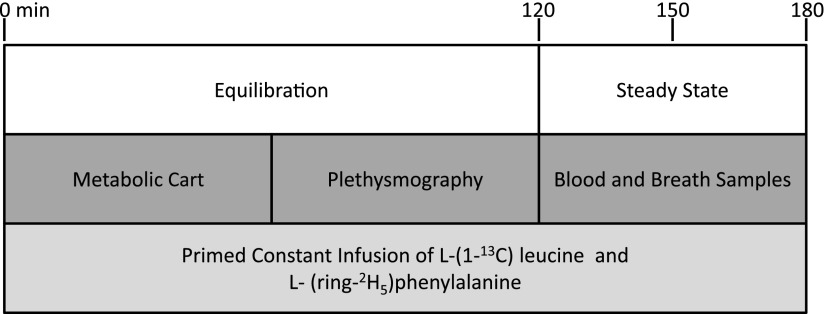
The primary outcome was the change in forearm muscle protein breakdown measured at 12 weeks from baseline. Metabolic studies were performed on a nondialysis day and each metabolic study consisted of a 2-hour equilibration phase followed by a 1-hour sampling period (Figure 2). A detailed description of these metabolic studies, the relevant calculations and the analytic procedures can be found elsewhere in detail (11,12). In brief, an intravenous catheter was placed at the venous site of the dialysis access to collect baseline blood samples and to initiate isotope infusions. The arterial site of the dialysis access was used to sample arterial blood. Another intravenous catheter was placed in the forearm vein of the nonaccess arm to collect blood samples from the forearm muscle bed. After baseline sampling, subjects received a bolus injection of stable isotopes followed by continuous infusion of labeled leucine and phenylalanine. Blood and breath samples were collected during the 1-hour sampling period. Forearm blood flow was estimated by using capacitance plethysmography (model 2560 with URI/CP software version 3.0; Moro Bay, CA).
Figure 2.
Schematic diagram of the metabolic study. A primed constant infusion of L-(1-13C) leucine and L-(ring-2H5) phenylalanine was maintained throughout the metabolic study. Metabolic cart and plethysmography measurements were performed during the equilibration period. Blood and breath sampling were performed during the steady state period.
Amino-acid concentrations were determined by reverse-phase high-performance liquid chromatography after derivatization with phenyliosthiocynate (13). Net skeletal muscle protein balance (synthesis-breakdown) was determined by dilution and enrichment of phenylalanine across the forearm as described previously (14). The steady-state rates of total whole-body leucine rate of appearance were calculated by dividing the (13C) leucine infusion rate by the plasma (13C) ketoisocaproic acid enrichment (15).
Other Measurements.
Glucose concentrations were measured using the glucose oxidase method (Glucose Analyzer 2; Beckman Coulter, Brea, CA). Insulin was measured by using a double-antibody radioimmunoassay (EMD Millipore, Billerica, MA). hs-CRP levels were measured using the high-sensitivity particle-enhanced turbidimetric UniCel Dxl Immunoassay System (Beckman Coulter). The extent of insulin resistance (IR) was estimated by using homeostatic model assessment (HOMA)-IR, insulin (microunits per millimeter) × glucose (milligrams per deciliter)/405 (16). Body composition was assessed by dual-energy x-ray absorptiometry on a nondialysis day using a Lunar Prodigy machine v.11.40.004 (software versions 2003–2011, General Electric, Madison, WI).
Statistical Analyses
Data are presented as mean±SD or as median with interquartile range (IQR) depending on their distribution for continuous variables and percentage for categoric variables. Characteristics were compared using the Wilcoxon rank sum test for baseline continuous variables and chi-squared test for categoric variables. Multivariable linear regression analysis was used to compare outcome variables at 12 weeks between treatment groups by adjusting for corresponding baseline outcome variables. Limited by sample size and to avoid over-fitting, a propensity score was created by fitting a logistic regression model with the treatment arms as dependent variables, and with age, sex, race, hs-CRP, diabetes mellitus, and fat mass as independent variables. The logit of the fitted model was extracted and included in our multivariable linear regression model. The 95% confidence intervals (95% CIs) were calculated with bootstrap resampling techniques. In this approach, statistical significance is declared when the 95% CI does not include zero for variables that have not been log-transformed. Inflammatory markers IL-6 and hs-CRP were natural log-transformed to meet the normality assumption of the multivariable linear regression model. The statistical software package R Studio version 3.1.2 (http://www.rstudio.com/) was used for analyses, and two-sided P values of <0.05 were considered statistically significant.
Results
Patient Characteristics, Body Composition and Laboratory Values
The details of overall patient enrollment for this clinical trial have been published previously. In brief, we screened 330 prevalent maintenance hemodialysis patients and 38 patients were randomly assigned to receive either ω-3 or placebo in the original trial (9). Of those, 20 provided informed consent for the metabolic study and completed the protocol at baseline and at the end of the intervention period (12 weeks) (Figure 1). The inclusion of the patients for this particular substudy was completely based on the patients’ willingness to perform metabolic studies. In order to maintain original randomization, patients were kept at the initially assigned arm rather than an additional randomization protocol.
Characteristics of the study population included in the metabolic studies are shown in Table 1. Overall, patients were similar for age, sex, race, and laboratory assessments between the study groups. Serum hs-CRP (7 mg/dl [4,9] in the intervention group versus 15 mg/dl [9,17] in the placebo group; P=0.003) and fat mass (24±15 kg in the intervention group and 44±18 kg in the placebo group; P=0.01) were statistically different between treatment arms. The placebo group had more diabetic patients compared with the intervention group (44% versus 9%).
Table 1.
Demographical characteristics and biochemical parameters of study population
| Variables | ω-3 (n=11) | Placebo (n=9) | P Value |
|---|---|---|---|
| Age (yr) | 53±9 | 53±13 | 0.74 |
| Sex, male/female; n, (%) | 9/2 (82/18) | 8/1 (89/11) | 0.66 |
| Race black/white; n, (%) | 8/3 (73/27) | 6/3 (67/33) | 0.77 |
| Diabetes mellitus (%) | 1 (9) | 4 (44) | 0.07 |
| Duration of hemodialysis (months) | 32 (16, 132) | 45 (16, 94) | 0.85 |
| Etiology of ESRD n, (%) | |||
| Diabetes mellitus (%) | 1 (9) | 1 (11) | |
| Glomerulonephritis (%) | 8 (73) | 1 (11) | |
| Hypertension (%) | 2 (18) | 7 (78) | 0.41 |
| Body mass index (kg/m2) | 28±7 | 35±9 | 0.05 |
| Fat mass by DEXA (kg) | 24±15 | 44±18 | 0.01 |
| Lean mass by DEXA (kg) | 53±8 | 61±12 | 0.07 |
| BUN (mg/dl) | 30±7 | 34±8 | 0.23 |
| Creatinine (mg/dl) | 7±2 | 8±3 | 0.25 |
| Albumin (g/dl) | 3.5±0.9 | 4±0.4 | 0.53 |
| Glucose (mg/dl) | 88 (80, 92) | 88 (75, 100) | 0.74 |
| HOMA-IR | 2.1 (1.4–2.7) | 4.7 (2.4–6.8) | 0.04 |
| HCT (%) | 41±7 | 41±4 | 0.83 |
| hs-CRP (mg/dl) | 7 (4–9) | 15 (9–17) | 0.003 |
| IL-6 (pg/ml) | 9 (5–11) | 6 (4–15) | 0.85 |
Continuous variables were described as mean with SD or median with interquartile range. Categoric variables were presented as percentages. P value was calculated using the Wilcoxon rank sum test for continuous variables and χ2 test for categoric variables. DEXA, dual-energy x-ray absorptiometry; HOMA-IR, homeostatic model assessment-insulin resistance; HCT, hematocrit; hs-CRP, high sensitivity C-reactive protein.
Insulin Resistance and Inflammatory Parameters
Baseline fasting glucose levels were similar between the study groups (P=0.74) (Table 1), whereas baseline HOMA-IR levels were significantly higher in the placebo group (4.7 [IQR, 2.4–6.8] versus 2.1 [IQR, 1.4–2.7]; P=0.04). At 12 weeks, there were no significantly different changes between arms for glucose (P=0.17) and HOMA-IR levels (P=0.63) (data not shown). Baseline hs-CRP levels were higher in the placebo group whereas IL-6 levels were not significantly different between groups (Table 1). As previously reported, neither hs-CRP nor IL-6 concentrations were affected by ω-3 supplementation (P=0.74 and P=0.58, respectively) (9).
Plasma Amino Acids
The arterial plasma amino acid (AA) concentrations at baseline and after intervention are summarized in Table 2 by groups of total AA, nonessential AA, essential AA and branched chain AA. Plasma AA concentrations were similar between study groups at baseline (P=0.28 for branched chain AA, P=0.74 for essential AA, P=0.17 for nonessential AA, and P=0.19 for total AA). There were no statistically significant changes in plasma AA concentrations between arms at 12 weeks or from baseline to 12 weeks (all P values >0.05, Table 2).
Table 2.
Plasma amino acid concentrations at baseline and after intervention by randomization group
| ω-3 (n=11) | Placebo (n=9) | P Value | ||
|---|---|---|---|---|
| Branched chain AA (μmol/L) | ||||
| Baseline | 225 (195–275) | 248 (233–280) | 0.28 | |
| 12 weeks | 225 (209–296) | 283 (263–301) | 0.14 | |
| δ | 16 (−6–41) | 29 (−2–78) | 0.58 | |
| Essential AA (μmol/L) | ||||
| Baseline | 646 (552–777) | 649 (624–690) | 0.74 | |
| 12 weeks | 648 (609–746) | 691 (668–793) | 0.25 | |
| δ | 46 (−108–132) | 75 (−9–158) | 0.40 | |
| Nonessential AA (μmol/L) | ||||
| Baseline | 1047 (885–1330) | 786 (721–955) | 0.17 | |
| 12 weeks | 1305 (975–1325) | 1003 (842–1035) | 0.44 | |
| δ | 155 (−19–334) | 67 (51–286) | 0.85 | |
| Total AA (μmol/L) | ||||
| Baseline | 1693 (1573–2058) | 1441 (1360–1628) | 0.19 | |
| 12 weeks | 1901 (1710–2095) | 1748 (1533–1851) | 0.32 | |
| δ | 80 (−85–587) | 131 (42–545) | 0.80 | |
Data are presented as median with interquartile range. Unadjusted P value was estimated with Wilcoxon rank sum test. AA, amino acids; δ, difference from baseline to 12 weeks.
Forearm Muscle Protein Metabolism
Forearm Muscle Protein Synthesis.
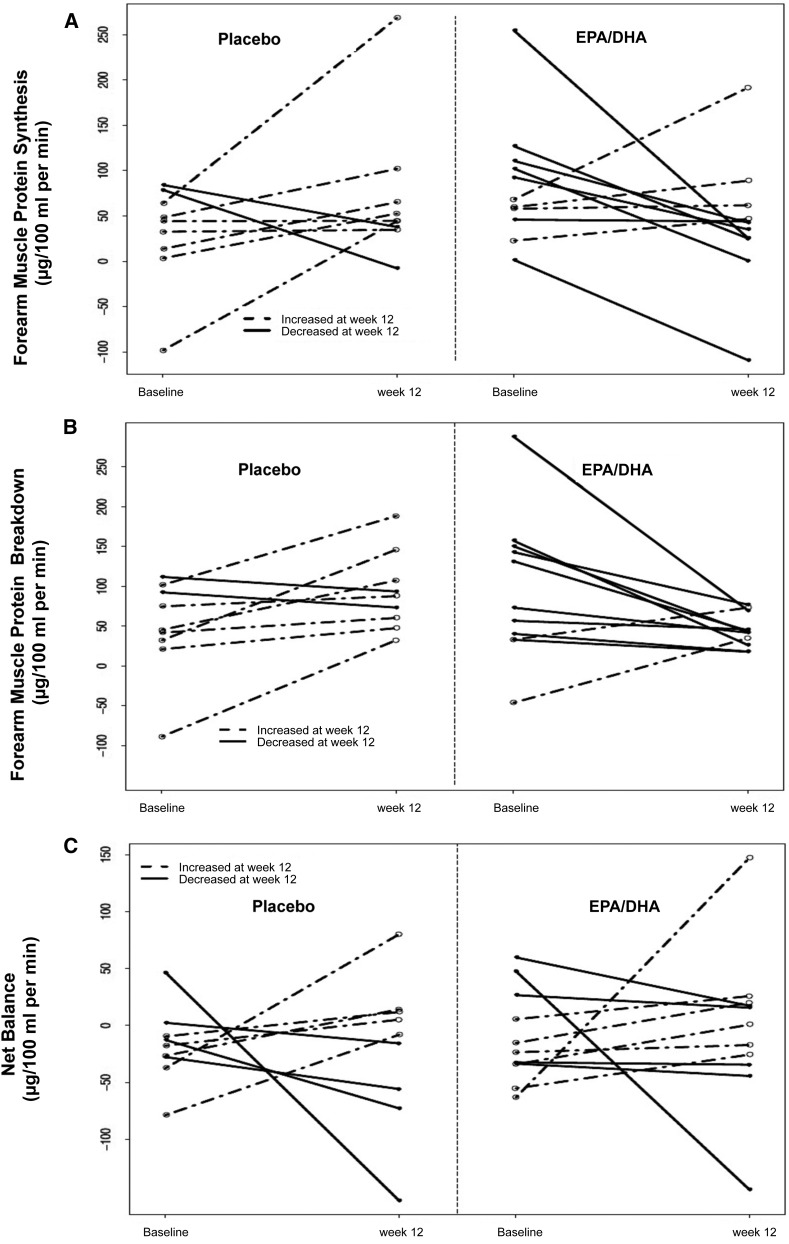
At baseline, the forearm muscle protein synthesis rates were similar between treatment groups (P=0.08) (Table 3). However, from baseline to 12 weeks, changes in forearm muscle protein synthesis rates were significantly different between intervention and placebo. The rates in the intervention arm decreased, while the rates in the placebo arm increased (−58 [IQR, −102–14] versus 49 [IQR, 0.5–54] µg/100 ml per minute, respectively; P=0.04) (Figure 3A, Table 3). However, at 12 weeks this difference was not statistically significant after adjusting for baseline values (−37 [95% CI, −126 to 45]) nor after further adjusting for other potential confounders using a propensity score that included age, sex, race, hs-CRP, diabetes mellitus and fat mass (−25 [95% CI, −163 to 87]) (Table 4).
Table 3.
Forearm muscle protein metabolism components at baseline and after intervention by randomization group
| ω-3 (n=11) | Placebo (n=9) | P Value | Difference Between Groups (95% CI) | |
|---|---|---|---|---|
| Forearm muscle protein metabolism (μg/100 ml per min) | ||||
| Synthesis | ||||
| Baseline | 68 (52–106) | 44 (14–64) | 0.08 | 56 (0.7 to 111) |
| 12 weeks | 43 (25–54) | 45 (38–65) | 0.37 | −30 (−96 to 36) |
| δ | −58 (-102–14) | 49 (0.5–54) | 0.04 | −86 (−167 to −5) |
| Breakdown | ||||
| Baseline | 73 (37–147) | 45 (32–92) | 0.26 | 48 (−20 to 117) |
| 12 weeks | 42 (31–58) | 88 (60–107) | 0.01 | −48 (−80 to −16) |
| δ | −31 (−98–−13) | 26 (13–87) | 0.01 | −97 (−160 to −34) |
| Net Balance | ||||
| Baseline | −24 (−34–16) | −17 (-28–−10) | 0.94 | 7 (−26 to 40) |
| 12 weeks | 0.6 (−31–19) | −8 (−56–12) | 0.50 | 18 (−42 to 78) |
| δ | 6 (−11–32) | 22 (−28–41) | 0.97 | 11 (−70 to 92) |
| Whole body protein metabolism (mg/kg per fat free mass per min) | ||||
| Synthesis | ||||
| Baseline | 4.2 (3.6–4.9) | 5.5 (4.1–5.8) | 0.12 | −1.0 (−2.0 to 0.0) |
| 12 weeks | 4.0 (3.3–4.7) | 4.2 (4.1–4.8) | 0.25 | −0.5 (−1.1 to 0.2) |
| δ | −0.08 (−0.72–0.22) | −0.84 (−0.89–−0.01) | 0.32 | 0.5 (−0.3 to 1.4) |
| Breakdown | ||||
| Baseline | 4.1 (3.5–4.9) | 5.5 (4.1–5.7) | 0.08 | −1.0 (−2.0 to 0.0) |
| 12 weeks | 4.1 (3.2–4.6) | 4.2 (4.2–4.8) | 0.17 | −0.5 (−1.1 to 0.1) |
| δ | −0.04 (−0.77–0.15) | −0.65 (−0.88–0.11) | 0.28 | 0.5 (−0.4 to 1.3) |
| Net Balance | ||||
| Baseline | −0.01 (−0.04–0.02) | 0.00 (−0.04–0.08) | 0.58 | 0.0 (−0.1 to 0.1) |
| 12 weeks | −0.02 (−0.07–0.14) | −0.07 (−0.11–0.02) | 0.10 | 0.1 (0.0 to 0.2) |
| δ | −0.04 (−0.09–0.11) | −0.10 (−0.14–0.003) | 0.25 | 0.1 (0.0 to 0.2) |
Data are presented as median with interquartile range. Unadjusted P value was estimated with Wilcoxon rank sum test. δ, difference from baseline to 12 weeks.
Figure 3.
Effects of intervention on forearm muscle protein turnover. Forearm muscle protein kinetics was calculated before and after 12 weeks of supplementation with either ω-3 or placebo. (A) Forearm muscle protein synthesis rates. (B) Forearm muscle protein breakdown rates. (C) Forearm muscle protein turnover net balances. DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid.
Table 4.
Treatment effect size on forearm and whole body muscle protein metabolism at week 12
| Difference Between Groups at 12 Weeksa (95% CI) | Difference Between Groups at 12 Weeksb (95% CI) | |
|---|---|---|
| Forearm muscle protein metabolism (μg/100 ml per min) | ||
| Protein synthesis | −37 (−126 to 45) | −25 (−163 to 87) |
| Protein breakdown | c−57 (−93 to −27) | c−46 (−102 to −1) |
| Net balance | 24 (−32 to 71) | −2 (−94 to 69) |
| Whole body protein metabolism (mg/kg per fat free mass per min) | ||
| Protein synthesis | −0.1 (−0.6 to 0.4) | 0.2 (−0.4 to 1.1) |
| Protein breakdown | −0.2 (−0.6 to 0.2) | 0.2 (−0.5 to 0.9) |
| Net Balance | 0.08 (−0.01 to 0.16) | 0.06 (−0.09 to 0.17) |
Multivariable linear regression model was used to estimate difference between groups on outcomes at week 12 after adjusting for baseline values only.
Multivariable linear regression model was used to estimate difference between groups at week 12 after adjusting for baseline values, age, sex, race, hs-CRP, diabetes and fat mass through the logit of propensity score. 95% CI was calculated using bootstrap resampling.
The effect was statistically significant.
Forearm Muscle Protein Breakdown.
The forearm muscle protein breakdown rates were similar between the two groups at baseline (P=0.26) (Table 3). However, from baseline to 12 weeks, changes in muscle protein breakdown rates were significantly different between intervention and placebo. The rates in the intervention arm decreased, while the rates in the placebo arm increased (−31 [IQR, −98–−13] versus 26 [IQR, 13–87] µg/100 ml per min, respectively; P=0.01) (Figure 3B, Table 3). Moreover, at 12 weeks this effect remained statistically significant after adjusting for baseline values (−57 [95% CI, −93 to −27]), as well as after multivariate adjustment (−46 [95% CI, −102 to −1]) (Table 4).
Forearm Net Protein Balance.
The change of forearm net balance was not statistically significantly different between treatments for any of the analyses (Figure 3C, Tables 3 and 4).
Whole Body Protein Metabolism
ω-3 Supplementation had no significant effect on the difference of whole body protein synthesis (−0.08 [IQR, −0.72–0.22]) in ω-3 group versus −0.84 (IQR, −0.89–−0.01) mg/kg per fat free mass per min in placebo group; P=0.32], whole body protein breakdown (−0.04 [IQR, −0.77–0.15] in ω-3 group versus −0.65 [IQR, −0.88–0.11] mg/kg per fat free mass per min in placebo group; P=0.28) or whole body protein net balance (−0.04 [IQR, −0.09–0.11] in ω-3 group versus −0.10 [IQR, −0.14–0.003) mg/kg per fat free mass per min in placebo group; P=0.25) at 12 weeks compared with baseline (Tables 3 and 4).
In order to provide additional statistical insight due to imbalances at baseline, we also performed adjustments with independent variables separately. The results revealed data similar to the multivariate model adjusting for propensity score for all of the individual components of whole body and skeletal muscle turnover. Additional analysis indicated that none of the risk factors in the propensity score model had a statistically significant association with treatment assignment (data not shown).
Discussion
The results of this study show that 12 weeks of 2.9 g of ω-3 fatty acid administration was associated with a statistically significant decrease in skeletal muscle protein breakdown in MHD patients with underlying systemic inflammation. Skeletal muscle protein synthesis also was associated with a numerical decrease, which lead to no difference in net skeletal muscle protein turnover. There were no differences in any components of the whole-body protein turnover in association with ω-3 fatty acid or placebo administration.
PEW of kidney disease is primarily characterized by loss of skeletal muscle mass along with a decrease in visceral protein concentrations (1). Among several etiological factors, unrecognized systemic inflammation seems to be a major determinant of the extent of protein catabolism. While a body of evidence associates the extent of systemic inflammation to worsened markers of nutrition status, only a limited number of physiologic studies in humans have shown that systemic inflammation is associated with increased protein breakdown in muscle tissue (17). Similarly, only a few studies have adequately examined the effects of anti-inflammatory interventions of muscle protein turnover (18). The current study using ω-3 fatty acids further extends these data to suggest that a potential beneficial effect in muscle breakdown can be observed despite the lack of a reduction in systemic markers of inflammation in MHD patients.
The potential mechanisms by which ω-3 fatty acids may modulate muscle protein turnover are not well understood. Although some studies indicate a more pronounced effect on protein synthesis (4,5), others report a decrease in protein breakdown (6) or no effect on protein turnover overall (19). We hypothesized that ω-3 supplementation would reduce the forearm muscle protein breakdown in MHD patients with no effect on protein synthesis, since metabolic studies were performed in a postabsorptive state. While we originally hypothesized that anti-inflammatory effects of ω-3 supplementation would be the underlying mechanism, we did not observe any statistically significant changes in systemic inflammatory markers, such as hs-CRP and IL-6, although levels of several other chemokines were improved in response to the intervention as previously reported (9). It is possible that the effects of ω-3 fatty acids on protein turnover are independent of its effects on inflammatory pathways, as suggested in previous studies (4,20). Since we hypothesized that the beneficial effects would be mediated though inflammatory pathways, lower CRP concentrations in the intervention arm at baseline could have led to a reduced effect of fish oil supplementation on protein breakdown. However, because the main study did not find a reduction in systemic markers of inflammation, other possibilities need to be considered. Similarly, baseline HOMA-IR and fat mass values were lower in the intervention arm, potentially limiting the effects of the intervention in a more suitable population. A statistically significant difference despite these unfavorable phenotypic characteristics raises the possibility that the intervention might have effects that extend beyond these metabolic pathways alone. Finally, since the variability in most markers of inflammation is very high, our sample size could be inadequate to detect an otherwise present biologic link between the effect on the highly variable inflammatory markers and muscle protein turnover.
In the unadjusted analysis, forearm muscle protein synthesis decreased in the intervention arm and increased in the placebo group. However, after adjustment, there were not significant differences between the groups. These findings could be considered as surprising by some and are not readily explained. The reason why ω-3 fatty acid administration was not significantly associated with protein synthesis could potentially be related to the fasting state in which the studies were performed. During physiologic assessment, protein synthesis is not expected to increase in a fasting state. Studies have shown that ω-3 supplementation improves skeletal muscle protein synthesis during a hyperinsulinemic-hyperaminoacidemic clamp where patients are in an anabolic state as opposed to under fasting conditions (4,5). Since there is a physiologic balance between protein synthesis and protein breakdown designed to compensate for each other to maintain a net nitrogen balance, our results are physiologically appropriate in that fish oil did decrease steady-state protein breakdown, which was in turn compensated for by a decrease in protein synthesis resulting in no appreciable change in net balance. Although the improvement in protein breakdown might seem relatively small (31 μg/100 ml per min), the lack of change in net balance makes it uncertain if longer term treatment would result in measurable changes in body composition.
A notable observation is that at baseline the placebo arm was more inflamed than the intervention arm. This could lead to an alternative explanation for the results such that the increased breakdown in the control group is due to greater exposure to an inflammatory state. Nevertheless, the concomitant decrease in protein breakdown in the intervention group suggests that there may be beneficial effects of ω-3 fatty acid administration. Another finding in this study was that any noticeable effects on whole body protein metabolism did not accompany the changes in forearm muscle protein breakdown. As noted above, this discrepancy is somewhat expected and may be related to compartmentalization of amino acid pools because of competing demands for muscle and visceral protein synthesis. In addition, changes in protein metabolism in specific tissues often may not be reflected in the total flux of leucine into plasma (21).
We achieved our goal of assessing and demonstrating the changes in protein turnover using a physiologically relevant marker. Although our samples size is relatively small, we used tracer methodology to directly measure protein synthesis and breakdown, providing more accurate estimates. This complex but established technique is considered to be one of the best physiologic markers of protein metabolism. Additionally, our results and effect size were consistent with our a priori sample size and power calculation.
There are number of other limitations beyond those previously mentioned. The short duration of the supplementation period and the lack of dietary protein control in the intervention arm might limit the interpretation of the effect of ω-3 supplementation on forearm protein turnover kinetics, particularly on protein synthesis. In addition, there were a disproportionally high percentage of black and male participants in the study, limiting the generalizability of our findings. There were several imbalances at baseline including lower levels of hs-CRP, HOMA-IR and fat mass in the intervention group. Our sample size was small and subgroup analysis was not feasible. These baseline differences were adjusted for by using a propensity score as a covariate, although our overall sample size limits the soundness of this approach. Given the lack of balanced randomization, these results have to be interpreted cautiously.
In conclusion, we have shown that 12 weeks ω-3 fatty acid supplementation in MHD patients is associated with statistically significant decrease in forearm muscle protein breakdown but did not influence skeletal muscle protein synthesis, skeletal muscle net protein balance or any component of whole-body protein balance. Longer-term studies with larger sample size are necessary to examine whether these observed effects can be translated into more clinically applicable outcomes such as lean body mass, physical performance, hospitalization, and death.
Disclosures
None.
Acknowledgments
A.H.'s work was supported by a Career Development Award (2-031-09S) from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Clinical Sciences Research. S.M.D was supported in part by an International Society of Nephrology and Turkish Society of Nephrology Fellowship Award and is currently a fellow of the American Heart Association.
This study was supported in part by grants R21 AT003844 from the National Institutes of Health/National Center for Complementary & Alternative Medicine, Clinical Translational Science Award UL1 TR000445 from the National Center for Research Resources, K24 DK062849, Vanderbilt Diabetes Research and Training Center grant P30 DK020593, and Vanderbilt O’Brien Mouse Kidney Center grant P30 DK079341 from the National Institute of Diabetes and Digestive and Kidney Diseases, Vanderbilt Center in Molecular Toxicology grant P30 ES000267 from the National Institute of Environmental Health Sciences, 1I01CX000414 from the Department of Veterans Affairs and Vanderbilt Center for Kidney Disease.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Studying Muscle Protein Turnover in CKD,” on pages 1131–1132.
References
- 1.Carrero JJ, Stenvinkel P, Cuppari L, Ikizler TA, Kalantar-Zadeh K, Kaysen G, Mitch WE, Price SR, Wanner C, Wang AY, ter Wee P, Franch HA: Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J Ren Nutr 23: 77–90, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Pupim LB, Caglar K, Hakim RM, Shyr Y, Ikizler TA: Uremic malnutrition is a predictor of death independent of inflammatory status. Kidney Int 66: 2054–2060, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R: Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38: 1154–1163, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B: Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr 93: 402–412, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B: Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond) 121: 267–278, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamolrat T, Gray SR: The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun 432: 593–598, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Gingras AA, White PJ, Chouinard PY, Julien P, Davis TA, Dombrowski L, Couture Y, Dubreuil P, Myre A, Bergeron K, Marette A, Thivierge MC: Long-chain omega-3 fatty acids regulate bovine whole-body protein metabolism by promoting muscle insulin signalling to the Akt-mTOR-S6K1 pathway and insulin sensitivity. J Physiol 579: 269–284, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Wang H, Lee IH, Du J, Mitch WE: Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 119: 3059–3069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung AM, Booker C, Ellis CD, Siew ED, Graves AJ, Shintani A, Abumrad NN, Himmelfarb J, Ikizler TA: Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol Dial Transplant 30: 266–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M: N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int 69: 1450–1454, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Siew ED, Ikizler TA: Insulin resistance and protein energy metabolism in patients with advanced chronic kidney disease. Semin Dial 23: 378–382, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Pupim LB, Flakoll PJ, Majchrzak KM, Aftab Guy DL, Stenvinkel P, Ikizler TA: Increased muscle protein breakdown in chronic hemodialysis patients with type 2 diabetes mellitus. Kidney Int 68: 1857–1865, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Heinrikson RL, Meredith SC: Amino acid analysis by reverse-phase high-performance liquid chromatography: precolumn derivatization with phenylisothiocyanate. Anal Biochem 136: 65–74, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Gelfand RA, Barrett EJ: Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest 80: 1–6, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe RR: Radioactive and Stable Isotope Tracers in Biomedicine: Principles and Practice of Kinetic Analysis, New York, Wiley-Liss, 1992, pp 283–316 [Google Scholar]
- 16.Shoji T, Emoto M, Nishizawa Y: HOMA index to assess insulin resistance in renal failure patients. Nephron 89: 348–349, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Raj DS, Moseley P, Dominic EA, Onime A, Tzamaloukas AH, Boyd A, Shah VO, Glew R, Wolfe R, Ferrando A: Interleukin-6 modulates hepatic and muscle protein synthesis during hemodialysis. Kidney Int 73: 1054–1061, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Biolo G, Ciocchi B, Bosutti A, Situlin R, Toigo G, Guarnieri G: Pentoxifylline acutely reduces protein catabolism in chronically uremic patients. Am J Kidney Dis 40: 1162–1172, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Palmer RM, Wahle KW: Protein synthesis and degradation in isolated muscle. Effect of omega 3 and omega 6 fatty acids. Biochem J 242: 615–618, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodacki CL, Rodacki AL, Pereira G, Naliwaiko K, Coelho I, Pequito D, Fernandes LC: Fish-oil supplementation enhances the effects of strength training in elderly women. Am J Clin Nutr 95: 428–436, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Rooyackers OE, Nair KS: Hormonal regulation of human muscle protein metabolism. Annu Rev Nutr 17: 457–485, 1997 [DOI] [PubMed] [Google Scholar]