Abstract
Background and objectives
Nephrotoxicity remains the dose–limiting side effect of cisplatin, an effective chemotherapeutic agent with applications across diverse tumor types. This study presents data on renal outcomes across multiple tumor types in 821 adults. We report on incidence of AKI, initial and long-term changes in eGFR after cisplatin, and relationships between cumulative dose, initial eGFR, age, sex, and long–term renal function.
Design, setting, participants, & measurements
This was a retrospective study of adult patients treated with cisplatin from January 1, 2000 to September 21, 2011 who had survived ≥5 years after initial dose. The Modification of Diet in Renal Disease equation was used to calculate eGFR. AKI was defined as an increase from the baseline creatinine of >25% within 30 days after the first cycle of cisplatin. Chi-squared tests were done to evaluate the relationships between categorical or ordinal variables; ANOVAs or t tests were used to evaluate continuous or categorical variables. Changes in eGFR over time were evaluated in a growth curve model.
Results
Mean follow-up was 6 years (25th and 75th percentiles, 4 and 9 years). AKI occurred in 31.5% of patients, with a median initial decline in eGFR of 10 ml/min per 1.73 m2 (25th and 75th percentiles, −41.5 and −23.3 ml/min per 1.73 m2). At any time point after the first cycle of cisplatin, <3% of patients progressed to eGFR<29 ml/min per 1.73 m2, and none were known to be on dialysis. Age was associated with a higher risk for AKI after cisplatin. Compared with age <25 years old, the odds ratios for AKI versus no AKI are 1.22 for >26–44 years old (95% confidence interval [95% CI], 0.60 to 2.4), 1.54 for >45–65 years old (95% CI, 0.78 to 3), and 2.96 for >66 years old (95% CI, 1.4 to 6.1). The lowest dose categories of cisplatin (≤100 and 101–250 mg/m2) are associated with increases in eGFR (P=0.06 and P=0.02, respectively) compared with the highest dose category (>701 mg/m2).
Conclusions
This is the largest study of adult patients with cancer who received cisplatin for treatment across multiple tumor types. Most patients experience small but permanent declines in eGFR, but none progressed to ESRD requiring hemodialysis.
Keywords: nephrotoxicity, chronic kidney failure, cisplatin nephrotoxicity, cisplatin, glomerular filtration rate, Acute Kidney Injury, creatinine, Follow-Up Studies, Humans, renal dialysis
Introduction
Cisplatin is associated with cure rates as high as 80% for testicular cancer (1) and prolonged survival for those with tumors of the head and neck, ovary, lung, cervix, endometrium, and bladder (2–4). Nephrotoxicity remains the dose–limiting side effect (5–7) of cisplatin, accounting for up to 20% of all episodes of AKI in hospitalized patients with cancer (8). A detailed understanding of the mechanisms by which cisplatin induces renal toxicity is incomplete. Increased generation of reactive oxygen species and production of proinflammatory cytokines seem to play significant roles (9,10,11).
Although its acute nephrotoxicity has been well recognized for decades, information on cisplatin’s long-term effects on renal function in adults is limited by lack of long–term renal follow-up and small sample sizes in the available studies (12–15). We took advantage of the large adult population that is treated with cisplatin across multiple tumor types at our institution and performed a study on adults to determine the incidence of AKI and the initial and long-term changes in eGFR after cisplatin. We analyzed the relationships between age of first cisplatin, cumulative dose (CD) of cisplatin, sex, baseline eGFR, and subsequent eGFR.
Materials and Methods
A retrospective chart analysis was performed after obtaining approval from the Institutional Review Board at Memorial Sloan Kettering Cancer Center. Protected health information was coded in accordance with the requirements of Health Insurance Portability and Accountability Act. We collected clinical information for patients ≥18 years old treated with intravenous cisplatin during the period from January 1, 2000 to September 20, 2011 who had survived for at least 5 years after their initial dose. Patients were excluded if they received ifosfamide concomitantly or subsequent to cisplatin administration or if a baseline creatinine was unavailable. All patients received hydration with 1.5–2 L normal saline with cisplatin. No patients received amifostine. There are no exclusion criteria for dosing cisplatin on the basis of serum creatinine (SCr) at our institution, but our dosing guidelines recommend caution when the eGFR is <50 ml/min.
The demographic and treatment data collected are shown in Table 1 and Supplemental Table 1. Data on type of primary malignancy (per International Classification of Diseases 9 [ICD 9] codes), concomitant and subsequent chemotherapeutic agents, dates of first and last cisplatin dose, and CD dose for each patient were collected. Baseline SCr was the value within and closest to 30 days before the start of cisplatin. The SCr value used after the first cycle of cisplatin was the level closest to 30 days after the infusion was completed. Yearly SCr levels were obtained ±6 months from the completion of treatment. The four–variable Modification of Diet in Renal Disease (MDRD) equation was used to calculate the eGFR. The MDRD equation was chosen, because we were specifically interested in renal outcomes in patients with mild to moderate renal impairment, and this equation tends to be more accurate and less biased when the GFR is <60 ml/min per 1.73 m2 (16). CKD stages were assigned solely on the basis of the calculated eGFR. We did not collect data on the presence of hematuria, proteinuria, or structural abnormalities. AKI was defined as an increase from the baseline creatinine of ≥25% within 30 days after infusion of the first cycle of cisplatin. ESRD requiring hemodialysis status was on the basis of ICD9 coding and review of the available medical records in patients with eGFR<29 ml/min per 1.73 m2. Before completing the data analysis, our expectation was that a significant proportion of patients would experience a small but permanent decline in renal function but that most would not progress to ESRD.
Table 1.
Patient demographics
| Data Collected | Total No., 821 | Percentage |
|---|---|---|
| Sex | ||
| Men | 520 | 63 |
| Women | 301 | 37 |
| Race | ||
| White | 697 | 85 |
| Asian | 56 | 7 |
| Black | 50 | 6 |
| Other/unknown | 18 | 2 |
| Ethnicity | ||
| Non-Hispanic | 312 | 38 |
| Hispanic | 53 | 6 |
| Unknown | 456 | 56 |
| Age, yr | ||
| <25 | 56 | 7 |
| ≥26–44 | 250 | 30 |
| ≥45–65 | 392 | 48 |
| ≥66 | 123 | 15 |
| Cumulative dose of cisplatin, mg/m2 | ||
| ≤100 | 57 | 7 |
| 101–250 | 420 | 51 |
| 251–400 | 215 | 26 |
| 401–550 | 106 | 13 |
| 551–700 | 15 | 2 |
| >701 | 8 | 1 |
| Minimum | Maximum | |
| Age at first cisplatin dose, yr | 19 | 85 |
| Mean | Median | |
| Age at first cisplatin dose, yr | 50 | 51 |
| Serum creatinine, mg/dl | Mean (SD) | Minimum, Maximum |
| Baseline | 0.99 (0.22) | 0.4, 2.1 |
| After first cisplatin dose | 1.18 (0.51) | 0.5, 9.5 |
| Year 1 | 1.14 (0.47) | 0.4, 9 |
| Year 5 | 1.18 (0.42) | 0.5, 5 |
Chi-squared tests were used to evaluate the relationships between categorical and ordinal variables; ANOVA or t tests were used to evaluate continuous or categorical variables. These analyses were done to evaluate relationships between eGFR and covariates. Trends in eGFR were investigated using summary measures, such as the mean, SD, median, and range, as well as graphically to evaluate patient-specific trends. We fit a repeated measures growth curve model to investigate whether there was a trend in eGFR after adjusting for age, ethnicity, sex, and CD. Time was entered into the model as year ranging from baseline (coded 0), last cisplatin dose (coded 1), year 1 (coded 2), and so forth to year 12 (coded 13). Time was reparameterized in this way so that the intercept represents the average eGFR value at baseline. We fit the model assuming different correlation structures, including variance components and compound symmetry. All analyses were conducted in SAS, version 9.4 (SAS Institute Inc., Cary, NC). P values <0.05 were considered to be statistically significant.
Results
Initially, 859 patients met inclusion criteria. After excluding 38 (4%) patients with no baseline or follow-up SCr, analysis was performed on the remaining 821 patients. Patient demographic and treatment information is shown in Table 1. The majority of patients were men (63%) and white (85%), and the mean age was 50 years old. The CD of cisplatin ranged from ≤100 to >701 mg/m2. Most patients received a CD of 101–250 mg/m2 (51%), 26% received a dose of 251–400 mg/m2, 16% received a dose of 401 mg/m2, and 77% received a dose of 101–400 mg/m2. On the basis of death notices and other entries in the chart, 684 (83%) were alive, and 137 (17%) were deceased at the time of analysis. The most commonly treated cancers were buccal cavity, larynx, and pharynx (21%); testicular (18%); lung (10%); esophagus (8%); cervix (6%); stomach (5%); and urinary bladder (5%). Eleven patients had more than one tumor type recorded for which cisplatin was given.
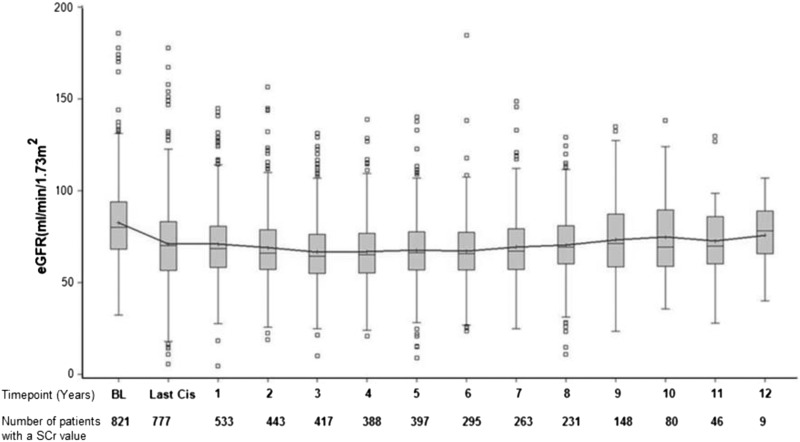
For patients who had at least a year 1 SCr, the mean and median follow-up were 6 and 7 years, respectively (25th and 75th percentiles, 4 and 9 years). The median initial decline in eGFR was 10 ml/min per 1.73 m2 (25th and 75th percentiles, −41.5 and −23.3 ml/min per 1.73 m2). The most significant decline in median eGFR (10 ml/min per 1.73 m2) occurred in the period from baseline to the completion of cisplatin treatment. Thereafter, the median eGFR remained stable to year 6, with a slight upward slope after year 7. AKI occurred in 31.5% of patients (n=245) after the first cycle of cisplatin, and this was seen most frequently among tumors of the head and neck (7.8%), gastrointestinal tract (5.4%), testicles (3.7%), and lung (3.5%).
Figure 1 depicts the number of patients with an SCr value at various time points: 777 patients (95%) had follow-up SCr values in their charts within 30 days of the end of cisplatin treatment, 533 (65%) had SCr at year 1, 443 (54%) had SCr at year 2, 417 (50%) had SCr at year 3, 388 (47%) had SCr at year 4, 397 (48%) had SCr at year 5, 295 (35%) had SCr at year 6, and 263 (32%) had SCr at year 7. Thereafter, <30% of SCr values were available.
Figure 1.
Box plot (10th and 90th percentiles) of change in median eGFR (milliliters per minute per 1.73 m2) from baseline to year 12. Time points and numbers of total patients with a serum creatinine (SCr) at each time point are shown. Time points are in years. BL, baseline; Last Cis, at completion of the first cycle of cisplatin.
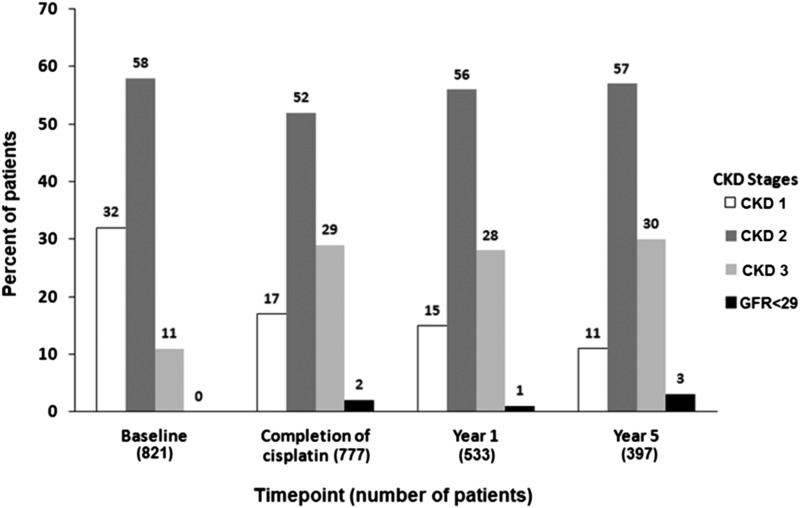
Figure 2 summarizes the number and percentage of patients found in each CKD group at baseline, completion of first cisplatin cycle, and years 1 and 5. At baseline, 58% were CKD stage 2, 32% were CKD stage 1, and 11% were CKD stage 3, and none had an eGFR<29 ml/min per 1.73 m2. At the completion of cisplatin treatment, 52% were CKD stage 2, 29% were CKD stage 3, 17% were CKD stage 1, and 2% had an eGFR<29 ml/min per 1.73 m2. At year 1, 56% were CKD stage 2, 28% were CKD stage 3, 15% were CKD stage 1, and 1% had an eGFR<29 ml/min per 1.73 m2. At year 5, 57% were CKD stage 2, 30% were CKD stage 3, 11% were CKD stage 1, and 3% had an eGFR<29 ml/min per 1.73 m2. At each time point after the completion of the first cycle of cisplatin, between 0.76% and 2.77% of those patients who had an SCr were CKD stage 4 and between 0.19% and 0.87% were CKD stage 5.
Figure 2.
Percentages of patients within each stage of CKD at baseline, completion of cisplatin, year 1, and year 5. CKD stage (eGFR): CKD stage 1 (≥90 ml/min per 1.73 m2), CKD stage 2 (≥60–89 ml/min per 1.73 m2), CKD stage 3 (≥30–59 ml/min per 1.73 m2), and <29 ml/min per 1.73 m2.
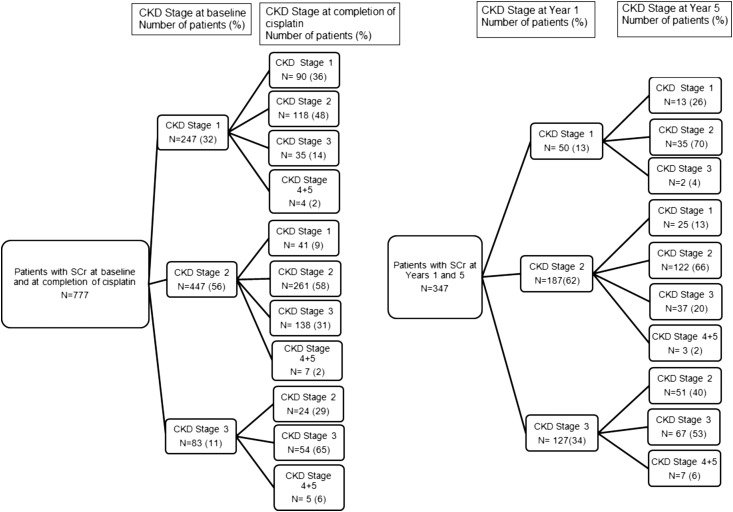
We did additional analysis to determine how individual patients’ CKD stages changed over time (Figure 3). There were 777 patients who had an SCr at baseline and the completion of cisplatin treatment. Of the 247 patients who were CKD stage 1 before treatment, 48% progressed to CKD stage 2, 36% remained at CKD stage 1, and 14% progressed to CKD stage 3 at completion; <2% (n=4) progressed to an eGFR<29 ml/min per 1.73 m2. Of the 447 patients at CKD stage 2 before treatment, at the completion, 58% remained at CKD stage 2, 31% progressed to CKD stage 3, and 9% moved to CKD stage 1; <2% progressed to CKD stage 4, and none progressed to CKD stage 5. Of the 83 patients at CKD stage 3 before cisplatin, at completion, 65% remained at CKD stage 3, 29% moved to CKD stage 2, and 6% (n=5) progressed to an eGFR<29 ml/min per 1.73 m2. The changes in CKD staging over time for those with SCr values at years 1 and 5 are also shown in Figure 3.
Figure 3.
For patients with a SCr at baseline and at completion of cisplatin and for those with a SCr at Year 1 and Year 5, fewer than 6% progressed to a GFR <29 ml/min per 1.73 m2. Changes in individual patient’s CKD stage over time: from baseline to the completion of cisplatin (left panel) and from year 1 to year 5 (right panel). SCr, serum creatinine.
In Table 2, we fit a repeated measures model to investigate whether there was a trend in eGFR after adjusting for age, ethnicity, sex, and CD. Time was entered into the model as year ranging from 0 (baseline) to 13, which is year 12 after cisplatin. We found a trend in time, with an average reduction in eGFR of 0.73 ml/min per 1.73 m2 with each increasing year (P<0.001). The lowest dose categories of cisplatin (≤100 and 101–250 mg/m2) are associated with increases in eGFR (P=0.06 and P=0.02, respectively) compared with the highest dose category (>701 mg/m2). Women were associated with decline in eGFR over time (P<0.02). When evaluating age as a four–level categorical variable (<25, ≥26–45, ≥46–65, and ≥66 years old), the incidence of AKI was found to be higher in the older compared with the younger age groups (P<0.001). Compared with age <25 years old, the odds ratios for AKI versus no AKI are 1.22 for age >26–44 years old (95% confidence interval [95% CI], 0.60 to 2.4), 1.54 for >45–65 years old (95% CI, 0.78 to 3), and 2.96 for >66 years old (95% CI, 1.4 to 6.1). In subset analysis, the observed frequency of AKI was 1.5% for age <25 years old, 7.6% for 26–44 years old, 14.1% for 45–65 years old, and 6.7% for >66 years old. Subset analysis on patients with 5-year data showed that patients with a lower eGFR before cisplatin administration were prescribed a lower CD of cisplatin (P=0.01) and that younger age categories were prescribed higher CDs compared with older age categories (P<0.001).
Table 2.
Multivariable repeated measures analysis of potential risk factors of trends in eGFR for 821 patients
| Variable | Parameter Estimate | 95% Confidence Interval | P Value |
|---|---|---|---|
| Intercept | 92.36 | 82.3 to 102.4 | <0.001 |
| Time, yra (coded as 0–13, continuous) | −0.73 | −0.87 to −0.59 | <0.001 |
| Sex | |||
| Women | −2.50 | −4.58 to −0.43 | 0.02 |
| Men (reference) | |||
| Ethnicity | |||
| Asian versus white | 4.54 | 0.58 to 8.49 | 0.03 |
| Black versus white | 7.84 | 3.74 to 11.95 | 0.002 |
| Unknown/other versus white | 0.54 | −6.40 to 7.48 | 0.88 |
| White (reference) | |||
| Age at first cisplatin | −0.51 | −0.59 to −0.44 | 0.001 |
| Cisplatin dose (mg/m2) | |||
| 101–250 versus >701 | 11.10 | 1.65 to 20.55 | 0.02 |
| 251–400 versus >701 | 6.93 | −2.61 to 16.46 | 0.16 |
| 401–550 versus >701 | 5.52 | −4.24 to 15.27 | 0.23 |
| 551–700 versus >701 | 6.51 | −5.27 to 18.28 | 0.28 |
| ≤100 versus >701 | 9.58 | −0.50 to 19.66 | 0.06 |
| >701 (reference) |
Model was fit using a compound symmetry covariance structure.
Time 0 is baseline; time 1 is last cisplatin date; time 2 is year 1 after cisplatin, and so on until time 13, which is year 12 after cisplatin.
Discussion
Since receiving Food and Drug Administration approval in the late 1970s, despite its characteristic nephrotoxicity, cisplatin has markedly improved the overall survival for scores of patients. To the best of our knowledge, this study represents the largest analysis on the long–term renal outcomes in adults treated with cisplatin across diverse solid tumors. Overall, 30.5% of treated patients experienced AKI. The higher incidence of AKI observed in some tumor types may be related to higher CDs needed to treat the specific tumor as opposed to the tumor type. Fewer than 3% of patients at any time point had an eGFR<29 ml/min per 1.73 m2. No patients required dialysis on long–term follow-up. However, the true long–term incidence of advanced CKD and ESRD could not be reliably ascertained because of data attrition after 5 years. Indeed, a limitation of this study is the loss of follow-up data in approximately 50% of patients after year 5. Consequently, most of the results of this analysis rely disproportionately on 5 years of data. The most frequent reason for loss of follow-up is that patients chose to follow-up with their local oncologists.
In defining AKI as an increase in SCr of ≥25% from baseline, we knowingly departed from the established classification schemes for AKI. Although we agree that there is a need for a single definition of AKI for practice and research, we opted for this definition for several reasons. First, because of the retrospective nature of the study, we were unable to obtain values for timed SCr and urine volume. Second, although AKI classification schemes define AKI as a >1.5 times increase in baseline SCr, studies have shown that increases in SCr of >25% are not insignificant and in fact, are associated with CKD on long–term follow-up (17,18). For the treating oncologist, it is a much easier clinical decision to discontinue cisplatin and consider alternative therapies if the patient has a sustained rise in the SCr of >1.5 times baseline after the first cycle and is expected to have ongoing AKI with successive dosing. The more challenging clinical decision is what to do with those patients with more modest elevations in SCr who may have nephrotoxicity from the drug but may also derive significant benefits.
We recognize that eGFR equations are predicated on steady–state renal function, and patients who experience AKI after cisplatin are not in steady state, especially because they continue to be exposed to the drug at regular intervals. We tried to minimize fluctuations in SCr by using the value as close to the end of the 30-day time point to allow for stabilization of renal function. Because most dosing protocols are 3–4 weeks, it was not possible to allow the renal function to stabilize over a longer period before recalculating the eGFR.
Our findings of an initial decline in eGFR after completion of cisplatin therapy with stabilization thereafter (Figure 1) are consistent with findings of smaller studies in adults with predominantly testicular or ovarian cancer (19,20). Our study, which included a greater diversity of tumor types, supports this initial decline in eGFR. In previous studies in which GFR and renal plasma flow were measured using exogenous filtration markers and 24-hour creatinine clearance, the observed acute and chronic declines in GFR were of larger magnitude, ranging from 12% to 23% (12,13,15,20–22). In our study, a lower median decline in eGFR of 10 ml/min per m2 was observed. These differences may be because of the methodology used (calculated versus measured GFR) and the routine use of aggressive hydration protocols at our institution. Different hydration protocols used elsewhere may produce different short- and long-term outcomes. Indeed, the single-center design of this study limits its generalizability. Other studies have reported improvement in renal function at long–term follow-up, implying that renal recovery is possible in some patients (15,22). In our cohort, there was a trend toward a slight improvement in eGFR after 7 years. However, no generalizations about long-term improvements in renal function can be made from our cohort, because <30% of SCr values were available after year 7, and this observation may be confounded by drop out of older patients and those with lower GFRs over time.
It is clear that, for the majority of patients with CKD stages 2 and 3 at baseline, CKD staging remained unchanged after 5 years of treatment. In contrast, for those at CKD stage 1, >70% had a higher CKD stage after year 5. Although very few patients were identified as having an eGFR<29 ml/min per 1.73 m2 beyond year 5, these findings have to be interpreted with caution, because incident ESRD may not have be reliably captured using ICD9 coding and review of the medical records given that a significant proportion of patients received follow-up external to our institution after this time. At baseline, no patients in this cohort had an eGFR<29 ml/min per 1.73 m2. Consequently, the effect of cisplatin in this group remains unknown.
Prior studies have found inconsistent relationships between CD of cisplatin and long-term declines in GFR (1,12,19,22–24). Dose intensity and frequency have been previously shown to correlate with cisplatin nephrotoxicity (24). The advent of aggressive hydration protocols, prolonged drug infusion times, and splitting the total dose over several days could have affected this relationship. In this study, compared with those given a CD>701 mg/m2, only the groups given the lowest CD of 101–250 mg/m2 had an associated significant decrease in eGFR (P<0.05) over time. A possible explanation for this surprising finding is that the treating physicians may have dosed cisplatin on the basis of the baseline eGFR and/or age of the individual patient. Indeed, subset analysis showed that patients with relatively lower eGFRs before cisplatin administration were prescribed a lower CD (P=0.01) and that younger patients were prescribed a higher CD relative to older patients (P<0.001). Older patients generally have less muscle mass and will consequently have a lower calculated eGFR. Additionally, if the physician lowered the cisplatin dose in those patients who experienced AKI, this would affect the CD and possibly, the renal outcomes. Lower muscle mass may also explain why women were associated with declines in eGFR over time (P<0.02). Because the most dramatic decline in eGFR occurred after the first cycle of cisplatin, an interesting but unanswered question in this analysis is whether the CD given with the first cycle alone correlates with long-term GFR.
In our cohort, older age groups were associated with higher frequencies and odds ratios for AKI after cisplatin treatment. Whether patient age at the time of onset of cisplatin treatment correlates with the incidence of AKI and long–term renal function is not well established. Some studies report a negative correlation between age and renal function (12,24,25), and others show no relationship (26). A possible explanation for this finding is that older patients had more subclinical renal injury that was not detected by SCr values compared with younger ones or that they may have other comorbidities that made them more susceptible to AKI from cisplatin.
The retrospective nature of this study does not allow us to comment on the development of microalbuminuria and hypertension, both known markers of more progressive CKD, among survivors. Urinalyses were not routinely obtained either pre- or postcisplatin treatment. In regards to hypertension, many cancer survivors in our program returned to their local physicians after treatment, thereby limiting the completeness of the available medical records with respect to hypertension diagnosis and treatment.
This represents the largest study to date of adults who received cisplatin to treat a wide range of tumor types for which the drug is first-line therapy. In this large cohort, about one in three patients treated with cisplatin experience AKI, and the majority of patients who survived for at least 5 years after treatment had a permanent but small decline in eGFR. None required dialysis. Cisplatin is first-line therapy for a variety of tumor types. Therefore, for oncologists confronted with the decision regarding whether to offer cisplatin to a patient with CKD stage 2 or 3, it is invaluable to know that, in >500 patients treated with this level of CKD in our group, most had only a small decline in eGFR, and only 3% progressed to an eGFR<29ml/min per 1.73 m2.
Disclosures
None.
Supplementary Material
Acknowledgments
This study was supported by Memorial Sloan Kettering Cancer Center Support grant/core grant P30CA008748 and Byrne Research Funds from the Department of Medicine at Memorial Sloan Kettering Cancer Center.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08070715/-/DCSupplemental.
References
- 1.Osanto S, Bukman A, Van Hoek F, Sterk PJ, De Laat JA, Hermans J: Long-term effects of chemotherapy in patients with testicular cancer. J Clin Oncol 10: 574–579, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Lord RV, Taron M, Reguart N: DNA repair and cisplatin resistance in non-small-cell lung cancer. Lung Cancer 38: 217–227, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Meyer KB, Madias NE: Cisplatin nephrotoxicity. Miner Electrolyte Metab 20: 201–213, 1994 [PubMed] [Google Scholar]
- 4.Taguchi T, Nazneen A, Abid MR, Razzaque MS: Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol 148: 107–121, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Sastry J, Kellie SJ: Severe neurotoxicity, ototoxicity and nephrotoxicity following high-dose cisplatin and amifostine. Pediatr Hematol Oncol 22: 441–445, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Arany I, Safirstein RL: Cisplatin nephrotoxicity. Semin Nephrol 23: 460–464, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Boulikas T, Vougiouka M: Cisplatin and platinum drugs at the molecular level. (Review). Oncol Rep 10: 1663–1682, 2003 [PubMed] [Google Scholar]
- 8.Berns JS, Ford PA: Renal toxicities of antineoplastic drugs and bone marrow transplantation. Semin Nephrol 17: 54–66, 1997 [PubMed] [Google Scholar]
- 9.Madias NE, Harrington JT: Platinum nephrotoxicity. Am J Med 65: 307–314, 1978 [DOI] [PubMed] [Google Scholar]
- 10.Kaeidi A, Rasoulian B, Hajializadeh Z, Pourkhodadad S, Rezaei M: Cisplatin toxicity reduced in human cultured renal tubular cells by oxygen pretreatment. Ren Fail 35: 1382–1386, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Ju SM, Pae HO, Kim WS, Kang DG, Lee HS, Jeon BH: Role of reactive oxygen species in p53 activation during cisplatin-induced apoptosis of rat mesangial cells. Eur Rev Med Pharmacol Sci 18: 1135–1141, 2014 [PubMed] [Google Scholar]
- 12.Brillet G, Deray G, Jacquiaud C, Mignot L, Bunker D, Meillet D, Raymond F, Jacobs C: Long-term renal effect of cisplatin in man. Am J Nephrol 14: 81–84, 1994 [DOI] [PubMed] [Google Scholar]
- 13.Meijer S, Sleijfer DT, Mulder NH, Sluiter WJ, Marrink J, Koops HS, Brouwers TM, Oldhoff J, van der Hem GK, Mandema E: Some effects of combination chemotherapy with cis-platinum on renal function in patients with nonseminomatous testicular carcinoma. Cancer 51: 2035–2040, 1983 [DOI] [PubMed] [Google Scholar]
- 14.Boyer M, Raghavan D, Harris PJ, Lietch J, Bleasel A, Walsh JC, Anderson S, Tsang CS: Lack of late toxicity in patients treated with cisplatin-containing combination chemotherapy for metastatic testicular cancer. J Clin Oncol 8: 21–26, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Hansen SW, Groth S, Daugaard G, Rossing N, Rørth M: Long-term effects on renal function and blood pressure of treatment with cisplatin, vinblastine, and bleomycin in patients with germ cell cancer. J Clin Oncol 6: 1728–1731, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Earley A, Miskulin D, Lamb EJ, Levey AS, Uhlig K: Estimating equations for glomerular filtration rate in the era of creatinine standardization: A systematic review. Ann Intern Med 156: 785–795, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, Slinin Y, Ensrud KE: The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 171: 226–233, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Dentino M, Luft FC, Yum MN, Williams SD, Einhorn LH: Long term effect of cis-diamminedichloride platinum (CDDP) on renal function and structure in man. Cancer 41: 1274–1281, 1978 [DOI] [PubMed] [Google Scholar]
- 20.Hamilton CR, Bliss JM, Horwich A: The late effects of cis-platinum on renal function. Eur J Cancer Clin Oncol 25: 185–189, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Meijer S, Mulder NH, Sleijfer DT, Donker AJ, Sluiter WJ, de Jong PE, Schraffordt Koops H, van der Hem GK: Influence of combination chemotherapy with cis-diamminedichloroplatinum on renal function: Long-term effects. Oncology 40: 170–173, 1983 [DOI] [PubMed] [Google Scholar]
- 22.Fjeldborg P, Sørensen J, Helkjaer PE: The long-term effect of cisplatin on renal function. Cancer 58: 2214–2217, 1986 [DOI] [PubMed] [Google Scholar]
- 23.Lam M, Adelstein DJ: Hypomagnesemia and renal magnesium wasting in patients treated with cisplatin. Am J Kidney Dis 8: 164–169, 1986 [DOI] [PubMed] [Google Scholar]
- 24.Skinner R, Parry A, Price L, Cole M, Craft AW, Pearson AD: Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: Relevance of age and dose as risk factors. Eur J Cancer 45: 3213–3219, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Skinner R, Pearson AD, English MW, Price L, Wyllie RA, Coulthard MG, Craft AW: Cisplatin dose rate as a risk factor for nephrotoxicity in children. Br J Cancer 77: 1677–1682, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hrushesky WJ, Shimp W, Kennedy BJ: Lack of age-dependent cisplatin nephrotoxicity. Am J Med 76: 579–584, 1984 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.