Abstract
Background and objectives
The high risk of cardiovascular disease (CVD) and premature death in patients with CKD associates with a plethora of elevated circulating biomarkers that may reflect distinct signaling pathways or simply, are epiphenomena of CKD. We compared the predictive strength of 12 biomarkers analyzed concomitantly in patients with stage 5 CKD.
Design, setting, participants, & measurements
From 1994 to 2014, 543 patients with stage 5 CKD (median age =56 years old; 63% men; 199 patients had CVD) took part in our study on malnutrition, inflammation, and CVD in incident dialysis patients. Circulating levels of albumin, ferritin, high–sensitivity C–reactive protein (hsCRP), IGF-1, IL-6, orosomucoid, troponin T (TnT), TNF, soluble intracellular adhesion molecule, soluble vascular cellular adhesion molecule 1 (sVCAM-1), and platelet and white blood cell (WBC) counts were analyzed as predictors of the presence of clinically overt CVD at baseline, protein-energy wasting (PEW), and subsequent all–cause mortality. During follow-up for a median of 28 months, there were 149 deaths, 81 of which were caused by CVD.
Results
Most biomarkers were elevated compared with reference values and–—except for albumin, ferritin, and IGF-1—higher in patients with CVD. In receiver operating characteristic analysis, age, IL-6, TnT, hsCRP, and IGF-1 were classifiers of baseline CVD and predictors of all-cause mortality. In addition to age, diabetes mellitus, smoking (for CVD), and PEW, only IL-6, relative risk (RR) 1.10 and 95% confidence interval ([95% CI], 1.02 to 1.19), sVCAM-1 RR 1.09 (95% CI, 1.01 to 1.17), and serum albumin RR 0.89 (95% CI, 0.83 to 0.95) associated with baseline CVD, and only WBC, hazard ratio (HR) 1.94 (95% CI, 1.34 to 2.82), IL-6 HR 1.79 (95% CI, 1.20 to 2.67), and TNF HR 0.65 (95% CI, 0.44 to 0.97) predicted all-cause mortality.
Conclusions
In addition to age and comorbidities, only IL-6, sVCAM-1, and albumin could—independently of other biomarkers—classify clinical CVD, and only IL-6, WBC, and TNF could—independently of other biomarkers—predict all–cause mortality risk. These data underscore the robustness of IL-6 as a classifier of clinically overt CVD and predictor of all-cause mortality in patients with stage 5 CKD.
Keywords: Inflammation; chronic kidney disease; cardiovascular disease; Interleukin-6; mortality; Biomarkers; C-Reactive Protein; Follow-Up Studies; Humans; Renal Insufficiency, Chronic
Introduction
Cardiovascular disease (CVD) is the major cause of death in patients with ESRD, and even a mildly reduced renal function results in a dramatically increased risk of premature CVD and death (1–6). Identifying and intervening against common risk factors are priorities, but traditional Framingham risk factors are poor predictors in patients with late-stage CKD (7–11), suggesting that the pathophysiologic mechanisms may differ. Over the last decade, multiple studies have attempted to identify better risk prediction tools specific to the CKD population (7,12,13). Many of these studies have evaluated the use of various peptide- biomarkers measured by proprietary or commercial ELISA. However, most small- to medium-sized polypeptides circulate in elevated concentrations in CKD and appear to rise contemporaneously with a drop in GFR (14–16). Thus, elevated levels of many biomarkers may reflect not only increased production as in other patient populations but also, a prolonged half-life caused by impaired clearance in the proximal tubules.
As reviewed by Zoccali (11), many biomarkers have been tested in the dialysis population, including ferritin (17), high–sensitivity C–reactive protein (hsCRP) (18–22), IGF-1 (23,24), IL-6 (18,22,25), soluble intracellular adhesion molecule (sICAM-1), soluble vascular cellular adhesion molecule 1 (sVCAM-1) (26,27), troponin T (TnT) (28), TNF (29), platelet count (PLT) (30), and white blood cell (WBC) (31). These risk biomarkers have each been shown to independently predict both clinical CVD and mortality in ESRD, but they often rely on relatively small materials, and multivariate testing and adjustment for common confounders, such as residual renal function, have not been performed consistently.
In this post hoc analysis, we used data from a study of 543 incident patients with CKD starting dialysis to compare the relative predictive power of 12 biomarkers—for which data were available in at least 300 patients—as classifiers of presence of clinically overt CVD at baseline and predictors of all–cause mortality risk during up to 60 months follow-up.
Materials and Methods
Patients
We performed a post hoc analysis of data from an ongoing longitudinal study conducted at the Karolinska University Hospital Huddinge (20). Recruitment of 543 incident patients on dialysis ages between 19 and 87 years old included in this study occurred from December of 1994 to January of 2014. The majority (n=403; 74%) of the patients investigated at baseline had not yet started on dialysis and were investigated 13 (range =0–54) days before dialysis start, whereas 140 patients (26%) had been treated by hemodialysis (HD) or peritoneal dialysis for a median of 9 (range =1–31) days before the baseline investigation. Patients were followed for a median of 28 months (range =7–60) or until December of 2014. Each patient’s medical chart was thoroughly reviewed by a nephrologist, and data were extracted on underlying kidney disease, prescribed medications, presence of clinically overt CVD, and other comorbid conditions, such as diabetes mellitus (DM). On the basis of the 10th revision of the International Statistical Classification of Diseases and Related Health Problems codes, baseline CVD diagnoses included myocardial infarction (I21–I23; n=54), heart failure (I11, I42, and I50; n=64), peripheral vascular disease (I70–I73; n=84), ischemic stroke (I63; n=26), unspecified stroke (I64; n=7), hemorrhagic stroke (I60–I62; n=6), and presence of coronary angioplasty implant and graft (Z95.5; n=43). The causes of CKD were chronic GN in 126 patients, diabetic nephropathy in 159 patients (including 125 patients with diabetes who were treated with insulin), hypertension/renal vascular disease in 113 patients, and other or unknown etiologies in 145 patients. The majority of patients were on antihypertensive medications and other commonly used drugs in CKD, such as phosphate and potassium binders, diuretics, erythropoiesis-stimulating agents, iron substitution, and vitamins B, C, and D supplementation. In accordance with current therapy recommendations, most patients with diabetes had been switched to insulin therapy before being included in the study. The Ethics Committee of the Karolinska Institute at the Karolinska University Hospital Huddinge (Stockholm, Sweden) approved the study protocol, and informed consent was obtained from each patient. Parts of this patient material have been presented previously (18,20,32,33).
The 12 biomarkers analyzed in this study (Table 1) were chosen on the basis of availability (data from at least 300 patients were required) and clinical utility (commercial kits are available for analysis, and each is well studied and reported to predict either CVD or mortality in the stage 5 CKD population).
Table 1.
Basal clinical and biochemical characteristics of 543 patients with stage 5 CKD by the presence or absence of clinically overt cardiovascular disease
| Characteristics | No CVD, n=344 | CVD, n=199 | P Valuea |
|---|---|---|---|
| Age, yr, n=543 | 50 (31–67) | 62 (49–70) | <0.001 |
| Men, %, n=543 | 56 | 73 | <0.001 |
| Diabetes mellitus, %, n=543 | 21 | 50 | <0.001 |
| PEW (SGA>1), %, n=496 | 24 | 47 | <0.001 |
| Smoking (yes/no), %, n=441 | 42/58 | 72/28 | <0.001 |
| BMI,b kg/m2, n=543 | 24.2 (19.5–30.8) | 24.6 (20.7–31.2) | 0.30 |
| Handgrip, % of control, n=483 | 97.6 (64.1–138.3) | 75.1 (50.1–112.6) | <0.001 |
| mBP,b mmHg, n=488 | 109 (91–126) | 106 (88–127) | 0.18 |
| eGFR, ml/min per 1.73 m2, n=497 | 6.7 (4.0–10.5) | 7.2 (4.0–12.0) | 0.03 |
| Medications | |||
| ACE inhibitors or AngII receptor blockers, %, n=543 | 63 | 54 | 0.05 |
| HMG-CoA reductase inhibitors (statins), %, n=543 | 19 | 41 | <0.001 |
| Markers of metabolism | |||
| Creatinine, mg/dl, n=543 | 8.5 (5.4–11.7) | 7.3 (4.3–11.1) | <0.001 |
| fP-parathyroid hormone, pg/ml, n=530 | 249 (52–643) | 207 (44–578) | 0.12 |
| fP-triglycerides, mg/dl, n=538 | 159 (80–283) | 150 (80–310) | 0.79 |
| HbA1c,c mmol/mol, n=366 | 36 (28–50) | 38 (31–51) | 0.05 |
| HOMA-IR Index,b,c n=323 | 3.0 (1.6–7.9) | 3.1 (1.4–6.7) | 0.35 |
| U-albumin, mg/L per 24 h, n=352 | 1646 (161–5986) | 2000 (168–5431) | 0.56 |
| Circulating biomarkers, n=12 | |||
| Albumin, g/dl, n=543 | 3.4 (2.7–4.0) | 3.3 (2.5–3.8) | 0.002 |
| Ferritin, ng/ml, n=531 | 124 (39–309) | 121 (35–320) | >0.99 |
| hsCRP, mg/L, n=543 | 3.8 (0.6–23.0) | 8.1 (1.0–44.0) | <0.001 |
| IGF-1, μg/ml, n=375 | 194 (84–355) | 138 (68–265) | <0.001 |
| IL-6, pg/ml, n=502 | 5.0 (1.9–13.2) | 8.8 (3.4–22.4) | <0.001 |
| Orosomucoid, g/L, n=393 | 1.0 (0.7–1.6) | 1.1 (0.8–1.8) | 0.002 |
| sICAM-1, ng/ml, n=329 | 234 (167–352) | 267 (195–403) | <0.001 |
| sVCAM-1, ng/ml, n=329 | 1226 (814–1800) | 1429 (893–2127) | <0.001 |
| Platelet count, 103/μl, n=497 | 245 (152–370) | 260 (156–400) | 0.13 |
| TNF, pg/ml, n=461 | 11.8 (7.0–20.4) | 12.7 (7.5–25.3) | 0.03 |
| Troponin T, μg/L, n=394 | 0.03 (0.01–0.17) | 0.07 (0.02–0.34) | <0.001 |
| White blood cells, 103/μl, n=521 | 7.3 (4.9–11.2) | 8.1 (5.4–12.0) | 0.002 |
Data are presented as medians (range of 10th to 90th percentiles) or percentages. CVD, cardiovascular disease; PEW, protein energy wasting; SGA, subjective global assessment of nutritional status; BMI, body mass index; mBP, mean arterial BP; ACE, angiotensin-converting enzyme; AngII, Angiotensin II; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA; fP, fasting plasma; HbA1c, hemoglobin A1c; HOMA-IR, homeostatic model assessment of insulin resistance; U-albumin, urine albumin; hsCRP, high–sensitivity C–reactive protein; sICAM-1, soluble intracellular adhesion molecule; sVCAM-1, soluble vascular cellular adhesion molecule 1.
For continuous variables, we used nonparametric Wilcoxon tests, and for nominal variables, we used Fischer exact tests.
Formulas: BMI (weight in kilograms divided by the square of the height in meters), HOMA-IR (fasting serum insulin [microunits per milliliter] × fasting plasma glucose [micromoles per liter]/22.5), and mBP (diastolic pressure + [systolic BP − diastolic BP]/3).
Only in patients with no diabetes.
Anthropometric and Metabolic Evaluation
Height and body weight were obtained at the baseline, and body mass index was recorded. Subjective global assessment (SGA) was used to evaluate overall protein-energy wasting (PEW) as previously described (24). PEW was defined as an SGA score >1. In patients who were not diabetic, insulin resistance was calculated by the homeostasis model assessment for insulin resistance (34). Insulin resistance was not assessed in patients with diabetes. Arterial systolic and diastolic BPs were measured three times in the morning after a 15-minute resting period, and the mean pressure was used.
Handgrip strength (HGS) was measured using a Harpenden Handgrip Dynamometer (Yamar, Jackson, MI) in the dominant hand. The individual values for HGS were expressed as percentage of healthy individuals adjusting for the sex. Eighty healthy individuals from the Stockholm region who were randomly selected by Statistics Sweden (a government agency) and accepted to participate were recruited from February of 2003 to January of 2007. Their median age was 63 years old, 71% were men, 5% had CVD, 9% had DM, 3% were malnourished (SGA>1), and median eGFR was 81 ml/min per 1.73 m2.
Biochemical Analyses
Blood samples were collected at baseline evaluation after overnight fasting. The plasma was separated within 30 minutes, and samples were kept frozen at −70°C if not analyzed immediately.
Concentrations of hemoglobin, serum albumin (bromcresol purple), creatinine, ferritin, hemoglobin A1c, hsCRP (nephelometry), orosomucoid, PLT, TnT, urea, and WBC and plasma concentrations of cystatin C and glucose were determined by routine methods at the Department of Laboratory Medicine, Karolinska University Hospital. Commercial ELISA kits were used to determine sVCAM-1 and sICAM-1 (R&D Systems, Minneapolis, MN). Plasma concentrations of insulin, IGF-1, IL-6, and TNF were measured on an Immulite TM Automatic Analyzer (Siemens Healthcare; Diagnostics Products Ltd.) according to the manufacturer’s instructions using assays manufactured for this analyzer. The coefficient of variation for investigated biomarkers is shown in Supplemental Table 1.
In the majority of the patients (n=441), eGFR was calculated by the mean of renal urea and creatinine clearances from a 24-hour urine collection, which is thought to provide accurate measurement of GFR in patients with stage 5 CKD, and in others (n=56), eGFR was calculated by a cystatin C–based equation (35). Thus, data on eGFR were available in 497 of 543 patients.
Statistical Analyses
Data are expressed as medians (10th to 90th percentiles), percentages, relative risks (RR) ratio (95% confidence intervals [95% CIs]), or hazard ratios (HRs; 95% CIs) as appropriate. Statistical significance was set at the level of P<0.05. Comparisons between two groups were assessed with the nonparametric Wilcoxon test for continuous variables and Fischer exact test for nominal variables. Adjudication of outcomes included (1) determination of optimal cutoff values for each biomarker to be used for dichotomous analysis followed by (2) multivariable regression analysis by the generalized linear model (GENMOD) procedure of SAS Institute Inc. (Cary, NC; www.sas.com), and (3) multinomial logistic regression analysis. (1) The best possible cutoff value for each biomarker was determined by plotting separate receiver operating characteristics (ROC) curves for each biomarker, calculating areas under the curves (AUCs) before each dichotomous analysis, and using the same outcome variable (e.g., CVD). ROC–derived AUC values were used as cutoffs for statistical analyses rather than using an arbitrary cutoff level (e.g., median or tertiles). (2) To study the associations of biomarkers and other parameters with the presence of clinically overt CVD at baseline and all–cause mortality risk, respectively, multivariable GENMOD regression analyses were performed. Age, sex, DM, eGFR, SGA, smoking, calendar years period (only for all-cause mortality; separated as 1994–1999, 2000–2005, and 2006–2009; the period from 2010 to 2014 was the reference), albumin, ferritin, hsCRP, IGF-1, IL-6, orosomucoid, sVCAM-1, sICAM-1, PLT, TnT, TNF, and WBC were included in the model. A multiple imputation of missing values was performed using the function PROC MI, with all variables in the covariate section used to produce the values for imputation. The original n for each variable is given throughout. The results for each imputation were generated using PROC GENMOD and PHREG and then, combined using PROC MIANALYZE. We used five imputed datasets for this study to ensure that our effect estimates were not overly inaccurate because of Monte Carlo variability. (3) A multinomial logistic regression model was used to assess classifiers of presence of baseline CVD and predictors of all-cause mortality. The predictive strength, expressed as pseudo-r, of biomarkers to classify presence of clinically overt CVD and all–cause mortality risk during follow-up of up to 60 months censored for transplantation was calculated. Statistical analyses were performed using statistical software SAS, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics According to Presence or Absence of Clinically Overt CVD
Baseline characteristics of the included patients, grouped according to the presence or absence of CVD, are shown in Table 1. As expected, patients with CVD were older, were more often men, were patients with diabetes, were smokers, were wasted (SGA>1), and had lower HGS and eGFR. The circulating levels of hsCRP, IL-6, orosomucoid, sICAM-1, sVCAM-1, TNF, TnT, and WBC were higher, whereas IGF-1 as well as serum albumin and creatinine were significantly lower in patients with clinically overt CVD at baseline. There were no statistical differences in ferritin and PLT levels or insulin resistance as assessed by homeostasis model assessment of insulin resistance between the groups. N–terminal probrain natriuretic peptide (n=194) was higher in patients with CVD (Supplemental Figure 1).
Predictive Power of Investigated Biomarkers
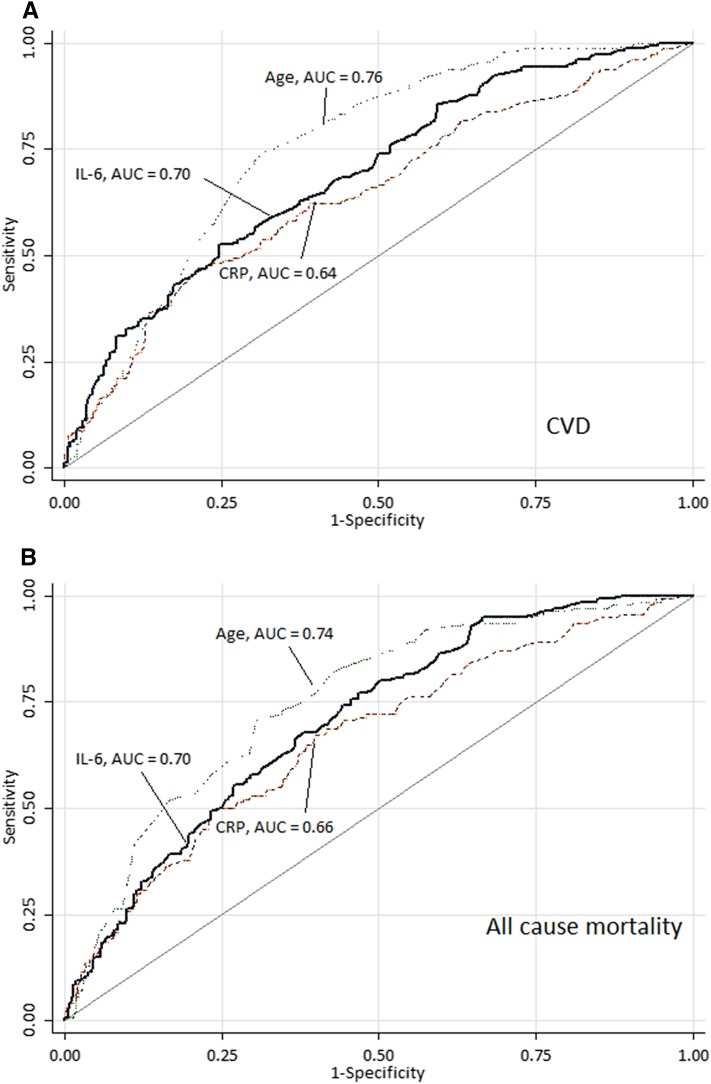
For each biomarker, the appropriate optimal predictive cutoff value was analyzed by ROC curves. As an example, Figure 1 shows ROC curves for age, hsCRP, and IL-6 versus CVD and all-cause mortality. Table 2 shows the predictive power (AUC) and cutoff levels of the investigated biomarkers assessed using ROC curves. Briefly, in addition to age, high IL-6, high TnT, high hsCRP, and low IGF-1 were the strongest predictors of baseline CVD and all-cause mortality. Thus, for baseline CVD, IL-6 and TnT followed by IGF-1 and hsCRP were the strongest predictors, whereas AUC values for PLT and ferritin were not significantly increased. As predictors of mortality, IGF-1 and IL-6 followed by hsCRP and TnT performed best, whereas AUC values for TNF and ferritin were not significantly increased. Of note, eGFR, added for comparison and representing a limited range (10th to 90th percentiles) of 4–12 ml/min per 1.73 m2, was also a poor predictor of baseline CVD.
Figure 1.
The optimal predictive cutoff value for each biomarker was defined using receiver operating characteristics curves. In the example shown here, age appeared as a stronger predictor of outcomes than IL-6 and high-sensitivity CRP, a finding which was confirmed by the subsequent analyses. Areas under the curves (AUCs) of receiver operating characteristics in 543 patients with stage 5 CKD for patient age, IL-6, and high–sensitivity C–reactive protein (CRP) in relation to (A) presence of clinically overt cardiovascular disease (CVD) and (B) 60 months all-cause mortality censored for transplantation. (A) The AUC of age, IL-6, and high-sensitivity CRP for CVD in patients with stage 5 CKD (n=543). (B) The AUC of age, IL-6, and high-sensitivity CRP for all-cause mortality in patients with stage 5 CKD (n=543).
Table 2.
Cutoff levels and areas under the curve of receiver operating characteristic curves for selected biomarkers as classifiers of the presence of clinically overt cardiovascular disease at baseline and all-cause mortality, respectively, in 543 patients with stage 5 CKD initiating dialysis therapy
| Variable | CVD | All-Cause Mortality | ||
|---|---|---|---|---|
| Cutoff Level | AUC (SEM) | Cutoff Level | AUC (SEM) | |
| Age, yr, n=543 | 58 | 0.76 (0.01) | 59 | 0.74 (0.01) |
| IL-6, pg/ml, n=502 | 7.0 | 0.70 (0.02) | 6.7 | 0.70 (0.01) |
| TnT, μg/L, n=394 | 0.05 | 0.67 (1.05) | 0.06 | 0.66 (0.92) |
| IGF-1, μg/ml, n=375 | 163 | 0.66 (0.01) | 155 | 0.72 (0.01) |
| hsCRP, mg/L, n=543 | 5.3 | 0.64 (0.01) | 6.4 | 0.66 (0.01) |
| sVCAM-1, ng/ml, n=329 | 1355 | 0.63 (0.01) | 1356 | 0.64 (0.01) |
| sICAM-1, ng/ml, n=32 | 249 | 0.62 (0.01) | 249 | 0.64 (0.01) |
| Orosomucoid, g/L, n=393 | 1.1 | 0.59 (0.25) | 1.1 | 0.57 (0.26) |
| Albumin, g/dl, n=543 | 3.3 | 0.58 (0.02) | 3.3 | 0.64 (0.02) |
| WBC, 103/μl, n=521 | 7.7 | 0.58 (0.03) | 8.0 | 0.64 (0.03) |
| TNF, pg/ml, n=461 | 12.2 | 0.56 (0.01) | 12.1 | 0.52 (0.01) |
| eGFR, ml/min per 1.73 m2, n=497 | 7.0 | 0.56 (0.03) | 6.9 | 0.51 (0.03) |
| PLT, 103/μl, n=497 | 253 | 0.54 (0.01) | 261 | 0.60 (0.01) |
| Ferritin, ng/ml, n=531 | 127 | 0.50 (0.01) | 122 | 0.53 (0.01) |
The order of variables is according to AUC for classification of the presence of CVD. CVD, cardiovascular disease; AUC, area under the curve; TnT, troponin T; hsCRP, high–sensitivity C–reactive protein; sVCAM-1, soluble vascular cellular adhesion molecule 1; sICAM-1, soluble intracellular adhesion molecule; WBC, white blood cell; PLT, platelet count.
Adjusted RRs for the Investigated Biomarkers as Predictors of CVD
Table 3 shows RRs for baseline CVD associated with biomarkers adjusted for age, sex, DM, smoking, PEW, and eGFR as well as all of the other investigated biomarkers. Apart from age (RR, 1.31; 95% CI, 1.22 to 1.41; P<0.001), DM (RR, 1.24; 95% CI, 1.14 to 1.34; P<0.001), smoking (RR, 1.15; 95% CI, 1.07 to 1.23; P=0.001), and PEW (RR, 1.10; 95% CI, 1.02 to 1.19; P=0.02), only high concentrations of IL-6 (RR, 1.10; 95% CI, 1.02 to 1.19; P=0.01) and sVCAM-1 (RR, 1.09; 95% CI, 1.01 to 1.17; P=0.04) and a low concentration of albumin (RR, 0.89; 95% CI, 0.83 to 0.95; P=0.001) were associated with presence of CVD at baseline, whereas IGF-1 (P=0.05) and orosomucoid (P=0.08) did not reach statistical significance for being associated with CVD.
Table 3.
Presence of baseline clinical overt cardiovascular disease
| Variable | Relative Risk Ratio (95% CI) | P Value |
|---|---|---|
| Age, >58 versus ≤58 yr | 1.31 (1.22 to 1.41) | <0.001 |
| Sex, men versus women | 1.06 (0.99 to 1.15) | 0.11 |
| Diabetes mellitus, present versus absent | 1.24 (1.14 to 1.34) | <0.001 |
| Smoking, yes versus no | 1.15 (1.07 to 1.23) | <0.001 |
| SGA, malnourished versus well nourished | 1.10 (1.02 to 1.19) | 0.02 |
| eGFR, ≤7.0 versus >7.0 ml/min per 1.73 m2 | 0.97 (0.91 to 1.04) | 0.46 |
| IL-6, >7.0 versus ≤7.0 pg/ml | 1.10 (1.02 to 1.19) | 0.01 |
| sVCAM-1, >1355 versus ≤1355 ng/ml | 1.09 (1.01 to 1.17) | 0.04 |
| Orosomucoid, >1.1 versus ≤1.1 g/L | 1.08 (0.99 to 1.17) | 0.08 |
| Troponin T, >0.05 versus ≤0.05 μg/L | 1.06 (0.98 to 1.15) | 0.13 |
| WBC, >7.7×103 versus ≤7.7×103/μl | 1.05 (0.97 to 1.13) | 0.25 |
| hsCRP, >5.3 versus ≤5.3 mg/L | 1.02 (0.93 to 1.11) | 0.71 |
| PLT, >253×103 versus ≤253×103/μl | 1.01 (0.94 to 1.08) | 0.87 |
| TNF, >12.2 versus ≤12.2 pg/ml | 0.97 (0.91 to 1.05) | 0.48 |
| sICAM-1, >249 versus ≤249 ng/ml | 0.97 (0.91 to 1.04) | 0.43 |
| Ferritin, >127 versus ≤127 ng/ml | 0.96 (0.90 to 1.03) | 0.30 |
| IGF-1, >163 versus ≤163 μg/ml | 0.93 (0.86 to 1.00) | 0.05 |
| Albumin, >3.3 versus ≤3.3 g/dl | 0.89 (0.83 to 0.95) | 0.001 |
The output from an imputed multivariate generalized linear model (GENMOD) of classifiers of baseline clinical cardiovascular disease incorporating multiple factors associated with cardiovascular disease in univariate analysis plus each of the studied biomarkers. Results are presented as relative risk ratios with 95% CIs. 95% CI, 95% confidence interval; SGA, subjective global assessment of nutritional status; sVCAM-1, soluble vascular cellular adhesion molecule 1; WBC, white blood cell; hsCRP, high–sensitivity C–reactive protein; PLT, platelet count; sICAM-1, soluble intracellular adhesion molecule.
Adjusted HRs for the Investigated Biomarkers as Predictors of All-Cause Mortality
There were 149 deaths over the follow-up period of 60 months, including 81 (54%) cardiovascular-related deaths and 68 (46%) noncardiovascular-related deaths (Supplemental Table 2). Table 4 shows HRs for all-cause mortality calculated for each of the investigated biomarkers adjusted for age, sex, DM, smoking, PEW, and eGFR as well as concomitantly taking all other investigated biomarkers into account. In addition to age (HR, 1.58; 95% CI, 1.10 to 2.28; P=0.01), PEW (HR, 2.28; 95% CI, 1.56 to 3.33; P<0.001), and DM (HR, 1.56; 95% CI, 1.07 to 2.26; P=0.01), only elevated WBC (HR, 1.94; 95% CI, 1.34 to 2.82; P<0.001) and IL-6 (HR, 1.79; 95% CI, 1.20 to 2.67; P=0.01) predicted higher risk of all-cause mortality, whereas high concentration of TNF (HR, 0.65; 95% CI, 0.44 to 0.97; P=0.04) unexpectedly predicted lower risk of all-cause mortality. In addition, low eGFR (P=0.09), high sICAM-1 (P=0.06), and low levels of orosomucoid (P=0.07) showed borderline significance for trend to be associated with higher risk of all-cause mortality.
Table 4.
All–cause mortality risk
| Variables | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age, >58 versus ≤58 yr | 1.58 (1.10 to 2.28) | 0.01 |
| Sex, men versus women | 1.07 (0.73 to 1.56) | 0.53 |
| Diabetes mellitus, present versus absent | 1.56 (1.07 to 2.26) | 0.01 |
| Smoking, yes versus no | 1.10 (0.75 to 1.61) | 0.47 |
| SGA, malnourished versus well nourished | 2.28 (1.56 to 3.33) | <0.001 |
| eGFR, ≤6.9 versus >6.9 ml/min per 1.73 m2 | 1.42 (0.99 to 2.04) | 0.09 |
| Calendar year period, 2010–2014 versus 2006–2009 | 1.21 (0.62 to 2.37) | 0.66 |
| Calendar year period, 2010–2014 versus 2000–2005 | 0.83 (0.42 to 1.65) | 0.64 |
| Calendar year period, 2010–2014 versus 1994–1999 | 0.75 (0.37 to 1.56) | 0.58 |
| WBC, >8.0×103 versus ≤8.0×103/μl | 1.94 (1.34 to 2.82) | <0.001 |
| IL-6, >6.7 versus ≤6.7 pg/ml | 1.79 (1.20 to 2.67) | 0.01 |
| Troponin T, >0.06 versus ≤0.06 μg/L | 1.49 (1.03 to 2.16) | 0.14 |
| hsCRP, >6.4 versus ≤6.4 mg/L | 1.25 (0.80 to 1.95) | 0.42 |
| PLT, >261×103 versus ≤261×103/μl | 1.22 (0.85 to 1.75) | 0.30 |
| sVCAM-1, >1356 versus ≤1356 ng/ml | 1.15 (0.78 to 1.69) | 0.43 |
| sICAM-1, >249 versus ≤249 ng/ml | 1.07 (0.76 to 1.49) | 0.06 |
| Albumin, >3.3 versus ≤3.3 g/dl | 1.07 (0.75 to 1.55) | 0.59 |
| Ferritin, >122 versus ≤122 ng/ml | 0.96 (0.69 to 1.34) | 0.77 |
| IGF-1, >155 versus ≤155 μg/ml | 0.78 (0.54 to 1.14) | 0.27 |
| TNF, >12.1 versus ≤12.1 pg/ml | 0.65 (0.44 to 0.97) | 0.04 |
| Orosomucoid, >1.1 versus ≤1.1 g/L | 0.62 (0.41 to 0.95) | 0.07 |
The output from an imputed multivariate generalized linear model (GENMOD) of all–cause mortality hazard ratios (95% CIs) estimated during 60 months of follow-up calculated for each evaluated biomarker and adjusted for other variables. Patient outcomes were censored for renal transplantation. 95% CI, 95% confidence interval; SGA, subjective global assessment of nutritional status; WBC, white blood cell; hsCRP, high–sensitivity C–reactive protein; PLT, platelet count; sVCAM-1, soluble vascular cellular adhesion molecule 1; sICAM-1, soluble intracellular adhesion molecule.
Adjusted HRs for the Investigated Biomarkers as Predictors of CVD-Related Mortality
In a separate analysis (Table 5), HRs for CVD-related mortality were calculated for each of the investigated biomarkers adjusted for age, sex, DM, smoking, PEW, and eGFR, while concomitantly taking all other investigated biomarkers into account. The results showed that, in addition to baseline CVD, DM, PEW, and eGFR, only WBC and low TNF predicted CVD-related mortality (Table 5).
Table 5.
Cardiovascular disease–related mortality
| Variables | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age, >58 versus ≤58 yr | 1.23 (0.75 to 2.05) | 0.41 |
| Sex, men versus women | 0.97 (0.58 to 1.63) | 0.92 |
| CVD, present versus absent | 2.53 (1.43 to 4.46) | 0.001 |
| Diabetes mellitus, present versus absent | 2.14 (1.27 to 3.60) | 0.004 |
| Smoking, yes versus no | 1.14 (0.65 to 2.01) | 0.65 |
| SGA, malnourished versus well nourished | 2.39 (1.42 to 4.03) | 0.001 |
| eGFR, ≤6.9 versus >6.9 ml/min per 1.73 m2 | 2.42 (1.44 to 4.07) | <0.001 |
| Calendar year period, 2010–2014 versus 2006–2009 | 2.63 (0.73 to 9.48) | 0.14 |
| Calendar year period, 2010–2014 versus 2000–2005 | 1.91 (0.54 to 6.75) | 0.32 |
| Calendar year period, 2010–2014 versus 1994–1999 | 2.34 (0.62 to 8.86) | 0.21 |
| WBC, >8.0×103 versus ≤8.0×103/μl | 2.53 (1.50 to 4.27) | <0.001 |
| IL-6, >6.7 versus ≤6.7 pg/ml | 1.43 (0.83 to 2.48) | 0.20 |
| Troponin T, >0.06 versus ≤0.06 μg/L | 1.28 (0.76 to 2.16) | 0.36 |
| hsCRP, >6.4 versus ≤6.4 mg/L | 1.33 (0.73 to 2.44) | 0.35 |
| PLT, >261×103 versus ≤261×103/μl | 1.08 (0.64 to 1.83) | 0.76 |
| sVCAM-1, >1356 versus ≤1356 ng/ml | 1.09 (0.63 to 1.89) | 0.75 |
| sICAM-1, >249 versus ≤249 ng/ml | 1.10 (0.69 to 1.74) | 0.70 |
| Albumin, >3.3 versus ≤3.3 g/dl | 0.98 (0.60 to 1.59) | 0.92 |
| Ferritin, >122 versus ≤122 ng/ml | 1.30 (0.81 to 2.07) | 0.27 |
| IGF-1, >155 versus ≤155 μg/ml | 1.03 (0.61 to 1.74) | 0.92 |
| TNF, >12.1 versus ≤12.1 pg/ml | 0.52 (0.30 to 0.92) | 0.02 |
| Orosomucoid, >1.1 versus ≤1.1 g/L | 0.65 (0.38 to 1.11) | 0.12 |
The output from an imputed multivariate generalized linear model (GENMOD) of hazard ratios (95% CIs) for CVD-related mortality (81 deaths) (Supplemental Table 2) estimated during 60 months of follow-up calculated for each evaluated biomarker and adjusted for other variables. Patient outcomes were censored for renal transplantation. 95% CI, 95% confidence interval; CVD, cardiovascular disease; SGA, subjective global assessment of nutritional status; WBC, white blood cell; hsCRP, high–sensitivity C–reactive protein; PLT, platelet count; sVCAM-1, soluble vascular cellular adhesion molecule 1; sICAM-1, soluble intracellular adhesion molecule.
Effect of Adjustment for eGFR
In univariate analysis, eGFR was significantly higher in patients with CVD. However, the differences in eGFR were rather small and likely of no clinical significance. eGFR was included in both multiple regression analyses for baseline CVD risk and the all–cause mortality HRs. Neither model showed any risk modification by baseline eGFR after adjustment for the studied biomarkers and clinical characteristics (Tables 3 and 4).
Relative Contributions of Factors Predicting CVD and Mortality
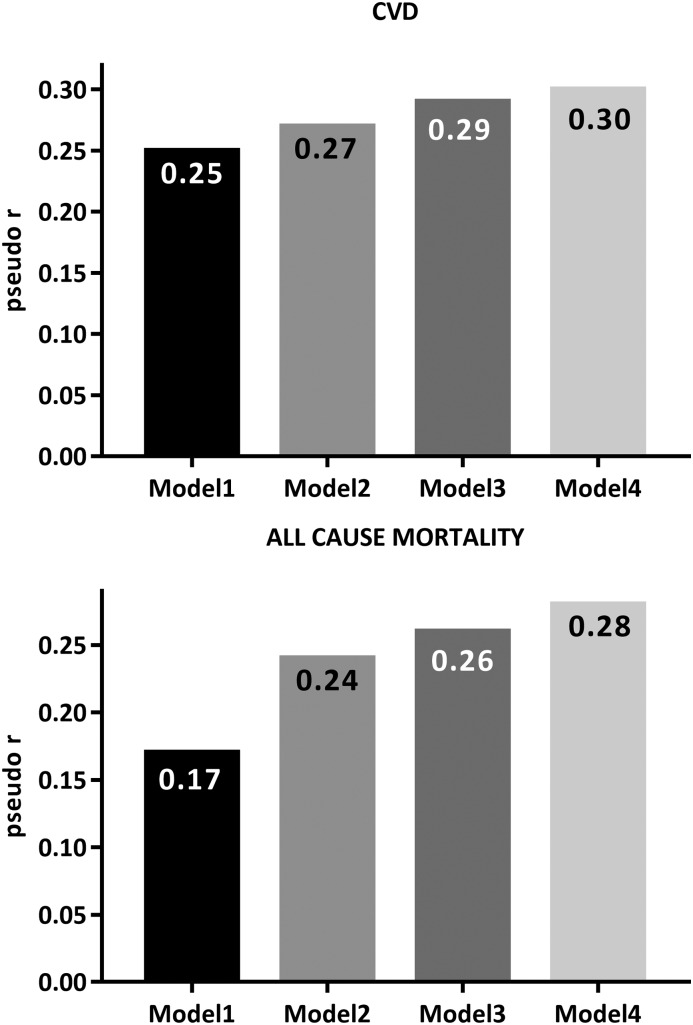
In a separate analysis, we analyzed how well combinations of different factors (clinical and demographic data and different biomarkers) could predict the variation of the different estimates of risk for CVD and all-cause mortality (Figure 2). The predictive strength, expressed as pseudo-r, of using biomarkers to predict presence of clinically overt CVD (model 1: 0.25, model 2: 0.27, model 3: 0.29, and model 4: 0.30) and all–cause mortality risk (model 1: 0.17, model 2: 0.24, model 3: 0.26, and model 4: 0.28) during follow-up of up to 60 months censored for transplantation was calculated using models adding progressively more biomarkers. The results show that the added predictive value of using biomarkers over and above prediction obtained by using only information about age, sex, DM, smoking, and eGFR is negligible.
Figure 2.
The predictive strength, expressed as pseudo-r, of using biomarkers to predict presence of clinically overt cardiovascular disease (CVD; upper panel) and all–cause mortality risk (lower panel) during follow-up of up to 60 months censored for transplantation in 543 patients with stage 5 CKD. Model 1: age, sex, diabetes mellitus, smoking, and eGFR (calendar year for all-cause mortality). Model 2: model 1 as well as subjective global assessment of nutritional status, albumin, IGF-1, TNF, soluble intracellular adhesion molecule, and soluble vascular cellular adhesion molecule 1. Model 3: model 2 as well as high–sensitivity C–reactive protein, troponin T, IL-6, and orosomucoid. Model 4: model 3 as well as ferritin, platelet count, and white blood cell count.
Discussion
The chief finding of this study is that IL-6 is the only biomarker that consistently and independently could classify the presence of clinical overt CVD at baseline and predict subsequent mortality over 60 months. Importantly, IL-6 withstood the scrutiny of being assessed together with a panel of other biomarkers that—using somewhat less rigorous approaches—repeatedly have been reported to predict CVD and mortality. The relative uselessness—compared with age and well established risk factors, including DM, smoking, and PEW—of many of the studied biomarkers, even under conditions optimized by applying separate ROC–calculated cutoffs for each model, suggests that these other biomarkers do not necessarily reflect pathophysiologic pathways mediating CVD or leading to premature death in CKD.
In this study, after adjustment for age, sex, DM, PEW, smoking, eGFR, and concomitant analysis of all other biomarkers, only high IL-6, high sVCAM-1, and low serum albumin could classify presence of clinical CVD, and only high WBC and high IL-6 levels were associated with higher all–cause mortality risk. Thus, this comparative analysis identified IL-6 as the only biomarker that, in competition with the other biomarkers, could classify the presence of clinical overt CVD at baseline and subsequent mortality after adjustments for confounders, such as age, DM, smoking, PEW, and eGFR, and moreover, concomitantly taking into account the effect of all other investigated biomarkers. Thus, we confirm several previous reports showing that IL-6 is a strong independent predictor of clinical outcomes in patients with CKD (25,36) as well as that it is involved in the high cardiovascular risk in the population (36). We speculate that one reason for the notable strong performance of IL-6 may be because of its reported links to the premature ageing phenotype on patients with CKD that is thought to be mediated by uremic toxins driving vascular smooth muscle cell damage and phenotypic changes that promote vascular calcification, a hallmark of vascular ageing, which associates with the senescence–associated secretory phenotype (2,37).
Surprisingly and in contrast to previous reports (38,39), we found that high TNF was associated with lower mortality risk. In prevalent patients on HD, although soluble TNF receptor 1 (TNFR1) and soluble TNFR2 (40) did not associate with clinical outcome, high TNF associated with higher risk of mortality (41), indicating that high TNF, but not TNFR1 and TNFR2, predicts poor outcome in this patient population (40,41). However, in contrast to the finding of a positive association of high TNF with higher risk of mortality in prevalent patients on HD (41), high TNF seemed to be protective among incident patients on dialysis, thus adding to the mixed results in the literature (42). Also, when including presence of baseline CVD as a variable, high TNF associated with lower mortality risk (Supplemental Table 3). Although the reason for this intriguing discrepancy is not clear, both TNF (and IL-6) are related to local and tissue–specific inflammatory processes (43,44), and it is possible that differences in local activity and tissue specificity may influence risk profiles in different patient populations.
Notably, almost all of the investigated biomarkers appeared as both classifiers of CVD and predictors of mortality in the ROC analysis, although AUC values were, in general, low. Most of them—in particular, IL-6 and hsCRP—were related to the other biomarkers (Supplemental Table 4). Because it seems unlikely that each of these molecules is uniquely involved in the pathogenic process of uremic CVD, the results of this study may reflect a general breakdown of signaling homeostasis in the uremic milieu and/or senescence–associated secretory phenotype. Alternatively, a reduced renal clearance resulting in an increased half-life would also lead to elevated circulating levels of either active or inactive epitopes recognized by our kits. Supporting the first hypothesis, Kalantar-Zadeh (42) counted ≥45 published peptide markers of inflammation elevated in CKD, many correlating with outcomes as well. Our finding that thrombocytosis associated with high mortality is in agreement with the work by Molnar et al. (30) in patients on HD. It should be noted that concentrations of circulating biomarkers depend, to some degree, on the underlying etiology of renal disease (Supplemental Table 5).
This study is, to our knowledge, the first to attempt at a comparison of so many diverse biomarkers in incident patients on dialysis. As such, it illustrates the importance of validating reported correlations by comparing observed results with those of other biomarkers assessed in the same dataset as well as correcting reported correlations for the confounder of reduced renal function. Previously, Doi et al. (12) used a similar approach in a similar population to identify predictors of 12-months mortality risk after the start of dialysis. Only two biomarkers (albumin and C-reactive protein) were included in that analysis of 688 patients, and only one of these (albumin) was included in the proposed prediction algorithm comprising six variables (where it was assigned the lowest weight).
A number of limitations of this study should be acknowledged. First, this is a rather small study in a relatively homogenous population with a narrow range of eGFR. Thus, we could not, for example, adequately study the effect of residual renal function on the observed relationships and test the hypothesis that reduced tubular metabolism is an important determinant of the observed elevation in biomarker concentrations. Second, our epidemiologic study does not address the direct clinical usefulness of the biomarkers in diagnosing acute events, such as cardiac ischemia, although it was recently shown that residual renal function is an important confounder when using TnT for that purpose (45,46). Third, because we measured the circulating biomarkers using commercial ELISA kits, the unknown epitopes of most of these makes any meaningful interpretation difficult. For example, we are not able to say with confidence that we have assayed whole polypeptides, let alone the ones that are bioactive. However, the same weakness applies also to the previous studies that we have cited. Fourth, this study relies on measurement of biomarkers at one single time point, namely in conjunction with dialysis initiation, which may not be an ideal time point for risk prediction, because patients are subject to many concomitant disturbances affecting both concentrations of investigated biomarkers as well as the general health status of the patients. Indeed, a recent study by Eckardt et al. (47) showed that high cardiovascular event rates occur within the first weeks of starting HD. Serial measurements could provide more information than a single measurement (48), especially because the pathways of inflammation might affect PEW, which is a powerful predictor of clinical outcome (49). Fifth, our study relied on post hoc analysis of clinical events, and we did not, thus, validate our diagnosis of CVD in a consistent manner across patients using an objective method, such as cardiac ultrasound.
In summary, this exploratory post hoc analysis of data obtained in incident patients on dialysis shows that a number of biomarkers may serve as both classifiers of presence of clinically overt CVD and predictors of all-cause mortality over a subsequent period of up to 60 months. However, with the exception of IL-6, their predictive power—compared with patient age and other clinical characteristics, such as DM, smoking, and PEW—is in general relatively weak, and neither the presence of CVD nor mortality risk in patients with CKD may be accurately predicted solely by using biomarkers. Our data suggest that CKD per se may cause as yet undefined far–reaching and systematic alterations in the circulating proteome, explaining these observations. Thus, future studies should attempt to also study the pathophysiologic relevance and role of any proposed risk biomarkers specifically in the patient with CKD.
Disclosures
A.M. and B.L. are employed by Baxter Healthcare Corporation. None of the other authors declare any conflict of interest.
Supplementary Material
Acknowledgments
We thank those of our patients and clinical colleagues who have supported this research.
This study was supported by a grant from Baxter Healthcare to Baxter Novum, Department of Clinical Science, Intervention and Technology, Karolinska Institutet. The study also benefited from generous support from the Chinese Scholarship Council (J.S.), the Marianne & Marcus Wallenbergs Stiftelse (J.A.), the Swedish Research Council (J.A. and P.S.), the Swedish Heart and Lung Foundation (J.A.), the Marie-Curie Network Training Grant from the Marie Curie Initial Training Network Grant from the European Union for the project European Training & Research in Peritoneal Dialysis (EuTRiPD) (to A.M.), the Karolinska Institutet Diabetes Theme Center (P.S.), and the Martin Rind Foundation (A.R.Q.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10441015/-/DCSupplemental.
References
- 1.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB: Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol 41: 1364–1372, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P: Chronic kidney disease and premature ageing. Nat Rev Nephrol 10: 732–742, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Levin A, Foley RN: Cardiovascular disease in chronic renal insufficiency. Am J Kidney Dis 36[Suppl 3]: S24–S30, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Menon V, Gul A, Sarnak MJ: Cardiovascular risk factors in chronic kidney disease. Kidney Int 68: 1413–1418, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ross R: Atherosclerosis--an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C, Mallamaci F, Tripepi G: Inflammatory proteins as predictors of cardiovascular disease in patients with end-stage renal disease. Nephrol Dial Transplant 19[Suppl 5]: V67–V72, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Nishimura K, Okamura T, Watanabe M, Nakai M, Takegami M, Higashiyama A, Kokubo Y, Okayama A, Miyamoto Y: Predicting coronary heart disease using risk factor categories for a Japanese urban population, and comparison with the Framingham risk score: The Suita study. J Atheroscler Thromb 21: 784–798, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Stenvinkel P, Lindholm B: Cardiovascular biomarkers in chronic kidney disease. J Ren Nutr 22: 120–127, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Stenvinkel P: Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. J Intern Med 268: 456–467, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Weiner DE, Tighiouart H, Griffith JL, Elsayed E, Levey AS, Salem DN, Sarnak MJ: Kidney disease, Framingham risk scores, and cardiac and mortality outcomes. Am J Med 120: 552.e1–552.e8, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Zoccali C: Traditional and emerging cardiovascular and renal risk factors: An epidemiologic perspective. Kidney Int 70: 26–33, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Doi T, Yamamoto S, Morinaga T, Sada KE, Kurita N, Onishi Y: Risk score to predict 1-year mortality after haemodialysis initiation in patients with stage 5 chronic kidney disease under predialysis nephrology care. PLoS One 10: e0129180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsushita K, Sang Y, Ballew SH, Shlipak M, Katz R, Rosas SE, Peralta CA, Woodward M, Kramer HJ, Jacobs DR, Sarnak MJ, Coresh J: Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol 26: 439–447, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Axelsson J, Bergsten A, Qureshi AR, Heimbürger O, Bárány P, Lönnqvist F, Lindholm B, Nordfors L, Alvestrand A, Stenvinkel P: Elevated resistin levels in chronic kidney disease are associated with decreased glomerular filtration rate and inflammation, but not with insulin resistance. Kidney Int 69: 596–604, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Manzano-Fernández S, Januzzi JL, Boronat-García M, Pastor P, Albaladejo-Otón MD, Garrido IP, Bayes-Genis A, Valdés M, Pascual-Figal DA: Impact of kidney dysfunction on plasma and urinary N-terminal pro-B-type natriuretic peptide in patients with acute heart failure. Congest Heart Fail 16: 214–220, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, Walukiewicz A, Gugala M, Krivoshei L, Marti N, Moreno Weidmann Z, Hillinger P, Puelacher C, Rentsch K, Honegger U, Schumacher C, Zurbriggen F, Freese M, Stelzig C, Campodarve I, Bassetti S, Osswald S, Mueller C: Optimal cutoff levels of more sensitive cardiac troponin assays for the early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation 131: 2041–2050, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Don BR, Rodriguez RA, Humphreys MH: Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis 37: 564–572, 2001 [PubMed] [Google Scholar]
- 18.Honda H, Qureshi AR, Heimbürger O, Barany P, Wang K, Pecoits-Filho R, Stenvinkel P, Lindholm B: Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am J Kidney Dis 47: 139–148, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K: Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next? Semin Dial 18: 365–369, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Stenvinkel P, Heimbürger O, Paultre F, Diczfalusy U, Wang T, Berglund L, Jogestrand T: Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 55: 1899–1911, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Kao WH, Berthier-Schaad Y, Plantinga L, Fink N, Smith MW, Coresh J: C-reactive protein haplotype predicts serum C-reactive protein levels but not cardiovascular disease risk in a dialysis cohort. Am J Kidney Dis 49: 118–126, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Zoccali C, Tripepi G, Mallamaci F: Dissecting inflammation in ESRD: Do cytokines and C-reactive protein have a complementary prognostic value for mortality in dialysis patients? J Am Soc Nephrol 17[Suppl 3]: S169–S173, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Jia T, Gama Axelsson T, Heimbürger O, Bárány P, Lindholm B, Stenvinkel P, Qureshi AR: IGF-1 and survival in ESRD. Clin J Am Soc Nephrol 9: 120–127, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi AR, Alvestrand A, Danielsson A, Divino-Filho JC, Gutierrez A, Lindholm B, Bergström J: Factors predicting malnutrition in hemodialysis patients: A cross-sectional study. Kidney Int 53: 773–782, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Pecoits-Filho R, Bárány P, Lindholm B, Heimbürger O, Stenvinkel P: Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol Dial Transplant 17: 1684–1688, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Suliman ME, Qureshi AR, Heimbürger O, Lindholm B, Stenvinkel P: Soluble adhesion molecules in end-stage renal disease: A predictor of outcome. Nephrol Dial Transplant 21: 1603–1610, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Stenvinkel P, Lindholm B, Heimbürger M, Heimbürger O: Elevated serum levels of soluble adhesion molecules predict death in pre-dialysis patients: Association with malnutrition, inflammation, and cardiovascular disease. Nephrol Dial Transplant 15: 1624–1630, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Löwbeer C, Stenvinkel P, Pecoits-Filho R, Heimbürger O, Lindholm B, Gustafsson SA, Seeberger A: Elevated cardiac troponin T in predialysis patients is associated with inflammation and predicts mortality. J Intern Med 253: 153–160, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH: Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236–244, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molnar MZ, Streja E, Kovesdy CP, Budoff MJ, Nissenson AR, Krishnan M, Anker SD, Norris KC, Fonarow GC, Kalantar-Zadeh K: High platelet count as a link between renal cachexia and cardiovascular mortality in end-stage renal disease patients. Am J Clin Nutr 94: 945–954, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto T, Qureshi AR, Anderstam B, Heimbürger O, Bárány P, Lindholm B, Stenvinkel P, Axelsson J: Clinical importance of an elevated circulating chemerin level in incident dialysis patients. Nephrol Dial Transplant 25: 4017–4023, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Gama-Axelsson T, Heimbürger O, Stenvinkel P, Bárány P, Lindholm B, Qureshi AR: Serum albumin as predictor of nutritional status in patients with ESRD. Clin J Am Soc Nephrol 7: 1446–1453, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuan J, Guo Q, Qureshi AR, Anderstam B, Eriksson M, Heimbürger O, Bárány P, Stenvinkel P, Lindholm B: Circulating vascular endothelial growth factor (VEGF) and its soluble receptor 1 (sVEGFR-1) are associated with inflammation and mortality in incident dialysis patients. Nephrol Dial Transplant 28: 2356–2363, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Grubb A, Horio M, Hansson LO, Björk J, Nyman U, Flodin M, Larsson A, Bökenkamp A, Yasuda Y, Blufpand H, Lindström V, Zegers I, Althaus H, Blirup-Jensen S, Itoh Y, Sjöström P, Nordin G, Christensson A, Klima H, Sunde K, Hjort-Christensen P, Armbruster D, Ferrero C: Generation of a new cystatin C-based estimating equation for glomerular filtration rate by use of 7 assays standardized to the international calibrator. Clin Chem 60: 974–986, 2014 [DOI] [PubMed] [Google Scholar]
- 36.Spoto B, Mattace-Raso F, Sijbrands E, Leonardis D, Testa A, Pisano A, Pizzini P, Cutrupi S, Parlongo RM, D’Arrigo G, Tripepi G, Mallamaci F, Zoccali C: Association of IL-6 and a functional polymorphism in the IL-6 gene with cardiovascular events in patients with CKD. Clin J Am Soc Nephrol 10: 232–240, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanahan CM: Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol 9: 661–670, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, Heimbürger O, Cederholm T, Girndt M: IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int 67: 1216–1233, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Tofik R, Swärd P, Ekelund U, Struglics A, Torffvit O, Rippe B, Bakoush O: Plasma pro-inflammatory cytokines, IgM-uria and cardiovascular events in patients with chest pain: A comparative study. Scand J Clin Lab Invest 75: 638–645, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Carlsson AC, Carrero JJ, Stenvinkel P, Bottai M, Barany P, Larsson A, Ärnlöv J: High levels of soluble tumor necrosis factor receptors 1 and 2 and their association with mortality in patients undergoing hemodialysis. Cardiorenal Med 5: 89–95, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meuwese CL, Snaedal S, Halbesma N, Stenvinkel P, Dekker FW, Qureshi AR, Barany P, Heimburger O, Lindholm B, Krediet RT, Boeschoten EW, Carrero JJ: Trimestral variations of C-reactive protein, interleukin-6 and tumour necrosis factor-α are similarly associated with survival in haemodialysis patients. Nephrol Dial Transplant 26: 1313–1318, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K: Inflammatory marker mania in chronic kidney disease: Pentraxins at the crossroad of universal soldiers of inflammation. Clin J Am Soc Nephrol 2: 872–875, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Carrero JJ, Park SH, Axelsson J, Lindholm B, Stenvinkel P: Cytokines, atherogenesis, and hypercatabolism in chronic kidney disease: A dreadful triad. Semin Dial 22: 381–386, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Pepys MB: C-reactive protein fifty years on. Lancet 1: 653–657, 1981 [DOI] [PubMed] [Google Scholar]
- 45.Hickman PE, McGill D, Potter JM, Koerbin G, Apple FS, Talaulikar G: Multiple biomarkers including cardiac troponins T and I measured by high-sensitivity assays, as predictors of long-term mortality in patients with chronic renal failure who underwent dialysis. Am J Cardiol 115: 1601–1606, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Walsh M, Wang CY, Ong GS, Tan AS, Mansor M, Shariffuddin II, Hashim NH, Lai HY, Undok AW, Kolandaivel UN, Vajiravelu V, Garg AX, Cuerden M, Guyatt G, Thabane L, Mooney J, Lee V, Chow C, Devereaux PJ: Kidney function alters the relationship between postoperative troponin T level and death. J Am Soc Nephrol 26: 2571–2577, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckardt KU, Gillespie IA, Kronenberg F, Richards S, Stenvinkel P, Anker SD, Wheeler DC, de Francisco AL, Marcelli D, Froissart M, Floege J ARO Steering Committee : High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int 88: 1117–1125, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snaedal S, Heimbürger O, Qureshi AR, Danielsson A, Wikström B, Fellström B, Fehrman-Ekholm I, Carrero JJ, Alvestrand A, Stenvinkel P, Bárány P: Comorbidity and acute clinical events as determinants of C-reactive protein variation in hemodialysis patients: Implications for patient survival. Am J Kidney Dis 53: 1024–1033, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Meuwese CL, Carrero JJ, Stenvinkel P: Recent insights in inflammation-associated wasting in patients with chronic kidney disease. Contrib Nephrol 171: 120–126, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.