Abstract
Background and objectives
Atrial fibrillation frequently complicates CKD and is associated with adverse outcomes. Progression to ESRD is a major complication of CKD, but the link with atrial fibrillation has not been fully delineated. In this study, we examined the association of incident atrial fibrillation with the risk of ESRD in patients with CKD.
Design, setting, participants, & measurements
We studied participants in the prospective Chronic Renal Insufficiency Cohort Study without atrial fibrillation at entry. Incident atrial fibrillation was identified by study visit ECGs, self-report, and hospital discharge diagnostic codes, with confirmation by physician adjudication. ESRD through 2012 was ascertained by participant self-report, medical records, and linkage to the US Renal Data System. Data on potential confounders were obtained from self-report, study visits, and laboratory tests. Marginal structural models were used to study the potential association of incident atrial fibrillation with risk of ESRD after adjustment for time-dependent confounding.
Results
Among 3091 participants, 172 (5.6%) developed incident atrial fibrillation during follow-up. During mean follow-up of 5.9 years, 43 patients had ESRD that occurred after development of incident atrial fibrillation (11.8/100 person-years) compared with 581 patients without incident atrial fibrillation (3.4/100 person-years). In marginal structural models with inverse probability weighting, incident atrial fibrillation was associated with a substantially higher rate of ESRD (hazard ratio, 3.2; 95% confidence interval, 1.9 to 5.2). This association was consistent across important subgroups by age, sex, race, diabetes status, and baseline eGFR.
Conclusions
Incident atrial fibrillation was associated with higher risk of developing ESRD in CKD. Additional study is needed to identify potentially modifiable pathways through which atrial fibrillation was associated with a higher risk of progression to ESRD. More aggressive monitoring and treatment of patients with CKD and atrial fibrillation may improve outcomes in this high-risk population.
Keywords: chronic kidney disease; cardiovascular disease; renal progression; Atrial Fibrillation; diabetes mellitus; Electrocardiography; Humans; Kidney Failure, Chronic; Prospective Studies; Risk
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide (1). Patients with CKD comprise nearly 14% of the population (2) and have even greater burden of AF. The incidence of AF is estimated to be two- to threefold higher (3) in patients with CKD compared with the general population. Moreover, AF is associated with poor outcomes, such as greater risks of ischemic stroke, myocardial infarction, and death (4–8).
Although it is generally accepted that CKD is associated with a higher risk of developing AF, few studies have evaluated the potential relationship between AF and risk of poor kidney–specific outcomes (9). One study of Japanese participants found that prevalent AF at entry was associated with a nearly twofold higher risk of developing incident CKD or proteinuria (10). Among a high–risk CKD clinical practice population, we found that incident AF was associated with a 67% (95% confidence interval 1.46 to 1.91) greater relative risk of ESRD among patients enrolled in a large, integrated health care delivery system (11). Limitations of this prior work included reliance on clinically obtained measures of kidney function and inability to account systematically for proteinuria, a potentially important confounder (11).
Given the growing population with CKD, a better understanding of the long-term effect of AF on the risk of adverse kidney outcomes in patients with CKD would have potentially important implications for the management of this high-risk group of patients by internists, cardiologists, and nephrologists. Thus, to address limitations of prior studies, we examined the association of incident AF with the risk of developing ESRD among a large, diverse cohort of participants in the largest, multicenter prospective cohort study of adults with CKD.
Materials and Methods
Study Population
We studied participants with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study. In total, 3939 adults were enrolled into the CRIC Study between June of 2003 and August of 2008 at seven clinical centers across the United States (Ann Arbor/Detroit, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland/San Francisco, CA). Men and women were eligible for the study if they were between 21 and 74 years of age and met the following age–specific eGFR criteria: 20–70 ml/min per 1.73 m2 for age 21–44 years old, 20–60 ml/min per 1.73 m2 for age 45–64 years old, and 20–50 ml/min per 1.73 m2 for age 65–74 years old. Exclusion criteria included severe (New York Heart Association class 3/4) heart failure and polycystic kidney disease among others previously described (12). Details on study design and baseline characteristics of the participants were previously published (12,13). Informed consent was obtained from all study participants, and local institutional review boards approved this study.
In total, 3091 participants were included in this analysis after excluding persons with prevalent AF at cohort entry (n=848), which was determined by self-report or presence of AF on 12-lead electrocardiogram (ECG) at cohort entry (14). All study participants provided written informed consent, and the study protocol was approved by institutional review boards at each of the participating sites.
Predictor Variable
Incident AF was the main predictor of interest. Incident AF was determined by identification of hospitalizations involving AF during study follow-up. Participants were asked twice yearly if they were hospitalized, and electronic health records from selected hospitals or health care systems were additionally queried for qualifying encounters. Diagnostic codes for AF and other arrhythmias prompted retrieval of medical records and centralized adjudicated review for the presence of AF. At least two study physicians reviewed all possible AF events by manual review of relevant medical records and ECGs. All discordances were discussed by the two reviewers and resolved. AF was confirmed when both reviewers agreed on the diagnosis of AF. Incident AF that developed after the onset of ESRD was excluded.
Outcomes
The primary study outcome was ESRD, defined as receipt of chronic dialysis or kidney transplant, from study entry through March 31, 2012. ESRD was identified through participant self-report, medical records review, and data from the US Renal Data System. For this analysis, patients were censored at death, loss to follow-up, or end of administrative follow-up in March of 2012. Deaths were identified from report from next of kin, retrieval of death certificates or obituaries, review of hospital or outpatient records, and searching Social Security Death vital status and state death certificate files, if available.
Covariates
At the baseline study visit, participants provided information on their sociodemographic characteristics, medical history, medication usage, and lifestyle behaviors. For this analysis, race/ethnicity was categorized as non-Hispanic white or non-Hispanic black. Anthropometric measurements and BP were assessed using standard protocols (15). Body mass index was derived as weight in kilograms divided by height in meters squared. Serum creatinine concentration was measured using an enzymatic method on an Ortho Vitros 950 at the CRIC Central Laboratory and standardized to isotope dilution mass spectrometry–traceable values (16–18). Additional assays measured serum cystatin C, serum phosphorus, 24-hour urine total protein, glucose, LDL cholesterol (mathematically derived), HDL cholesterol, fibroblast growth factor 23, and total parathyroid hormone. The aforementioned assays were performed at the central laboratory with the exception of parathyroid hormone (measured at Scantibodies Laboratory Inc.) and hemoglobin (locally measured). Diabetes mellitus was defined as a fasting glucose >126 mg/dl, a nonfasting glucose >200 mg/dl, or use of insulin or other antidiabetic medication. eGFR was calculated from serum creatinine and cystatin C using a CRIC Study equation (18). Transthoracic echocardiograms were performed 1 year after enrollment; they provided data on left ventricular ejection fraction and left ventricular mass index (19–22) and were quantified centrally by a highly trained Registered Diagnostic Cardiac Sonographer.
Statistical Analyses
All analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC). We compared baseline characteristics of participants who did versus did not develop incident AF using t tests or chi-squared methods as appropriate. Crude rates of ESRD were calculated per 100 person-years for those with and without incident AF.
We performed multivariable Cox proportional hazards regression to examine the association between development of incident AF during follow-up and risk of ESRD. Follow-up for each subject started on the index date and continued until loss to follow-up, death, end of study period, or occurrence of the outcome event (i.e., ESRD). AF was a time-updated exposure. Thus, if a patient developed AF during follow-up, they contributed time to the no AF exposure group before being diagnosed with incident AF. After being diagnosed with AF, they would contribute person-time to the incident AF exposure group. Baseline variables included in models were sociodemographics, clinical center, tobacco use, history of heart failure, history of coronary heart disease, history of hypertension, history of diabetes, systolic BP, body mass index, hemoglobin level, diuretic use, and angiotensin converting enzymes inhibitor/angiotensin receptor blocker use. Quadratic spline terms for continuous urine proteinuria and eGFR were included to account for nonlinear relationships.
Given the concern for time-dependent confounding and our interest in a potential causal relation between incident AF and ESRD, we performed marginal structural models (MSMs), which apply inverse probability weighting in a discrete time failure model (23). A substantial body of work has emerged showing the usefulness of statistical tools, like MSM, in the areas of HIV and CKD (24,25). Briefly, MSM is a two-step approach, wherein models were first fit to predict the probability of AF during follow-up (i.e., the exposure of interest) and the probability of noncensoring, and second, inverse probability–weighted models were fit for the outcomes. Inverse probability weighting was also used to handle censored events and missing data. In the MSM, both AF and covariates were time updated with the exception of sex, race/ethnicity, and education level (26). Additional details of the MSM method specific to this analysis are provided in Supplemental Appendix. Hazards ratios (HRs) and 95% confidence intervals (95% CIs) were reported for all models.
On the basis of a priori hypotheses about potential differences across key patient subgroups, we conducted stratified analyses by age (<60 and ≥60 years old), sex (men and women), race (white and black), diabetes status (yes or no), and eGFR level at entry (<45 and ≥45 ml/min per 1.73 m2). We also tested for potential interactions between incident AF and these covariates. Details are provided in Supplemental Appendix.
Results
Among the 3091 participants in our study, 172 developed incident AF, with the mean timing of detection of 3.79 years from cohort entry in those who developed incident AF. Participants who developed incident AF were older, more likely to be white, more likely to have lower entry eGFR, more likely to have prior cardiovascular disease, and more likely to be receiving β-blockers and diuretics at entry (Table 1). Participants who developed incident AF were also more likely to have left ventricular hypertrophy, higher left atrial diameter, and lower left ventricular ejection fraction by echocardiogram.
Table 1.
Baseline characteristics of 3091 Chronic Renal Insufficiency Cohort Study participants
| Characteristic | Incident Atrial Fibrillation, n=172 | No Incident Atrial Fibrillation, n=2919 | P Value |
|---|---|---|---|
| Age, yr, mean (SD) | 63.4 (8.0) | 57.2 (11.3) | <0.001 |
| Men, N (%) | 103 (59.9) | 1597 (54.7) | 0.20 |
| Self-reported race/ethnicity, N (%) | |||
| Non-Hispanic white | 106 (61.6) | 1196 (41.0) | <0.001 |
| Non-Hispanic black | 47 (27.3) | 1184 (40.6) | |
| Hispanic | 11 (6.4) | 417 (14.3) | |
| Other | 8 (4.7) | 122 (4.2) | |
| eGFR, ml/min per 1.73 m2, N (%) | |||
| <30 | 35 (20.3) | 563 (19.3) | <0.001 |
| 30–44 | 70 (40.7) | 942 (32.3) | |
| 45–59 | 54 (31.4) | 838 (28.7) | |
| ≥60 | 13 (7.6) | 576 (19.7) | |
| 24-h Urine protein, g/24 h, median (IQR) | 0.2 (0.1–0.6) | 0.2 (0.1–1.0) | 0.70 |
| Current smoker, N (%) | 21 (12.2) | 377 (12.9) | 0.80 |
| Current alcohol use, N (%) | 101 (58.7) | 1876 (64.3) | 0.10 |
| Total Met score from physical activity, mean (SD) | 185.4 (120.4) | 203.6 (151.9) | 0.10 |
| Diabetes mellitus, N (%) | 91 (52.9) | 1379 (47.2) | 0.20 |
| Hypertension, N (%) | 157 (91.3) | 2486 (85.2) | 0.03 |
| Coronary heart disease, N (%) | 59 (34.3) | 489 (16.8) | <0.001 |
| Stroke, N (%) | 23 (13.4) | 252 (8.6) | 0.03 |
| Heart failure, N (%) | 21 (12.2) | 162 (5.5) | <0.001 |
| Peripheral vascular disease, N (%) | 11 (6.4) | 173 (5.9) | 0.80 |
| Dyslipidemia, N (%) | 154 (89.5) | 2356 (80.7) | <0.001 |
| Medications on entry, N (%) | |||
| ACE inhibitor or ARB, N (%) | 124 (72.1) | 1961 (67.7) | 0.20 |
| Angiotensin receptor blocker, N (%) | 39 (22.7) | 735 (25.4) | 0.40 |
| β-Blocker, N (%) | 97 (56.4) | 1313 (45.3) | <0.001 |
| Calcium channel blocker, N (%) | 75 (43.6) | 1142 (39.4) | 0.30 |
| Diuretic, N (%) | 118 (68.6) | 1628 (56.2) | 0.001 |
| Statin, N (%) | 109 (63.4) | 1546 (53.4) | 0.01 |
| Aspirin, N (%) | 94 (54.7) | 1193 (41.2) | <0.001 |
| Antiplatelet agent, N (%) | 100 (58.1) | 1280 (44.2) | <0.001 |
| Systolic BP, mmHg, mean (SD) | 129.0 (21.9) | 128.4 (22.2) | 0.70 |
| Diastolic BP, mmHg, mean (SD) | 68.4 (12.0) | 72.2 (12.9) | <0.001 |
| Body mass index, kg/m2, mean (SD) | 32.8 (6.9) | 31.8 (7.7) | 0.09 |
| Hemoglobin, g/dl, mean (SD) | 12.8 (1.8) | 12.6 (1.8) | 0.30 |
| Hemoglobin A1C, mean (SD) | 6.8 (1.6) | 6.6 (1.5) | 0.06 |
| Serum phosphate, mg/dl, mean (SD) | 3.7 (0.8) | 3.7 (0.7) | 0.40 |
| Serum calcium, mg/dl, mean (SD) | 9.2 (0.5) | 9.2 (0.5) | 0.40 |
| Fibroblast growth factor 23, RU/ml, median (IQR) | 154.3 [103.0, 229.7] (434.9) | 136.4 [93.2, 222.9] (388.2) | 0.20 |
| Total parathyroid hormone, pg/ml, median (IQR) | 57.3 (40.5–88.8) | 52.0 (33.3–86.3) | 0.10 |
Values in brackets are median (interquartile range [IQR]). Met, Metabolic Equivalent Task; ACE, angiotensin converting enzymes; ARB, angiotensin receptor blocker.
During mean follow-up of 5.9 years, there were 624 patients with ESRD (43 for those with incident AF and 581 for those without incident AF). The rate of ESRD after development of incident AF was 11.8/100 person-years compared with 3.4/100 person-years among participants who did not develop incident AF.
In unadjusted Cox regression models, where incident AF was treated as a time-updated exposure, incident AF was associated with a greater than threefold greater relative rate of ESRD (HR, 3.4; 95% CI, 2.5 to 4.7). After adjustment for baseline covariates, this association remained statistically significant and strong (adjusted HR, 3.3; 95% CI, 2.4 to 4.6) (Table 2). Using MSM with inverse probability weighting that further accounted for possible time–dependent confounding, we still found that incident AF with associated with a notably higher adjusted rate of ESRD (adjusted HR, 3.2; 95% CI, 1.9 to 5.2).
Table 2.
Multivariable association of incident atrial fibrillation with risk of ESRD among participants with CKD in the Chronic Renal Insufficiency Cohort Study
| Statistical Approach | N/Rate (Per 100 person-yr) of ESRD Events | Hazard Ratio (95% Confidence Interval) of AF with ESRD |
|---|---|---|
| Cox regression model | ||
| No incident AF | 581/3.4 | Reference |
| Incident AF | 43/11.8 | 3.3 (2.4 to 4.6) |
| Marginal structural model | ||
| No incident AF | 581/3.4 | Reference |
| Incident AF | 43/11.8 | 3.2 (1.9 to 5.2) |
Adjusted for demographics, clinical site, proteinuria, eGFR, tobacco use, heart failure, coronary heart disease, hypertension, diabetes, systolic BP, body mass index, hemoglobin, diuretic use, and angiotensin converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) use. AF, atrial fibrillation.
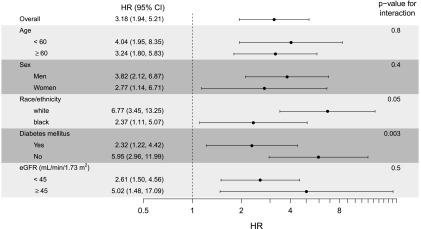
In addition, in MSMs stratified by important patient subgroups, we found a strong association of incident AF with risk of ESRD across different ranges of age, sex, race, diabetes status, and baseline eGFR category (Figure 1). However, interaction by race was marginally significant and interaction by presence of diabetes mellitus was statistically significant, with non-Hispanic whites (versus non-Hispanic blacks) and participants without diabetes mellitus (versus participants with diabetes mellitus) having greater risk of ESRD with development of incident AF (Figure 1).
Figure 1.
Multivariable association of incident atrial fibrillation with risk of ESRD among subgroups with CKD in the Chronic Renal Insufficiency Cohort Study (adjusted for demographics, clinical site, proteinuria, eGFR, tobacco use, heart failure, coronary heart disease, hypertension, diabetes, systolic BP, body mass index, hemoglobin, diuretic use, and angiotensin converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) use). The P values are for the interactions between the incident atrial fibrillation indicator and the covariates defining these subgroups. 95% CI, 95% confidence interval; HR, hazard ratio.
Discussion
Among a well characterized, large, prospective cohort of participants with CKD longitudinally followed for up to 9 years, we found that development of incident AF was associated with a greater than twofold higher rate of ESRD, independent of baseline measures of kidney function and a wide range of other possible explanatory factors. This association was robust among important subgroups by categories of age, sex, race/ethnicity, diabetes status, and baseline level of eGFR and significantly stronger among white participants and those without diabetes. These findings support that AF is strongly associated with adverse kidney–specific outcomes, which are particularly relevant in the high–risk CKD population, and add to the set of other known complications of AF (e.g., ischemic stroke).
This study extends our previous work, which also showed a strong association between incident diagnosed AF and excess risk of ESRD among patients with CKD enrolled in Kaiser Permanente Northern California, a large integrated health care delivery system (11). Interestingly, this study using a more rigorous prospective cohort design revealed a stronger association of incident AF with risk of ESRD. We found that incident AF was associated with a greater than twofold higher risk of ESRD compared with a 67% greater risk in our prior study (11). Important differences in the study populations likely explain the differences in the strength of the association: this study examined a well characterized prospective cohort of patients with CKD from multiple centers throughout the United States who had standardized annual measures of eGFR (rather than eGFR measures obtained for clinical indications) and had systematic quantification of proteinuria and other possible confounders. The findings of this study were also consistent when using multiple statistical approaches for evaluation of this association and carefully considering important issues of time-dependent confounding. Interestingly, even with careful adjustment and weighting of time–updated eGFR measures, BP, and comorbid disease, such as heart failure, incident AF more than doubled the risk of ESRD. Overall, these two complimentary studies support a growing body of evidence that incident AF may contribute to the risk of progression to ESRD among patients with CKD, independent of baseline eGFR. This is consistent with prior work that has established strong associations of other types of cardiovascular disease, such as heart failure and myocardial infarction, with adverse kidney outcomes (27,28), highlighting the complex and potentially bidirectional interactions between kidney and cardiac diseases.
We found that the observed associations of incident AF with risk of ESRD were stronger among white participants and participants without diabetes. These findings are interesting, because white patients are at significantly higher risk for developing AF compared with black patients (29,30). Our results are consistent with previous work that suggests that white patients with AF are at higher risk of death compared with black participants with AF (31). Our data do differ from some prior studies suggesting a lower incidence of AF but a higher risk of AF-related complications in blacks compared with whites. For example, in the Reasons for Geographic and Racial Differences in Stroke Study, the association of AF with myocardial infarction was significantly greater in blacks compared with in whites (5). Among prevalent patients with dialysis, chronic AF was significantly associated with higher risk of ischemic stroke, and this association was stronger in blacks compared with in whites (6). However, the differences in our findings from these other studies may be related to our focus on kidney-specific outcomes. We also noted a higher risk of ESRD with incident AF in participants without diabetes compared with in participants with diabetes. The reasons for this finding are unclear, but it is plausible that AF may have a greater contribution in nonpatients with diabetes, because they may be less likely to have other concomitant risk factors (e.g., uncontrolled hypertension or more severe heart failure) that may contribute to risk of ESRD. These findings warrant additional exploration in future studies.
Several possible mechanisms may explain how AF could contribute to greater risk of ESRD. AF contributes to decline of left ventricular systolic and diastolic function over time (32,33), which may promote progression of CKD through altered cardiac hemodynamics (33,34). Animal studies have also suggested that AF may directly and adversely affect renal hemodynamics. In a study of dogs, induction of AF caused prolonged renal vasoconstriction and decreased renal blood flow, even greater than the effects seen on cardiac output and mean arterial pressure (35). AF can also induce fibrosis within the myocardium (36), and animal studies have suggested that AF may also lead to renal fibrosis as well. In a study of pigs, AF directly led to increased renal expression of proteins involved in fibrosis, such as TGF-β (37). Other possible mechanisms may be related to inflammation, which is higher in the setting of AF and may lead to kidney dysfunction (38–42). It is also plausible that AF may be prothrombotic, leading to renal microinfarcts, similar to silent cerebral infarcts that have been noted in patients with AF (43). Given the strong associations in our study, additional understanding of mechanisms to explain our findings may help lead to targeted therapies to mitigate adverse renal outcomes.
Observational data suggest that treatment of AF may be associated with improvement in kidney function. In a small study of patients with AF undergoing catheter ablation, eGFR level increased 115 days after ablation, particularly so in those with CKD (44). In another prospective study of patients with AF undergoing ablation, kidney function improved 1 year after ablation, and the greatest improvement in kidney function was seen in those with the lowest baseline eGFR (45). Specifically, a 6-ml/min per 1.73 m2 increase in eGFR was seen in those with eGFR<59 ml/min per 1.73 m2 at baseline (45). Although these studies are intriguing, they are observational, are small in size, and study primarily patients with preserved kidney function. Additional studies of AF treatment and its effect on progression of kidney disease in patients with CKD are needed.
Our study had several notable strengths. We prospectively examined a very large, diverse sample of well characterized patients with CKD. The CRIC Study is the largest prospective CKD cohort to date, with almost 10 years of follow-up and systematic data collection. Incident AF was captured through rigorous processes, including physician adjudication of ECGs obtained during hospitalizations and presence of AF on a research protocol–driven outpatient ECG obtained annually and centrally adjudicated by the CRIC ECG Reading Center. Our primary end point was ESRD requiring dialysis or renal transplant, which was comprehensively captured. We were able to adjust for a large array of important confounders in our analyses, including proteinuria, and address the potential problem of time-dependent confounding. Our study also had some limitations. Classification of types of AF (paroxysmal, persistent, and permanent) was not available in the CRIC Study. We were not able to comprehensively capture all outpatient AF, because ambulatory ECGs performed as part of usual clinical care were unavailable. We were also unable to capture patients with undiagnosed paroxysmal AF in this study. However, if anything, this would likely have biased our results toward the null, because we are likely misclassifying some patients with AF and underestimating the burden of AF in this population. We were not able to examine interim changes of eGFR before ESRD because of only annual measurement of eGFR per the study protocol. In this observational study, we were also unable to determine the exact mechanisms explaining the association between AF and ESRD. We cannot completely rule out residual confounding, although we were able to statistically adjust for a wide range of potential explanatory factors, including measures of left atrial and ventricular disease from research-grade echocardiograms, C-reactive protein, and mineral metabolism markers. Finally, we conducted our study among research study volunteers, and therefore, our findings may not be completely generalizable to all CKD populations.
In conclusion, incident AF is associated with a greater than twofold higher rate of ESRD among patients with CKD, independent of known clinical risk factors and baseline measures of kidney function. Additional study is needed to delineate the contributing factors leading to the development of AF in the setting of CKD and potentially modifiable pathways through which AF is associated with a higher rate of progression to ESRD. Our study highlights the clinical importance of identifying AF in the growing population of adults with CKD, and more aggressive monitoring and treatment of patients with CKD and AF may be warranted to improve outcomes in this high–risk patient population.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by NIDDK grant K23DK088865 (to N.B.). Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under a cooperative agreement from the National Institute of Diabetes and Digestive and Kidney Diseases (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003, Johns Hopkins University grant UL1 TR-000424, University of Maryland grant GCRC M01 RR-16500, the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH Roadmap for Medical Research, Michigan Institute for Clinical and Health Research grant UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Awards grant UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology grant P30GM103337, and Kaiser Permanente NIH/National Center for Research Resources University of California, San Francisco Clinical Translational Science Institute grant UL1 RR-024131.
The CRIC Study investigators are Lawrence Appel, Johns Hopkins University, Department of Medicine, Baltimore, MD; Harold Feldman, University of Pennsylvania, Departments of Medicine and Epidemiology, Philadelphia, PA; Alan S. Go, Kaiser Permanente Division of Research, Oakland, CA; Jiang He, Tulane University, Department of Epidemiology, New Orleans, LA; John Kusek, NIDDK, National Institutes of Health, Bethesda, MD; James Lash, University of Illinois, Chicago, Department of Medicine, Chicago, IL; Akinlolu Ojo, University of Michigan, Departments of Medicine and Epidemiology, Ann Arbor, MI; Mahboob Rahman, Case Western Reserve University, Department of Medicine, Cleveland, OH; Raymond Townsend, University of Pennsylvania, Department of Medicine, Philadelphia, PA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10921015/-/DCSupplemental.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr., Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ: Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 129: 837–847, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alonso A, Lopez FL, Matsushita K, Loehr LR, Agarwal SK, Chen LY, Soliman EZ, Astor BC, Coresh J: Chronic kidney disease is associated with the incidence of atrial fibrillation: The Atherosclerosis Risk in Communities (ARIC) study. Circulation 123: 2946–2953, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Fan D, Hsu CY, Ordonez JD, Go AS: Incident atrial fibrillation and risk of death in adults with chronic kidney disease. J Am Heart Assoc 3: e001303, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman EZ, Safford MM, Muntner P, Khodneva Y, Dawood FZ, Zakai NA, Thacker EL, Judd S, Howard VJ, Howard G, Herrington DM, Cushman M: Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med 174: 107–114, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis M, Rigler SK, Mukhopadhyay P, Spertus JA, Zhou X, Hou Q, Shireman TI: Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 23: 112–118, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wetmore JB, Ellerbeck EF, Mahnken JD, Phadnis MA, Rigler SK, Spertus JA, Zhou X, Mukhopadhyay P, Shireman TI: Stroke and the “stroke belt” in dialysis: Contribution of patient characteristics to ischemic stroke rate and its geographic variation. J Am Soc Nephrol 24: 2053–2061, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wizemann V, Tong L, Satayathum S, Disney A, Akiba T, Fissell RB, Kerr PG, Young EW, Robinson BM: Atrial fibrillation in hemodialysis patients: Clinical features and associations with anticoagulant therapy. Kidney Int 77: 1098–1106, 2010 [DOI] [PubMed] [Google Scholar]
- 9.O’Neal WT, Tanner RM, Efird JT, Baber U, Alonso A, Howard VJ, Howard G, Muntner P, Soliman EZ: Atrial fibrillation and incident end-stage renal disease: The REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Int J Cardiol 185: 219–223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y: Close bidirectional relationship between chronic kidney disease and atrial fibrillation: The Niigata preventive medicine study. Am Heart J 158: 629–636, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS: Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation 127: 569–574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, Ojo A, Teal VL, Jensvold NG, Robinson NL, Dries DL, Bazzano L, Mohler ER, Wright JT, Feldman HI Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic kidney disease and prevalent atrial fibrillation: The Chronic Renal Insufficiency Cohort (CRIC). Am Heart J 159: 1102–1107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics (NCHS) : National Health and Nutrition Examination Survey Anthropometry Procedures Manual, Hyattsville, MD, Centers for Disease Control and Prevention, 2000 [Google Scholar]
- 16.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL Chronic Renal Insufficiency Cohort (CRIC) Study Group : Variability of creatinine measurements in clinical laboratories: Results from the CRIC study. Am J Nephrol 31: 426–434, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI CRIC Study Investigators : Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group American Society of Echocardiography’s Guidelines and Standards Committee European Association of Echocardiography : Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440–1463, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal NKM, Delafontaine P, Dries D, Foster E, Gadegbeku CA, Go AS, Hamm LL, Kusek J, Ojo A, Rahman M, Tao K, Wright JT, Xie D, Hsu CY: Evolution of left ventricular structure from CKD through ESRD: The CRIC Study. Clin J Am Soc Nephrol 8: 355–362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I: Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 2: 358–367, 1989 [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Hernán MA, Brumback B: Marginal structural models and causal inference in epidemiology. Epidemiology 11: 550–560, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Hernán MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Brunelli SM, Joffe MM, Israni RK, Yang W, Fishbane S, Berns JS, Feldman HI: History-adjusted marginal structural analysis of the association between hemoglobin variability and mortality among chronic hemodialysis patients. Clin J Am Soc Nephrol 3: 777–782, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cole SR, Hernán MA: Constructing inverse probability weights for marginal structural models. Am J Epidemiol 168: 656–664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Investigators : Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 113: 671–678, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Hillege HL, van Gilst WH, van Veldhuisen DJ, Navis G, Grobbee DE, de Graeff PA, de Zeeuw D CATS Randomized Trial : Accelerated decline and prognostic impact of renal function after myocardial infarction and the benefits of ACE inhibition: The CATS randomized trial. Eur Heart J 24: 412–420, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Dewland TA, Olgin JE, Vittinghoff E, Marcus GM: Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation 128: 2470–2477, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Marcus GM, Alonso A, Peralta CA, Lettre G, Vittinghoff E, Lubitz SA, Fox ER, Levitzky YS, Mehra R, Kerr KF, Deo R, Sotoodehnia N, Akylbekova M, Ellinor PT, Paltoo DN, Soliman EZ, Benjamin EJ, Heckbert SR Candidate-Gene Association Resource (CARe) Study : European ancestry as a risk factor for atrial fibrillation in African Americans. Circulation 122: 2009–2015, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wattigney WA, Mensah GA, Croft JB: Increased atrial fibrillation mortality: United States, 1980-1998. Am J Epidemiol 155: 819–826, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Grogan M, Smith HC, Gersh BJ, Wood DL: Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol 69: 1570–1573, 1992 [DOI] [PubMed] [Google Scholar]
- 33.Naito M, David D, Michelson EL, Schaffenburg M, Dreifus LS: The hemodynamic consequences of cardiac arrhythmias: Evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model. Am Heart J 106: 284–291, 1983 [DOI] [PubMed] [Google Scholar]
- 34.Chen SC, Su HM, Hung CC, Chang JM, Liu WC, Tsai JC, Lin MY, Hwang SJ, Chen HC: Echocardiographic parameters are independently associated with rate of renal function decline and progression to dialysis in patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 2750–2758, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katholi RE, Oparil S, Urthaler F, James TN: Mechanism of postarrhythmic renal vasoconstriction in the anesthetized dog. J Clin Invest 64: 17–31, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burstein B, Qi XY, Yeh YH, Calderone A, Nattel S: Atrial cardiomyocyte tachycardia alters cardiac fibroblast function: A novel consideration in atrial remodeling. Cardiovasc Res 76: 442–452, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Bukowska A, Lendeckel U, Krohn A, Keilhoff G, ten Have S, Neumann KH, Goette A: Atrial fibrillation down-regulates renal neutral endopeptidase expression and induces profibrotic pathways in the kidney. Europace 10: 1212–1217, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR: C-reactive protein elevation in patients with atrial arrhythmias: Inflammatory mechanisms and persistence of atrial fibrillation. Circulation 104: 2886–2891, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Hatzinikolaou-Kotsakou E, Tziakas D, Hotidis A, Stakos D, Floros D, Papanas N, Chalikias G, Maltezos E, Hatseras DI: Relation of C-reactive protein to the first onset and the recurrence rate in lone atrial fibrillation. Am J Cardiol 97: 659–661, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Dudley SC Jr., Hoch NE, McCann LA, Honeycutt C, Diamandopoulos L, Fukai T, Harrison DG, Dikalov SI, Langberg J: Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: Role of the NADPH and xanthine oxidases. Circulation 112: 1266–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Friedrichs K, Klinke A, Baldus S: Inflammatory pathways underlying atrial fibrillation. Trends Mol Med 17: 556–563, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Negi S, Sovari AA, Dudley SC Jr.: Atrial fibrillation: The emerging role of inflammation and oxidative stress. Cardiovasc Hematol Disord Drug Targets 10: 262–268, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Das RR, Seshadri S, Beiser AS, Kelly-Hayes M, Au R, Himali JJ, Kase CS, Benjamin EJ, Polak JF, O’Donnell CJ, Yoshita M, D’Agostino RB Sr., DeCarli C, Wolf PA: Prevalence and correlates of silent cerebral infarcts in the Framingham offspring study. Stroke 39: 2929–2935, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Navaravong L, Barakat M, Burgon N, Mahnkopf C, Koopmann M, Ranjan R, Kholmovski E, Marrouche N, Akoum N: Improvement in estimated glomerular filtration rate in patients with chronic kidney disease undergoing catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 26: 21–27, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Takahashi Y, Takahashi A, Kuwahara T, Okubo K, Fujino T, Takagi K, Nakashima E, Kamiishi T, Hikita H, Hirao K, Isobe M: Renal function after catheter ablation of atrial fibrillation. Circulation 124: 2380–2387, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.