Abstract
Objective
This meta-analysis aimed to compare the superiority of loop electrosurgical excision procedure (LEEP) or large loop excision of the transformation zone (LLETZ) versus cold-knife conization (CKC) in the surgical treatment of cervical intraepithelial neoplasia (CIN).
Methods
Systematic searches were performed in the MEDLINE, EMBASE, Cochrane databases, and the China National Knowledge Infrastructure Databases to identify all potential articles involving patients with CIN treated with LEEP/LLETZ or CKC published up to February 2016. Risk ratios (RRs) or weighted mean difference (MD) with a 95% confidence interval (95% CI) were calculated.
Results
Seven randomized controlled trials, one prospective cohort study, and twelve retrospective cohort studies were included in this meta-analysis. There were no significant differences following LEEP/LLETZ compared with CKC in recurrence rate (RR =1.75, 95% CI =0.99–3.11, P=0.06), positive margin rate (RR =1.45; 95% CI =0.85–2.49, P=0.17), residual disease rate (RR =1.15, 95% CI =0.73–1.81, P=0.48), secondary hemorrhage (RR =1.16, 95% CI =0.74–1.81; P=0.46), or cervical stenosis. Moreover, subgroup analyses based on randomized trials also revealed that no statistical significance was observed in the above outcomes. However, women treated with CKC had a significantly deeper cervical cone than those treated with LLETZ/LEEP (MD =−5.71, 95% CI =−7.45 to −3.96; P<0.001).
Conclusion
LEEP/LLETZ is as effective as CKC with regard to recurrence rate, positive margin rate, residual disease rate, secondary hemorrhage, and cervical stenosis for the surgical treatment of CIN. Further large-scale studies are needed to confirm our findings.
Keywords: cervical intraepithelial neoplasia, cold-knife conization, loop electrosurgical excision procedure, meta-analysis
Introduction
Cervical intraepithelial neoplasia (CIN) is a precursor lesion of cervical cancer and is classified by histology as CIN 1, CIN 2, or CIN 3. Widespread cervical screening using cytology combined with human papilloma virus testing has resulted in a considerable increase in the number of women diagnosed with CIN in recent decades.1 According to laboratory surveys from the College of American Pathologists,2 more than 1 million women are found to have CIN 1 each year, and 500,000 are diagnosed with CIN 2 and CIN 3, which are referred to as high-grade CIN. As recommended by the American Society for Colposcopy and Cervical Pathology (ASCCP) guidelines,3 patients with CIN 1 are usually monitored by continued follow-up because the regression rates are high and progression to CIN 2+ is uncommon. Excisional treatment is mainly used to treat CIN 2/3, which might progress to invasive cervical cancer if left untreated. The management of CIN has been a public health burden in many parts of the world.
Currently, the two main excisional strategies for CIN treatment are loop electrosurgical excision procedure (LEEP) or large loop excision of the transformation zone (LLETZ) and cold-knife conization (CKC), which offers deep excision of the cervical transformation zone with minimal damage. CKC has been the traditional procedure for CIN and is typically performed in a hospital setting under general or local anesthesia. First described in 1989 by Prendiville,4 LEEP has been the most commonly used method for CIN and has several advantages, including shorter operative time, ease of performance, and low cost.5
Recent years have seen an increase in studies reporting CIN treatments with success rates exceeding 90%.6–9 However, these studies are inconsistent regarding the therapeutic efficacy and complications associated with the two procedures, and the 2012 ASCCP guidelines3 do not make any recommendations indicating CKC or LEEP as the optimal therapy option. Although two reviews10,11 have been published previously, a more comprehensive meta-analysis focusing on treatment failures or operative morbidity and other previously unanalyzed factors is needed. The objective of this meta-analysis is to evaluate the superiority of LLETZ/LEEP versus CKC in the surgical treatment of CIN.
Materials and methods
Search strategy
Systematic searches were performed using the MEDLINE, EMBASE, Cochrane databases, and the China National Knowledge Infrastructure Databases (CNKI) to identify all articles published up to February 2016 involving patients with CIN treated with CKC or LEEP. The searches were restricted to English or Chinese language and included only human studies. The following terms were used to identify studies: “cervical intraepithelial neoplasia”, or “cervical dysplasia”, “large loop excision of the transformation zone” or “loop electrosurgical excisional procedure” or “cold knife conization”. Because of the lack of details regarding research methods and results, abstracts and unpublished works were not included. Searches of the title and abstract of each article were independently conducted by YMJ and LL to determine potentially relevant studies.
Study selection and data extraction
Studies comparing the efficacy of LEEP and CKC to patients with CIN were included. Studies in which more than one treatment procedure was used but the outcomes for each treatment procedure were not reported separately were excluded. We also excluded case reports and studies undertaken during pregnancy. The primary outcome included the rates of residual disease, recurrent disease, positive margins, secondary hemorrhage, cervical stenosis at follow-up, cone depth, and pregnancy outcomes. To determine the validity of the studies, a modified Jadad scale was used to assess the quality of the included randomized studies.12 For nonrandomized studies, the Newcastle–Ottawa score was determined.13 The data were independently extracted from each included study by YMJ and LL, and any disagreements were resolved by consensus with a third review author (CXC) as necessary.
Data synthesis
We extracted data from the experimental and the control group for every observed outcome. Relative risks (RRs) or weighted mean difference (MD) with 95% confidence intervals (CIs) were calculated with Revman 5.3 software (Cochrane Informatics & Knowledge Management Department, Copenhagen, Denmark), and heterogeneity was quantified using the I2 statistic.14 If heterogeneity is accepted at I2<50%, a fixed-effects model was used for the meta-analysis. Otherwise, a random effects model was used. Statistical significance was defined as a P-value less than 0.05. Sensitivity analyses based on randomized or nonrandomized trials were performed.
Results
Study identification and selection
We reviewed 112 potentially relevant eligible studies. Based on the inclusion criteria, seven randomized controlled studies, one prospective cohort study, and twelve retrospective cohort studies were included (Figure 1). The vast majority of the participants had CIN 2–3, although some patients with CIN 1 were included because the ASCCP consensus guidelines were not updated until 2006 with the suggestion that CIN 1 could be managed conservatively in adults.15 The follow-up period ranged from 3 months to 23 years. The mean age ranged from 27.3 to 43.8 years. Table 1 lists the main characteristics of the 20 included studies involving 5,709 patients.5–9,16–30 Table 1 shows the Newcastle–Ottawa scores and the modified Jadad scale for the quality assessment of the nonrandomized studies and randomized studies. The quality was not high for any of the studies.
Figure 1.
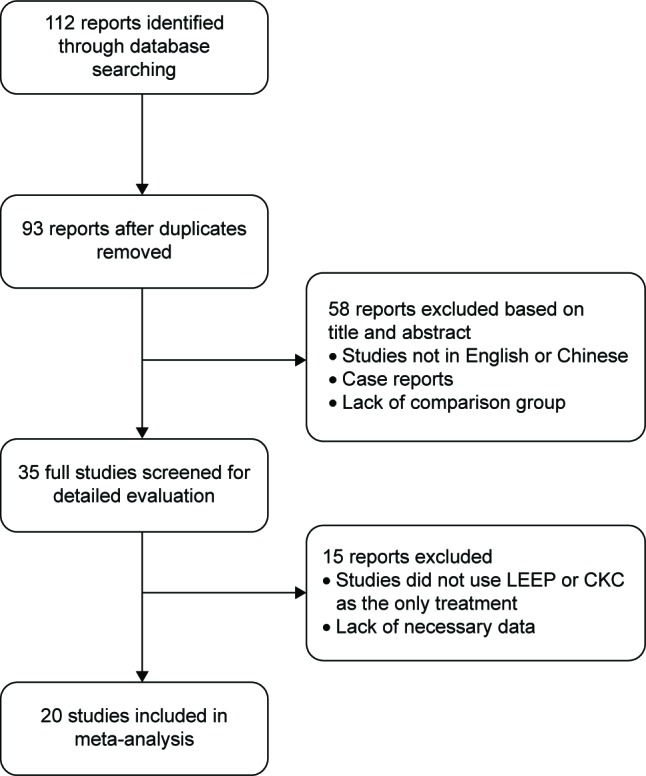
Study selection and exclusion process.
Abbreviations: LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization.
Table 1.
Characteristics of the included studies
| Source | Intervention
|
Histology | Study design | Study period | Follow-up period | Outcomes | Quality scorea | |
|---|---|---|---|---|---|---|---|---|
| LEEP | CKC | |||||||
| Giacalone et al5 | 38 | 28 | CIN 2–3 | RCT | 1997–1998 | 3 months | Residual disease, secondary hemorrhage | 4 |
| Mathevet et al6 | 36 | 37 | CIN 1–3 | RCT | 1990–1992 | 6 months | Residual disease, secondary hemorrhage, cervical stenosis | 5 |
| Duggan et al7 | 89 | 85 | CIN 1–3 | RCT | 1992–1994 | 12 months | Recurrence, positive margin, residual disease, secondary hemorrhage, cervical stenosis | 5 |
| Takač and Gorišek8 | 120 | 120 | CIN 1–3 | RCT | 1993–1996 | 3 months | Secondary hemorrhage | 3 |
| Bozanovic et al9 | 72 | 100 | CIN 1–3 | Retrospective study | NA | Over 2 years | Positive margin, secondary hemorrhage, cervical stenosis | 7 |
| Mathevet et al16 | 29 | 28 | CIN 2–3 | RCT | NA | 38–118 months | Recurrence, cervical stenosis, cesarean section, miscarriage rate, preterm delivery | 5 |
| Gonzalez-Bosquet et al17 | 58 | 25 | CIN 1–3 | Prospective multicenter project | 1991–1994 | NA | Recurrence | 9 |
| Zeng et al18 | 74 | 869 | NA | Retrospective study | 2002–2007 | 12–78 months | Recurrence, secondary hemorrhage | 7 |
| Bornstein et al19 | 52 | 22 | CIN 2–3 | Retrospective study | 1983–1993 | NA | Cone size, recurrence, positive margin, cervical stenosis | 8 |
| Serati et al20 | 214 | 68 | CIN 1–3 | Retrospective cohort study | 1999–2009 | 6–100 months | Recurrence | 6 |
| Murta et al21 | 101 | 142 | CIN 1–3 | Retrospective study | 1981–2003 | ≥16 months | Recurrence | 8 |
| Chen et al22 | 453 | 660 | NA | Retrospective cohort study | 2006–2009 | 8 months to 8 years | Positive margin | 8 |
| Grimm et al23 | 412 | 392 | CIN 1–3 | Retrospective multicenter study | 2004–2009 | NA | Positive margin | 7 |
| Shin et al24 | 79 | 39 | CIN 1–3 | Retrospective study | 2005–2006 | NA | Positive margin | 7 |
| Miroshnichenko et al25 | 96 | 61 | NA | Retrospective study | 2003–2007 | NA | Positive margin | 7 |
| Panna and Luanratanakorn26 | 269 | 194 | NA | Retrospective study | 2002–2007 | NA | Positive margin | 9 |
| Huang and Hwang27 | 73 | 43 | NA | Retrospective study | 1992–1997 | NA | Positive margin, residual disease, secondary hemorrhage | 9 |
| Liu et al28 | 124 | 120 | CIN 3 | RCT | 2006–2009 | NA | Cesarean delivery, preterm delivery, low birth weight, miscarriage rate | 3 |
| Michelin et al29 | 95 | 102 | CIN 1–3 | Retrospective study | 1981–2004 | 1–23 years | Preterm delivery, low birth weight, miscarriage rate | 9 |
| Girardi et al30 | 38 | 52 | CIN 1–3 | RCT | NA | 10 months | Cone depth, secondary hemorrhage | 3 |
Notes:
Quality assessment: the modified Jadad scale was used for RCTs, and the Newcastle–Ottawa score was used for nonrandomized studies.
Abbreviations: LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; CIN, Cervical intraepithelial neoplasia; RCT, randomized controlled trial; NA, not available.
Recurrence rate
Seven studies, including two randomized controlled trials (RCTs)6,7 and five non-RCTs,17–21 reported recurrence rates, and the results of the individual studies varied. A meta-analysis based on all these seven studies revealed that patients in CKC group had slightly lower recurrence rate than those in LLETZ/LEEP group (RR =1.75, 95% CI =0.99–3.11, P=0.06; Figure 2). Moreover, subgroup analyses based on RCTs or non-RCTs also showed similar results (Figure 2).
Figure 2.
Comparison of LLETZ/LEEP and CKC in recurrence rate.
Abbreviations: LLETZ, large loop excision of the transformation zone; LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel test.
Positive margin rate
One RCT7 and eight non-RCTs9,19,22–26 described positive margin rate. The prevalence of positive margins was 22% (343/1,595) after LLETZ/LEEP and 13% (200/1,596) after CKC. Pooled results exhibited no statistical significance based on all nine studies (RR =1.45; 95% CI =0.85–2.49, P=0.17) (Figure 3).
Figure 3.
Comparison of LLETZ/LEEP and CKC in positive margin.
Abbreviations: LLETZ, large loop excision of the transformation zone; LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel test.
Residual disease rate
Three RCTs5–7 and one non-RCT27 described the rate of residual disease. Results from individual studies showed no differences between LLETZ/LEEP and CKC group. Pooled results of all four studies (RR =1.15, 95% CI =0.73–1.81, P=0.48) or all three RCTs confirmed that there was no evidence of significant differences in residual disease rate (Figure 4).
Figure 4.
Comparison of LLETZ/LEEP and CKC in residual disease.
Abbreviations: LLETZ, large loop excision of the transformation zone; LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel test.
Pregnancy outcomes
The differences in pregnancy outcomes between LLETZ/LEEP and CKC were evaluated in two RCTs6,28 and one non-RCT29 with small sample size. Michelin et al29 reported that miscarriages and preterm pregnancies were more frequent in CKC cases versus LEEP: 26% and 5.2%, and 23% and 5.5%, respectively. Liu et al28 concluded that the rates of preterm premature rupture of membranes (P=0.03), preterm delivery (P=0.04) and low-birth-weight infants (<2,500 g) (P=0.04) were higher in the CKC group than in the LEEP group, but there were no differences in the mean birth weight, cesarean delivery, labor induction, or neonatal intensive care unit admission. Mathevet et al6 reported that there was no major difference in obstetrical outcomes between CKC and LLETZ/LEEP techniques.
Secondary hemorrhage
The results regarding secondary hemorrhage were reported by five RCTs5–8,30 and three non-RCTs.9,18,27 The results of the included individual studies did not differ significantly. After the pooled meta-analysis of the eight studies, the RR for secondary hemorrhage was not different between LLETZ/LEEP and CKC groups based on all eight studies (RR =1.16, 95% CI =0.74–1.81; P=0.46) (Figure 5).
Figure 5.
Comparison of LLETZ/LEEP and CKC in secondary hemorrhage.
Abbreviations: LLETZ, large loop excision of the transformation zone; LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel test.
Cervical stenosis
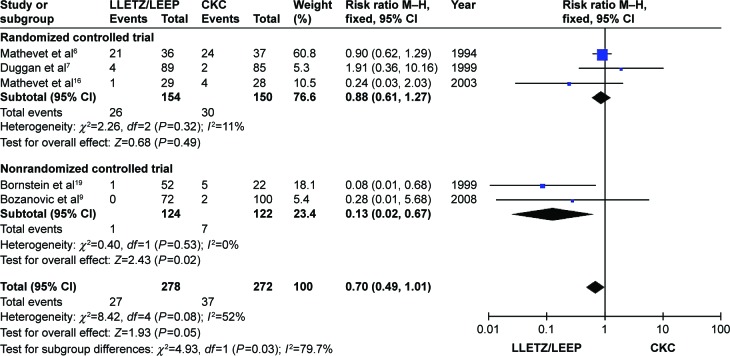
Cervical stenosis results were based on three RCTs6,7,16 and two non-RCTs.9,19 As shown in Figure 6, the pooled meta-analyses showed that the results were not significantly different across all studies or the RCT subgroup (all P>0.05). However, the opposite result was found in the non-RCT subgroup (RR =0.13, 95% CI =0.02–0.67; P=0.02).
Figure 6.
Comparison of LLETZ/LEEP and CKC in cervical stenosis.
Abbreviations: LLETZ, large loop excision of the transformation zone; LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; CI, confidence interval; df, degrees of freedom; M–H, Mantel–Haenszel test.
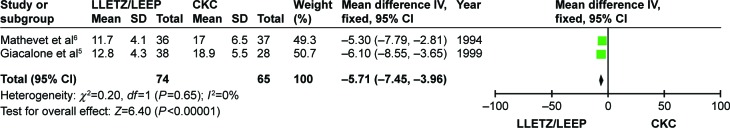
Cone depth
As shown in Figure 7, the cone depth was reported in two RCTs.5,6 The two individual studies showed that women treated with CKC had a significantly deeper cervical cone than those treated with LLETZ/LEEP. The pooled results also indicated that cone depth of CKC was statistically deeper (MD =−5.71, 95% CI =−7.45 to −3.96; P<0.001).
Figure 7.
Comparison of LLETZ/LEEP and CKC in cone depth.
Abbreviations: LLETZ, large loop excision of the transformation zone; LEEP, loop electrosurgical excision procedure; CKC, cold-knife conization; SD, standard deviation; CI, confidence interval; df, degrees of freedom; IV, independent variable.
Discussion
In this review, we found 20 studies in which LLETZ/LEEP was compared with CKC for the treatment of CIN. Differences between the included studies in terms of their setting, research protocol, patient characteristics, and efficacy were observed. No significant heterogeneity was detected across studies for any of the data evaluated, except for the rates of positive surgical margins and recurrences.
A previous review31 reported that the prevalence of residual and recurrent disease after incomplete LEEP did not differ significantly (22%; 766/3,476) compared with the results after knife-cone biopsy (27%; 445/1,661). That review did not differentiate between residual and recurrent disease. The availability of the new studies increased the statistical power and sample size and, to the best of our knowledge, enabled us to compare for the first time the differences in recurrence rates and positive margin rates. With respect to efficacy, we concluded that the recurrence rate is not significantly different between the two methods.
Incomplete excision of CIN exposes women to a high risk of high-grade cervical disease posttreatment.31 High-grade posttreatment disease occurred in 18% (597/3,335) of women who had incomplete excision compared with 3% (318/12,493) who had complete excision.31 This poses a challenge for doctors in choosing appropriate options so as to avoid residual disease resulting from incomplete excision during conization. Disease recurrence is the main problem during the 5-year follow-up period because the risk of recurrence remains elevated for 8 years or more after treatment for CIN.32 Serati et al20 found that 22.7% of women developed histologically confirmed recurrence, which does not appear to depend on the surgical technique used. Another study showed that recurrences occurred after 5–31 months in 7.1% and 11.2% of the patients who underwent LEEP and CKC, respectively, and had negative histological findings on surgical specimens.21 In this meta-analysis, no significant differences in the rates of recurrence or residual disease between the LLETZ/LEEP and CKC groups were observed. The results appear to suggest that LLETZ/LEEP is as effective as CKC in the surgical treatment of CIN and that there is no significant difference in recurrence or residual disease. However, larger sample size and longer follow-up randomized studies are necessary to further confirm these findings.
Increasing concerns have been raised regarding the rate of positive margins after the treatment of CIN, and the effects of different surgical treatments on the positive margin rate remain unclear. In previous years, there was no significant difference in the rates of positive margins reported between the two groups, but in recent years, as the case numbers increased, the results have varied. In our analysis, the rate of positive margins after LLETZ/LEEP was 22% (343/1,595); the rate of positive margins after CKC was 13% (200/1,596). These rates were both lower than previous reports; a possible explanation for these lower rates is that more importance was assigned to the problem of positive margins later. Our pooled analysis indicated that LLETZ/LEEP was associated with a higher incidence of positive margins, which might be because of the significantly deeper conization of CKC and the removal of occult endocervical lesions. The age of the woman is another important factor in determining surgical options. In one study included in this meta-analysis, Shin et al24 found that in patients aged >45 years, the LLETZ/LEEP group had significantly higher rate of nonnegative surgical margins compared with the CKC group. This result suggests that the use of LLETZ/LEEP might not be recommended if achieving complete negative margin is the only consideration, especially for older women. However, heterogeneity and bias were noted in these data and could be ascribed to the effects of small studies. It was recently reported that for adenocarcinoma in situ of the cervix, positive margins were found in 18% of the women treated with CKC versus 40% of the women treated with LLETZ/LEEP.33 Based on these results, further research should be performed to evaluate whether LLETZ/LEEP can increase the positive margin rate for cervical precancerous lesions.
In theory, a higher positive margin rate after treatment of CIN should lead to greater recurrence during the follow-up period. A positive surgical margin was a high risk factor for residual disease or relapse after conization of CIN.34 However, an inconsistent relationship appears to exist between positive surgical margins and recurrence in the two groups in our meta-analysis. Evidence-based research has reported a similar discrepancy; the rate of disease recurrence/persistence in women with positive margins who were followed up was only 9.3%.22 A possible explanation for this result is that not all of the women with positive surgical margins had residual disease, and longer follow-up periods would be beneficial.
Kyrgiou et al35 focused on pregnancy outcomes in a previous meta-analysis and found that CKC and LLETZ/LEEP were significantly associated with preterm delivery and low birth weight and that CKC was associated with higher relative risks than LLETZ/LEEP. However, this meta-analysis compared groups with or without a previous conservative intervention on the cervix and did not compare CKC and LLETZ/LEEP groups. We did not identify any meta-analyses comparing pregnancy outcomes between these two procedures. The pooled analysis in our study was not evaluated because of the small sample size. The main findings regarding pregnancy outcomes are consistent with the results of the previous meta-analysis. The available evidence suggested that differences in pregnancy outcomes, such as the rate of miscarriage and low birth weight, between CKC and LLETZ/LEEP were observed in our study. Women with a history of CKC treatment were found to have an increased risk of preterm delivery compared to those with a history of LLETZ/LEEP treatment.28,29 Few studies on the effects of LLETZ/LEEP and CKC have adequate power to detect a significant difference on subsequent pregnancy. LLETZ/LEEP is more appropriate for patients with CIN for future pregnancies compared with CKC. Moreover, we must take note of the new recommendation by tailoring excision treatment according to the type of the transformation zone to try to avoid unnecessary excision of healthy cervical tissue.36
Limitations
Several limitations of this meta-analysis should be considered. First, some of the included studies were retrospective in nature; therefore, there might have been confounders that were not recognized or controlled. However, a subgroup analysis of the data extracted from the studies revealed similar results. Second, the follow-up time, patient age, and disease degree varied among the included studies, and these differences may have affected the results. Finally, it is possible that the exclusion of some missing and unpublished data might have led to a bias in the effect.
Conclusion
The present meta-analysis showed that there was no significant difference regarding residual and recurrence rate in LLETZ/LEEP compared with CKC for treating CIN. A woman should select the surgical procedure after discussing the benefits and risks with her surgeon. Further large-scale and high-quality RCTs are needed to confirm the best procedure.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Prendiville W. The treatment of CIN: what are the risks? Cytopathology. 2009;20:145–153. doi: 10.1111/j.1365-2303.2009.00669.x. [DOI] [PubMed] [Google Scholar]
- 2.Jones BA, Davey DD. Quality management in gynecologic cytology using interlaboratory comparison. Arch Pathol Lab Med. 2000;124:672–681. doi: 10.5858/2000-124-0672-QMIGCU. [DOI] [PubMed] [Google Scholar]
- 3.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1–S27. doi: 10.1097/LGT.0b013e318287d329. [DOI] [PubMed] [Google Scholar]
- 4.Prendiville W. Appropriate use of leep interventions. J Low Genit Tract Dis. 1998;2:254. doi: 10.1097/00128360-199810000-00034. [DOI] [PubMed] [Google Scholar]
- 5.Giacalone PL, Laffargue F, Aligier N, Roger P, Combecal J, Daures JP. Randomized study comparing two techniques of conization: cold knife versus loop excision. Gynecol Oncol. 1999;75:356–360. doi: 10.1006/gyno.1999.5626. [DOI] [PubMed] [Google Scholar]
- 6.Mathevet P, Dargent D, Roy M, Beau G. A randomized prospective study comparing three techniques of conization: cold knife, laser, and LEEP. Gynecol Oncol. 1994;54:175–179. doi: 10.1006/gyno.1994.1189. [DOI] [PubMed] [Google Scholar]
- 7.Duggan BD, Felix JC, Muderspach LI, et al. Cold-knife conization versus conization by the loop electrosurgical excision procedure: a randomized, prospective study. Am J Obstet Gynecol. 1999;180(2 Pt 1):276–282. doi: 10.1016/s0002-9378(99)70200-0. [DOI] [PubMed] [Google Scholar]
- 8.Takač I, Gorišek B. Cold knife conization and loop excision for cervical intraepithelial neoplasia. Tumori. 1999;85:243–246. doi: 10.1177/030089169908500406. [DOI] [PubMed] [Google Scholar]
- 9.Bozanovic T, Ljubic A, Momcilov P, Milicevic S, Mostić T, Atanacković J. Cold-knife conization versus the loop electrosurgical excision procedure for treatment of cervical dysplasia. Eur J Gynaecol Oncol. 2008;29:83–85. [PubMed] [Google Scholar]
- 10.Martin-Hirsch PP, Paraskevaidis E, Bryant A, Dickinson HO, Keep SL. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev. 2010;16:CD001318. doi: 10.1002/14651858.CD001318.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebisch R, Rovers MM, Bosgraaf RP, et al. Evidence supporting see-and-treat management of cervical intraepithelial neoplasia: a systematic review and meta-analysis. BJOG. 2015;14:59–66. doi: 10.1111/1471-0528.13530. [DOI] [PubMed] [Google Scholar]
- 12.Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y. Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dement Geriatr Cogn Disord. 2001;12:232–236. doi: 10.1159/000051263. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 15.Apgar BS, Kittendorf AL, Bettcher CM, Wong J, Kaufman AJ. Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am Fam Physician. 2009;80:147–155. [PubMed] [Google Scholar]
- 16.Mathevet P, Chemali E, Roy M, Dargent D. Long-term outcome of a randomized study comparing three techniques of conization: cold knife, laser, and LEEP. Eur J Obstet Gynecol Reprod Biol. 2003;106:214–218. doi: 10.1016/s0301-2115(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Bosquet J, Centeno C, Bermejo B, et al. Treatment of cervical intraepithelial neoplasia in a prospective study comparing large-loop excision of the transformation zone, laser vaporization, and knife cone biopsy. J Low Genit Tract Dis. 1997;1:229–233. doi: 10.1097/00128360-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Zeng SY, Liang MR, Li LY, Wu YY. Comparison of the efficacy and complications of different surgical methods for cervical intraepithelial neoplasia. Eur J Gynaecol Oncol. 2012;33:257–260. [PubMed] [Google Scholar]
- 19.Bornstein J, Yaakov Z, Pascal B, et al. Decision-making in the colposcopy clinic – a critical analysis. Eur J Obstet Gynecol Reprod Biol. 1999;85:219–224. doi: 10.1016/s0301-2115(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 20.Serati M, Siesto G, Carollo S, et al. Risk factors for cervical intraepithelial neoplasia recurrence after conization: a 10-year study. Eur J Obstet Gynecol Reprod Biol. 2012;165:86–90. doi: 10.1016/j.ejogrb.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Murta EF, Silva AO, Silva EA. Clinical significance of a negative loop electrosurgical excision procedure, conization and hysterectomy for cervical intraepithelial neoplasia. Eur J Gynaecol Oncol. 2006;27:50–52. [PubMed] [Google Scholar]
- 22.Chen Y, Lu H, Wan X, Lv W, Xie X. Factors associated with positive margins in patients with cervical intraepithelial neoplasia grade 3 and post-conization management. Int J Gynaecol Obstet. 2009;107:107–110. doi: 10.1016/j.ijgo.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Grimm C, Brammen L, Sliutz G, et al. Impact of conization type on the resected cone volume: results of a retrospective multi-center study. Arch Gynecol Obstet. 2013;288:1081–1086. doi: 10.1007/s00404-013-2873-1. [DOI] [PubMed] [Google Scholar]
- 24.Shin JW, Rho HS, Park CY. Factors influencing the choice between cold knife conization and loop electrosurgical excisional procedure for the treatment of cervical intraepithelial neoplasia. J Obstet Gynaecol Res. 2009;35:126–130. doi: 10.1111/j.1447-0756.2008.00834.x. [DOI] [PubMed] [Google Scholar]
- 25.Miroshnichenko GG, Parva M, Holtz DO, Klemens JA, Dunton CJ. Interpretability of excisional biopsies of the cervix: cone biopsy and loop excision. J Low Genit Tract Dis. 2009;13:10–12. doi: 10.1097/LGT.0b013e31817ff940. [DOI] [PubMed] [Google Scholar]
- 26.Panna S, Luanratanakorn S. Positive margin prevalence and risk factors with cervical specimens obtained from loop electrosurgical excision procedures and cold knife conization. Asian Pac J Cancer Prev. 2009;10:637–640. [PubMed] [Google Scholar]
- 27.Huang LW, Hwang JL. A comparison between loop electrosurgical excision procedure and cold knife conization for treatment of cervical dysplasia: residual disease in a subsequent hysterectomy specimen. Gynecol Oncol. 1999;73:12–15. doi: 10.1006/gyno.1998.5300. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Qiu HF, Tang Y, Chen J, Lv J. Pregnancy outcome after the treatment of loop electrosurgical excision procedure or cold-knife conization for cervical intraepithelial neoplasia. Gynecol Obstet Invest. 2014;77:240–244. doi: 10.1159/000360538. [DOI] [PubMed] [Google Scholar]
- 29.Michelin MA, Merino LM, Franco CA, Murta EFC. Pregnancy outcome after treatment of cervical intraepithelial neoplasia by the loop electrosurgical excision procedure and cold knife conization. Clin Exp Obstet Gynecol. 2009;36:17–19. [PubMed] [Google Scholar]
- 30.Girardi F, Heydarfadai M, Koroschetz F, Pickel H, Winter R. Cold-knife conization versus loop excision: histopathologic and clinical results of a randomized trial. Gynecol Oncol. 1994;55:368–370. doi: 10.1006/gyno.1994.1308. [DOI] [PubMed] [Google Scholar]
- 31.Ghaem-Maghami S, Sagi S, Majeed G, Soutter WP. Incomplete excision of cervical intraepithelial neoplasia and risk of treatment failure: a meta-analysis. Lancet Oncol. 2007;8:985–993. doi: 10.1016/S1470-2045(07)70283-8. [DOI] [PubMed] [Google Scholar]
- 32.Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer. 2006;118:2048–2055. doi: 10.1002/ijc.21604. [DOI] [PubMed] [Google Scholar]
- 33.Baalbergen A, Molijn AC, Quint WG, Smedts F, Helmerhorst TJ. Conservative treatment seems the best choice in adenocarcinoma in situ of the cervix uteri. J Low Genit Tract Dis. 2015;4:4. doi: 10.1097/LGT.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 34.Jin J, Li L, Zhang F. Meta-analysis of high risk factors of residue or relapse of cervical intraepithelial neoplasia after conization. J Biol Regul Homeost Agents. 2015;29:451–458. [PubMed] [Google Scholar]
- 35.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367:489–498. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 36.Bornstein J, Bentley J, Bösze P, et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet Gynecol. 2012;120:166–172. doi: 10.1097/AOG.0b013e318254f90c. [DOI] [PubMed] [Google Scholar]