Abstract
During 2000 research on the pharmacology of marine chemicals involved investigators from Australia, Brazil, Canada, Egypt, France, Germany, India, Indonesia, Israel, Italy, Japan, the Netherlands, New Zealand, Phillipines, Singapore, Slovenia, South Korea, Spain, Sweden, Switzerland, United Kingdom, and the United States. This current review, a sequel to the authors’ 1998 and 1999 reviews, classifies 68 peer-reviewed articles on the basis of the reported preclinical pharmacologic properties of marine chemicals derived from a diverse group of marine animals, algae, fungi, and bacteria. Antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antituberculosis, or antiviral activity was reported for 35 marine chemicals. An additional 20 marine compounds were shown to have significant effects on the cardiovascular and nervous system, and to possess anti-inflammatory or immunosuppressant properties. Finally, 23 marine compounds were reported to act on a variety of molecular targets and thus could potentially contribute to several pharmacologic classes. Thus, as in 1998 and 1999, during 2000 pharmacologic research with marine chemicals continued to contribute potentially novel chemical leads to the ongoing global search for therapeutic agents in the treatment of multiple disease categories.
Keywords: marine pharmacology, 2000, toxicology, review, secondary metabolites
Introduction
The purpose of this article is to review the 2000 primary literature on pharmacologic and toxicologic studies of marine natural products using a format similar to the one used in our previous 1998 and 1999 reviews of the marine pharmacology peer-reviewed literature (Mayer and Lehmann, 2000; Mayer and Hamann, 2002). The 2000 review of reports on marine-derived compounds with antitumor and cytotoxic activity has been published elsewhere (Mayer and Gustafson, 2003). Consistent with our 1998 and 1999 reviews, the present review includes only those articles reporting on the bioactivity or pharmacology of marine chemicals whose structures have been established. We have used Schmitz’s chemical classification (Schmitz et al., 1993) to assign each marine compound to a major chemical class: namely, polyketides, terpenes, nitrogen-containing compounds, or polysaccharides. Publications reporting on antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antituberculosis, or antiviral properties of marine chemicals have been tabulated (Table 1) and corresponding structures are shown in Figure 1. The articles reporting on marine compounds affecting the cardiovascular and nervous system, as well as those with anti-inflammatory and immunosuppressant effects, are grouped in Table 2, and the structures are presented in Figure 2. Finally, marine compounds targeting a number of distinct cellular and molecular targets and mechanisms are shown in Table 3, and their structures are depicted in Figure 3. Publications on the biological or pharmacological activity of marine extracts or as yet structurally uncharacterized marine compounds are not included in the present review, though several reports were published during 2000 (Khudyakova et al., 2000; Matsubara et al., 2000).
Table 1.
Marine Compounds with Antibacterial, Anticoagulant, Antifungal, Antimalarial, Antiplatelet, Antituberculosis, and Antiviral Activities
| Drug class | Compound/organisma | Chemistry | Pharmacologic activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|
| Antibacterial | Acetylenic acid/sponge | Fatty acidd | Gram-positive and negative inhibition | Undetermined | JPN, NETH | Matsunaga et al., 2000 |
| Antibacterial | Discorhabdin R/sponge | Alkaloidf | Gram-positive and negative inhibition | Undetermined | AUS | Ford and Capon et al. 2000 |
| Anticoagulant | Fucoidan/alga | Sulfated Polysaccharideg | Inhibition of microvascular thrombus | No effect on P- and L-selectin | SWE, GER | Thorlacius et al., 2000 |
| Anticoagulant | Proteoglycan/alga | Polysaccharideg | Anticoagulant | Inhibition of thrombin and potentiation of antithrombin III | JPN | Matsubara, 2000 |
| Anticoagulant | Sulfated D-galactan/alga | Sulfated Polysaccharideg | Anticoagulant | Inhibition of thrombin and factor Xa | BRA | Farias et al., 2000 |
| Antifungal | Lyngbyabellin B/bacterium | Depsipeptidef | C. albicans inhibition | Undetermined | USA | Milligan et al., 2000 |
| Antifungal | Naamine D/sponge | Alkaloidf | C. neoformans inhibition | Nitric oxide inhibition | USA, NZ | Dunbar et al., 2000 |
| Antimalarial | Ascosalipyrrolidinone A/fungus | Polyketided | P. falciparum inhibition | p56lck tryrosine kinase inhibition | GER, SWI | Osterhage et al., 2000 |
| Antimalarial | Homofascaplysin A & fascaplysin/sponge | Sesterterpenee | P. falciparum inhibition | Undetermined | GER, SWZ | Kirsch et al., 2000 |
| Antimalarial | Manzamine A/sponge | Alkaloidf | In vivo P. berghei inhibition | Undetermined | SING, JPN, USA | Ang et al., 2000 |
| Antiplatelet | Eryloside F/sponge | Sterol glycosidee | Platelet aggregation inhibition | Thrombin receptor antagonist | USA, UK | Stead et al., 2000 |
| Antituberculosis | Axisonitrile-3/sponge | Sesquiterpenee | M. tuberculosis inhibition | Undetermined | GER | Konig et al., 2000 |
| Antituberculosis | Elisapterosin B/soft coral | Diterpenee | M. tuberculosis inhibition | Undetermined | USA | Rodriguez et al., 2000 |
| Antiviral | Cyclodidemniserinol trisulfate/ascidian | Polyketided | In vitro HIV infection inhibition | HIV-1 integrase inhibition | USA | Mitchell et al., 2000 |
| Antiviral | Dragmacidin F/sponge | Alkaloidf | In vitro HSV-1 and HIV-1 inhibition | Undetermined | ITA | Cutignano et al., 2000 |
| Antiviral | Lobohedleolide, 17-dimethylamino/soft coral | Diterpenee | In vitro HIV infection inhibition | Undetermined | USA | Rashid et al., 2000 |
| Antiviral | Mololipids/sponge | Alkaloidf | In vitro HIV-1 infection inhibition | Undetermined | USA | Ross et al., 2000 |
Kingdom Animalia: brittle star and cucumber (phylum Echinodermata), clam and mussel (phylum Mollusca), sponge (phylum Porifera), tunicate (phylum Chordata). Kingdom Fungi: fungus; Kingdom Plantae: alga; Kingdom Monera: bacterium (phylum Cyanobacteria).
MMOA is molecular mechanism of action.
AUS is Australia; BRA, Brazil; GER, Germany; ITA, Italy; JPN, Japan; NETH, The Netherlands; NZ, NewZealand; SING, Singapore; SWE, Sweden; SWZ, Switzerland; UK, United Kingdom.
Polyketides;
Terpene;
Nitrogen-containing compound;
Polysaccharide.
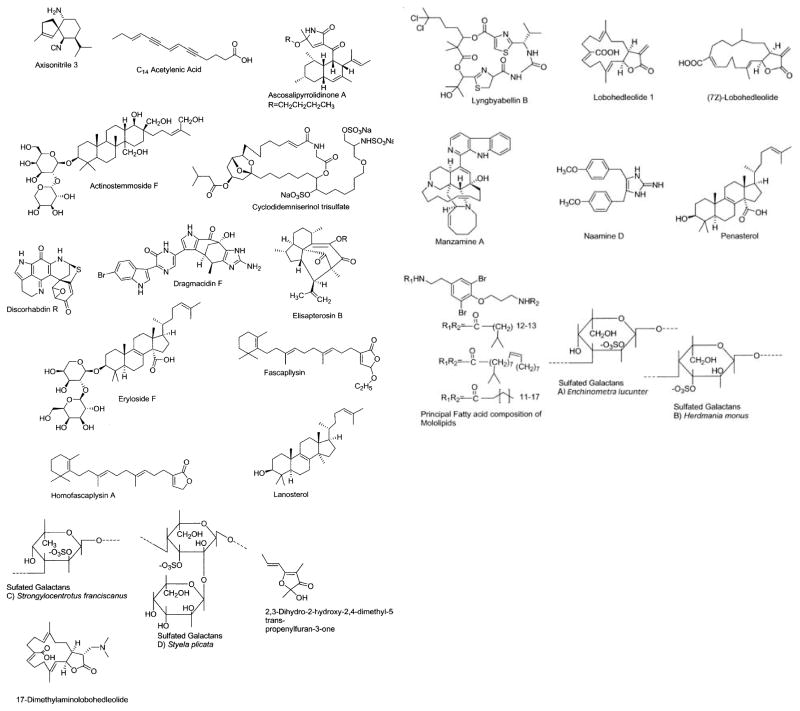
Figure 1.
Marine compounds with antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antituberculosis and antiviral activities.
Table 2.
Marine Compounds with Anti-inflammatory and Immunosuppressant Effects and Affecting the Cardiovascular and Nervous Systems
| Drug class | Compound/organisma | Chemistry | Pharmacological Activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|---|
| Anti-inflammatory | Cavernolide/coral | Terpenee | In vitro TNF-α, NO and PGE2 inhibition | sPLA2, iNOS and COX2 inhibition | ITA, SPA | Posadas et al., 2000 |
| Anti-inflammatory | Contignasterol/sponge | Sterole | In vivo allergen-induced plasma protein exhudation inhibition | Undetermined | AUS | Coulson et al. 2000 |
| Anti-inflammatory | Cyclolinteinone/sponge | Sesterterpenee | In vitro NO and PGE2 inhibition | NF-κB binding, iNOS and COX2 expression inhibition | ITA | D’Acquisto et al., 2000 |
| Anti-inflammatory | Oxepinamide A/fungus | Alkaloidf | In vivo neurogenic inflammation assay | Undetermined | GER, USA | Belofsky et al., 2000 |
| Anti-inflammatory | Sterol/alga | Sterol glycosidee | In vivo inflammation assay | Undetermined | EGYP | Awad 2000 |
| Cardiovascular | Docosahexaenoic acid/fish | Fatty acidd | In vivo vascular reactivity assays and in vitro biochemical assays | Undetermined | AUS | Mori et al., 2000 |
| Immunosuppressant | Theonellapeptolides/sponge | Peptidef | In vitro mixed lymphocyte reaction assay | Undetermined | JPN, IND | Roy et al., 2000 |
| Nervous system | Conantokin G, T/snail | Peptidef | In vivo Parkinson’s disease model | Alterations of striatal efferent neurons function | USA | Adams et al., 2000 |
| Nervous system | Conantokin-G/snail | Peptidef | In vivo block of dopamine-enhancing drug methamphetamine | NMDA receptor antagonism | USA | Bush et al., 2000 |
| Nervous system | Conantokin-G/snail | Peptidef | In vitro neuronal and oocyte whole-cell electrophysiology | Competitive antagonist of NR2B NMDA receptors | USA | Donevan et al., 2000 |
| Nervous system | Conantokin-G/snail | Peptidef | In vivo and in vitro neuroprotective assays | Decrease Ca2+ responses to NMDA | USA | Williams et al., 2000 |
| Nervous system | Conantokin-R/snail | Peptidef | In vitro NMDA receptor antagonist and in vivo anticonvulsant assays | NR2 NMDA receptor selectivity | PHIL, USA | White et al., 2000 |
| Nervous system | Conantokin-R/snail | Peptidef | In vitro binding assays, spectroscopy, and NMR | Disulfide loop not essential for receptor and cation binding | USA | Blandl et al., 2000 |
| Nervous system | C. marmoreus conotoxin/snail | Peptidef | In vitro and in vivo electrophysiology & binding assays | Undetermined | PHIL, USA | McIntosh et al., 2000a |
| Nervous system | α-Conotoxin MII/snail | Peptidef | In vitro nicotinic, receptor-transfected, oocyte electrophysiology | Blocks β-3 nicotinic receptor subunit | USA | McIntosh et al., 2000b |
| Nervous system | Halitoxins/bacterium | Alkaloidsf | In vitro neuronal electrophysiology and calcium imaging | Pore formation in biological membranes | UK | Scott et al., 2000 |
| Nervous system | Lembehyne A/sponge | Fatty acidd | In vitro neuritogenic assay | Actin polymerization and protein synthesis dependent | JPN, INDO | Aoki et al., 2000 |
| Nervous system | Stolonidiol/soft coral | Diterpenee | In vitro choline acetyltransferase activity assay | Undetermined | JPN | Yabe et al., 2000 |
Kingdom Animalia: coral (phyllum Cnidaria); snail (phylum Mollusca); sponge (phylum Porifera); seastar and sea cucumber (phylum Echinodermata); seal (phylum Chordata). Kingdom Plantae: dinoflagellate and alga. Kingdom Monera: bacterium (phylum Cyanobacteria).
MMOA is molecular mechanism of action.
AUS is Australia; EGYP, Egypt; GER, Germany; IND, India; INDO, Indonesia; ITA, Italy; JPN, Japan; PHIL, Phillipines; SPA, Spain; UK, United Kingdom.
Polyketides;
Terpenes;
Nitrogen-containing compounds.
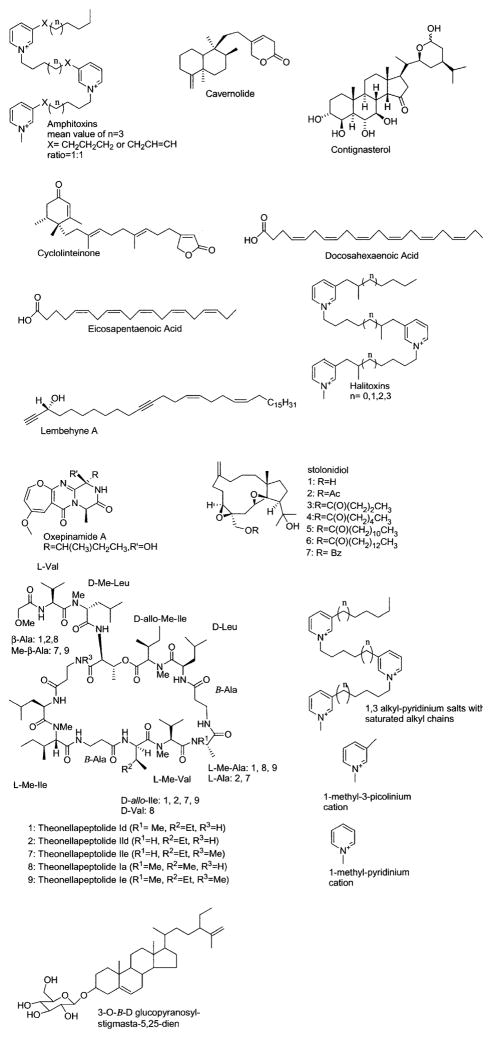
Figure 2.
Marine compounds with anti-inflammatory and immunosuppressant properties and effects on the cardiovascular and nervous systems.
Table 3.
Marine Compounds with Miscellaneous Mechanisms of Action
| Compound/organisma | Chemistry | Pharmacologic activity | MMOAb | Countryc | References |
|---|---|---|---|---|---|
| Adociasulfate 10/sponge | Sulfated triterpenee | Undetermined | Kinesin motor inhibition | USA | Blackburn et al., 2000 |
| Agelasine F/sponge | Alkaloidf | Antituberculosis activity | Undetermined | PHIL, USA | Mangalindan et al., 2000 |
| Anthraquinone/betaenone/fungus | Polyketided | Undetermined | Protein kinase inhibition | GER | Brauers et al., 2000 |
| B-5354c/bacterium | Alkaloidf | Undetermined | Sphingosine kinase inhibition | JPN | Kono et al., 2000 |
| Bisprasin/sponge | Alkaloidf | In vitro sketeletal muscle sarcoplasmic reticulum assay | Ryanodine receptor Ca2+ release induction | JPN | Suzuki et al., 2000 |
| Ceratospongamide/alga-sponge | Depsipeptidef | Undetermined | Secretory Phospholipase A2 expression inhibition | USA | Tan et al., 2000 |
| 5α-Cholest-7-en-3β-ol/starfish | Sterole | Antigenotoxic and mutagenic | Undetermined | S.KOR | Han et al., 2000 |
| Clavosines A & B/sponge | Polyketided | Undetermined | Protein phosphatase-1 inhibition | USA, CAN | McCready et al., 2000 |
| Dysidotronic acid/sponge | Diterpenee | Undetermined | Phospholipase A2 inhibition | ITA, FRA, SPA | Giannini et al., 2000 |
| Equistatin/sea anemone | Proteinf | In vitro inhibition of papain and cathepsin D | Characterization of cysteine proteinase inhibitory domains | SLOV, NETH | Strukelj et al., 2000 |
| Fascaplysin/sponge | Alkaloidf | In vitro cyclin-dependent kinase assays | Cyclin-dependent kinase 4 catalytic subunit inhibition | SWZ | Soni et al., 2000 |
| Hymenialdisine/sponge | Alkaloidf | In vitro and In vivo inhibition of protein phosphorylation | Cyclin-dependent kinase 5 inhibition | FRA, GER, UK, USA | Meijer et al., 2000 |
| Jasplakinolide/sponge | Peptidef | Apoptosis | Caspase-3-like protease dependent pathway | JPN, USA | Odaka et al., 2000 |
| Latrunculin A/sponge | Polyketided | Antiprion effect | Actin cytoskeleton inhibition | USA | Bailleul-Winslett et al., 2000 |
| Latrunculin-B/sponge | Polyketided | In vivo antiglaucoma assay | G-actin sequestration | ISR, USA | Peterson et al., 2000 |
| Microginins/bacterium | Peptidef | Undetermined | Zinc metalloproteases inhibition | JPN | Ishida et al., 2000 |
| Miraziridine A/sponge | Peptidef | Undetermined | Cathepsin B inhibition | JPN | Nakao et al., 2000a |
| Penarolide sulfates A1 & A2 sponge | Lipopeptidef | Undetermined | A-glucosidase inhibition | JPN, NETH | Nakao et al., 2000b |
| Phomopsidin/fungus | Polyketided | Undetermined | Microtubule assembly inhibition | JPN | Namikoshi et al., 2000 |
| Pyridinium alkaloids/sponge | Alkaloidf | Undetermined | Phospholipase A2 inhibition | ITA, FRA, SPA | De Marino et al., 2000 |
| Scalarane & homoscalarane/nudibranchs | Sesquiterpenese | Undetermined | Phospholipase A2 inhibition | ITA, FRA, SPA | Fontana et al., 2000 |
| Sphingosines/tunicate | Fatty acidd | Undetermined | Phospholipase A2 inhibition | FRA | Loukaci et al., 2000 |
| Stelliferins/sponge | Triterpenese | In vitro cytotoxicity and morphology assays | Undetermined | JPN | Oku et al., 2000 |
| Sterols/sponge | Sterolse | Undetermined | Interleukin 8 receptor antagonist | AUS | Leone et al., 2000 |
| Turbotoxins A & B/gastropod | Alkaloidf | Undetermined | Acetylcholinesterase inhibition | JPN | Kigoshi et al., 2000 |
Kingdom Animalia: anemones, corals and hydroids (phylum Cnidaria), mollusk (phylum Mollusca), sea cucumber (phylum Echinodermata), sponge (phylum Porifera), Kingdom Fungi: fungus. Kingdom Plantae: alga; Kingdom Monera: bacterium (phylum Cyanobacteria).
MMOA is molecular mechanism of action.
AUS is Australia; CAN, Canada; FRA, France; GER, Germany; ISR, Israel; ITA, Italy; JPN, Japan; NETH, The Netherlands; PHIL, Phillipines; S.KOR, South Korea; SLO, Slovenia; SPA, Spain; SWZ, Switzerland; UK, United Kingdom.
Polyketides.
Terpenes.
Nitrogen-containing compounds.
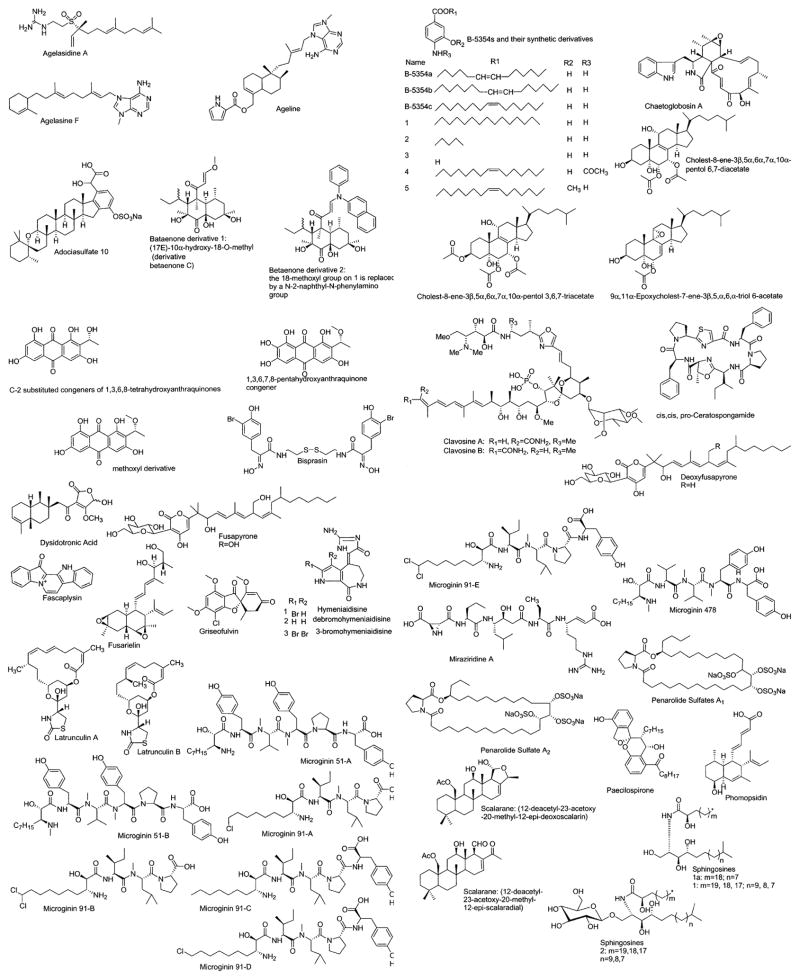
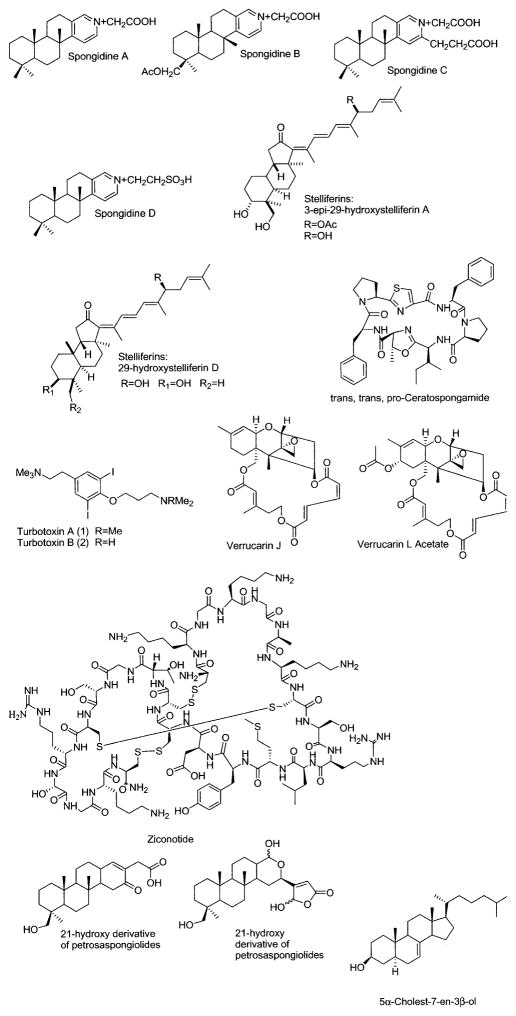
Figure 3.
Marine compounds with miscellaneous mechanisms of action.
Marine Compounds with Antibacterial, Anticoagulant, Antifungal, Antimalarial, Antiplatelet, Antiituberculosis, and Antiviral Activities
Table 1 summarizes the main findings of 17 papers that reported on the preclinical antibacterial, anticoagulant, antifungal, antimalarial, antiplatelet, antituberculosis, and antiviral pharmacology of the 35 marine natural products shown in Figure 1.
Antibacterial Compounds
During 2000 only 2 studies contributed to the antibacterial pharmacology of marine natural products. A novel C14 acetylenic acid with antibacterial activity was isolated from the marine sponge Oceanapia sp. (Matsunaga et al., 2000). This fatty acid is the first reported midchain acetylenic acid without a bromine atom that inhibited growth of the Gram-negative bacteria Escherichia coli and Pseudomonas aeruginosa and the Gram-positive bacteria Bacillus subtillis and Staphylococcus aureus. Discorhabdin R, a novel antibacterial pyrroloiminoquinone, was isolated from the southern Australian sponges Negombata sp. and an Antarctic Latrunculia sp. (Ford and Capon, 2000). Although discorhabdin R appeared to have been partially responsible for the antibacterial activity against both Gram-positive bacteria (Staphylococcus aureus and Micrococcus luteus) and Gram-negative bacteria (Serratia marcescens and Escherichia coli), the pertinent data were not reported. Furthermore, the authors reported neither a comparison with established clinically used antibiotics nor an investigation on the mechanism of action of either marine product.
Anticoagulant Compounds
Three papers were published during 2000 on the anticoagulant properties of marine polysaccharides. An investigation on the in vivo anticoagulant pharmacology of the sulfated polysaccharide fucoidan was completed during 2000 by Thorlacius et al. (2000). These researchers determined the effect of fucoidan on the function of P- and L-selectin, two members of the selectin family of adhesion molecules. The fact that fucoidan inhibited thrombus formation in arterioles and venules in vivo with no effect on P- and L-selectin function suggested that the anticoagulant effect of fucoidan was mainly responsible for its powerful antithrombotic property in vivo.
Matsubara et al. reported a novel sulfated proteoglycan isolated from the marine green alga Codium pugniformis collected in Japan, which helped extend current knowledge on the anticoagulants isolated from the genus Codium (Matsubara, 2000). Although the novel proteoglycan inhibited both the intrinsic and common pathways of coagulation, it showed a weaker anticoagulant activity than heparin and involved a mechanism of direct inhibition of thrombin as well as the potentiation of antithrombin III.
Farias et al. (2000) characterized a unique sulfated D-galactan from the red algae Botryocladia occidentalis. The algal sulfated D-galactan had potent anticoagulant activity, similar to that of unfractionated heparin, owing to enhanced inhibition of thrombin and factor Xa by antithrombin or heparin cofactor II. The presence of 2,3-di-O-sulfated galactose residues probably resulted in an “amplifying effect” of the anticoagulant activity of the algal sulfated galactans when compared to sulfated L-galactans from marine invertebrates, which have well-defined structures and were also studied in the authors’ experiments. The authors proposed that “sulfated galactans from B. occidentalis are natural candidate molecules for testing in experimental thrombosis.”
Antifungal Compounds
Two studies were published during 2000 on the antifungal properties of two novel marine natural products. Lyngbyabellin B, an antifungal depsipeptide from the marine cyanobacterium Lynbya majuscula, proved active against Candida albicans in a disk diffusion assay (Milligan et al., 2000). A novel imidazole alkaloid, naamine D, was isolated from the Red Sea sponge Leucetta cf. chagosensis along with 4 known alkaloids: namely, naamidine A, B, D, and G (Dunbar et al., 2000). Although no extensive studies on mechanism of action were completed, naamine D had a MIC of 6.25 μg/ml against Cryptococcus neoformans. Although a comparison with established antifungal agents was not included in these studies, thus precluding a comparative assessment of this compound’s antifungal potency, interestingly naamine D at 1 mM competitively inhibited murine macrophage-inducible nitric oxide synthase by 50%.
Antimalarial, Antiplatelet, and Antituberculosis Compounds
During 2000, 6 studies were reported in the area of antimalarial, antiplatelet, and antituberculosis pharmacology of structurally characterized marine natural products. Three compounds were shown to possess antimalarial activity. As a result of studies with fungal strains associated with marine algae, Osterhage et al. (2000) isolated the novel polyketide ascosalipyrrolidinone A from the obligate marine fungus Ascochyta salicorniae. Clearly significant is the fact that ascosalipyrrolidinone A was shown to have some antiplasmodial activity toward both Plasmodium falciparum strain K1, a strain resistant to chloroquine, and strain NF 54, a strain susceptible to standard antimalarials. In addition, the compound demonstrated limited antimicrobial activity and tyrosine kinase p56lck inhibition. Homofascaplysin A and fascaplysin, sesterterpenes isolated from the Fijian marine sponge Hyrtios cf. erecta, demonstrated potent activity in vitro against both chloroquine-susceptible (NF-54) and chloroquine-resistant (K-1) Plasmodium falciparum strains (IC50 = 14–24 ng/ml) (Kirsch et al., 2000). Their reduced cytotoxicity against muscle myoblast cells and mouse peritoneal macrophages suggests that these compounds might constitute potential leads for novel antimalarial compounds. Finally, manzamine A, a β-carboline alkaloid, was shown to inhibit the growth of the rodent malaria parasite Plasmodium berghei, not only in vitro but also in vivo (Ang et al., 2000). Remarkably, manzamine A caused reduction of parasitemia by either the oral or intraperitoneal route of administration, suggesting it is a potentially promising antimalarial agent.
Only a single paper reported on antiplatelet pharmacology of marine natural products during 2000. Eryloside F, a penasterol disaccharide isolated form the marine sponge Erylus formosus, demonstrated potent and relatively selective thrombin receptor antagonist activity (Stead et al., 2000). Eryloside F was identified upon completion of a study that involved screening over 50,000 compounds and natural product samples using a thrombin receptor antagonist high-throughput screen. Evaluation in a platelet aggregation assay revealed that eryloside F inhibited thrombin-induced human platelet aggregation in vitro in a concentration-dependent manner.
Two papers reported work on antituberculosis pharmacology with marine natural products. In a systematic study involving more than 30 selected marine compounds belonging to several structural classes and derived from both marine algae and invertebrates, antimycobaterial activities against either Mycobacterium tuberculosis or Mycobacterium avium were observed in approximately one third of the screened compounds (Konig et al., 2000). Of interest was the observation that molecules with an isonitrile group were the most active. In particular, axisonitrile-3, a compound isolated from the sponge Acanthella kletra, was shown to be extremely active against M. tuberculosis, with a concomitant low cytotoxicity, and constituting in the authors’ view, “a very good antimycobacterial lead compound for the first time.” Rodriguez and his collaborators (2000) isolated the novel diterpene elisapterosin B from the West Indian gorgonian Pseudopterogorgia elisabethae. Although no mechanistic studies were reported, the elisapterosin B demonstrated inhibitory activity against M. tuberculosis at a concentration of 12.5 μg/ml.
Antiviral Compounds
Interest in the antiviral pharmacology of marine natural products remained high during 2000 as evidenced by the 4 papers published, a number similar to those reported in our corresponding 1998 and 1999 reviews (Mayer and Lehmann, 2000; Mayer and Hamann, 2002). As a result of an ongoing program focused on the discovery of new nonsteroidal inhibitors of the human immunodeficiency virus (HIV)-1 integrase from marine invertebrates, Mitchell et al. (2000) reported on the isolation of cyclodidemniserinol trisulfate from the Palauan ascidian Didemnum guttatum. Although cyclodidemniserinol trisulfate inhibited the purified HIV-1 integrase (IC50 = 60 μg/ml), it also inhibited the topoisomerase enzyme of the Molluscum contagiosum virus (IC50 = 60 μg/ml) with a similar potency, thus demonstrating a limited selectivity for the HIV-1 integrase. Cutignano and collaborators (2000) reported on a new antiviral bromoindole alkaloid, dragmacidin F, isolated from the Mediterranean sponge Halicortex sp. In collaboration with French research groups, these investigators determined that dragmacidin F weakly inhibited herpes simplex virus (HSV)-1–infected cells from HSV-induced destruction (IC50 = 95.8 μM) and furthermore delayed syncytia formation by HIV-2 (IC50 = 0.91 μM). Bioassay-guided fractionation of aqueous extracts from the Phillipine soft coral Lobophytum sp. yielded 2 known cembranoid diterpenes, lobohedleolide and (7Z)-lobohedleolide, and a new compound, 17-dimethylaminolobohedleolide, with moderate HIV-inhibitory activity (IC50 = 9–10.2 μg/ml) in a cell-based in vitro anti-HIV assay (Rashid et al., 2000). Ross et al. (2000) reported on a new series of anti-HIV bromotyrosine-derived lipids, namely the mololipids, identified in an Hawaiian sponge of the order Verongida. Interestingly the mololipids appeared to be selectively but weakly active against HIV-1 (EC50 = 52.2 μM), with low cytotoxicity against human peripheral blood mononuclear cells, thus suggesting to the authors that this series had some potential “for future studies.”
Compounds with Anti-inflammatory and Immunosuppressant Effects and Affecting the Cardiovascular and Nervous Systems
Table 2 summarizes preclinical pharmacologic research completed on 20 marine chemicals shown in Figure 2 that were shown to affect the cardiovascular and nervous systems and to possess anti-inflammatory and immunosuppressant activities.
Anti-inflammatory Compounds
The anti-inflammatory pharmacology of carvernolide, contignasterol, cyclolinteinone, oxenamide A, and an algal sterol glycoside was reported during 2000, an increase of one compound over 1999 (Mayer and Hamann 2002). Posadas et al. (2000) reported on the mechanism of action of cavernolide, a novel C21 terpene lactone isolated from the sponge Fasciospongia cavernosa. Their results suggested that cavernolide’s potent inhibition of tumor necrosis factor-α, nitric oxide, and prostaglandin E2 in vitro was the result of both human synovial phospholipase A2 (IC50 = 8.8 μM) inhibition, and inhibition of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) gene expression in intact cells.
Coulson and O’Donnell (2000) extended the in vivo pharmacology of contignasterol, a highly oxygenated sterol isolated from the sponge Petrosia contignata that appears to be as potent as nedocromil in inhibiting allergen-induced bronchoconstriction in vivo. The study demonstrated that contignasterol dose-dependently inhibited plasma exudation in vivo in response to ovalbumin, indicating that this is a potential anti-inflammatory compound.
D’Acquisto et al. (2000) determined the effect of cyclolinteinone, a sesterterpene isolated from the sponge Cacospongia linteiformis, on iNOS synthase and COX-2 enzyme in vitro. Cyclolinteinone’s reported anti-inflammatory properties included inhibition of nuclear transcription factor-κB binding activity, a decrease of both iNOS and COX-2 expression, and the concomitant weak in vitro inhibition of both prostaglandin E2 (IC50 = 50 μM). and nitric oxide (IC50 = 50 μM).
Belofsky et al. (2000) reported on new oxepinamides and two fumiquinazolines, alkaloids isolated from cultures of the marine fungus Acremonium sp. Only oxepinamide A exhibited good in vivo anti-inflammatory activity in the resiniferatoxin-induced topical mouse ear edema assay, a test for neurogenic inflammation. Awad (2000) reported on a novel sterol glycoside from Ulva lactuca collected from Alexandrian shores in Egypt. The glycoside exhibited good in vivo anti-inflammatory activity in the phorbol-ester-induced topical mouse ear edema assay.
Immunosuppressant Compounds; Cardiovascular Pharmacology
Only 2 reports during 2000 contributed to the immunosuppressant and cardiovascular pharmacology of marine natural products. Roy et al. (2000) presented novel information on potential immunosuppresant activity of cyclic peptides isolated from the marine sponge Theonella swinhoei, which have some common structural features with the cyclosporins, agents currently used clinically as immunosuppressants. The tridecapeptide lactones theonellapeptolides Ia, Id, and IId were strongly immunosuppressive, possibly as a result of “their cytotoxic effect.” In a contribution to the cardiovascular pharmacology of marine-derived eicosapentaenoic and docosahexanoic acid, Mori et al. (2000) investigated the effect of these purified omega-3 fatty acids on vascular reactivity of the forearm circulation in hyperlipidemic and overweight men. The results of a double-blind, placebo-controlled trial involving 59 overweight, mildly hyperlipidemic men demonstrated that docosahexanenoic acid, but not eicosapentaenoic acid, enhanced the vasodilator mechanisms and constrictor responses in forearm circulation, thus contributing to selective blood-pressure-lowering effects. The authors proposed that these observations could be relevant to the food industry with respect to the possible inclusion of omega-3 fatty acids into foodstuffs and animal feeds, and “hence the human food chain.”
Nervous System Pharmacology
Reports on both central and autonomic nervous system pharmacology of marine natural products increased slightly over 1998 and 1999 (Mayer and Lehmann, 2000; Mayer and Hamann, 2002), with the 2000 studies involving the conantokins-G, T, and R, the marine C. marmoreus conotoxin and α-Conotoxin MII, and the halitoxins, lembehyne A and stolonidiol.
Several studies extended the pharmacology of the conantokins and conotoxins, a family of small peptide toxins derived from the venom of marine snails of the genus Conus that have been shown to bind to excitable tissue (Olivera, 1997). Adams et al. (2000) examined whether 2 peptides derived from the marine cone snail Conus geographus, and potent N-Methyl-D-asparate (NMDA) ionotropic glutamate receptor antagonists, conantokin-G and conantokin-T(G), would potentiate contralateral rotation induced by L-3,4-dihydroxyph-enylalanine (L-DOPA) in 6-hydroxydopamine-treated rats, an animal model used to search for antiparkinsonian compounds. The 2 conantokins potentiated the behavioral effects of L-DOPA, probably by alterations in the function of striatal efferent neurons, suggesting that further research with the conantokins may lead to useful adjuncts for the treatment of Parkinson’s disease.
Bush et al. (2000) reported that conantokin-G enhanced the behavioral effects of methamphetamine, a potent central nervous system stimulant that causes dopamine release, as well as attenuated tissue levels of 2 neuropeptides found in the striatum and substantia nigra: namely, neurotensin and dynorphin A. Donevan and McCabe (2000) contributed to the mechanism of action of conantokin G by demonstrating that it is a NR2B-selective competitive antagonist of the NMDA receptor. The unique subunit selectivity may explain its favorable in vivo profile compared to those of other nonselective NMDA antagonists. Williams et al. (2000) evaluated the in vivo and in vitro neuroprotective properties of conantokin-G. While in vivo conantokin-G reduced infarct volume and increased both neurologic recovery and electroencephalogram power scores when administered even 4 hours after occlusion of the middle cerebral artery, in vitro conantokin-G demonstrated neuroprotective properties against multiple forms of neuronal injury by blocking NMDA-induced calcium signaling. The authors concluded this extensive and detailed investigation by proposing that “conantokin-G may represent an excellent adjunct treatment with drug therapy targeting the penumbral tissue.”
Blandl et al. (2000) completed a study to determine the bioactivity and conformation properties of the 7-amino acid peptide conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. The study provided experimental evidence that the structural elements common to conantokin-R and other conantokins are the primary determinants for receptor function and cation binding or secondary structure stability. White et al. (2000) completed an extensive in vitro and in vivo characterization of conantokin-R. In in vitro studies conantokin-R showed selectivity for NR2 NMDA receptor subunits: NR2B ~ NR2A > NR2C ≫ NR2D, but no effect on AMPA and kainate ionotropic glutamate receptors. In a battery of in vivo seizure models, conantokin-R demonstrated potent anticonvulsant activity at doses devoid of behavioral toxicity. Thus, because conantokin-R could be used as a pharmacologic agent to differentiate between the anticonvulsant and toxic effects of NMDA antagonists, it might contribute to the ongoing search for novel subunit-selective NMDA antagonists to treat human epilepsy.
A new conotoxin was isolated and characterized from the venom of Conus marmoreus during 2000 (McIntosh et al., 2000a). The peptide sequence has a novel disulfide bond connectivity that appears highly divergent from all other known conotoxins, and it targets a yet undetermined antinociceptive receptor. In a short communication McIntosh and collaborators (2000b) extended the pharmacology of α-conotoxins, widely used to probe neuronal nicotinic receptors. The investigators demonstrated that α-conotoxin MII blocked α3β2β3 human nicotinic acetylcholine receptors expressed in Xenopus oocytes.
Three interesting papers appeared on the halitoxins, lembehyne A and stolonidiol, compounds that appear to affect nervous tissue in different ways. Analysis of structure and electrophysiologic actions of halitoxins, marine 1,3 alkyl-pyridinium salts isolated from the marine sponge Callyspongia ridleyi, were reported by Scott et al. (2000). Most notable is that because halitoxins form ion-permeable pores in biological and artificial membranes, allowing flux of monovalent (K+, Na+) and divalent (Ca2+) cations, they could be useful in a number of applications, including the development of novel cytotoxic molecules and possibly as agents for intracellular drug delivery. Aoki et al. (2000) reported on lembehyne A, a novel fatty acid derived from the sponge Haliclona sp., which induces neurite outgrowth. Although the molecular target of lembehyne A is unknown at this time, this is the first report that a linear polyacetylene can induce neurite outgrowth in vitro, a biological response that appears to be dependent on actin polymerization and de novo protein synthesis. A significant contribution to the search for choline acetyltransferase inducers as potential agents to improve cognitive function in persons with diseases exhibiting cholinergic deficits, such as Alzheimer’s disease, was reported by Yabe et al. (2000). The fact that a structure-activity relationship study with stolonidiol, a marine diterpenoid isolated from the soft coral Clavularia sp., demonstrated potent choline-acetyltransferase-inducible activity in neuronal cultures in vitro suggests that stolonidiol or some of its derivates may serve as leads in the discovery of more useful agents with potential benefit to the cholinergic nervous system.
Marine Compounds with Miscellaneous Mechanisms of Action
Table 3 lists 23 marine compounds with miscellaneous mechanisms of action, and their structures are shown in Figure 3. Interestingly, and in contrast with the chemicals included in Tables 1 and 2, this third group of marine compounds includes nitrogen-containing compounds (i.e. proteins, peptides), terpenes, and polyketides, but not polysaccharides.
For some of these marine chemicals—namely, bisprasin, equistatin, fascaplysin, hymenialdisine, jasplakinolide, and latrunculin-B—both pharmacologic activity and a molecular mechanism of action have been investigated. For agelasine F, 5α-cholest-7-en-3β-ol, and stelliferins, only the pharmacologic activity has been investigated so far, and little is known about their molecular mechanism of action. Finally, although adociasulfate 10, B-5354c, ceratospongamide, clavosines A and B, dysidotronic acid, microginins, miraziridine A, penarolide sulfates, phomopsidin, pyridinium alkaloids, scalarane, and homoscalarane, sphingosines, sterols, and turbotoxins have been explored at the molecular level, they have not been assigned to a particular pharmacologic class at this time.
Reviews on Marine Pharmacology
Several reviews covering selected aspects of marine pharmacology were published during 2000: structure-function studies of anticoagulant marine sulfated polysaccharides (Mulloy et al., 2000); heparinoid-active sulfated polysaccharides from marine algae as potential blood anticoagulant agents (Shanmugam and Mody, 2000); an evaluation of intrathecal ziconotide for the treatment of chronic pain (Jain, 2000); natural product leads for the chemotherapy of HIV infection (De Clercq, 2000); different approaches to the pharmaceutical application of marine lipids (Masson et al., 2000); and the chemistry of marine natural products (Faulkner, 2000).
Conclusions
Although during 2000 no new marine natural product was approved for patient care by the U.S. Food and Drug Administration, during 2000 preclinical pharmacologic research with marine chemicals continued to proceed at a very active pace, involving both natural product chemists and pharmacologists from 21 foreign countries and the United States.
Acknowledgments
This publication was made possible with support from the National Institutes of Health (R03 ES10138-01 to A.M.S.M.) and (1R01A136596, 5K02A101502 to M.T.H.) and UNDP/World Bank/WHO special Program for Research and Training in Tropical Diseases (TDR 990199 to M.T.H.) and Midwestern University. Its content is solely the responsibility of the authors and does not necessarily represent the official view of the NIEHS, NIH. Jennifer Allman and Shelley Fleming are gratefully acknowledged for their assistance with the preparation of figures. Excellent support for literature searches and article retrieval by library staff members as well as medical and pharmacy students of Midwestern University is most gratefully acknowledged. The authors specially thank Mrs. Victoria Sears and Ms. Mary Hall for their assistance in the preparation of this manuscript.
References
- Adams AC, Layer RT, McCabe RT, Keefe KA. Effects of conantokins on L-3,4-dihydroxyphenylalanine-induced behavior and immediate early gene expression. Eur J Pharmacol. 2000;404:303–313. doi: 10.1016/s0014-2999(00)00640-3. [DOI] [PubMed] [Google Scholar]
- Ang KKH, Holmes MJ, Higa T, Hamann MT, Kara UAK. In vivo antimalarial activity of the beta-carboline alkaloid manzamine A. Antimicrob Agents Chemother. 2000;44:1645–1649. doi: 10.1128/aac.44.6.1645-1649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Matsui K, Tanaka K, Satari R, Kobayashi M. Lembehyne a, a novel neuritogenic polyacetylene, from a marine sponge of Haliclona sp. Tetrahedron. 2000;56:9945–9948. [Google Scholar]
- Awad NE. Biologically active steroid from the green alga Ulva lactuca. Phytother Res. 2000;14:641–643. doi: 10.1002/1099-1573(200012)14:8<641::aid-ptr668>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Bailleul-Winslett PA, Newman GP, Wegrzyn RD, Chernoff YO. An antiprion effect of the anticytoskeletal drug Latrunculin A in yeast. Gene Expression. 2000;9:145–156. doi: 10.3727/000000001783992650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belofsky GN, Anguera M, Jensen PR, Fenical W, Kock M. Oxepinamides A-C and fumiquinazolines H–I: bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chem Eur J. 2000;6:1355–1360. doi: 10.1002/(sici)1521-3765(20000417)6:8<1355::aid-chem1355>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Blackburn CL, Faulkner DJ. Adociasulfate 10, a new merohexaprenoid sulfate from the sponge Haliclona (aka Adocia) sp. Tetrahedron. 2000;56:8429–8432. [Google Scholar]
- Blandl T, Warder SE, Prorok M, Castellino FJ. Structure-function relationships of the NMDA receptor antagonist peptide, conantokin-R. FEBS Lett. 2000;470:139–146. doi: 10.1016/s0014-5793(00)01309-0. [DOI] [PubMed] [Google Scholar]
- Brauers G, Edrada RA, Ebel R, Proksch P, Wray V, Berg A, Grafe U, Schachtele C, Totzke F, Finkenzeller G, Marme D, Kraus J, Munchbach M, Michel M, Bringmann G, Schaumann K. Anthraquinones and betaenone derivatives from the sponge-associated fungus Microsphaeropsis species: novel inhibitors of protein kinases. J Nat Prod. 2000;63:739–745. doi: 10.1021/np9905259. [DOI] [PubMed] [Google Scholar]
- Bush L, McCabe T, Hanson GR. Selective antagonism of nigral neuropeptide responses to methamphetamine by conantokin G, a naturally occurring conopeptide. Eur J Pharmacol. 2000;387:55–58. doi: 10.1016/s0014-2999(99)00806-7. [DOI] [PubMed] [Google Scholar]
- Coulson FR, O’Donnell SR. The effects of contignasterol (IZP-94,005) on allergen-induced plasma protein exudation in the tracheobronchial airways of sensitized guinea-pigs in vivo. Inflamm Res. 2000;49:123–127. doi: 10.1007/s000110050569. [DOI] [PubMed] [Google Scholar]
- Cutignano A, Bifulco G, Bruno I, Casapullo A, Gomez-Paloma L, Riccio R. Dragmacidin F: a new antiviral bromoindole alkaloid from the Mediterranean sponge Halicortex sp. Tetrahedron. 2000;56:3743–3748. [Google Scholar]
- D’Acquisto F, Lanzotti V, Carnuccio R. Cyclolinteinone, a sesterterpene from sponge Cacospongia linteiformis, prevents inducible nitric oxide synthase and inducible cyclooxygenase protein expression by blocking nuclear factor-kappaB activation in J774 macrophages. Biochem J. 2000;346(Pt 3):793–798. [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Current lead natural products for the chemotherapy of human immunodefiency virus (HIV) infection. Med Res Rev. 2000;20:323–349. doi: 10.1002/1098-1128(200009)20:5<323::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- De Marino S, lorizzi M, Zollo F, Debitus C, Menou JL, Ospina LF, Alcaraz MJ, Paya M. New pyridinium alkaloids from a marine sponge of the genus Spongia with human phospholipase A(2) inhibitor profile. J Nat Prod. 2000;63:322–326. doi: 10.1021/np990374+. [DOI] [PubMed] [Google Scholar]
- Donevan SD, McCabe RT. Conantokin G is an NR2B-selective competitive antagonist of N-methyl-D-aspartate receptors. Mol Pharmacol. 2000;58:614–623. doi: 10.1124/mol.58.3.614. [DOI] [PubMed] [Google Scholar]
- Dunbar DC, Rimoldi JM, Clark AM, Kelly M, Hamann MT. Anti-cryptococcal and nitric oxide synthase inhibitory imidazole alkaloids from the calcareous sponge Leucetta cf chagosensis. Tetrahedron. 2000;56:8795–8798. [Google Scholar]
- Farias WRL, Valente AP, Pereira MS, Mourao PAS. Structure and anticoagulant activity of sulfated galactans—isolation of a unique sulfated galactan from the red algae Botryocladia occidentalis and comparison of its anticoagulant action with that of sulfated galactans from invertebrates. J Biol Chem. 2000;275:29299–29307. doi: 10.1074/jbc.M002422200. [DOI] [PubMed] [Google Scholar]
- Faulkner DJ. Marine natural products. Nat Prod Rep. 2000;17:7–55. doi: 10.1039/a809395d. [DOI] [PubMed] [Google Scholar]
- Fontana A, Mollo E, Ortea J, Gavagnin M, Cimino G. Scalarane and homoscalarane compounds from the nudibranchs Glossodoris sedna and Glossodoris dalli: chemical and biological properties. J Nat Prod. 2000;63:527–530. doi: 10.1021/np990506z. [DOI] [PubMed] [Google Scholar]
- Ford J, Capon RJ. Discorhabdin R: a new antibacterial Pyrroloiminoquinone from two latrunculiid marine sponges, Latrunculia sp. and Negombata sp. J Nat Prod. 2000;63:1527–1528. doi: 10.1021/np000220q. [DOI] [PubMed] [Google Scholar]
- Giannini C, Debitus C, Posadas I, Paya M, D’Auria MV. Dysidotronic acid, a new and selective human phospholipase A(2) inhibitor from the sponge Dysidea sp. Tetrahedron Lett. 2000;41:3257–3260. [Google Scholar]
- Han YH, Ham JH, Lee NJ, Park CH, Shin YH, Lee DU. Antimutagenic activity of 5alpha-cholest-7-en-3beta-ol, a new component from the starfish Asterina pectinifera. Biol Pharm Bull. 2000;23:1247–1249. doi: 10.1248/bpb.23.1247. [DOI] [PubMed] [Google Scholar]
- Ishida K, Kato T, Murakami M, Watanabe M, Watanabe MF. Microginins, zinc metalloproteases inhibitors from the cyanobacterium Microcystis aeruginosa. Tetrahedron. 2000;56:8643–8656. [Google Scholar]
- Jain KK. An evaluation of intrathecal ziconotide for the treatment of chronic pain. Expert Opin Investig Drugs. 2000;9:2403–2410. doi: 10.1517/13543784.9.10.2403. [DOI] [PubMed] [Google Scholar]
- Khudyakova YV, Pivkin MV, Kuznetsova TA, Svetashev VI. Fungi in sediments of the sea of Japan and their biologically active metabolites. Microbiology. 2000;69:608–611. [PubMed] [Google Scholar]
- Klgoshi H, Kanematsu K, Yokota K, Uemura D. Turbotoxins A and B, novel diiodotyramine derivatives from the Japanese gastropod Turbo marmorata. Tetrahedron. 2000;56:9063–9070. [Google Scholar]
- Kirsch G, Kong GM, Wright AD, Kaminsky R. A new bioactive sesterterpene and antiplasmodial alkaloids from the marine sponge Hyrtios cf. erecta. J Nat Prod. 2000;63:825–829. doi: 10.1021/np990555b. [DOI] [PubMed] [Google Scholar]
- Konig GM, Wright AD, Franzblau SG. Assessment of antimycobacterial activity of a series of mainly marine derived natural products. Planta Med. 2000;66:337–342. doi: 10.1055/s-2000-8534. [DOI] [PubMed] [Google Scholar]
- Kono K, Tanaka M, Mizuno T, Kodama K, Ogita T, Kohama T. B-5354a, b and c, new sphingosine kinase inhibitors, produced by a marine bacterium; taxonomy, fermentation, isolation, physico-chemical properties and structure determination. J Antibiot (Tokyo) 2000;53:753–758. doi: 10.7164/antibiotics.53.753. [DOI] [PubMed] [Google Scholar]
- Leone PD, Redburn J, Hooper JNA, Quinn RJ. Polyoxygenated Dysidea sterols that inhibit the binding of [I125] IL-8 to the human recombinant IL-8 receptor type A. J Nat Prod. 2000;63:694–697. doi: 10.1021/np9904657. [DOI] [PubMed] [Google Scholar]
- Loukaci A, Bultel-Ponce V, Longeon A, Guyot M. New lipids from the tunicate Cystodytes cf. dellechiajei, as PLA2 inhibitors. J Nat Prod. 2000;63:799–802. doi: 10.1021/np990443k. [DOI] [PubMed] [Google Scholar]
- Mangalindan GC, Talaue MT, Cruz LJ, Franzblau SG, Adams LB, Richardson AD, Ireland CM, Concepcion GP. Agelasine F from a Philippine Agelas sp. sponge exhibits in vitro antituberculosis activity. Planta Med. 2000;66:364–365. doi: 10.1055/s-2000-8554. [DOI] [PubMed] [Google Scholar]
- Masson W, Loftsson T, Haraldsson GG. Marine lipids for prodrugs, soft compounds and other pharmaceutical applications. Pharmazie. 2000;55:172–177. [PubMed] [Google Scholar]
- Matsubara K. An anticoagulant proteogylcan from the marine green alga, Codium pungniformis. J Appl Phycol. 2000;12:9–14. [Google Scholar]
- Matsubara K, Hori K, Matsura Y, Miyazawa K. Purification and characterization of a fibrinolytic enzyme and identification of fibrinogen clotting enzyme in a marine green alga, Codium divaricatum. Comp Biochem Physiol B Biochem Mol Biol. 2000;125:137–143. doi: 10.1016/s0305-0491(99)00161-3. [DOI] [PubMed] [Google Scholar]
- Matsunaga S, Okada Y, Fusetani N, van Soest RWM. An antimicrobial C14 acetylenic acid from a marine sponge Oceanapia species. J Nat Prod. 2000;63:690–691. doi: 10.1021/np990577y. [DOI] [PubMed] [Google Scholar]
- Mayer AM, Gustafson KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer. 2003;105:291–299. doi: 10.1002/ijc.11080. [DOI] [PubMed] [Google Scholar]
- Mayer AMS, Hamann MT. Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities; affecting the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 2002;132:315–339. doi: 10.1016/s1532-0456(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist. 2000;42:62–69. [Google Scholar]
- Mayer AMS, Lehmann VKB. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Res. 2001;21:2489–2500. [PubMed] [Google Scholar]
- McCready TL, Islam BF, Schmitz FJ, Luu HA, Dawson JF, Holmes CF. Inhibition of protein phosphatase-1 by clavosines A and B: novel members of the calyculin family of toxins. J Biol Chem. 2000;275:4192–4198. doi: 10.1074/jbc.275.6.4192. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Corpuz GO, Layer RT, Garrett JE, Wagstaff JD, Bulaj G, Vyazovkina A, Yoshikami D, Cruz LJ, Olivera BM. Isolation and characterization of a novel Conus peptide with apparent antinociceptive activity. J Biol Chem. 2000a;275:32391–32397. doi: 10.1074/jbc.M003619200. [DOI] [PubMed] [Google Scholar]
- McIntosh JM, Gardner S, Luo SQ, Garrett JE, Yoshikami D. Conus peptides: novel probes for nicotinic acetylcholine receptor structure and function. Eur J Pharmacol. 2000b;393:205–208. doi: 10.1016/s0014-2999(99)00887-0. [DOI] [PubMed] [Google Scholar]
- Meijer L, Thunnissen AMWH, White AW, Garnier M, Nikolic M, Tsai LH, Walter J, Cleverley KE, Salinas PC, Wu YZ, Biernat J, Mandelkow EM, Kim SH, Pettit GR. Inhibition of cyclin-dependent kinases, GSK-3 beta and CK1 by hymenialdisine, a marine sponge constituent. Chem Biol. 2000;7:51–63. doi: 10.1016/s1074-5521(00)00063-6. [DOI] [PubMed] [Google Scholar]
- Milligan KE, Marquez BL, Williamson RT, Gerwick WH. Lyngbyabellin B, a toxic and antifungal secondary metabolite from the marine cyanobacterium Lyngbya majuscula. J Nat Prod. 2000;63:1440–1443. doi: 10.1021/np000133y. [DOI] [PubMed] [Google Scholar]
- Mitchell SS, Rhodes D, Bushman FD, Faulkner DJ. Cyclodidemniserinol trisulfate, a sulfated serinolipid from the Palauan ascidian Didemnum guttatum that inhibits HIV-1 integrase. Org Lett. 2000;2:1605–1607. doi: 10.1021/ol005866o. [DOI] [PubMed] [Google Scholar]
- Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Bellin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102:1264–1269. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- Mulloy B, Mourao PAS, Gray E. Structure/function studies of anticoagulant sulphated polysaccharides using NMR. J Biotechnol. 2000;77:123–135. doi: 10.1016/s0168-1656(99)00211-4. [DOI] [PubMed] [Google Scholar]
- Nakao Y, Fujita M, Warabi K, Matsunaga S, Fusetani N. Miraziridine A, a novel cysteine protease inhibitor from the marine sponge Theonella aff. mirabilis. J Am Chem Soc. 2000a;122:10462–10463. [Google Scholar]
- Nakao Y, Maki T, Matsunaga S, van Soest RWM, Fusetani N. Penarolide sulfates A(1) and A(2) new alpha-glucosidase inhibitors from a marine sponge Penares sp. Tetrahedron. 2000b;56:8977–8987. [Google Scholar]
- Namikoshi M, Kobayashi H, Yoshimoto T, Meguro S, Akano K. Isolation and characterization of bioactive metabolites from marine-derived filamentous fungi collected from tropical and sub-tropical coral reefs. Chem Pharm Bull (Tokyo) 2000;48:1452–1457. doi: 10.1248/cpb.48.1452. [DOI] [PubMed] [Google Scholar]
- Odaka C, Sanders ML, Crews P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin Diagn Lab Immunol. 2000;7:947–952. doi: 10.1128/cdli.7.6.947-952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oku N, Matsunaga S, Wada S, Watabe S, Fusetani N. New isomalabaricane triterpenes from the marine sponge Stelletta globostellata that induce morphological changes in rat fibroblasts. J Nat Prod. 2000;63:205–209. doi: 10.1021/np990333d. [DOI] [PubMed] [Google Scholar]
- Olivera BM. E.E. Just Lecture (1996) Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhage C, Kaminsky R, Konig GM, Wright AD. Ascosalipyrrolidinone A, an antimicrobial alkaloid, from the obligate marine fungus Ascochyta salicorniae. J Org Chem. 2000;65:6412–6417. doi: 10.1021/jo000307g. [DOI] [PubMed] [Google Scholar]
- Peterson JA, Tian BH, Geiger B, Kaufman PL. Effect of latrunculin-B on outflow facility in monkeys. Exp Eye Res. 2000;70:307–313. doi: 10.1006/exer.1999.0797. [DOI] [PubMed] [Google Scholar]
- Posadas I, Terencio MC, De Rosa S, Paya M. Cavernolide: a new inhibitor of human sPLA2 sharing unusual chemical features. Life Sci. 2000;67:3007–3014. doi: 10.1016/s0024-3205(00)00875-4. [DOI] [PubMed] [Google Scholar]
- Rashid MA, Gustafson KR, Boyd MR. HIV-inhibitory cembrane derivatives from a Philippines collection of the soft coral Lobophytum species. J Nat Prod. 2000;63:531–533. doi: 10.1021/np990372p. [DOI] [PubMed] [Google Scholar]
- Rodriguez AD, Ramirez C, Rodriguez II, Barnes CL. Novel terpenoids from the West Indian sea whip Pseudopterogorgia elisabethae (Bayer). Elisapterosins A and B: rearranged diterpenes possessing an unprecedented cagelike framework. J Org Chem. 2000;65:1390–1398. doi: 10.1021/jo9914869. [DOI] [PubMed] [Google Scholar]
- Ross SA, Weete JD, Schinazi RF, Wirtz SS, Thamish P, Scheuer PJ, Hamann MT. Mololipids, a new series of anti-HIV bromotyramine-derived compounds from a sponge of the order Verongida. J Nat Prod. 2000;63:501–503. doi: 10.1021/np980414u. [DOI] [PubMed] [Google Scholar]
- Roy MC, Ohtani II, Ichiba T, Tanaka J, Satari R, Higa T. New cyclic peptides from the Indonesian sponge Theonella swinhoei. Tetrahedron. 2000;56:9079–9092. [Google Scholar]
- Schmitz FJ, Bowden BF, Toth SI. Antitumor and cytotoxic compounds from marine organisms. In: Attaway DH, Zaborsky OR, editors. Marine Biotechnology. New York, N.Y: Plenum Press; 1993. pp. 197–308. [Google Scholar]
- Scott RH, Whyment AD, Foster A, Gordon KH, Milne BF, Jaspars M. Analysis of the structure and electrophysiological actions of halitoxins: 1,3 alkyl-pyridinium salts from Callyspongia ridleyi. J Membr Biol. 2000;176:119–131. doi: 10.1007/s00232001078. [DOI] [PubMed] [Google Scholar]
- Shanmugam M, Mody KH. Heparinoid-active sulphated polysaccharides from marine algae as potential blood anticoagulant agents [Review] Curr Sci. 2000;79:1672–1683. [Google Scholar]
- Soni R, Muller L, Furet P, Schoepfer J, Stephan C, Zumstein-Mecker S, Fretz H, Chaudhuri B. Inhibition of cyclin-dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem Biophys Res Commun. 2000;275:877–884. doi: 10.1006/bbrc.2000.3349. [DOI] [PubMed] [Google Scholar]
- Stead P, Hiscox S, Robinson PS, Pike NB, Sidebottom PJ, Roberts AD, Taylor NL, Wright AE, Pomponi SA, Langley D. Eryloside F, a novel penasterol disaccharide possessing potent thrombin receptor antagonist activity. Bioorg Med Chem Lett. 2000;10:661–664. doi: 10.1016/s0960-894x(00)00063-9. [DOI] [PubMed] [Google Scholar]
- Struket B, Lenarcic B, Gruden K, Pungercar J, Rogelj B, Turk V, Bosch D, Jongsma MA. Equistatin, a protease inhibitor from the sea anemone Actinia equina, is composed of three structural and functional domains. Biochem Biophys Res Commun. 2000;269:732–736. doi: 10.1006/bbrc.2000.2356. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Matsunaga K, Shin H, Tabudrav J, Shizuri Y, Ohizumi Y. Bisprasin, a novel Ca(2+) releaser with caffeine-like properties from a marine sponge, Dysidea spp. acts on Ca(2+)-induced Ca(2+) release channels of skeletal muscle sarcoplasmic reticulum. J Pharmacol Exp Ther. 2000;292:725–730. [PubMed] [Google Scholar]
- Tan LT, Williamson RT, Gerwick WH, Watts KS, Mc-Gough K, Jacobs R. Cis,cis- and trans,trans-ceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J Org Chem. 2000;65:419–425. doi: 10.1021/jo991165x. [DOI] [PubMed] [Google Scholar]
- Thorlacius H, Vollmar B, Seyfert UT, Vestweber D, Menger MD. The polysaccharide fucoidan inhibits microvascular thrombus formation independently from P- and L-selectin function in vivo. Eur J Clin Invest. 2000;30:804–810. doi: 10.1046/j.1365-2362.2000.00704.x. [DOI] [PubMed] [Google Scholar]
- White HS, McCabe RT, Armstrong H, Donevan SD, Cruz LJ, Abogadie FC, Torres J, Rivier JE, Paarmann I, Hollmann M, Olivera BM. In vitro and in vivo characterization of conantokin-R, a selective NMDA receptor antagonist isolated from the venom of the fish-hunting snail Conus radiatus. J Pharmacol Exp Ther. 2000;292:425–432. [PubMed] [Google Scholar]
- Williams AJ, Dave JR, Phillips JB, Lin Y, McCabe RT, Tortella FC. Neuroprotective efficacy and therapeutic window of the high-affinity N-methyl-D-aspartate antagonist conantokin-G: in vitro (primary cerebellar neurons) and in vivo (rat model of transient focal brain ischemia) studies. J Pharmacol Exp Ther. 2000;294:378–386. [PubMed] [Google Scholar]
- Yabe T, Yamada H, Shimomura M, Miyaoka H, Yamada Y. Induction of choline acetyltransferase activity in cholinergic neurons by stolonidiol: structure-activity relationship. J Nat Prod. 2000;63:433–435. doi: 10.1021/np990263a. [DOI] [PubMed] [Google Scholar]